Abstract
T-2 toxin is a dangerous natural pollutant and widely exists in animal feed, often causing toxic damage to poultry, such as slow growth and development, immunosuppression, and death. Although geese are considered the most sensitive poultry to T-2 toxin, the exact damage caused by T-2 toxin to geese is elusive. In the present study, a total of forty two 1-day-old healthy Yangzhou male goslings were randomly allotted seven diets contaminated with 0, 0.2, 0.4, 0.6, 0.8, 1.0, or 2.0 mg/kg T-2 toxin for 21 d, and the effects of T-2 toxin exposure on growth performance, feather quality, tibia development, and blood parameters were investigated. The results showed that T-2 toxin exposure significantly inhibited feed intake, body weight gain, shank length growth, and organ development (e.g., ileum, cecum, liver, spleen, bursa, and tibia) in a dose-dependent manner. In addition, the more serious feathering abnormalities and feather damage were observed in goslings exposed to a high dose of T-2 toxin (0.8, 1.0, and 2.0 mg/kg), which were mainly sparsely covered with short, dry, rough, curly, and gloss-free feathers on the back. We also found that hypertrophic chondrocytes of the tibial growth plate exhibited abnormal morphology and nuclear consolidation or loss, accompanied by necrosis and excessive apoptosis under 2.0 mg/kg T-2 toxin exposure. Moreover, 2.0 mg/kg T-2 toxin exposure triggered erythropenia, thrombocytosis, alanine aminotransferase, and aspartate aminotransferase activity, as well as high blood urea nitrogen, uric acid, and lactic dehydrogenase levels. Collectively, these data indicate that T-2 toxin had an adverse effect on the growth performance, feather quality, and tibia development, and caused liver and kidney damage and abnormal blood parameters in Yangzhou goslings, providing crucial information toward the prevention and control of T-2 toxin contamination in poultry feed.
Key words: T-2 toxin, goslings, growth performance, feather quality, tibia development
INTRODUCTION
T-2 toxin is a virulent type A trichothecene secreted by Fusarium spp., and is widely present in maize, barley, wheat, oats, and other cereal crops, which are common animal feed ingredients (Escrivá et al., 2015). Of 420 Chinese feedstuff samples, T-2 toxin incidence was 79.5%, and levels ranged 10 to 735 µg/kg, posing a potential threat to animals (Wang et al., 2013). T-2 toxin ingestion by animals can produce acute or chronic effects, with serious toxic effects on feed intake (FI), growth and development, reproductive capacity, and health (Binder et al., 2007; Schuhmacher-Wolz et al., 2010). Lower FI, reduced weight gain, growth retardation (Wei et al., 2019), lower egg production and quality, decreased hatchability (Dazuk et al., 2020), coagulopathy (Singh et al., 2020), and feather abnormalities (Wyatt et al., 1975; Manafi et al., 2015) are visible signs of T-2 toxin poisoning in poultry (Sokolović et al., 2008). The toxic effects of T-2 toxin include inhibition of protein, DNA and RNA synthesis, and immunoglobulin production (Szabó et al., 2019). In addition, T-2 toxin accelerates the generation of reactive oxygen species (ROS) that induces oxidative stress, which further leads to inflammatory responses and apoptosis (Mackei et al., 2018; Huang et al., 2021; Zhang et al., 2021). The hepatic apoptosis rate and pathology of chickens were aggravated with the increase of T-2 concentration that ranged 0.5 to 2.0 mg/kg, which induced the mitochondria-mediated apoptosis by producing ROS and promoting cytochrome c translocation and apoptosomes formation (Yin et al., 2020). It has been reported that 4.0 mg/kg of T-2 toxin triggers oxidative stress and inflammatory responses to renal damage in mice (Huang et al., 2021). In addition, 0.5 mg/kg T-2 toxin disturbed various endogenous metabolic changes and pathways and oxidative stress, causing the accumulation of amino acids and nucleotides in the liver, kidney, and spleen of broiler chickens (Wan et al., 2016). T-2 toxin targets tissues in an actively and rapidly dividing state through several toxic mechanisms that cause severe damage (Sokolović et al, 2008), such as the intestines, liver, kidneys, spleen, bursa, feathers, and bones. Thus, as T-2 toxin is widely present in animal feed, it often causes a decrease in animal performance and tissue damage.
Some studies have concluded that T-2 toxin is extremely hazardous to poultry. Among poultry, geese are more sensitive to T-2 toxin than chickens, ducks (Mézes et al., 1999; Fernye et al., 2018), and other mammals such as rodents (Li et al., 2011) and ruminants (Gholampour Azizi et al., 2014). Considering the effects of T-2 toxin on lipid peroxidation and the glutathione redox system, geese are the most sensitive poultry species, followed by ducks and chickens, and the liver is the most sensitive tissue, followed by plasma and red blood cells (Mézes et al., 1999). Geese showed upper alimentary distress and died after foraging on moldy barley, which was associated with T-2 mycotoxicosis; however, experimental mice exhibited only slight symptoms under long-term exposure (Puls and Greenway, 1976). Ruminants are more resistant to the T-2 toxin than non-ruminants because of microbial degradation within rumen microorganisms and the lack of phosphatidylcholine in the membrane (DeLoach et al., 1989; Kuca et al., 2008). Different doses of toxins affect the degree of toxicity in animals. For chickens, the Lethal Dose 50% (LD50) of T-2 toxin for broilers was 4.97 mg/(kg·b.w) and the LD50 for laying hens was 6.27 mg/(kg·b.w) (Chi et al., 1977b; Li et al., 2022). T-2 toxin causes impaired performance, DNA fragmentation in spleen leukocytes, and elevated serum immune globulin A (IgA) levels in a dose-dependent manner in broilers, especially with 13.5 mg/kg contamination (Rezar et al., 2007). For geese, 0.1, 0.2, and 0.3 mg/kg/day T-2 toxin reduced hatching rates and was dose-dependent, with a significant increase in mortality at levels above 0.8 mg/kg/day (Vanyi et al., 1994).
As the effects of T-2 toxin on geese production performance and tissue damage have been rarely reported, and studies have mainly focused on European geese and reproduction performance, it was unclear whether Chinese geese have similar or different responses at different T-2 toxin concentrations. Therefore, the objective of this study was to investigate the effect of T-2 toxin with different concentrations on growth performance, feather quality, tibia development, and blood parameters in Yangzhou goslings.
MATERIALS AND METHODS
Ethics Statement
All animal protocols were approved by the Animal Care and Use Committee of the Yangzhou University.
Animals and Sample Collection
Forty-two healthy 1-day-old Yangzhou male goslings were randomly divided and raised in stainless-steel cages (90 cm × 60 cm × 45 cm) in an animal housing facility at Yangzhou University (Yangzhou, Jiangsu, China) with insulation lamps, and the temperature was controlled at 25°C to 30°C. Goslings had free access to the growing diet (Table 1) supplemented with 0, 0.2, 0.4, 0.6, 0.8, 1.0, and 2.0 mg/kg T-2 toxin and water during the growing period (1–21 d of age). T-2 toxin (CAS NO: 21259-20-1, 50 mg, purity >98%, Wuhan Grint Biological Technology Co., Ltd., Wuhan, China) was dissolved in 10 mL of non-aqueous ethanol to obtain a primary solubility of 5 mg/mL of mother liquor, which was divided into ten tubes and stored at −20°C. Before usage, enzyme-free water was added to achieve the target concentration and then sprayed onto the feed, stirred well, evaporated naturally for 30 min and stored at room temperature for later use. The real T-2 toxin concentration in each diet (0.009, 0.208, 0.412, 0.621, 0.814, 1.020, and 2.033 mg/kg) was determined using enzyme-linked immunosorbent assay (ELISA; CAT NO: EKT-060, Qingdao Pribolab Biotech Co., Ltd., Qingdao, China) to ensure the accuracy of T-2 toxin supplementation. After 21 d of daily feeding management and recording, goslings were fasted overnight for 6 h before sample collection. On the 21st day, all goslings from each group were sacrificed. After capturing images and scoring feathering, live body weight (BW) and shank length (SL) were measured, followed by blood collection. After cleaning with 75% alcohol, 4 mL of blood was collected from the right wing vein. Half (2 mL) was mixed with K2EDTA in a plasma collection tube, placed on ice for 10 min, and sent to the Yangzhou Centers for Disease Control and Prevention (CDC) for routine blood tests. Another 2 mL sample was centrifuged and sent to the Yangzhou University Animal Hospital for serum biochemical tests. The weights of the heart, liver, spleen, lungs, kidneys, gizzard, proventriculus, bursa, and pancreas were measured. The weight and length of intestines (duodenum, jejunum, ileum, cecum, and rectum) were also measured after cleaning to remove intestinal contents and adherent fat. After removal of adhesions, the left tibial phenotypes were observed, and the length and weight were measured. For histological analysis, tibial growth plate (TGP) samples (approximately 1.5 cm3) were fixed in 10% formalin overnight, followed by storage in fresh 10% formalin, hematoxylin and eosin (HE) staining, and histomorphological analysis.
Table 1.
Ingredient composition and nutritional level of the growing diet for 1–21 day-old Yangzhou goslings.
| Ingredient composition (%) | Nutritional level (%) | ||
|---|---|---|---|
| Corn | 66.60 | ME (MJ/kg) | 11.64 |
| Soybean meal | 26.50 | Crude protein | 17.04 |
| Rice husk | 0.50 | Crude fiber | 3.66 |
| Stone powder | 0.50 | Crude fat | 2.48 |
| Calcium hydrogen phosphate | 1.25 | Ca | 0.68 |
| Methionine | 0.35 | Total phosphorus | 0.66 |
| Salt | 0.50 | Available phosphorus | 0.41 |
| Premix | 3.80 | Lysine | 0.86 |
| Total | 100.00 | Methionine | 0.60 |
Note: The premix per kilogram contains 1,200, 000 IU VA; 400,000 IU VD; 1,800 IU VE; 150 mg VK; 60 mg VB1; 600 mg VB2; 200 mg VB6; 1 mg VB12; 3,000 mg niacin; 900 mg D-pantothenic acid; 50 mg folic acid; 4 mg biotin; 35 g choline; 6 g Fe; 1 g Cu; 9.5 g Mn; 9 g Zn; 30 mg Se; and 50 mg I. Nutrient levels were calculated values.
