Abstract
Objectives
To evaluate the risk of haematological malignancies in patients with psoriatic arthritis (PsA) overall, and in relation to treatment with tumour necrosis factor inhibitors (TNFi).
Methods
We identified that patients with PsA starting a first TNFi from the clinical rheumatology registers (CRR) in the five Nordic countries (n=10 621) and biologics-naïve PsA patients from (1) the CRR (n=18 705) and (2) the national patient registers (NPR, n=27 286, Sweden and Denmark) from 2006 through 2019. For Sweden and Denmark, general population comparators were matched 5:1 to PsA patients on birth year, year at start of follow-up and sex. By linkage to the national cancer registers in all countries, we collected information on haematological malignancies overall, and categorised into lymphoid or myeloid types. We estimated incidence rate ratios (IRRs) with 95% CIs using modified Poisson regression for TNFi-treated versus biologics-naïve PsA patients and versus the general population adjusted for age, sex, calendar period and country.
Results
During 59 827 person-years, 40 haematological malignancies occurred among TNFi-treated patients with PsA resulting in a pooled IRR of 0.96 (0.68–1.35) versus biologics-naïve PsA from CRR and an IRR of 0.84 (0.64–1.10) versus biologics-naïve PsA from NPR. The IRR of haematological malignancies in PsA overall versus general population comparators was 1.35 (1.17–1.55). The estimates were largely similar for lymphoid and myeloid malignancies.
Conclusions
Treatment with TNFi in patients with PsA was not associated with an increased incidence of haematological malignancies. Conversely, a moderately increased underlying risk was seen in patients with PsA compared with the general population.
Keywords: arthritis, psoriatic; tumor necrosis factor inhibitors; epidemiology; biological therapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
In psoriasis and psoriatic arthritis (PsA), there is limited and inconclusive evidence regarding potential associations with haematological malignancy, and a recently published meta-analysis observed a 1.5-fold increased risk of malignant lymphoma in patients with psoriasis, but no increased risk in PsA. Further, studies assessing the risk of myeloid malignancies in PsA are scarce and the impact of treatment with tumour necrosis factor inhibitors (TNFi) in PsA on the risk of haematologic malignancy is largely unknown.
WHAT THIS STUDY ADDS
In a five-nation collaborative cohort study of patients with PsA and >2 70 000 person-years of follow-up, the incidence of haematologic malignancies was similar among 10 621 TNFi-treated and 18 387 biologics-naïve patients. However, patients with PsA had a 35% increased relative risk compared with the general population, and this increase was seen for both lymphoid and myeloid malignancies.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Results showing no increased incidence of haematological malignancy in TNFi-treated compared with biological disease-modifying anti-rheumatic drug-naïve PsA patients, which is largely in line with findings from RA cohorts, provide reassurance to patients and physicians. However, the underlying increased risk of haematological malignancies observed in patients with PsA in general compared with matched comparators merits further investigation into potential explanatory factors such as accumulated inflammation, autoimmune mechanisms, genetics, immunosuppression, viral infections and lifestyle factors.
Introduction
The link between autoimmune conditions and haematological malignancy is complex.1 2 Several autoimmune diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and Sjogren’s syndrome have repeatedly been associated with increased risks of both lymphoid and myeloid malignancies as compared with the general population.1–8 In psoriasis and psoriatic arthritis (PsA), data on haematological malignancy are more limited and inconclusive.9–14 A recently published meta-analysis observed a 1.5-fold increased risk of malignant lymphoma in psoriasis, but found no association with PsA.15 Studies specifically assessing the risk of myeloid malignancies in PsA are scarce.
Although the pathological mechanisms behind an association between autoimmune diseases and haematological malignancy remains unclear, there are indications of a strong link between longstanding disease activity and severity, and lymphoma development in patients with RA and SLE.16–18 Other potential mechanisms are that immunosuppressive treatment may lead to especially myeloid malignancies, through loss of immunosurveillance, via mutagenic effects2 or through a shared susceptibility between the two disease entities.1 Notably, the factor(s) behind haematological malignancy in patients with autoimmune diseases may vary both by type of autoimmune disease and by type of haematological malignancies developed.
Today, treatment with biological disease-modifying anti-rheumatic drugs (bDMARDs), especially tumour necrosis factor inhibitor (TNFi) is a mainstay in several inflammatory diseases including PsA.19 Whereas, emerging evidence do not indicate an excess risk of malignant lymphoma following TNFi treatment in patients with RA, corresponding data for PsA are sparse and limited by low power.20–24
Overall, this highlights the importance of international collaboration to assemble large cohorts with sufficiently long follow-up time combined with data from national cancer registries of high quality to enable robust risk evaluations. We have previously used this Nordic collaborative effort to report on the risk of solid cancer in patients with PsA.25
In this population-based cohort study, we aimed to evaluate risks of haematological malignancy overall and of lymphoid and myeloid malignancies separately, in TNFi-treated versus bDMARD-naïve patients with PsA. Additionally, we investigated the underlying risk of haematological malignancy in PsA compared with the general population.
Patients and methods
Setting and data sources
In the Nordic countries, healthcare systems are tax funded, and individual level information on healthcare use is available from administrative registers. Using personal identification numbers assigned to all residents, data from different registers can be linked to each other within each country and used for research. For this study, patients with PsA were identified in the five Nordic clinical rheumatology registries (CRR): DANBIO (Denmark), ICEBIO (Iceland), NOR-DMARD (Norway), ROB-FIN (Finland) and SRQ (Sweden).26 Within those registries, the diagnosis of PsA is made, registered and continuously re-evaluated by a rheumatologist. For Denmark and Sweden, we additionally selected bDMARD-naïve patients with PsA from the national patient registers (NPR) that hold data on hospital-based inpatient and outpatient specialist contacts.27–29
To further put the incidence in context, we assembled general population comparator groups from the Danish and Swedish population registers. In each country, information on malignancy was assembled from the national cancer registers (NCR) containing information on date and type of cancer according to the International Statistical Classification of Diseases (ICD-10). Reporting of incident cancers to the NCR is mandatory and thus the coverage is high.30–34
We obtained information on date of emigration and death from the population and cause of death registers in each country.
In a sensitivity analysis, the bDMARD-naïve PsA groups from the Danish and Swedish NPRs were linked to the national prescribed drug registers (PDR) in both countries.35 36
The study period was from 2006 to the end of 2016 for Denmark, end of 2017 for Finland and Iceland and the end of 2019 for Norway and Sweden. All registers have been described in detail elsewhere.25 26
Study population, exposure and follow-up
For this study, up to five groups were identified in each country:
TNFi-treated group with PsA from CRR (all countries)
All individuals≥18 years of age diagnosed with PsA in each CRR and starting their first TNFi during the study period. Follow-up started at the start date of their first ever TNFi (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab). Patients who had received treatment with a non-TNFi bDMARD prior to starting their first TNFi were excluded.
bDMARD-naïve group with PsA from CRR (all countries except Iceland)
All individuals≥18 years of age diagnosed with PsA in each CRR and bDMARD-naïve at start of follow-up (ie, time of first visit registered in the CRR).
bDMARD-naïve group with PsA from NPR (Denmark and Sweden)
All individuals≥18 years of age registered with≥2 ICD-10 codes for PsA (M07.0-3 or L40.5) from a department of rheumatology or internal medicine in the NPR of Sweden and Denmark. Patients were followed from the date of the second registered PsA diagnosis.
For both bDMARD-naïve groups (CRR and NPR), patients were excluded at start of any bDMARD during follow-up and, if starting a TNFi, instead followed up in the TNFi-treated group.
All patients with PsA from CRR and NPR (Denmark and Sweden)
To estimate the underlying incidence and risk of haematological malignancy in PsA compared with the general population, data from all patients with PsA in each of the three above PsA groups was combined into one, and a unique record was kept for each patient. Patients were followed from the date of first registered CRR visit, date of second visit with a diagnosis of PsA registered in NPR, or date of starting a first TNFi, whichever occurred first.
General population comparator groups (Denmark and Sweden)
Two comparator groups were defined:
First, for all TNFi-treated patients (group 1 above), up to five individuals matched on year of birth, calendar year of start of follow-up, sex and county of residence (for Swedish data), were randomly selected from the general population.
Second, for all patients with PsA (group 4 above) up to five individuals were randomly selected from the general population using the same matching factors as above. For both comparator groups, individuals had to be free from PsA at the diagnosis of their index PsA patient.
Individuals from both comparator groups were followed from the date when their index PsA counterpart started follow-up. All study individuals were followed until the date of any haematological malignancy, emigration, death, bDMARD treatment start (for the bDMARD-naïve group) or end of the study period in the respective countries, whichever occurred first.
Outcome
By linkage to NCR in each country, we identified the first incident haematological malignancy for each study participant according to ICD-10 codes, online supplemental table S1.37 38 We divided haematological malignancies into lymphoid and myeloid types, see online supplemental table S1 for details. All study participants were allowed to have a previous history of solid and/or skin cancer.
rmdopen-2022-002776supp001.pdf (208.4KB, pdf)
Covariates
For all groups, we obtained information on sex, age (years) and calendar period of start of follow-up (2006–2010 and ≥2011). For descriptive purposes, we collected information on comorbidities from the NPR. For TNFi-treated and bDMARD-naïve patients with PsA identified from the CRR, we also obtained information on Disease Activity Score 28 with C reactive protein (DAS28-CRP), Health Assessment Questionnaire and concomitant use of methotrexate and oral steroid use (yes/no) at start of follow-up, see online supplemental table S1 for definitions.
Statistics
Country-specific analyses
For each group in each country, we estimated number of person-years, and events, and the crude incidence rates (IRs) with 95% CI of haematological malignancy overall per 100 000 person-years. We applied Cox regression using attained age as time scale to calculate crude, as well as sex and calendar period-adjusted hazard ratios with 95% CI of haematological malignancy for TNFi-treated compared with bDMARD-naïve patients with PsA from CRR. If the number of events within a country and for the individual groups involved in the comparison was <3, incidences and HRs were not presented.
For Denmark and Sweden, country-specific Cox regression were performed comparing TNFi-treated versus bDMARD-naïve PsA groups from NPR and versus matched population comparator groups, respectively. Additionally, HR of haematological malignancy was estimated for all Danish and Swedish patients with PsA (group 4) compared with their matched general population comparators.
Pooled analyses
We constructed Lexis matrices39 for each country splitting time on attained age (18–55 years, 56–65 years, 66–70 years and >70 years) and calendar time (2006–2010 and ≥2011), stratified by sex, and calculated number of events and person-years in each of the categories obtained by the Lexis function. We performed a modified Poisson regression with this pooled data to estimate incidence rate ratio (IRR) with 95% CI for TNFi-treated compared with bDMARD-naïve patients from CRR. This model included country as a covariate and a robust SE.
For Sweden and Denmark, the same and with similar adjustments (except for age now categorised in 10-year groups) was used to compare TNFi-treated patients with bDMARD-naïve patients from NPR and with matched general population comparators, respectively, as well as comparing the entire PsA population (group 4) with their matched general population comparator group. For all pooled analyses, we estimated IRR of haematological malignancy overall and divided into lymphoid or myeloid malignancies.
Drug-specific incidence
Using two different exposure models, we estimated crude IRs of haematological malignancy for each of the TNFi agents used for the treatment of PsA during the study period: adalimumab, certolizumab pegol, etanercept, golimumab and infliximab. Originators and their biosimilars were considered the same drug. Patients could contribute with person-years to all the TNFi they had received treatment with. The exposure models were defined as:
‘Ever treated’, patients were followed from the first start of any TNFi until end of follow-up. In this way, stopping the TNFi treatment and/or starting another TNFi was ignored in the follow-up of the individual for that treatment.
‘Most recent drug’, follow-up began at the first start of any TNFi and ended at the date of starting another bDMARD or end of follow-up.
In both models, one patient could thereby contribute with exposed person-time to ≥1 TNFi.
Sensitivity analyses
To minimise the risk of protopathic bias, we excluded all person-years and all events from the first year of follow-up in all groups under study. Second, for Denmark and Sweden, we excluded all patients ever diagnosed with RA in the NPR prior to start of follow-up; and censored patients if, during follow-up, they had an RA diagnosis recorded in the NPR to account for the possible misclassification of diagnosis between RA and PsA.
Third, to evaluate if baseline disease activity was associated with the outcome and hence should be adjusted for in the analyses, we performed Cox models including all patients, regardless of TNFi exposure status, who had data available on various disease activity parameters to see if these were associated with haematological malignancies. We performed both crude and sex-adjusted and calendar period-adjusted analyses. Further, we explored if disease activity at baseline differed in patients with PsA that did (vs did not) develop a haematological malignancy in TNFi-treated and bDMARD-naïve patients, respectively.
Fourth, to increase the comparability between TNFi-treated and bDMARD-naïve patients, we created an alternative bDMARD-naïve PsA group: by linkage to the PDR we identified a subset of NPR-bDMARD-naïve PsA patients treated with conventional synthetic (cs) DMARDs who either started or switched from one to another csDMARD between the date of second PsA diagnosis in NPR and end of follow-up. Those fulfilling these criteria, were followed from the date of their first redeemed csDMARD prescription in the period, thus corresponding to a new user active comparator study design.
Finally, to explore if confounding by comorbidities explained any potential association between exposure and outcome, we performed a sensitivity analysis on Danish and Swedish data with Cox models that included adjustment for the following comorbidities: chronic obstructive pulmonary disease, cardiovascular disease, diabetes mellitus and arterial hypertension.
All Cox and Poisson analyses only incorporated variables that had no missingness, and hence all patients were included in the regression models. Specifically, age, sex and calendar period were adjusted for in some way in all regression models. Data analyses were performed in R, V.3.4.0 and in SAS, V.9.4.