Growth Performance and Organ Indexes
The average FI was recorded daily on a pen basis during the 21-d exposure to the T-2 toxin. After 6 h of fasting, live individual BW and SL were measured, and the goslings were sacrificed on the 21st day. Different organs were collected sequentially, and the relevant contents and adhesive substances were removed for weighing. Organ indices were calculated as organ weight (g)/live weight (g) × 100%.
Feather Scoring and Injury Degree
On the 21st day, the feather scoring of the neck, back, thorax-abdomen, wing, and tail for all goslings was counted, and back-feather damage scoring of individuals was also calculated. The feather scoring system for evaluating cleanliness and damage was adapted from previous studies (Morrissey et al., 2014; Mahmoud et al., 2015; Liu et al., 2021). Briefly, a score of 0 indicates that the feather was completely clean and covered. A score of 1 indicates less clean or damaged feathers, with no bare skin. A score of 2 indicates unclean feathers or less than 3 × 3 cm injured regions. A score of 3 indicates less dirty feathers or a denuded area of more than 3 × 3 cm. A score of 4 represents dirty feathers or visible skin. The total score for various parts of the body indicates the total father cleanliness. The lower the feather scoring, the less feathers were contaminated or damaged, and the better the welfare condition.
HE Staining and Histomorphological Analysis of the Tibial Growth Plate
HE staining was performed following the guidelines of the HE dye solution kit sets (CAT NO: GP1012, GP1001, GP1010, and GP1031, Wuhan Servicebio Biotechnology Co., Ltd., Wuhan, China). Briefly, TGP samples were fixed in 10% formalin, decalcified for 1 month, dehydrated until transparent, and embedded in paraffin. Subsequently, sections were prepared, placed in water for extension, and dried. The sections were then placed in xylene, ethanol, and 75% ethanol, and washed with water. Next, the sections were stained with hematoxylin, followed by staining with eosin. Finally, the sections were placed in ethanol and xylene and sealed with neutral gum. TGP histological images were captured using a micro-image analysis system (CaseViewer 2.4, 3DHISTECH Ltd., Budapest, Hungary).
Hematological Parameters Tests
After blood collection, half the blood was transferred to a K2EDTA-tube for routine blood parameter analysis performed using an automatic blood cell counter (MEK-8222K, NIHON KOHDEN CORP., Tokyo, Japan), including white blood cells (WBC), red blood cells (RBC), hematocrit (HCT), mean corpuscular volume (MCV), red blood cell distribution width (RDW), hemoglobin (HGB), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT), platelet (PCT), mean platelet volume (MPV), platelet distribution width (PDW), and platelet-larger cell ratio (P-LCR). The remaining non-anticoagulant blood samples were processed through centrifugation at 3,000 rpm for 10 min at 4°C to determine serum biochemical indicators using a fully automatic biochemical analyzer (AU480, Beckman Coulter K.K, Tokyo, Japan), containing alanine aminotransferase (ALT), aspartate aminotransferase (AST), AST/ALT, blood urea nitrogen (BUN), uric acid (UA), total protein (TP), albumin (ALB), globulin (GLOB), ALB/GLOB, calcium (Ca), phosphorus (P), Ca/P, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH).
Statistical Analysis
SPSS software (version 25.0, IBM Corp, Armonk, NY) was used to analyze the statistical significance of the difference between the T-2 toxin contamination and control groups. All data were tested using the Shapiro-Wilk (N < 50) and homogeneity tests to ensure the normality and homogeneity of variances among the various groups. The data of average daily FI were analyzed using one-way repeated measures analysis of variance (ANOVA). After the Mauchly's test of sphericity, a simple effect analysis was executed and the significant comparison of different T-2 toxin dosages at the same age was presented. Other data were expressed as the mean ± standard error (SEM) and subjected to one-way ANOVA followed by Tukey's honestly significant difference (HSD) multiple range test. If the data (e.g., feather scoring, RBC, PLT, and Ca/P) were not normal and homogeneous, it was subjected to the non-parametric test (Kruskal-Wallis tests) and results were expressed as medians (interquartile range) and presented in figures with box plots (median, first, and third quartiles). Statistical significance was set at P < 0.05.
RESULTS
Effect of T-2 Toxin on Growth Performance and Organ Development
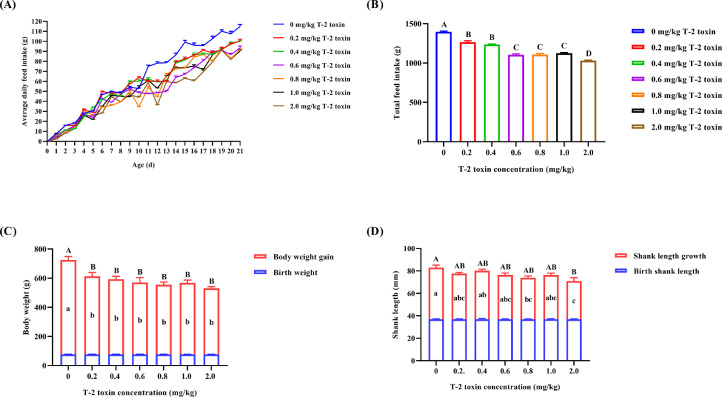
To determine the effect of T-2 toxin on the growth performance of goslings, the relevant parameters were measured. Goslings in the control group (0 mg/kg) appeared active with tidy plumage and normal feed intake, droppings, and body weight. Compared with the control, the average daily FI (P < 0.05; Figure 1A; Supplementary table 1) and 21-d total FI (P < 0.05; Figure 1B) changed in a dose-dependent manner during T-2 toxin exposure. This was consistent with the fact that BWG and final BW significantly decreased in all T-2 toxin groups during the 21-d exposure period (P < 0.05; Figure 1C). SL is closely related to BW, meat production, and slaughter performance. In terms of shank growth, SLG and the final SL significantly decreased in the 2.0 mg/kg T-2 toxin-contaminated groups (P < 0.05; Figure 1D).
Figure 1.
Average daily feed intake (A), total feed intake (B), body weight, body weight gain (C), shank length and shank length growth (D) of Yangzhou goslings exposed to 0, 0.2, 0.4, 0.6, 0.8, 1.0, and 2.0 mg/kg T-2 toxin from birth to 21-day-old. Note: The values were presented as the means ± SEM. N = 6. The significance analysis of Figure 1A was shown in Supplementary table 1. In others, no superscripts or the same superscripts in the shoulder or heading mark of peer data indicated that the differences were not significant (P > 0.05) and different superscripts indicated significant differences (P < 0.05). Lowercase letters represented partial significance, and uppercase letters represented overall significance. It was the same in the following figures.
Goslings were in a state of delayed organ development under 21-d T-2 toxin exposure. Compared with the control group, the weights of the liver, spleen, gizzard, and bursa decreased in all T-2 toxin groups, in contrast to the increased weight indexes of the heart, lungs, kidneys, proventriculus, and pancreas in some groups (P < 0.05; Table 2). In addition, the weights of the jejunum, ileum, cecum, and rectum; the lengths of the ileum, cecum, and rectum; and the indices of the ileum and cecum in some T-2 toxin groups were significantly lower than those in the control, especially with higher T-2 toxin doses (P < 0.05; Table 3).
Table 2.
Organ weight and index of 21-day-old Yangzhou goslings under T-2 toxin exposure from birth.
| Organ | Item | 0 mg/kg T-2 | 0.2 mg/kg T-2 | 0.4 mg/kg T-2 | 0.6 mg/kg T-2 | 0.8 mg/kg T-2 | 1.0 mg/kg T-2 | 2.0 mg/kg T-2 |
|---|---|---|---|---|---|---|---|---|
| Heart | Weight (g) | 5.56 ± 0.21 | 5.46 ± 0.25 | 5.35 ± 0.05 | 5.27 ± 0.23 | 5.20 ± 0.36 | 5.29 ± 0.17 | 5.05 ± 0.10 |
| Index (%) | 0.77 ± 0.01b | 0.90 ± 0.04ab | 0.91 ± 0.03ab | 0.93 ± 0.03a | 0.94 ± 0.06a | 0.93 ± 0.02a | 0.95 ± 0.02a | |
| Liver | Weight (g) | 24.05 ± 0.23a | 17.61 ± 0.12bc | 18.00 ± 0.71b | 18.22 ± 0.82b | 17.30 ± 0.29bc | 18.32 ± 0.16b | 15.86 ± 0.22c |
| Index (%) | 3.34 ± 0.13 | 2.90 ± 0.14 | 3.05 ± 0.15 | 3.26 ± 0.25 | 3.13 ± 0.07 | 3.24 ± 0.10 | 3.00 ± 0.05 | |
| Spleen | Weight (g) | 1.22 ± 0.06a | 0.74 ± 0.02b | 0.74 ± 0.08b | 0.82 ± 0.12b | 0.66 ± 0.07b | 0.67 ± 0.10b | 0.84 ± 0.01b |
| Index (%) | 0.17 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.02 | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.16 ± 0.01 | |
| Lung | Weight (g) | 4.03 ± 0.09bc | 3.94 ± 0.25c | 3.93 ± 0.32c | 5.45 ± 0.06a | 5.19 ± 0.18a | 5.04 ± 0.10ab | 4.78 ± 0.39abc |
| Index (%) | 0.56 ± 0.01b | 0.65 ± 0.06b | 0.67 ± 0.07b | 0.97 ± 0.06a | 0.94 ± 0.02a | 0.89 ± 0.03a | 0.90 ± 0.06a | |
| Kidney | Weight (g) | 7.11 ± 0.14 | 7.16 ± 0.06 | 7.54 ± 0.49 | 7.20 ± 0.29 | 7.07 ± 0.39 | 6.92 ± 0.47 | 6.85 ± 0.38 |
| Index (%) | 0.98 ± 0.03b | 1.18 ± 0.04ab | 1.27 ± 0.08a | 1.29 ± 0.09a | 1.27 ± 0.04a | 1.21 ± 0.05ab | 1.29 ± 0.06a | |
| Gizzard | Weight (g) | 40.93 ± 3.14a | 30.32 ± 0.64bc | 30.36 ± 0.57bc | 32.61 ± 1.55b | 23.90 ± 1.70c | 30.85 ± 0.12bc | 26.45 ± 1.43bc |
| Index (%) | 5.62 ± 0.31a | 4.99 ± 0.22ab | 5.15 ± 0.21ab | 5.78 ± 0.29a | 4.31 ± 0.24b | 5.46 ± 0.17a | 4.99 ± 0.23ab | |
| Proventriculus | Weight (g) | 5.22 ± 0.04ab | 5.00 ± 0.10ab | 5.74 ± 0.27a | 5.56 ± 0.01ab | 4.84 ± 0.35b | 5.31 ± 0.07ab | 5.71 ± 0.24a |
| Index (%) | 0.72 ± 0.03c | 0.82 ± 0.04bc | 0.98 ± 0.07ab | 0.99 ± 0.06ab | 0.87 ± 0.04bc | 0.94 ± 0.03ab | 1.08 ± 0.03a | |
| Bursa | Weight (g) | 1.00 ± 0.08a | 0.65 ± 0.03b | 0.69 ± 0.08b | 0.57 ± 0.06b | 0.71 ± 0.02b | 0.74 ± 0.02b | 0.76 ± 0.04b |
| Index (%) | 0.14 ± 0.01ab | 0.11 ± 0.01b | 0.12 ± 0.01ab | 0.10 ± 0.01b | 0.13 ± 0.00ab | 0.13 ± 0.01ab | 0.14 ± 0.01a | |
| Pancreas | Weight (g) | 2.62 ± 0.08 | 3.04 ± 0.04 | 2.76 ± 0.18 | 2.72 ± 0.16 | 2.69 ± 0.06 | 2.81 ± 0.06 | 2.60 ± 0.16 |
| Index (%) | 0.36 ± 0.01b | 0.50 ± 0.02a | 0.47 ± 0.04a | 0.48 ± 0.02a | 0.49 ± 0.01a | 0.50 ± 0.02a | 0.49 ± 0.02a |
Note: The values used for ANOVA analysis are presented as the means ± SEM and the values used for the non-parametric test are presented as medians (interquartile range). N = 6.
Means within a row with no common superscript differ significantly (P < 0.05).
Table 3.
Intestinal weight, index, and length of 21-day-old Yangzhou goslings under T-2 toxin exposure from birth.
| Index | Item | 0 mg/kg T-2 | 0.2 mg/kg T-2 | 0.4 mg/kg T-2 | 0.6 mg/kg T-2 | 0.8 mg/kg T-2 | 1.0 mg/kg T-2 | 2.0 mg/kg T-2 |
|---|---|---|---|---|---|---|---|---|
| Duodenum | Weight (g) | 4.55 ± 0.26a | 4.47 ± 0.10ab | 4.32 ± 0.40ab | 3.54 ± 0.19b | 3.97 ± 0.15ab | 3.80 ± 0.22ab | 3.70 ± 0.15ab |
| Index (%) | 0.63 ± 0.02b | 0.73 ± 0.03ab | 0.72 ± 0.04ab | 0.81 ± 0.06a | 0.71 ± 0.01ab | 0.67 ± 0.04ab | 0.70 ± 0.01ab | |
| Length (cm) | 23.00 ± 0.22 | 23.13 ± 1.48 | 21.67 ± 0.88 | 20.00 ± 0.96 | 20.33 ± 0.67 | 19.50 ± 0.48 | 20.50 ± 1.20 | |
| Jejunum | Weight (g) | 15.64 ± 0.34a | 13.63 ± 0.52ab | 14.00 ± 0.79ab | 13.61 ± 0.52ab | 11.73 ± 0.41bc | 12.31 ± 0.61b | 9.69 ± 0.36c |
| Index (%) | 2.16 ± 0.03ab | 2.23 ± 0.04ab | 2.39 ± 0.19a | 2.42 ± 0.14a | 2.12 ± 0.09ab | 2.16 ± 0.05ab | 1.83 ± 0.06b | |
| Length (cm) | 58.58 ± 3.10 | 56.75 ± 1.97 | 55.08 ± 1.07 | 56.00 ± 2.92 | 51.17 ± 0.46 | 57.50 ± 1.14 | 52.00 ± 1.46 | |
| Ileum | Weight (g) | 15.13 ± 0.42a | 12.68 ± 0.94ab | 12.21 ± 0.72b | 11.93 ± 0.46b | 11.78 ± 0.65b | 10.05 ± 0.41bc | 8.45 ± 0.39c |
| Index (%) | 2.09 ± 0.01ab | 2.07 ± 0.11ab | 2.05 ± 0.08ab | 2.12 ± 0.10a | 2.13 ± 0.11a | 1.77 ± 0.01bc | 1.59 ± 0.04c | |
| Length (cm) | 55.67 ± 3.13a | 52.00 ± 1.10ab | 53.25 ± 1.09ab | 49.17 ± 1.70ab | 49.67 ± 2.07ab | 50.33 ± 0.42ab | 46.67 ± 1.31b | |
| Cecum | Weight (g) | 3.41 ± 0.13a | 2.79 ± 0.04ab | 2.34 ± 0.30bc | 1.92 ± 0.21c | 2.05 ± 0.16bc | 1.91 ± 0.13c | 1.99 ± 0.17c |
| Index (%) | 0.47 ± 0.01a | 0.46 ± 0.02a | 0.39 ± 0.05ab | 0.33 ± 0.03c | 0.37 ± 0.03bc | 0.33 ± 0.02c | 0.37 ± 0.02bc | |
| Length (cm) | 33.33 ± 1.12a | 30.00 ± 0.37ab | 29.50 ± 0.80ab | 28.42 ± 0.70b | 28.50 ± 1.28ab | 29.83 ± 1.60ab | 26.50 ± 1.38b | |
| Rectum | Weight (g) | 3.34 ± 0.18a | 2.80 ± 0.09ab | 2.84 ± 0.09ab | 2.87 ± 0.18ab | 2.90 ± 0.10ab | 2.39 ± 0.09bc | 2.01 ± 0.05c |
| Index (%) | 0.46 ± 0.03abc | 0.46 ± 0.01abc | 0.48 ± 0.01ab | 0.51 ± 0.04ab | 0.52 ± 0.02a | 0.42 ± 0.02bc | 0.38 ± 0.01c | |
| Length (cm) | 10.67 ± 0.53a | 9.00 ± 0.41b | 8.00 ± 0.52bc | 6.33 ± 0.31d | 6.50 ± 0.26cd | 7.17 ± 0.21cd | 6.33 ± 0.17d |
Note: The values used for ANOVA analysis are presented as the means ± SEM and the values used for the non-parametric test are presented as medians (interquartile range). N = 6.
Means within a row with no common superscript differ significantly (P < 0.05).
Effect of T-2 Toxin on Feather Quality
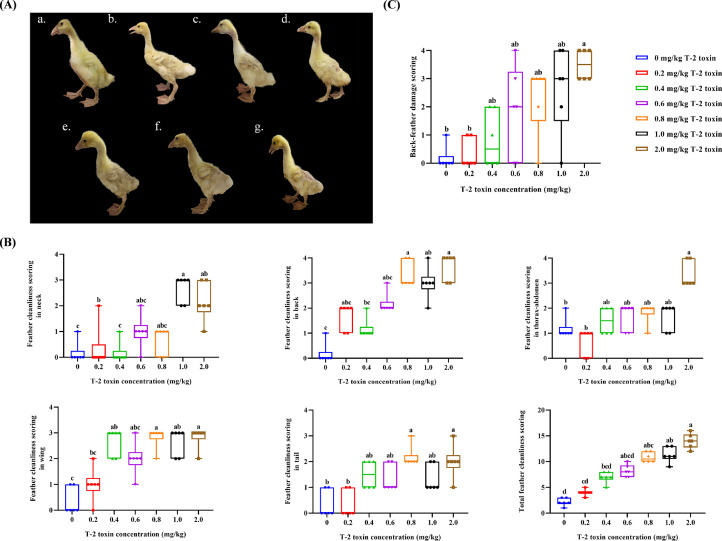
Overall, it was clear that feather quality varied with T-2 toxin exposure in a dose-dependent manner in goslings. Based on appearance, T-2 toxin caused feathering abnormalities, especially through short, dry, rough, curly, and gloss-free feathers sparsely covering the back (Figure 2A). Interestingly, the feather cleanliness scoring (Figure 2B) and back-feather damage scoring (Figure 2C) also increased in a dose-dependent manner (P < 0.05), and the most serious damage appeared in the 2.0 mg/kg T-2 toxin group.
Figure 2.
Lateral view of feathering patterns (A), feather cleanliness scoring (B) and back-feather damage scoring (C) of 21-day-old goslings exposed to 0 (a.), 0.2 (b.), 0.4 (c.), 0.6 (d.), 0.8 (e.), 1.0 (f.), and 2.0 (g.) mg/kg T-2 toxin from birth.