Results
In total, 10 621 TNFi-treated patients with PsA were identified, contributing a total of 59 827 person-years of follow-up. The baseline characteristics for TNFi-treated and bDMARD-naïve patients are presented in tables 1 and 2 divided by country. For TNFi-treated patients, the median age and sex distributions as well as DAS28-CRP (median ranging from 3.5 to 4.4) were comparable between the countries. Within each country, the sex distribution of TNFi-treated patients was roughly similar to bDMARD-naïve patients from the CRR, but in most countries, TNFi-treated patients were slightly younger and had higher DAS28-CRP at start of follow-up, tables 1 and 2.
Table 1.
Characteristics of TNFi-treated patients with psoriatic arthritis at start of follow-up, grouped by country
| Sweden 2006–2019 |
Denmark 2006–2016 |
Finland 2006–2017 |
Norway 2006–2019 |
Iceland 2006–2017 |
|
| All, n | 6505 | 2429 | 474 | 879 | 334 |
| Male, n (%) | 3158 (48.6) | 1098 (45.2) | 256 (54.0) | 425 (48.7) | 136 (40.7) |
| Age (years), mean (SD) | 49.4 (12.9) | 47.2 (12.3) | 48.3 (11.6) | 47.5 (12.2) | 48.4 (13.2) |
| BMI (kg/m2) | N/A | 27.7 (24.2 to 30.9) | 27.8 (25.1 to 31.2) | N/A | 29.6 (26.1 to 33.1) |
| BMI missing (n, %) | 6505 (100) | 2036 (83.8) | 86 (18.1) | 879 (100) | 206 (61.7) |
| Smoking status, n (%) | |||||
| Current | 295 (4.5) | 296 (12.2) | 12 (2.5) | 176 (20.0) | 33 (9.9) |
| Previous | 833 (12.8) | 240 (9.9) | N/A | 271 (30.8) | 59 (17.7) |
| Never | 915 (14.1) | 487 (20.0) | 88 (18.6) | 299 (34.0) | 98 (29.3) |
| Missing | 4462 (68.6) | 1406 (57.9) | 374 (78.9) | 133 (15.1) | 144 (43.1) |
| Disease-related characteristics at start of first TNFi | |||||
| DAS28-CRP (0–10) | 4.1 (3.3–4.8) | 4.0 (3.0–5.0) | 3.7 (2.5–4.7) | 3.5 (2.7–4.3) | 4.4 (3.9–5) |
| Missing n (%) | 2613 (40.2) | 745 (30.7) | 128 (27.0) | 110 (12.5) | 189 (56.6) |
| CRP (mg/L) | 5 (2–13) | 6 (2–15) | 7 (3–15) | 5 (2–11) | N/A |
| Swollen joint count (0–28) | 2 (0–5) | 2 (0–4) | 1 (0–4) | 1 (0–3) | 4 (2–6) |
| Tender joint count (0–28) | 4 (2–8) | 5 (2–11) | 1 (0–4) | 2 (1–6) | 5 (2–8) |
| HAQ Score (0–3) | 0.9 (0.5–1.3) | 1 (0.6–1.5) | 0.8 (0.4–1.2) | 0.6 (0.3–0.9) | 1.2 (0.6–1.6) |
| Missing n (%) | 2415 (37.1) | 1174 (48.3) | 100 (21.1) | 51 (5.8) | 192 (57.5) |
| First TNFi, n (%) | |||||
| Adalimumab | 1996 (30.7) | 939 (38.7) | 188 (39.7) | 147 (16.7) | 25 (7.5) |
| Certolizumab pegol | 248 (3.8) | 215 (8.9) | 15 (3.2) | 184 (20.9) | 1 (0.3) |
| Etanercept | 2697 (41.5) | 527 (21.7) | 148 (31.2) | 302 (34.4) | 65 (19.5) |
| Golimumab | 450 (6.9) | 217 (8.9) | 38 (7.9) | 142 (16.2) | 42 (12.6) |
| Infliximab | 1114 (17.1) | 531 (21.9) | 85 (17.9) | 104 (11.8) | 199 (59.6) |
| Other antirheumatic treatment, n (%) at start of first TNFi | |||||
| Methotrexate, n (%) | 2726 (41.9) | 1233 (50.8) | 269 (56.8) | 520 (59.2) | 147 (44) |
| Oral steroid, n (%) | 909 (14.0) | 506 (20.8) | 128 (26.7) | 300 (34.1) | 12 (3.6) |
| Calendar year at start of first TNFi, n (%) | |||||
| 2006–2010 | 1536 (23.6) | 1034 (42.6) | 165 (34.8) | 258 (29.4) | 73 (21.9) |
| 2011—end of follow-up | 4969 (76.4) | 1395 (57.4) | 309 (65.2) | 621 (70.6) | 261 (78.1) |
| Comorbidities and extra-articular manifestations, as registered in national patient registers up to 10 years prior to start of first TNFi, n (%) | |||||
| Cardiovascular disease | 360 (5.5) | 167 (6.9) | 33 (6.9) | N/A | N/A |
| COPD | 97 (1.5) | 39 (1.6) | 2 (0.4) | N/A | N/A |
| Diabetes mellitus | 430 (6.6) | 104 (4.3) | 36 (7.6) | N/A | N/A |
| Hypertension | 964 (14.8) | 231 (9.5) | 73 (15.4) | N/A | N/A |
| Inflammatory bowel disease | 120 (1.8) | 44 (1.8) | 14 (2.9) | N/A | N/A |
| Uveitis | 170 (2.6) | 32 (1.3) | 14 (2.9) | N/A | N/A |
| Urethritis | 38 (0.6) | ≤3 | 0 (0) | N/A | N/A |
| Hospitalisations, n, mean (range) | 1 (0–2) | 4 (1–7) | 2 (1–4) | N/A | N/A |
| Hip and/or knee replacement any time prior to start of first TNFi | 286 (4.4) | 72 (3.0) | 48 (10.1) | N/A | N/A |
| Mean time of follow-up in years (SD) | 5.7 (3.8) | 5.1 (2.9) | 6.1 (3.3) | 6.8 (3.5) | 4.9 (3.4) |
| Median time of follow-up in years (Q1–Q3) | 5.2 (2.3–8.7) | 5.1 (2.7–7.4) | 6.2 (3.2–9.0) | 6.6 (3.9–9.6) | 4.2 (1.9–7.2) |
Values are median and IQR if not stated otherwise. Patients who shifted from biological disease-modifying anti-rheumatic drug (bDMARD)-naïve to TNFi-treated appear in both TNFi-treated and bDMARD-naïve groups because all switchers have two baseline records.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; DAS28, Disease Activity Score in 28 joints; HAQ, Health Assessment Questionnaire; N/A, not available; Q1, percentile 25; Q3, percentile 75; TNFi, tumour necrosis factor inhibitor; VAS, visual analogue scale.