Effect of T-2 Toxin on Tibia Growth and Development
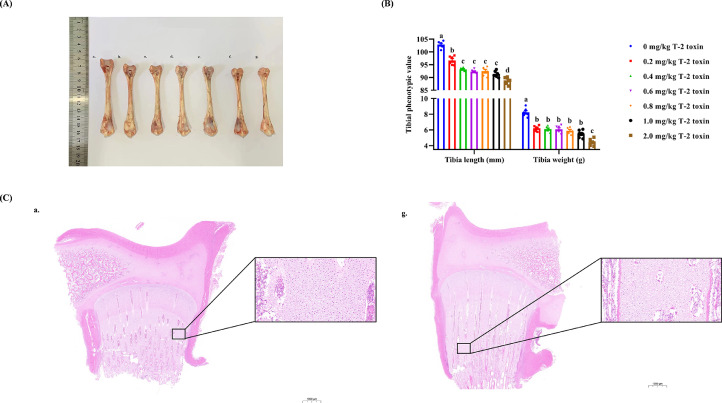
Furthermore, the tibial growth plate and morphological structure can reflect longitudinal bone growth and health to a certain extent, and tibial development is also a major contributor to support BW. T-2 toxin clearly slowed tibial growth (length and weight) in a dose-dependent manner (P < 0.05), especially in the 2.0 mg/kg T-2 toxin group (Figure 3A, B). As shown through HE staining and histomorphological analysis (Figure 3C), the TGP was subdivided into resting, proliferative, hypertrophic, and calcified zones, with clear cell morphology. Compared with the control group, the TGP exhibited abnormal morphological structure in the 2.0 mg/kg T-2 toxin group. Chondrocytes were arranged in a crowded and stagnant state with increasing density. Importantly, some chondrocytes in the hypertrophic zones manifested abnormal morphology and nuclear consolidation or loss, accompanied by necrosis and excessive apoptosis.
Figure 3.
The tibia phenotype (A), length and weight (B) and tibial growth plate morphological structure (C) of 21-day-old goslings exposed to 0 (a.), 0.2 (b.), 0.4 (c.), 0.6 (d.), 0.8 (e.), 1.0 (f.), and 2.0 (g.) mg/kg T-2 toxin from birth.
Effect of T-2 toxin on Blood Parameters
Blood is the main and most important component of the internal environment of the body, and changes in blood composition reflect the metabolic status and health status of the animal body. In routine blood tests (Table 4), compared with the control group, RBC and RDW decreased in the 2.0 mg/kg T-2 toxin group, along with an increase in MCH (P < 0.05). In addition, PLT rose in the 2.0 mg/kg T-2 toxin group, accompanied by a reduction in MPV and P-LCR (P < 0.05).
Table 4.
Routine blood parameters of 21-day-old Yangzhou goslings under T-2 toxin exposure from birth.
| Index | 0 mg/kg T-2 | 0.2 mg/kg T-2 | 0.4 mg/kg T-2 | 0.6 mg/kg T-2 | 0.8 mg/kg T-2 | 1.0 mg/kg T-2 | 2.0 mg/kg T-2 |
|---|---|---|---|---|---|---|---|
| WBC (109/L) | 334.78 ± 8.66 | 326.06 ± 11.41 | 334.46 ± 8.75 | 328.78 ± 7.84 | 334.17 ± 11.49 | 312.75 ± 19.73 | 356.77 ± 17.84 |
| RBC (1012/L) | 1.52 (1.46–1.57)a | 1.37 (1.32–1.52)a | 1.39 (1.19–1.44)a | 1.23 (1.18–1.23)a | 1.29 (1.17–1.41)a | 1.12 (1.12–1.15)a | 1.16 (1.11–1.24)b |
| HCT (%) | 20.55 ± 2.03 | 21.67 ± 1.20 | 20.22 ± 1.36 | 20.66 ± 1.11 | 21.62 ± 1.45 | 18.63 ± 0.81 | 18.41 ± 0.47 |
| MCV (fL) | 153.68 ± 4.91 | 150.61 ± 3.04 | 154.40 ± 3.87 | 164.57 ± 1.76 | 167.22 ± 5.10 | 153.90 ± 2.64 | 160.18 ± 7.69 |
| RDW (%) | 29.33 ± 1.38a | 28.22 ± 0.78ab | 26.32 ± 0.50abc | 27.62 ± 0.72ab | 23.97 ± 0.40bc | 27.40 ± 0.84ab | 22.62 ± 1.57c |
| HGB (g/L) | 110.40 ± 2.89ab | 110.20 ± 2.28ab | 95.13 ± 2.93c | 104.52 ± 2.95abc | 114.90 ± 1.73a | 102.65 ± 2.73bc | 112.62 ± 2.80ab |
| MCH (pg) | 73.67 ± 1.05b | 77.65 ± 3.39b | 80.02 ± 5.05b | 84.73 ± 5.29ab | 90.50 ± 3.17ab | 85.53 ± 4.55ab | 97.64±2.95a |
| MCHC (g/L) | 476.00 ± 18.46 | 516.17 ± 24.51 | 545.33 ± 45.83 | 513.33 ± 27.16 | 555.17 ± 40.76 | 561.50 ± 36.06 | 614.83±27.49 |
| PLT (109/L) | 132.00 (115.75–178.00)ac | 188.00 (155.25–206.25)c | 121.50 (119.00–125.25)abc | 113.00 (105.00–130.50)abc | 147.00 (138.00–148.50)abc | 147.00 (128.75–175.25)ab | 177.00 (176.25–181.00)b |
| PCT | 0.14 ± 0.01ab | 0.18 ± 0.02a | 0.12 ± 0.01bc | 0.10 ± 0.01c | 0.15 ± 0.00ab | 0.13 ± 0.01bc | 0.18± 0.00a |
| MPV (fL) | 10.80 ± 0.16a | 10.62 ± 0.07ab | 10.47 ± 0.13abc | 9.45 ± 0.08d | 10.23 ± 0.05abc | 10.12 ± 0.22bc | 9.88±0.13cd |
| PDW (%) | 9.80 ± 0.62a | 9.40 ± 0.24ab | 9.48 ± 0.09ab | 8.18 ± 0.19b | 9.55 ± 0.07a | 9.60 ± 0.22a | 9.82±0.29a |
| P–LCR (%) | 27.66 ± 0.25a | 26.82 ± 0.34a | 26.10 ± 0.81ab | 20.25 ± 0.67c | 24.88 ± 0.41ab | 24.43 ± 1.39ab | 22.82±0.76bc |
Abbreviations: HCT, hematocrit; HGB, Hemoglobin; MCH (HGB/RBC), mean corpuscular hemoglobin; MCV (HCT/RBC), mean corpuscular volume; MCHC (HGB/HCT), mean corpuscular hemoglobin concentration; MPV, mean platelet volume; PDW, platelet distribution width; P-LCR, platelet-larger cell ratio; PLT, Platelets; PCT, plateletocrit; RDW, red blood cell distribution width; RBC, red blood cells; WBC, white blood cells.
Note: The values used for ANOVA analysis are presented as the means ± SEM and the values used for the non-parametric test are presented as medians (interquartile range). N = 6.
Means within a row with no common superscript differ significantly (P < 0.05).
Serum biochemical tests (Table 5) showed that, compared with the control, the liver function-related parameters (ALT, AST, and AST/ALT) increased significantly to varying degrees in most T-2 toxin groups (P < 0.05). Furthermore, the renal function relative parameter BUN increased in most T-2 toxin groups (P < 0.05), whereas UA increased only in the 2.0 mg/kg T-2 toxin group (P < 0.05). In addition, TP increased significantly only in the 2.0 mg/kg T-2 toxin group (P < 0.05), whereas there were no significant changes in the ALB, GLOB, and ALB/GLOB. Moreover, 0.4 to 0.8 mg/kg of T-2 toxin accelerated the rise of phosphorus and caused the Ca/P ratio to decrease (P < 0.05). Similarly, Ca decreased only in the 0.8 mg/kg T-2 toxin group (P < 0.05). However, ALP increased in 1.0 and 2.0 mg/kg T-2 toxin groups (P < 0.05). Finally, LDH showed a distinct improvement in most T-2 toxin groups (P < 0.05).
Table 5.
Serum biochemical parameters of 21-day-old Yangzhou goslings under T-2 toxin exposure from birth.