Table 2.
Characteristics of bDMARD-naïve patients with psoriatic arthritis from clinical rheumatology registers and national patient registers
| bDMARD-naïve patients with PsA from the clinical rheumatology registers (comparator group 1) n=18 387 | bDMARD-naïve patients with PsA from the national patient registers (comparator group 2)* n=27 286 | |||||
| Sweden 2006–2019 |
Denmark 2006–2016 |
Finland 2006–2017 |
Norway 2006–2019 |
Sweden 2006–2019 |
Denmark 2006–2016 |
|
| All, n | 9560 | 6779 | 1621 | 427 | 21 629 | 5657 |
| Male, n (%) | 4708 (49.2) | 2948 (43.5) | 791 (48.8) | 197 (46.2) | 9944 (46.0) | 2420 (42.8) |
| Age (years), mean (SD) | 53.4 (14.1) | 51.1 (13.7) | 50.5 (13.8) | 49.7 (12.7) | 53.6 (14.8) | 52.1 (13.9) |
| BMI (kg/m2) | N/A | 26.9 (24.1–30.5) | 27.7 (24.6–31.5) | N/A | N/A | N/A |
| BMI missing (n, %) | 9560 (100) | 5774 (85.2) | 116 (7.2) | 427 (100) | N/A | N/A |
| Smoking status, n (%) | ||||||
| Current | 425 (4.4) | 834 (12.3) | 252 (15.5) | 122 (28.6) | N/A | N/A |
| Previous | 1301 (13.6) | 746 (11.0) | N/A | 155 (36.3) | N/A | N/A |
| Never | 1279 (13.4) | 1512 (22.3) | 959 (59.2) | 142 (33.3) | N/A | N/A |
| Missing | 6555 (68.6) | 3687 (54.4) | 410 (25.3) | 8 (1.9) | N/A | N/A |
| Disease-related characteristics at start of follow-up | ||||||
| DAS28-CRP (0–10) | 3.3 (2.4–4.2) | 3.0 (2.2–4.1) | 2.5 (1.7–3.5) | 3.9 (3.1–4.5) | N/A | N/A |
| Missing, n (%) | 3416 (35.7) | 2168 (32.0) | 654 (40.3) | 28 (6.6) | ||
| CRP (mg/L) | 5 (2–10) | 5 (2–10) | 4 (2–8) | 5 (3–15) | N/A | N/A |
| Swollen joint count (0–28) | 1 (0–3) | 1 (0–2) | 0 (0–1) | 2 (1–4) | N/A | N/A |
| Tender joint count (0–28) | 2 (0–5) | 2 (0–6) | 0 (0–1) | 4 (1–8) | N/A | N/A |
| HAQ Score (0–3) | 0.6 (0.3–1.0) | 0.6 (0.1–1.0) | 0.8 (0.2–1.1) | 0.5 (0.3–0.9) | N/A | N/A |
| Missing, n (%) | 3079 (32.2) | 1998 (29.5) | 480 (29.6) | 18 (4.2) | ||
| Other antirheumatic treatment, n (%) at start of follow-up | ||||||
| Methotrexate at start of follow-up, n (%) | 5241 (54.8) | 3257 (48.0) | 703 (43.4) | 362 (84.8) | N/A | N/A |
| Oral steroid at start of follow-up, n (%) | 1280 (13.4) | 855 (12.6) | 219 (13.5) | 296 (69.3) | N/A | N/A |
| Calendar year of start of follow-up | ||||||
| 2006–2010 | 1732 (18.1) | 1498 (22.1) | 128 (7.9) | 350 (82.0) | 7552 (34.9) | 2244 (39.7) |
| 2011—end of follow-up | 7828 (81.9) | 5281 (77.9) | 1493 (92.1) | 77 (18.0) | 14 077 (65.0) | 3413 (60.3) |
| Comorbidities and extra-articular manifestations as registered up to 10 years prior to start of follow-up, n (%) | ||||||
| Cardiovascular disease | 729 (7.6) | 525 (7.7) | 117 (7.2) | N/A | 2117 (9.8) | 539 (9.5) |
| COPD | 207 (2.2) | 164 (2.4) | 23 (1.4) | N/A | 573 (2.6) | 184 (3.3) |
| Diabetes mellitus | 687 (7.2) | 370 (5.4) | 132 (8.1) | N/A | 1674 (7.7) | 378 (6.7) |
| Hypertension | 1694 (17.7) | 760 (11.2) | 263 (16.2) | N/A | 3946 (18.2) | 764 (13.5) |
| Inflammatory bowel disease | 121 (1.3) | 96 (1.4) | 21 (1.3) | N/A | 392 (1.8) | 105 (1.9) |
| Uveitis | 175 (1.8) | 51 (0.7) | 31 (1.9) | N/A | 387 (1.8) | 52 (0.9) |
| Urethritis | 54 (0.6) | 7 (0.1) | 0 (0) | N/A | 91 (0.4) | ≤3 |
| Hospitalisations, n, mean (range) | 1 (0–2) | 3 (1–6) | 1 (0–3) | N/A | 1 (0–2) | 3 (1–6) |
| Hip and/or knee replacement any time prior to start of follow-up | 462 (4.8) | 235 (3.5) | 120 (7.4) | N/A | 1007 (4.7) | 142 (2.5) |
| Number of patients censored during time of follow-up due to start of TNFi | 6513 (68.1) | 1268 (18.7) | 177 (10.9) | 31 (7.3) | 4953 (22.9) | 811 (14.3) |
| Mean time of follow-up in years (SD) | 4.1 (3.2) | 3.2 (2.4) | 3.0 (2.2) | 2.4 (1.9) | 5.6 (4.1) | 4.2 (3.0) |
| Median time of follow-up in years (Q1–Q3) | 3.5 (1.1–6.5) | 2.7 (1.4–2.9) | 2.5 (1.2–4.7) | 2.0 (0.8–3.4) | 5.1 ((1.9–8.8) | 3.8 (1.4–6.5) |
*For Finland, Norway and Iceland, this bDMARD-naïve comparator cohort was not available. Values are median and IQR except where stated otherwise. Patients who shifted from bDMARD-naïve to TNFi-treated appear in both TNFi treated and bDMARD-naïve groups, because all switchers have two baseline records.
bDMARD, biological disease-modifying anti-rheumatic drug; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; DAS28, Disease Activity Score in 28 joints; HAQ, Health Assessment Questionnaire; N/A, not available; TNFi, tumour necrosis factor inhibitor; VAS, visual analogue scale.