| Index | 0 mg/kg T-2 | 0.2 mg/kg T-2 | 0.4 mg/kg T-2 | 0.6 mg/kg T-2 | 0.8 mg/kg T-2 | 1.0 mg/kg T-2 | 2.0 mg/kg T-2 |
|---|---|---|---|---|---|---|---|
| ALT (U/L) | 24.63 ± 0.53d | 25.93 ± 0.88cd | 28.25 ± 0.37bcd | 33.62 ± 1.39ab | 30.83 ± 1.90abc | 30.13 ± 1.75abcd | 35.22 ± 1.90a |
| AST (U/L) | 14.44 ± 0.41d | 18.83 ± 0.72d | 33.82 ± 0.98ab | 36.45 ± 0.74a | 25.95 ± 2.39c | 28.12 ± 2.16bc | 37.10 ± 0.63a |
| AST/ALT | 0.59 ± 0.01e | 0.73 ± 0.02de | 1.20 ± 0.03a | 1.09 ± 0.04ab | 0.83 ± 0.04cd | 0.93 ± 0.03bc | 1.07 ± 0.06ab |
| BUN (mmol/L) | 0.54 ± 0.02d | 0.65 ± 0.04cd | 0.84 ± 0.03abc | 0.72 ± 0.05cd | 0.98 ± 0.07ab | 0.78 ± 0.02bc | 1.03 ± 0.10a |
| UA (µmol/L) | 242.57 ± 8.82b | 237.37 ± 12.81b | 239.33 ± 38.54b | 239.03 ± 25.17b | 222.18 ± 13.21b | 194.93 ± 14.19b | 354.20 ± 20.85a |
| TP (g/L) | 36.80 ± 0.06b | 37.60 ± 0.88ab | 41.13 ± 0.84ab | 41.67 ± 2.07a | 41.20 ± 0.58ab | 40.23 ± 1.12ab | 41.57 ± 0.50a |
| ALB (g/L) | 14.57 ± 0.10 | 13.48 ± 0.22 | 15.13 ± 0.88 | 15.20 ± 0.59 | 15.47 ± 0.40 | 14.67 ± 0.37 | 14.95 ± 0.13 |
| GLOB (g/L) | 22.23 ± 0.07 | 24.12 ± 0.71 | 26.00 ± 1.60 | 26.47 ± 2.00 | 25.73 ± 0.19 | 25.57 ± 1.28 | 26.62 ± 0.44 |
| A/G | 0.66 ± 0.01 | 0.56 ± 0.01 | 0.61 ± 0.08 | 0.59 ± 0.06 | 0.60 ± 0.01 | 0.58 ± 0.04 | 0.56 ± 0.01 |
| Ca (mmol/L) | 2.49 ± 0.05ab | 2.49 ± 0.06ab | 2.62 ± 0.05ab | 2.35 ± 0.14b | 1.97 ± 0.05c | 2.75 ± 0.11a | 2.74 ± 0.04a |
| P (mmol/L) | 2.45 ± 0.02b | 2.40 ± 0.04b | 3.17 ± 0.04a | 3.45 ± 0.09a | 3.37 ± 0.21a | 2.55 ± 0.23b | 2.52 ± 0.08b |
| Ca/P | 1.01 (0.99–1.01)ab | 1.01 (1.01–1.03)ab | 0.85 (0.83–0.85)abc | 0.66 (0.65–0.66)bc | 0.59 (0.54–0.94)c | 1.22 (0.99–1.22)a | 1.05 (1.03–1.13)a |
| ALP (U/L) | 432.00 ± 40.07bc | 442.00 ± 28.04bc | 449.67 ± 10.36bc | 496.50 ± 11.28abc | 408.17 ± 40.29c | 570.33 ± 42.99ab | 629.67 ± 51.27a |
| LDH (U/L) | 496.00 ± 8.43d | 601.67 ± 19.16cd | 923.50 ± 11.07ab | 1125.67 ± 41.39a | 810.67 ± 87.04bc | 739.00 ± 26.62bcd | 802.00 ± 105.30bc |
Abbreviations: A/G, ALB/GLOB; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca, calcium; GLOB, globulin; LDH, lactate dehydrogenase; P, phosphorus; TP, total protein; UA, uric acid.
Note: The values used for ANOVA analysis are presented as the means ± SEM and the values used for the non-parametric test are presented as medians (interquartile range). N = 6.
Means within a row with no common superscript differ significantly (P < 0.05).
DISCUSSION
The T-2 toxin is the most toxic secondary metabolite of all trichothecenes produced by diverse Fusarium spp. (for example, F. acuminatum, F. poae, and F. sporotrichioides), which widely occurs in cereal crops for animal feed production (Janik et al., 2021). Many agricultural technical and environmental factors induce T-2 toxin pollution in crop growth, harvest and processing, feed production, food processing, and animal production, which threatens animal health, production, and human food safety, resulting in significant economic losses and health issues (Magnoli et al., 2019; Janik et al., 2021). Geese, especially goslings, are the most to T-2 toxin damage among poultry, and relevant studies are limited to increasing the difficulties of prevention and control management in production.
Effect of T-2 Toxin on Growth Performance and Organ Development
In this study, the growth performance of goslings was significantly reduced by 21-d T-2 toxin consumption, including FI, BW, BWG, SL, and SLG, as well as by the delay of organ development. The lower growth performance under T-2 toxin toxicity in goslings was consistent with the changes observed in male broiler chicks (Wei et al., 2019), White Roman geese (Lin et al., 2018) and Brown Tsaiya and Kaiya ducklings (Tso et al., 2021). The effect of T-2 toxin on growth performance and health clearly depends on animal age, dosage, and exposure time (Li et al., 2011; Janik et al., 2021). T-2 toxin causes contact irritation and metabolic toxic damage to the rostrum, oral cavity, and gastrointestinal tract, thus negatively affecting FI and causing further injuries to the blood system, liver, kidneys, and other organs (Yohannes et al., 2012). Moreover, the decline in FI caused by the T-2 toxin is an iconic effect that is well explained by the previously observed food refusal phenomenon and strange feeding behaviors regulated by various appetitive central and peripheral modulators, gut satiety hormones, and proinflammatory cytokines (Gaigé et al., 2014; Sheng et al., 2019), as well as growth hormone deficiency (Liu et al., 2017; Zhang et al., 2020). In addition, T-2 toxin inhibits protein, DNA, and RNA synthesis, and subsequently affects physiological functions by influencing the synthesis and secretion of key molecular precursors and raw materials (Sokolović et al., 2008). In addition to affecting morphological structures, T-2 toxin also affects digestion, absorption, and metabolic utilization, thus causing the nutritional needs of the body for the development of organs and induction of immunotoxicity to be unmet (Rafai et al., 2000; Manafi et al., 2015). This also results in oxidative stress (Rezar et al., 2007; Chen et al., 2019; Yin et al., 2020), inflammation (Sun et al., 2022), apoptosis (Chen et al., 2019; Yin et al., 2020), and autophagy (Yin et al., 2020; Sun et al., 2022). Consequently, the organ weights and indices measured in our study also changed significantly in a malnourished and stunted environment owing to the decreased FI and weakened digestion, absorption, and metabolism through T-2 toxin exposure. Atrophy of the liver, spleen, gizzard, and bursa and the elevated organ indexes of the heart, lungs, kidneys, proventriculus, and pancreas, as well as the weight loss of the jejunum, ileum, cecum, and rectum, and the descended organ indexes of the ileum, cecum, and the shortened lengths of the ileum, cecum, and rectum were observed. Interestingly, these results are similar to those of previous studies, especially in chicks (Rezar et al., 2007; Manafi et al., 2015) and ducklings (Rafai et al., 2000; Tso et al., 2021).
Effect of T-2 Toxin on Feather Quality
Feathers play a role in regulating fowl body temperature, conserving heat, and resisting external damage, thus affecting appearance evaluation, health, productivity, and animal welfare. We discovered that T-2 toxin induced a significant decrease in feather quality in a dose-dependent manner compared to the control, particularly on the back, which is consistent with the delayed development of plumage in young ducks (Hayes and Wobeser, 1983; Rafai et al., 2000) and chickens sparsely covered in short protruding feathers (Wyatt et al., 1975; Yohannes et al., 2012). Similar to beak/oral lesions in other studies, T-2 toxin causes contact corrosion and irritation and generates malformed feathers in birds that are thin, uneven, shedding, and dirty (Yohannes et al., 2012). Additionally, T-2 toxin has adverse effects on nutritional imbalances (arginine, pyridoxine, and zinc deficiency), causing abnormal feathering; a more direct influence (necrosis) is specifically observed on the follicular tissue (Wyatt et al., 1975; Leeson and Walsh, 2004), the layer of regenerative cells in the feather base, and the basilar layer of the ramus (Hoerr et al., 1981; Yohannes et al., 2012). Moreover, T-2 toxin inhibits keratin synthesis and causes feather abnormalities (Nguansangiam et al., 2003). In practice, feather lesions and low growth performance are the main characteristics of T-2 toxin toxicity, as an indication of poisoning in poultry production (Yohannes et al., 2012).
Effect of T-2 Toxin on Bone Growth and Development
Leg (femur, tibia, and metatarsus) growth, development, and health are key concerns for growth performance, health, animal welfare, and profits in the poultry industry (Guo et al., 2019). SL, the indicator of metatarsus development, is a monitoring body size parameter of growth and development because of its close correlation with BW (Gao et al., 2010; Ukwu et al., 2014). In addition to BW, the inhibitory effect of T-2 toxin on growth was directly reflected in the slow growth of SL. Interestingly, there was a significant SL shortening as well as a significant drop in SLG after 21-d 2.0 mg/kg T-2 toxin exposure. Leg bones grow similarly and synchronously with BW to support BWG and life activities, thus directly influencing meat quality and skeletal abnormalities in meat geese (Yu et al., 2022). It is well known that the T-2 toxin is an environmental risk factor related to bone malformation, especially chondrocyte damage in the TGP, which are central sites regulating endochondral growth of chondrocyte formation, maturation, and turnover for bone elongation and repair (Farquharson and Jefferies, 2000; He et al., 2012). For instance, T-2 toxin is implicated in tibial dyschondroplasia (TD), which is characterized by the accumulation of immature, poorly differentiated, unmineralized, and avascular cartilage owing to intoxication and growth retardation of long bones in chickens (Nascimento et al., 2001; He et al., 2012). Furthermore, T-2 toxin participates in the pathological process of human Kashin-Beck disease (KBD) with chondrocyte damage to bone formation and morphogenesis (proliferation, differentiation, migration, and apoptosis) and imbalanced extracellular matrix (ECM) homeostasis (inhibition of synthesis and increase in degradation) (Li et al., 2016; Chang et al., 2017; Ning et al., 2021). Similarly, compared to the control, 2.0 mg/kg of T-2 toxin significantly inhibited the growth and development of tibia in goslings, reducing their length and weight. Breakage, necrosis, and excessive apoptosis of TGP chondrocytes in the hypertrophic zones were also observed. Our limited study could not confirm these TGP chondrocyte injuries as a TD or KBD model, which requires further investigation. However, we focused on these similar histopathological features under T-2 toxin exposure. Nutrient intake, hormone balance, and blood flow are key factors for normal bone growth and development (Waldenstedt, 2006; Marenzana and Arnett, 2013), and are toxicological targets of T-2 toxin. In this study, we speculated that T-2 toxin as a neurotoxin may lead to anorexia nervosa, reduce nutrient delivery, and cause hematological disorders such as anemia and variations in serum enzyme activities (e.g., Ca, P, and ALP), which may cause bone marrow necrosis and hematopoietic dysfunction, further aggravating blood burden, abnormal long bone (tibia and metatarsus) growth and development, and forming a recurring cycle, which was partly shown in chickens (Hoerr et al., 1982a,b; Janssens et al., 2000). T-2 toxin also reduces DNA synthesis in chondrocytes because of a basic depression of protein, DNA, and RNA (Wright Jr et al., 1987; Sokolović et al., 2008; He et al., 2012). Furthermore, T-2 toxin facilitates growth retardation and anomalous structural lesions of the TGP, with some unusual apoptotic and necrotic areas interfering with normal proliferation, differentiation, migration, mineralization of chondrocytes and synthesis, degradation of ECM, and the specific molecular expression mechanism (Ning et al., 2021). In addition, inflammatory cytokines (mainly interleukin 1β) potently induce matrix degrading enzyme MMP expression in chondrocytes, inhibit the expression of matrix components, and evoke cell apoptosis, affecting chondrocyte growth under T-2 toxin exposure (Chang et al., 2017). Moreover, the T-2 toxin enhances catabolic activity and inhibits anabolic reactivity in hypertrophic chondrocytes through the ROS-NF-κB-HIF-2α pathway (Tian et al., 2012). Finally, leg bone development abnormalities (defective tibia and metatarsus) may affect walking and balance, cause paralysis (clearly observed in the 2.0 mg/kg T-2 toxin group in the final week), and increase mutual contact and the occurrence of feather pecking.