TNFi-treated versus bDMARD-naïve patients
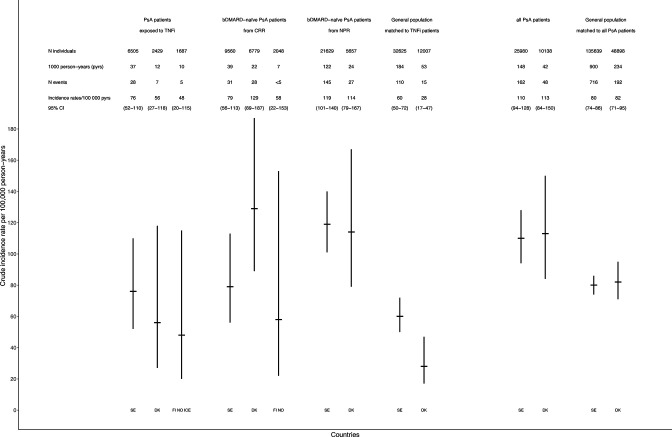
We identified 40 cases of haematological malignancies among TNFi-treated patients amounting to crude IRs (per 100 000 person-years) ranging from 48 (Finland, Iceland and Norway combined) to 76 (Sweden). Corresponding IR range in bDMARD-naïve patients from CRRs were 48 (Finland, Iceland and Norway combined) to 129 (Denmark) and for bDMARD-naïve patients from the NPR, 114 (Denmark) to 119 (Sweden), figure 1. Since <5 events were observed in TNFi-treated and bDMARD-naïve groups in Finland, Iceland and Norway, the pooled crude incidence is presented for these 3 countries.
Figure 1.
Occurrence and crude incidence rates of haematological malignancy per Nordic country in TNFi-treated and bDMARD-naïve patients with psoriatic arthritis and matched general population comparators, and for patients with PsA overall compared with matched general population comparators. General population TNFi was matched by sex, year of birth and calendar period to the TNFi-exposed PsA patients. And for SE also by area of residence. General population PsA was matched by sex, year of birth and calendar period to all PsA patients. And in SE also by area of residence. Data from FI, NO, ICE are presented together due to few cancer events per country. bDMARDs, biological disease-modifying anti-rheumatic drugs; CRR, clinical rheumatology register; DK, Denmark; event, first ever haematological cancer, that is, previous other malignancies are allowed; FI, Finland; ICE, Iceland; NO, Norway; NPR, national patient register (only available in Sweden and Denmark); PsA, psoriatic arthritis; SE, Sweden; TNFi, tumour necrosis factor inhibitor.
The age, sex and calendar period-adjusted HR for any haematological malignancy in TNFi-treated versus bDMARD-naïve PsA patients from CRR was 1.33 (0.79–2.22) in Sweden and 0.54 (0.23–1.29) in Denmark. Excluding the first year of follow-up resulted in largely similar point estimates, table 3.
Table 3.
HR of haematological malignancy in TNFi-treated versus bDMARD-naïve patients and general population comparators, respectively, for Sweden and Denmark
| HR (95% CI)* of haematological malignancy in TNFi-treated versus bDMARD-naïve PsA from the clinical rheumatology register (comparator group 1) | HR (95% CI)* of haematological malignancy in TNFi-treated versus bDMARD-naïve PsA from the national patient register (comparator group 2) |
HR (95% CI)* of haematological malignancy in TNFi-treated PsA versus the general population group 1† | HR (95% CI)* of haematological malignancy in PsA overall versus the general population group 2‡ |
|||||
| Sweden | Denmark | Sweden | Denmark | Sweden | Denmark | Sweden | Denmark | |
| Subjects, n | 6505 vs 9560 |
2429 vs 6779 |
6505 vs 21 629 |
2429 vs 5657 |
6505 vs 32 625 |
2429 vs 12 007 |
25 980 vs 135 639 |
10 204 vs 48 898 |
| Person-years | 36 950 vs 39 058 |
12 426 vs 21 693 |
36 950 vs 121 663 | 12 426 vs 23 595 |
36 950 vs 184 252 |
12 426 vs 53 334 |
147 713 vs 900 016 |
42 379 vs 234 151 |
| Haematological cancer overall, n | 28 vs 31 | 7 vs 28 | 28 vs 145 | 7 vs 27 | 28 vs 110 | 7 vs 15 | 162 vs 716 | 48 vs 192 |
| HR (95% CI) | ||||||||
| Crude | 1.33 (0.79 to 2.22) |
0.59 (0.25 to 1.36) |
0.89 (0.59 to 1.33) |
0.72 (0.31 to 1.69) |
1.25 (0.83 to 1.89) |
2.28 (0.89 to 5.86) |
1.30 (1.10 to 1.55) |
1.37 (0.99 to 1.90) |
| Adjusted for sex, age and calendar time | 1.33 (0.79 to 2.22) |
0.54 (0.23 to 1.29) |
0.90 (0.60 to 1.35) |
0.71 (0.30 to 1.67) |
1.25 (0.83 to 1.89) |
2.28 (0.89 to 5.86) |
1.30 (1.09 to 1.54) |
1.37 (0.99 to 1.90) |
| Adjusted for sex, age and calendar time and excluding first year of follow-up | 1.19 (0.68 to 2.08) | 0.56 (0.22 to 1.44) | 0.93 (0.59 to 1.47) | 0.78 (0.31 to 1.98) | 1.23 (0.78 to 1.94) | 1.96 (0.75 to 5.17) |
1.19 (0.99 to 1.44) | 1.24 (0.86 to 1.79) |
Bold values = statistically significant with 95% confidence intervals.
*Multivariate Cox regression with HR with 95% CI adjusted for sex and calendar period (2006–2010, 2011-end of follow-up) with attained age as time scale.
†General population group 1: Each TNFi treated patients with PsA are matched 1:5 on age, sex and calendar period to five comparators from the general population. For Sweden also matched to area of residence. The follow-up begins at the start of TNFi for the patients with PsA and at the same date for their general population comparators.
‡This PsA group includes all patients with PsA identified in the national patient register (NPR) and/or in clinical rheumatology register (CRR) irrespective of treatment. General population group 2: Each of the patients with PsA are matched 1:5 on age, sex, calendar period to the five comparators from the general population. For Sweden also matched to area of residence. Start of follow-up for the patients with PsA is whichever comes first of a first visit with PsA diagnosis in NPR, the inclusion date in CRR with a diagnosis of PsA or initiating a first TNFi according to CRR. Start of follow-up for the gen pop comparator was at the same date as start of follow-up of their index PsA patient.
N/A, Not available; PsA, psoriatic arthritis; TNFi, tumour necrosis factor inhibitor.
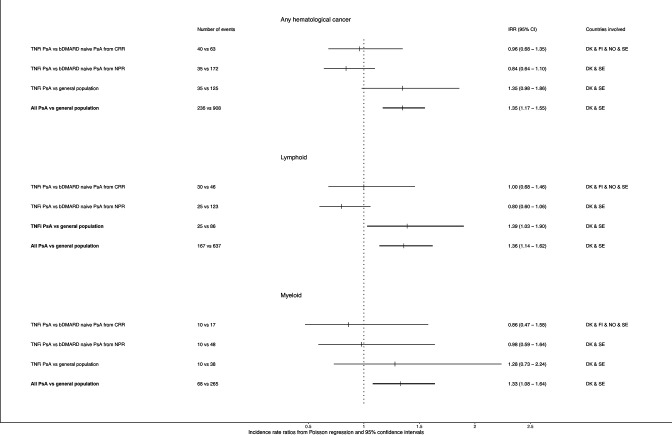
Pooling data from all five countries resulted in an IRR of 0.96 (0.68–1.35) of haematological malignancy in TNF-treated versus CRR-bDMARD-naïve patients, figure 2. Excluding everyone with an RA diagnosis gave largely similar pooled IRR (0.90; 0.61–1.32), online supplemental figure S1.
Figure 2.
Pooled incidence rate ratios (IRRs)1 of haematological malignancy overall and by lymphoid and myeloid types, in first ever TNFi-treated versus bDMARD-naïve patients with PsA and versus general population comparators 1IRR and 95% CI with modified Poisson regression adjusted for age (categories 18–55, 56–65, 66–70, >70 years), sex, calendar period (2006–2010, 2011-end of follow-up) and country. One event in the bDMARD-naïve PsA group from the national patient registers and one event from the general comparator group 1 (ie, the compactors to the TNFi group), as well as six events in the general comparator group 2 (ie, the comparators to the all PsA group) had ICD10 code C96.9 in the cancer registry defined as ‘other and unspecified tumours in hematopoietic and lymphoid tissue’. These are not included among the lymphoid or the myeloid malignancies For exact ICD-10 codes, see online supplemental table S1). bDMARDs, biological disease-modifying anti-rheumatic drugs; CRR, clinical rheumatology registers; DK, Denmark; FI, Finland; NO, Norway; NPR, national patient registers; PsA, psoriatic arthritis; SE, Sweden; TNFi, tumour necrosis factor inhibitor.
TNFi-treated patients had HRs of 0.89 (0.59–1.33) and 0.71 (0.30–1.67) compared with bDMARD-naïve PsA patients from the NPR in Sweden and Denmark, respectively (table 3). The pooled IRR for this comparison was 0.84 (0.64–1.10), figure 2. Excluding the first year of follow-up did not substantially change the estimates (table 3), whereas excluding patients with an ever diagnosis of RA slightly decreased the pooled IRR (0.75, 95% CI 0.52 to 1.07, online supplemental figure S1).
Using an alternative bDMARD-naïve comparator group resulted in slightly decreased risk estimates, online supplemental table S2. Risk estimates for lymphoid and myeloid haematological malignancies were largely similar to estimates for haematological malignancies overall, figure 2.
In the Danish and Swedish data respectively, baseline DAS28-CRP was not statistically associated with haematological, lymphoid or myeloid malignancies. When pooling the two country-specific estimates, DAS28-CRP (as a continuous variable) was neither associated with haematological malignancy; HR 0.99 (95% CI 0.82 to 1.20), see online supplemental table S3. Additionally, there were no clear differences between the baseline disease activity and functional status parameters in TNFi-treated and CCR-bDMARD-naïve patients who developed a haematological malignancy (vs not). However, no formal hypothesis testing was performed, online supplemental table S4. Finally, adjusting for baseline presence of comorbidities at baseline did not alter the results (online supplemental table S5).
Haematological malignancy in TNFi-treated patients versus the general population
Point estimates for haematological malignancy in TNFi-treated patients from Denmark and Sweden versus their population comparators were increased to 2.28 (0.89–5.86) and 1.25 (0.83–1.89), respectively, and similar when excluding the first year of follow-up, table 3. Pooling these data resulted in IRRs of 1.35 (0.98–1.86), 1.39 (1.03–1.90) and 1.28 (0.73–2.24) for overall, lymphoid and myeloid haematological malignancies, respectively, figure 2. Excluding RA attenuated the estimates, online supplemental figure S1.
Haematological malignancy in patients with PsA versus the general population
Compared with the age and sex matched comparators from the general population, the HR was 1.37 (0.99–1.90) in Denmark and 1.30 (1.09–1.54) in Sweden for PsA patients overall (group 4). Pooling the data resulted in an IRR of 1.35 (1.17–1.55). Increased IRRs were seen for both lymphoid and myeloid malignancies, figure 2.
Haematological malignancy by TNFi agent
Overall, the different TNFi agents had comparable crude IRs for haematological malignancy applying both ever and most recent exposure models, and when taking the wide CIs into account. The IRs per 100 000 person-years ranged from 49.8 (golimumab) to 77.8 (adalimumab), online supplemental table S6.
Discussion
In this large observational study based on clinical data from five countries, we observed no increased incidence rates of haematological malignancy overall, nor of lymphoid or myeloid malignancies in TNFi-treated patients with PsA compared with bDMARD-naïve patients. By contrast, we observed a 35% increased incidence of haematological malignancy in patients with PsA overall as compared with the general population.
Some clinical trials have indicated an increased occurrence of lymphoma following TNFi exposure in PsA, based on few events and given the design of a clinical trial short time of follow-up,20 22 40 whereas most of the although few observational studies on the topic have not reported any increased risks in TNFi-treated versus naïve PsA patients.15 24 A previous Swedish–Danish study (from parts of our group and based in part on the data used in the present study) observed an HR of 1.0 (0.4–2.7) of lymphoma (n=5),24 thus almost identical with the HR of 1.01 (0.69–1.48) in the present study based on 30 lymphoid malignancies. Thus, the current study represents the largest study to date evaluating haematological malignancy following TNFi treatment in patients with PsA. Our risk estimates remained largely similar regardless of the data source used to identify bDMARD-naïve patients, as well as when using stricter comparator group definitions. Further, to our knowledge, the present study is the first to investigate the incidence of myeloid malignancy following TNFi treatment in PsA; and importantly, found no increased risks compared with bDMARD-naïve patients.
By contrast, we found a moderately increased risk of haematological malignancy, both of lymphoid and myeloid types, in PsA patients overall compared with the general population. Data on underlying risk of haematological malignancy in PsA are inconclusive, where some studies have shown increased point estimates12–14 and others have not.9–11 15 Some studies have been hampered by low precision.13 14 The British cohort study by Hagberg et al12 observed a statistically significant increased rate in PsA overall versus a non-PsA cohort (IRR, 1.52; 1.10–2.10). However, and interestingly, the risk among patients with PsA treated versus non-treated with DMARD/biologics was about threefold increased. This indirectly suggests an effect of disease severity and/or treatment on the risk. For RA, a link between disease activity and lymphoma development is well described.16 A similar association has not been shown for PsA. Notably, another study instead observed a significantly increased risk of lymphoma (HR=1.7) with use of csDMARD (but not with TNFi) in patients with PsA versus the general population.11 Thus, it may be that disease activity rather than type of treatment is of importance. In the present study, we set out to evaluate the impact of disease activity (DAS28-CRP) at start of follow-up among patients later developing haematological malignancy (vs not). Unfortunately, the high proportion of missing information on disease activity in our data made it difficult to draw any firm conclusions.