Effect of T-2 Toxin on Blood Parameters
The blood system is an important factor in maintaining homeostasis of the internal environment and is essential for the metabolism of tissues and cells throughout the body and for the sustainability of life, as it contains various nutrients, cellular metabolites, hormones, enzymes, and antibodies. In the present study, 2.0 mg/kg of T-2 toxin diminished blood RBC, RDW, MPV, and P-LCR, and elevated MCH and PLT, promoting the occurrence of hemorrhagic anemia syndrome, platelet dysfunction, and coagulopathy (Doerr et al., 1981; Parent-Massin, 2004). Notable changes in serum enzyme activity can reflect vital organ damage under toxic effects. Consistent with previous studies (Pande et al., 2006), T-2 toxin also triggered serum ALT, AST, and AST/ALT dependent on dosage, exhibiting abnormal hepatic dysfunction and damage owing to hepatic degeneration and subsequent leakage of enzymes into circulation (Wang et al., 2009). It has been reported that T-2 toxin causes liver glycolipid metabolism disruption (Kang et al., 2020), induces hepatic pathological symptoms (Garcia et al., 2003), provokes oxidative stress (Yang et al., 2016), and leads to apoptosis as well as CYP450 drug metabolism disorders (Osselaere et al., 2013) in animals. Furthermore, in line with previous reports (Pande et al., 2006), the increased serum BUN and UA levels, which is catabolized waste, indicated potential kidney injury caused by T-2 toxin (Gowda et al., 2010). BUN is the main end-product of protein metabolism and can be used as a diagnostic and screening indicator of glomerular filtration (Gowda et al., 2010). Under normal renal function conditions, small molecules are filtered out of the glomerulus. When glomerular filtration is reduced, particularly with renal insufficiency, BUN levels increase because of retention. UA is a waste product of purine catabolism, and high UA can readily lead to gout in the severe condition of long-term purine metabolism disorder and renal insufficiency, triggering immune responses (Rock et al., 2013). Few studies have reported the clinical symptoms of visceral gout in geese fed with moldy food containing T-2 toxin, but subsequent attempts at the experimental production of gout have failed (Chi et al., 1977b; Siller, 1981). However, another explanation is that this increase was dependent on the poor utilization of protein for the inhibition of protein synthesis by T-2 toxin rather than on kidney dysfunction (Chi et al., 1977c; Coffin and Combs Jr, 1981; Singh et al., 2020). Therefore, the causes and relationships between BUN, UA, and renal injury of T-2 toxin require further research in the future. In addition, serum ALB, GLOB, and A/G remained unchanged, whereas TP increased with T-2 toxin contamination in diets, which is inconsistent with earlier findings (Pande et al., 2006; Manafi et al., 2015; Tso et al., 2021), which were accompanied by decreased serum TP, ALB, and GLOB under hepatic injury and immunosuppression. Here, it appeared not to be a direct result of protein synthesis inhibition or immunosuppression, but a slight stimulatory effect. In addition, the Ca level was halved under 0.8 mg/kg T-2 toxin contamination and a stimulation of serum P and a decreased Ca/P ratio of 0.8 mg/kg T-2 toxin and a promotion of serum ALP in the presence of 1.0 and 2.0 mg/kg T-2 toxin were observed, which was consistent with the previous studies (Chi et al., 1977a; Kubena et al., 1989). The aberrant alterations of Ca, P, Ca/P, and ALP indicate that T-2 toxin had a significant negative effect on normal Ca absorption and ALP activation, thus disturbing the calcification and development of bones (Chi et al., 1977a; Sergeev et al., 1990; Yadav et al., 2011). Previous studies have shown that excessive phosphorus enhances bone fragility in laying fowls and the imbalance of calcium and phosphorus possibly triggers bone disease in growing poultry (Chi et al., 1977a; Waldenstedt, 2006; Elwinger et al., 2016). With multiple organ damage (for example, liver, kidney, and bones), serum LDH increased, similar to previous research because of the cytotoxicity of T-2 toxin on cell viability to induce the release of intracellular LDH into the circulatory system (Pande et al., 2006; Yang et al., 2016; Yang et al., 2019).
CONCLUSIONS
Collectively, T-2 toxin exhibits a negative influence on growth performance, feather quality, tibia development, and blood parameters in Yangzhou goslings, and is therefore hazardous to animal health and welfare and reduces economic efficiency.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant number KYCX22_3529) and the Modern Agro-industry Technology Research System (CARS-42-3) and the “JBGS” Project of Seed Industry Revitalization in Jiangsu Province (JBGS (2021) 023) and the Project in Ministry of Agriculture and Rural Affairs, PRC (19211168).
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.102382.
Appendix. Supplementary materials
REFERENCES
- Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007;137:265–282. [Google Scholar]
- Chang Y., Wang X., Sun Z., Jin Z., Chen M., Wang X., Lammi M.J., Guo X. Inflammatory cytokine of IL-1β is involved in T-2 toxin-triggered chondrocyte injury and metabolism imbalance by the activation of Wnt/β-catenin signaling. Mol. Immunol. 2017;91:195–201. doi: 10.1016/j.molimm.2017.08.019. [DOI] [PubMed] [Google Scholar]
- Chen Y., Han S., Wang Y., Li D., Zhao X., Zhu Q., Yin H. Oxidative stress and apoptotic changes in broiler chicken splenocytes exposed to T-2 toxin. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/5493870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi M.S., Mirocha C.J., Kurtz H.J., Weaver G., Bates F., Shimoda W. Effects of T-2 toxin on reproductive performance and health of laying hens. Poult. Sci. 1977;56:628–637. doi: 10.3382/ps.0560628. [DOI] [PubMed] [Google Scholar]
- Chi M.S., Mirocha C.J., Kurtz H.J., Weaver G., Bates F., Shimoda W., Burmeister H.R. Acute toxicity of T-2 toxin in broiler chicks and laying hens. Poult. Sci. 1977;56:103–116. doi: 10.3382/ps.0560103. [DOI] [PubMed] [Google Scholar]
- Chi M.S., Mirocha C.J., Kurtz H.J., Weaver G., Bates F., Shimoda W. Subacute toxicity of T-2 toxin in broiler chicks. Poult. Sci. 1977;56:306–313. doi: 10.3382/ps.0560306. [DOI] [PubMed] [Google Scholar]
- Coffin J.L., Combs G.F., Jr Impaired vitamin E status of chicks fed T-2 toxin. Poult. Sci. 1981;60:385–392. doi: 10.3382/ps.0600385. [DOI] [PubMed] [Google Scholar]
- Dazuk V., Boiago M.M., Rolim G., Paravisi A., Copetti P.M., Bissacotti B.F., Morsch V.M., Vedovatto M., Gazoni F.L., Matte F., Gloria E.M., Da Silva A.S. Laying hens fed mycotoxin-contaminated feed produced by fusarium fungi (T-2 toxin and fumonisin B1) and saccharomyces cerevisiae lysate: impacts on poultry health, productive efficiency, and egg quality. Microb. Pathog. 2020;149 doi: 10.1016/j.micpath.2020.104517. [DOI] [PubMed] [Google Scholar]
- DeLoach J.R., Gyongyossy-Issa M.I.C, Khachatourians G.G. Species-specific hemolysis of erythrocytes by T-2 toxin. Toxicol. Appl. Pharmacol. 1989;97:107–112. doi: 10.1016/0041-008x(89)90059-8. [DOI] [PubMed] [Google Scholar]
- Doerr J.A., Hamilton P.B., Burmeister H.R. T-2 toxicosis and blood coagulation in young chickens. Toxicol. Appl. Pharmacol. 1981;60:157–162. doi: 10.1016/0041-008x(91)90219-5. [DOI] [PubMed] [Google Scholar]
- Escrivá L., Font G., Manyes L. In vivo toxicity studies of fusarium mycotoxins in the last decade: a review. Food Chem. Toxicol. 2015;78:185–206. doi: 10.1016/j.fct.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Elwinger K., Fisher C., Jeroch H., Sauveur B., Tiller H., Whitehead C. A brief history of poultry nutrition over the last hundred years. World's Poult. Sci. J. 2016;72:701–720. [Google Scholar]
- Farquharson C., Jefferies D. Chondrocytes and longitudinal bone growth: the development of tibial dyschondroplasia. Poult. Sci. 2000;79:994–1004. doi: 10.1093/ps/79.7.994. [DOI] [PubMed] [Google Scholar]