Overall, our findings are not entirely in line with a recent meta-analysis by Vaengebjerg et al showing a 1.5-fold elevated risk of lymphoma in patients with psoriasis, but no increased risk in PsA.15 Using different PsA definitions in the studies included in the meta-analysis as compared with ours might explain these discrepancies. Previous studies have observed increased risks of cutaneous T-cell lymphoma in patients with severe psoriasis.15 Of note, this association may potentially be a misclassification, that is, early cutaneous lymphomas being misdiagnosed as cutaneous psoriasis. Alternatively, it may be that an overall increased risk of lymphoma in psoriasis or in PsA is explained by persistent immune activation or by longstanding inflammation leading to lymphoma development. Finally, we also assessed incidence by type of TNFi agent and found no signals of substantial differences.
The main strengths of our study include a large contemporary cohort, prospectively collected data and relatively long follow-up time (mean 5.7 years for TNFi treated). This, and the possibility to pool data from all Nordic countries, enabled us to assess risks not only of haematological malignancy overall but also of lymphoid and myeloid types separately. Because the diagnosis of PsA in all CRR is made by a rheumatologist and because of the complete coverage of the national cancer registers in the five countries, both exposure and outcome misclassification risk was minimised. Additionally, the PsA diagnosis in the NPR-based bDMARD-naïve group was based on ≥2 registered visits in NPR at a rheumatology or internal medicine department, thereby further minimising any risk of exposure misclassification. Additionally, the risk estimates were similar irrespective of what bDMARD-naïve PsA group was compared with. This shows the robustness of our results, as does the possibility to compare with an alternative active comparator (csDMARD treated) group. Further, access to matched general population comparators allowed estimations of the underlying risk of haematological malignancy in the entire PsA population.
Study limitations include missing data on smoking, disease activity, and other lifestyle risk factors. Further, we were unable to extract data on accumulated disease activity during the time of follow-up, and at the same time, we considered baseline disease activity in terms of DAS28-CRP an insufficient parameter, and thus abstained from adjusting for this in our models. This decision was indirectly backed by the sensitivity analysis showing no differences in baseline disease activity parameters between those that did or did not develop a haematological malignancy. Additionally, pooling Danish and Swedish data together, DAS28-CRP at baseline was not associated with future risk of haematological cancer neither overall, nor by subtype. Additionally, the high proportion of missing data on smoking is indeed a limitation. However, we were able to adjust for comorbidities including chronic obstructive pulmonary disease as a proxy of smoking; and the results remained nearly similar to those of the main analysis. Also, the use of a register based PsA diagnosis from the NPR could potentially include patients with other chronic inflammatory arthritides such as RA that carries an inherited increased lymphoma risk. This would overestimate the lymphoma risk in patients with PsA compared with the general population. However, excluding patients ever diagnosed with RA only attenuated the risk estimate slightly. Lastly, we did not have the statistical power to investigate any potential association between non-TNFi biologics and haematological malignancies; and nor to investigate potential associations between TNFi use and specific types of lymphoid malignancies.
To conclude, TNFi treatment did not increase the risk of haematological malignancy overall, nor of lymphoid and myeloid types, in patients with PsA. However, there were signals of a moderately increased risk in patients with PsA overall as compared with the general population. The findings contribute important clinical information for patients and physicians.
Acknowledgments
We thank the Nordic clinical rheumatology registers for allowing us to use their clinical data as well as the hospital departments of rheumatology and the private rheumatologic clinics of all countries for their contribution to these registers. We also would like to acknowledge the NordForsk and FOREUM and especially the patient representatives of the NordForsk collaboration for their valuable contribution to this study. This work was presented as an oral presentation (OP0257) at EULAR 2022.
Footnotes
RLC and JA contributed equally.
LD and KH contributed equally.
Contributors: KH, LD, RLC and JA designed the study. BD and RLC were responsible for the data management and performed the statistical analyses. KH and RLC drafted the first version of the manuscript. All authors contributed significantly in improving and optimising the design of the study and the manuscript. All authors signed off on the final manuscript. KH and RLC are the guarantors of the work.
Funding: This work was supported by NordForsk and FOREUM.
Competing interests: JA: Consultant and grant research support from: AbbVie, AstraZeneca, BMS, Eli Lilly, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi and UCB. PI: Speakers bureau: AbbVie, Eli Lilly and Pfizer; Consultant of: AbbVie, Eli Lilly, Pfizer, Roche and Vifor Pharma; Grant/research support from: Pfizer. BG: Speakers bureau and consultant of Novartis, not related to this work. TJL: Speakers bureau: AbbVie and Pfizer Advisory board: Galapagos, Vifor Pharma, Eli Lilly, Pfizer. BM: Grant/research support from: Novartis, not related to this work. LD: Speakers bureau: Eli Lilly, Galderma and Janssen; Grant/research support from: BMS not related to this work. BD is partly employed by the ARTIS/Swedish Biologics Register.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Due to GDPR regulations, all the data used for this study are kept within each country on encrypted servers, and are not available for the public.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Ethics Review Board in Stockholm, Sweden (2015/1844-31/2), Finland (73/13/03/00/14.), Norway (2017/2041, 2011/1339) and Iceland (VSNb2017010049/03.01). For Denmark, the Data Protection Committee approved the study but no ethical approval was required according to national legislation on registry research and data protection.