- Fernye C., Ancsin Z., Bócsai A., Balogh K., Mézes M., Erdélyi M. Role of glutathione redox system on the T-2 toxin tolerance of pheasant (phasianus colchicus) Toxicol. Res. 2018;34:249–257. doi: 10.5487/TR.2018.34.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaigé S., Djelloul M., Tardivel C., Airault C., Félix B., Jean A., Lebrun B., Troadec J.D., Dallaporta M. Modification of energy balance induced by the food contaminant T-2 toxin: a multimodal gut-to-brain connection. Brain Behav. Immun. 2014;37:54–72. doi: 10.1016/j.bbi.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Gao Y., Du Z.Q., Feng C.G., Deng X.M., Li N., Da Y., Hu X.X. Identification of quantitative trait loci for shank length and growth at different development stages in chicken. Anim. Genet. 2010;41:101–104. doi: 10.1111/j.1365-2052.2009.01962.x. [DOI] [PubMed] [Google Scholar]
- Garcia A.R., Avila E., Rosiles R., Petrone V.M. Evaluation of two mycotoxin binders to reduce toxicity of broiler diets containing ochratoxin A and T-2 toxin contaminated grain. Avian Dis. 2003;47:691–699. doi: 10.1637/7021. [DOI] [PubMed] [Google Scholar]
- Gholampour Azizi I., Azarmi M., Pouya N.Danesh, Rouhi S. T-2 toxin analysis in poultry and cattle feedstuff. Jundishapur J. Nat. Pharm. Prod. 2014;9:e13734. doi: 10.17795/jjnpp-13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda S., Desai P.B., Kulkarni S.S., Hull V.V., Math A.A.K., Vernekar S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010;2:170–173. [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Tang H., Wang X., Li W., Wang Y., Yan F., Kang X., Li Z., Han R. Clinical assessment of growth performance, bone morphometry, bone quality, and serum indicators in broilers affected by valgus-varus deformity. Poult. Sci. 2019;98:4433–4440. doi: 10.3382/ps/pez269. [DOI] [PubMed] [Google Scholar]
- Hayes M.A., Wobeser G.A. Subacute toxic effects of dietary T-2 toxin in young mallard ducks. Can. J. Comp. Med. 1983;47:180–187. [PMC free article] [PubMed] [Google Scholar]
- He S.J., Hou J.F., Dai Y.Y., Zhou Z.L., Deng Y.F. N-acetyl-cysteine protects chicken growth plate chondrocytes from T-2 toxin-induced oxidative stress. J. Appl. Toxicol. 2012;32:980–985. doi: 10.1002/jat.1697. [DOI] [PubMed] [Google Scholar]
- Hoerr F.J., Carlton W.W., Tuite J., Vesonder R.F., Rohwedder W.K., Szigeti G. Experimental trichothecene mycotoxicosis produced in broiler chickens by Fusarium sporotrichiella var. sporotrichioides. Avian Pathol. 1982;11:385–405. doi: 10.1080/03079458208436113. [DOI] [PubMed] [Google Scholar]
- Hoerr F.J., Carlton W.W., Yagen B. Mycotoxicosis caused by a single dose of T-2 toxin or diacetoxyscirpenol in broiler chickens. Vet. Pathol. 1981;18:652–664. doi: 10.1177/030098588101800510. [DOI] [PubMed] [Google Scholar]
- Hoerr F.J., Carlton W.W., Yagen B., Joffe A.Z. Mycotoxicosis produced in broiler chickens by multiple doses of either T-2 toxin or diacetoxyscirpenol. Avian Pathol. 1982;11:369–383. doi: 10.1080/03079458208436112. [DOI] [PubMed] [Google Scholar]
- Huang L., Zhu L., Ou Z., Ma C., Kong L., Huang Y., Chen Y., Zhao H., Wen L., Wu J., Yuan Z., Yi J. Betulinic acid protects against renal damage by attenuation of oxidative stress and inflammation via Nrf2 signaling pathway in T-2 toxin-induced mice. Int. Immunopharmacol. 2021;101 doi: 10.1016/j.intimp.2021.108210. [DOI] [PubMed] [Google Scholar]
- Janik E., Niemcewicz M., Podogrocki M., Ceremuga M., Stela M., Bijak M. T-2 toxin-the most toxic trichothecene mycotoxin: metabolism, toxicity, and decontamination strategies. Molecules. 2021;26:6868. doi: 10.3390/molecules26226868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens A.M., Offner F.C., Van Hove W.Z. Bone marrow necrosis. Cancer. 2000;88:1769–1780. [PubMed] [Google Scholar]
- Kang R., Perveen A., Li C. Effects of maternal T-2 toxin exposure on the hepatic glycolipid metabolism in young mice. Ecotoxicol. Environ. Saf. 2020;196 doi: 10.1016/j.ecoenv.2020.110530. [DOI] [PubMed] [Google Scholar]
- Kubena L.F., Harvey R.B., Huff W.E., Corrier D.E., Philips T.D., Rottinghaus G.E. Influence of ochratoxin A and T-2 toxin singly and in combination on broiler chickens. Poult. Sci. 1989;68:867–872. doi: 10.3382/ps.0680867. [DOI] [PubMed] [Google Scholar]
- Kuca K., Dohnal V., Jezkova A., Jun D. Metabolic pathways of T-2 toxin. Curr. Drug Metab. 2008;9:77–82. doi: 10.2174/138920008783331176. [DOI] [PubMed] [Google Scholar]
- Leeson S., Walsh T. Feathering in commercial poultry II. Factors influencing feather growth and feather loss. World's Poult. Sci. J. 2004;60:52–63. [Google Scholar]
- Li D., Han J., Guo X., Qu C., Yu F., Wu X. The effects of T-2 toxin on the prevalence and development of Kashin-Beck disease in China: a meta-analysis and systematic review. Toxicol. Res. 2016;5:731–751. doi: 10.1039/c5tx00377f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.J., Zhang G., Xue B., Ding Q., Han L., Huang J.C., Wu F., Li C., Yang C. Toxicity and detoxification of T-2 toxin in poultry. Food Chem. Toxicol. 2022;169 doi: 10.1016/j.fct.2022.113392. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang Z., Beier R.C., Shen J., Smet D.D., De Saeger S., Zhang S. T-2 toxin, a trichothecene mycotoxin: review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011;59:3441–3453. doi: 10.1021/jf200767q. [DOI] [PubMed] [Google Scholar]
- Lin M.J., Chang S.C., Tso K.H., Lin W.C., Chang C.L., Lee T.T. Effect of T-2 toxin and antioxidants on angel wing incidence and severity in White Roman geese. J. Appl. Anim. Res. 2018;46:340–348. [Google Scholar]
- Liu X., Guo P., Liu A., Wu Q., Xue X., Dai M., Hao H., Qu W., Xie S., Wang X., Yuan Z. Nitric oxide (NO)-mediated mitochondrial damage plays a critical role in T-2 toxin-induced apoptosis and growth hormone deficiency in rat anterior pituitary GH3 cells. Food Chem. Toxicol. 2017;102:11–23. doi: 10.1016/j.fct.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Liu Z.L., Xue J.J., Huang X.F., Chen Y., Wang Q.G., Zhang S., Wang C. Effect of stocking density on growth performance, feather quality, serum hormone, and intestinal development of geese from 1 to 14 days of age. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackei M., Matis G., Neogrady Z. The effects of T-2 toxin on animal health, focusing especially on poultry: literature review. Magy. Allatorv. Lapja. 2018;140:475–483. [Google Scholar]
- Magnoli A.P., Poloni V.L., Cavaglieri L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019;29:99–108. [Google Scholar]
- Mahmoud U.T., Abdel-Rahman M.A.M., Darwish M.H.A., Applegate T.J., Cheng H.W. Behavioral changes and feathering score in heat stressed broiler chickens fed diets containing different levels of propolis. Appl. Anim. Behav. Sci. 2015;166:98–105. [Google Scholar]
- Manafi M., Pirany N., Ali M.N., Hedayati M., Khalaji S., Yari M. Experimental pathology of T-2 toxicosis and mycoplasma infection on performance and hepatic functions of broiler chickens. Poult. Sci. 2015;94:1483–1492. doi: 10.3382/ps/pev115. [DOI] [PubMed] [Google Scholar]
- Marenzana M., Arnett T.R. The key role of the blood supply to bone. Bone Res. 2013;1:203–215. doi: 10.4248/BR201303001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mézes M., Barta M., Nagy G. Comparative investigation on the effect of T-2 mycotoxin on lipid peroxidation and antioxidant status in different poultry species. Res. Vet. Sci. 1999;66:19–23. doi: 10.1053/rvsc.1998.0233. [DOI] [PubMed] [Google Scholar]
- Morrissey K.L.H., Widowski T., Leeson S., Sandilands V., Arnone A., Torrey S. The effect of dietary alterations during rearing on feather condition in broiler breeder females. Poult. Sci. 2014;93:1636–1643. doi: 10.3382/ps.2013-03822. [DOI] [PubMed] [Google Scholar]
- Nascimento J.A.F.B., Nunes V.A., Guedes R.M.C., Rachid M.A. T-2 toxin and disturbed endochondral bone growth in broiler chicken. Arq. Bras. Med. Vet. Zootec. 2001;53:332–341. [Google Scholar]
- Nguansangiam S., Angsubhakorn S., Bhamarapravati S., Suksamrarn A. Effects of elephant garlic volatile oil (Allium ampeloprasum) and T-2 toxin on murine skin. Southeast Asian J. Trop. Med. Public Health. 2003;34:899–905. [PubMed] [Google Scholar]
- Ning Y., Hu M., Diao J., Gong Y., Huang R., Chen S., Zhang F., Liu Y., Chen F., Zhang P., Zhao G., Chang Y., Xu K., Zhou R., Li C., Zhang F., Lammi M., Wang X., Guo X. Genetic variants and protein alterations of selenium- and T-2 toxin-responsive genes are associated with chondrocytic damage in endemic osteoarthropathy. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.773534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osselaere A., Li S.J., De Bock L., Devreese M., Goossens J., Vandenbroucke V., Van Bocxlaer J., Boussery K., Pasmans F., Martel A., De Backer P., Croubels S. Toxic effects of dietary exposure to T-2 toxin on intestinal and hepatic biotransformation enzymes and drug transporter systems in broiler chickens. Food Chem. Toxicol. 2013;55:150–155. doi: 10.1016/j.fct.2012.12.055. [DOI] [PubMed] [Google Scholar]
- Pande V.V., Kurkure N.V., Bhandarkar A.G. Effect of T-2 toxin on growth, performance and haematobiochemical alterations in broilers. Indian J. Exp. Biol. 2006;44:86–88. [PubMed] [Google Scholar]
- Parent-Massin D. Haematotoxicity of trichothecenes. Toxicol. Lett. 2004;153:75–81. doi: 10.1016/j.toxlet.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Puls R., Greenway J.A. Fusariotoxicosis from barley in British Columbia. II. Analysis and toxicity of syspected barley. Can. J. Comp. Med. 1976;40:16–19. [PMC free article] [PubMed] [Google Scholar]
- Rafai P., Pettersson H., Bata A., Papp Z., Glávits R., Tuboly S., Ványi A., Soós P. Effect of dietary T-2 fusariotoxin concentrations on the health and production of white Pekin duck broilers. Poult. Sci. 2000;79:1548–1556. doi: 10.1093/ps/79.11.1548. [DOI] [PubMed] [Google Scholar]
- Rezar V., Frankic T., Narat M., Levart A., Salobir J. Dose-dependent effects of T-2 toxin on performance, lipid peroxidation, and genotoxicity in broiler chickens. Poult. Sci. 2007;86:1155–1160. doi: 10.1093/ps/86.6.1155. [DOI] [PubMed] [Google Scholar]
- Rock K.L., Kataoka H., Lai J.J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 2013;9:13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher-Wolz U., Heine K., Schneider K. Report on toxicity data on trichothecene mycotoxins HT-2 and T-2 toxins. EFSA Supporting Publ. 2010;7:65E. [Google Scholar]
- Sergeev I., Piliia N.M., Tutel'ian V.A., Spirichev V.B. Mechanism of impairment of calcium metabolism caused by toxin T-2; the role of the vitamin D-dependent endocrine system. Vopr. Med. Khim. 1990;36:45–48. [PubMed] [Google Scholar]
- Sheng K., Lu X., Yue J., Gu W., Gu C., Zhang H., Wu W. Role of neurotransmitters 5-hydroxytryptamine and substance P in anorexia induction following oral exposure to the trichothecene T-2 toxin. Food Chem. Toxicol. 2019;123:1–8. doi: 10.1016/j.fct.2018.10.041. [DOI] [PubMed] [Google Scholar]
- Siller W.G. Renal pathology of the fowl - a review. Avian Pathol. 1981;10:187–262. doi: 10.1080/03079458108418474. [DOI] [PubMed] [Google Scholar]
- Singh R., Park S., Koo J.S., Kim I.H., Balasubramanian B. Significance of varying concentrations of T-2 toxin on growth performance, serum biochemical and hematological parameters in broiler chickens. J. Anim. Sci. Technol. 2020;62:468–474. doi: 10.5187/jast.2020.62.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolović M., Garaj-Vrhovac V., Šimpraga B. T-2 toxin: incidence and toxicity in poultry. Arh. Hig. Rada Toksikol. 2008;59:43–52. doi: 10.2478/10004-1254-59-2008-1843. [DOI] [PubMed] [Google Scholar]
- Sun Y., Huang K., Long M., Yang S., Zhang Y. An update on immunotoxicity and mechanisms of action of six environmental mycotoxins. Food Chem. Toxicol. 2022;163 doi: 10.1016/j.fct.2022.112895. [DOI] [PubMed] [Google Scholar]
- Szabó R.T., Kovács-Weber M., Erdélyi M., Balogh K., Fazekas N., Horváth Á., Mézes M., Kovács B. Comet assay study of the genotoxic effect of T-2 and HT-2 toxins in chicken hepatocytes. Biol. Futura. 2019;70:330–335. doi: 10.1556/019.70.2019.37. [DOI] [PubMed] [Google Scholar]
- Tian J., Yan J., Wang W., Zhong N., Tian L., Sun J., Min Z., Ma J., Lu S. T-2 toxin enhances catabolic activity of hypertrophic chondrocytes through ROS-NF-κB-HIF-2α pathway. Toxicol. Vitro. 2012;26:1106–1113. doi: 10.1016/j.tiv.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Tso K.H., Lumsangkul C., Cheng M.C., Ju J.C., Fan Y.K., Chiang H.I. Differential effects of green tea powders on the protection of Brown Tsaiya and Kaiya ducklings against trichothecene T-2 toxin toxicity. Animals. 2021;11:2541. doi: 10.3390/ani11092541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukwu H.O., Okoro V.M.O., Nosike R.J. Statistical modelling of body weight and linear body measurements in Nigerian indigenous chicken. IOSR J. Agric. Vet. Sci. 2014;7:27–30. [Google Scholar]
- Vanyi A., Bata A., Kovacs F. Effects of T-2 toxin treatment on the egg yield and hatchability in geese. Acta Vet. Hung. 1994;42:79–85. [PubMed] [Google Scholar]
- Waldenstedt L. Nutritional factors of importance for optimal leg health in broilers: a review. Anim. Feed Sci. Technol. 2006;126:291–307. [Google Scholar]
- Wan Q., He Q., Deng X., Hao F., Tang H., Wang Y. Systemic metabolic responses of broiler chickens and piglets to acute T-2 toxin intravenous exposure. J. Agric. Food Chem. 2016;64:714–723. doi: 10.1021/acs.jafc.5b05076. [DOI] [PubMed] [Google Scholar]
- Wang G.H., Xue C.Y., Chen F., Ma Y.L., Zhang X.B., Bi Y.Z., Cao Y.C. Effects of combinations of ochratoxin A and T-2 toxin on immune function of yellow-feathered broiler chickens. Poult. Sci. 2009;88:504–510. doi: 10.3382/ps.2008-00329. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu S., Zheng H., He C., Zhang H. T-2 toxin, zearalenone and fumonisin B₁ in feedstuffs from China. Food. Addit. Contam. Part B, Surveill. 2013;6:116–122. doi: 10.1080/19393210.2013.764506. [DOI] [PubMed] [Google Scholar]
- Wei J.T., Wu K.T., Sun H., Khalil M.M., Dai J.F., Liu Y., Liu Q., Zhang N.Y., Qi D.S., Sun L.H. A novel modified hydrated sodium calcium aluminosilicate (HSCAS) adsorbent can effectively reduce T-2 toxin-induced toxicity in growth performance, nutrient digestibility, serum biochemistry, and small intestinal morphology in chicks. Toxins. 2019;11:199. doi: 10.3390/toxins11040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G.C., Jr, Marasas W.F.O., Sokoloff L. Effect of fusarochromanone and T-2 toxin on articular chondrocytes in monolayer culture. Fundam. Appl. Toxicol. 1987;9:595–597. doi: 10.1016/0272-0590(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Wyatt R.D., Hamilton P.B., Burmeister H.R. Altered feathering of chicks caused by T-2 toxin. Poult. Sci. 1975;54:1042–1045. doi: 10.3382/ps.0541042. [DOI] [PubMed] [Google Scholar]
- Yadav M.C., Simao A.M.S., Narisawa S., Huesa C., McKee M.D., Farquharson C., Millán J.L. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J. Bone Miner. Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Tu D., Wang N., Deng Z., Zhan Y., Liu W., Hu Y., Liu T., Tan L., Li Y., Guo S., Wang A. The protective effects of DL-Selenomethionine against T-2/HT-2 toxins-induced cytotoxicity and oxidative stress in broiler hepatocytes. Toxicol. Vitro. 2019;54:137–146. doi: 10.1016/j.tiv.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Yang L., Yu Z., Hou J., Deng Y., Zhou Z., Zhao Z., Cui J. Toxicity and oxidative stress induced by T-2 toxin and HT-2 toxin in broilers and broiler hepatocytes. Food Chem. Toxicol. 2016;87:128–137. doi: 10.1016/j.fct.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Yin H., Han S., Chen Y., Wang Y., Li D., Zhu Q. T-2 toxin induces oxidative stress, apoptosis and cytoprotective autophagy in chicken hepatocytes. Toxins. 2020;12:90. doi: 10.3390/toxins12020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohannes T., Sharma A.K., Sumi V., Rout S.S. Clinical manifestation and growth performance of broiler chickens fed with T-2 toxin and co-infected with infectious bronchitis virus. ISABB J. Food. Agric. Sci. 2012;2:30–35. [Google Scholar]
- Yu J., Wan Y., Yang H., Wang Z. Age- andsex-related changes in body weight, muscle, and tibia in growing chinese domestic geese (anser domesticus) Agriculture. 2022;12:463. [Google Scholar]
- Zhang J., You L., Wu W., Wang X., Chrienova Z., Nepovimova E., Wu Q., Kuca K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): current status and future perspectives. Food Chem. Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111676. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang M., Wang H., Zhang Y., Li Z., Feng Y., Liu Y., Liu Y., Liao Y., Wang W., Fang Q., Chen J. Decreased expression of heat shock protein 47 is associated with T-2 toxin and low selenium-induced matrix degradation in cartilages of Kashin-Beck disease. Biol. Trace Elem. Res. 2021;199:944–954. doi: 10.1007/s12011-020-02237-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.