References
- 1.Baecklund E, Smedby KE, Sutton L-A, et al. Lymphoma development in patients with autoimmune and inflammatory disorders – what are the driving forces? Semin Cancer Biol 2014;24:61–70. 10.1016/j.semcancer.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 2.Boddu PC, Zeidan AM. Myeloid disorders after autoimmune disease. Best Pract Res Clin Haematol 2019;32:74–88. 10.1016/j.beha.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smedby KE, Askling J, Mariette X, et al. Autoimmune and inflammatory disorders and risk of malignant lymphomas - an update. J Intern Med 2008;264:514–27. 10.1111/j.1365-2796.2008.02029.x [DOI] [PubMed] [Google Scholar]
- 4.Simon TA, Thompson A, Gandhi KK, et al. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther 2015;17:212. 10.1186/s13075-015-0728-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein A, Polliack A, Gafter‐Gvili A. Rheumatoid arthritis and lymphoma: incidence, pathogenesis, biology, and outcome. Hematol Oncol 2018;36:733–9. 10.1002/hon.2525 [DOI] [PubMed] [Google Scholar]
- 6.Kristinsson SY, Björkholm M, Hultcrantz M, et al. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol 2011;29:2897–903. 10.1200/JCO.2011.34.8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komrokji RS, Kulasekararaj A, Al Ali NH, et al. Autoimmune diseases and myelodysplastic syndromes. Am J Hematol 2016;91:E280–3. 10.1002/ajh.24333 [DOI] [PubMed] [Google Scholar]
- 8.Wilson AB, Neogi T, Prout M, et al. Relative risk of myelodysplastic syndromes in patients with autoimmune disorders in the general practice research database. Cancer Epidemiol 2014;38:544–9. 10.1016/j.canep.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 9.Gross RL, Schwartzman-Morris JS, Krathen M, et al. A comparison of the malignancy incidence among patients with psoriatic arthritis and patients with rheumatoid arthritis in a large US cohort. Arthritis Rheumatol 2014;66:1472–81. 10.1002/art.38385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohekar S, Tom BDM, Hassa A, et al. Prevalence of malignancy in psoriatic arthritis. Arthritis Rheum 2008;58:82–7. 10.1002/art.23185 [DOI] [PubMed] [Google Scholar]
- 11.Hellgren K, Smedby KE, Backlin C, et al. Ankylosing spondylitis, psoriatic arthritis, and risk of malignant lymphoma: a cohort study based on nationwide prospectively recorded data from Sweden. Arthritis Rheumatol 2014;66:1282–90. 10.1002/art.38339 [DOI] [PubMed] [Google Scholar]
- 12.Hagberg KW, Li L, Peng M, et al. Rates of cancers and opportunistic infections in patients with psoriatic arthritis compared with patients without psoriatic arthritis. J Clin Rheumatol 2016;22:241–7. 10.1097/RHU.0000000000000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilton KM, Crowson CS, Matteson EL. Malignancy incidence in patients with psoriatic arthritis: a comparison cohort-based incidence study. Clin Rheumatol 2016;35:2603–7. 10.1007/s10067-016-3396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polachek A, Muntyanu A, Lee K-A, et al. Malignancy in psoriatic disease: results from prospective longitudinal cohorts. Semin Arthritis Rheum 2021;51:144–9. 10.1016/j.semarthrit.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 15.Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol 2020;156:421–9. 10.1001/jamadermatol.2020.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006;54:692–701. 10.1002/art.21675 [DOI] [PubMed] [Google Scholar]
- 17.Bernatsky S, Ramsey-Goldman R, Joseph L, et al. Lymphoma risk in systemic lupus: effects of disease activity versus treatment. Ann Rheum Dis 2014;73:138–42. 10.1136/annrheumdis-2012-202099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellgren K, Smedby KE, Feltelius N, et al. Do rheumatoid arthritis and lymphoma share risk factors?: a comparison of lymphoma and cancer risks before and after diagnosis of rheumatoid arthritis. Arthritis Rheum 2010;62:1252–8. 10.1002/art.27402 [DOI] [PubMed] [Google Scholar]
- 19.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700.1–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foeldvari I, Constantin T, Vojinović J, et al. Etanercept treatment for extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis, or psoriatic arthritis: 6-year efficacy and safety data from an open-label trial. Arthritis Res Ther 2019;21:125. 10.1186/s13075-019-1916-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burmester GR, Gordon KB, Rosenbaum JT, et al. Long-Term safety of adalimumab in 29,967 adult patients from global clinical trials across multiple indications: an updated analysis. Adv Ther 2020;37:364–80. 10.1007/s12325-019-01145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis JR, Mariette X, Gaujoux-Viala C, et al. Long-Term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn's disease: a pooled analysis of 11 317 patients across clinical trials. RMD Open 2019;5:e000942. 10.1136/rmdopen-2019-000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo X, Deng C, Fei Y, et al. Malignancy development risk in psoriatic arthritis patients undergoing treatment: a systematic review and meta-analysis. Semin Arthritis Rheum 2019;48:626–31. 10.1016/j.semarthrit.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 24.Hellgren K, Dreyer L, Arkema EV, et al. Cancer risk in patients with spondyloarthritis treated with TNF inhibitors: a collaborative study from the ARTIS and DANBIO registers. Ann Rheum Dis 2017;76:105–11. 10.1136/annrheumdis-2016-209270 [DOI] [PubMed] [Google Scholar]
- 25.Hellgren K, Ballegaard C, Delcoigne B, et al. Risk of solid cancers overall and by subtypes in patients with psoriatic arthritis treated with TNF inhibitors - a Nordic cohort study. Rheumatology 2021;60:3656–68. 10.1093/rheumatology/keaa828 [DOI] [PubMed] [Google Scholar]
- 26.Chatzidionysiou K, Hetland ML, Frisell T, et al. Opportunities and challenges for real-world studies on chronic inflammatory joint diseases through data enrichment and collaboration between national registers: the Nordic example. RMD Open 2018;4:e000655. 10.1136/rmdopen-2018-000655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldenlind K, Eriksson JK, Grewin B, et al. Validation of the rheumatoid arthritis diagnosis in the Swedish national patient register: a cohort study from Stockholm County. BMC Musculoskelet Disord 2014;15:432. 10.1186/1471-2474-15-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish cancer register – a sample survey for year 1998. Acta Oncol 2009;48:27–33. 10.1080/02841860802247664 [DOI] [PubMed] [Google Scholar]
- 31.Gjerstorff ML. The Danish cancer registry. Scand J Public Health 2011;39:42–5. 10.1177/1403494810393562 [DOI] [PubMed] [Google Scholar]
- 32.Aaltonen KJ, Joensuu JT, Virkki L, et al. Rates of serious infections and malignancies among patients with rheumatoid arthritis receiving either tumor necrosis factor inhibitor or rituximab therapy. J Rheumatol 2015;42:372–8. 10.3899/jrheum.140853 [DOI] [PubMed] [Google Scholar]
- 33.Sigurdardottir LG, Jonasson JG, Stefansdottir S, et al. Data quality at the Icelandic cancer registry: comparability, validity, timeliness and completeness. Acta Oncol 2012;51:880–9. 10.3109/0284186X.2012.698751 [DOI] [PubMed] [Google Scholar]
- 34.Larsen IK, Småstuen M, Johannesen TB, et al. Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31. 10.1016/j.ejca.2008.10.037 [DOI] [PubMed] [Google Scholar]
- 35.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35. 10.1002/pds.1294 [DOI] [PubMed] [Google Scholar]
- 36.Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, et al. Existing data sources for clinical epidemiology: the Danish national database of Reimbursed prescriptions. Clin Epidemiol 2012;4:303–13. 10.2147/CLEP.S37587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campo E, Swerdlow SH, Harris NL, et al. The 2008 who classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019–32. 10.1182/blood-2011-01-293050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swerdlow SH, Campo E, Harris NL, et al. Who classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press, 2008. [Google Scholar]
- 39.Carstensen B. Age-Period-Cohort models for the Lexis diagram. Stat Med 2007;26:3018–45. 10.1002/sim.2764 [DOI] [PubMed] [Google Scholar]
- 40.Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013;72:517–24. 10.1136/annrheumdis-2011-201244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002776supp001.pdf (208.4KB, pdf)
Data Availability Statement
No data are available. Due to GDPR regulations, all the data used for this study are kept within each country on encrypted servers, and are not available for the public.