Abstract
Objectives
This study aimed to investigate the physical and emotional effects of the coronavirus disease 2019 pandemic in patients with fibromyalgia syndrome (FMS) and chronic low back pain (CLBP) patients.
Patients and methods
The cross-sectional controlled study was performed with 1,360 participants (332 males, 1,028 females; mean age: 42.3±12.5 years; range, 18 to 65 years) between September 2020 and February 2021. The participants were evaluated in three groups: the FMS group (n=465), the CLBP group (n=455), and the healthy control group (n=440). Physical activity, pain levels, and general health status before and during the pandemic were evaluated in all participants. Stress levels were analyzed with the perceived stress scale (PSS) in all groups, and disease activity was analyzed with the fibromyalgia impact questionnaire (FIQ) in patients with FMS.
Results
Patients with FMS had worsened general health status and pain levels during the pandemic compared to the other groups (p<0.01). The FMS group showed significantly higher PSS scores than those in other groups (p<0.01). There was a weak-positive correlation between FIQ and PSS parameters in patients with FMS (p<0.05, r=0.385).
Conclusion
The general health status, pain, and stress levels of the patients with FMS and CLBP tended to worsen during the pandemic. This high-stress level appeared to affect disease activity in patients with FMS.
Keywords: Chronic low back pain, coronavirus disease 2019 pandemic, fibromyalgia
Introduction
The effect of the coronavirus disease 2019 (COVID-19) pandemic on society has been becoming more profound every day.[1] Although ongoing vaccine studies and vaccination activities give us hope, additional precautions and restrictions due to emerging mutant coronavirus species prevent people from participating in active life. Limitation of actions can lead to the worsening of both physical and psychological well-being.[2] Exercise is the most effective approach to preventing this situation; however, there are challenges in accessing rehabilitation services during the pandemic.[3] Although the mentioned factors affect even the healthy population, it is inevitable to observe a more dramatic effect in patients with diseases that mainly manifest with pain, such as fibromyalgia syndrome (FMS) and chronic low back pain (CLBP).[4,5]
Regular outpatient follow-up has agreat importance in FMS, as the disease can be activated without regular treatment and is negatively affected by anxiety, stress, depression, and sedentary lifestyle. However, there is limited data on FMS during the pandemic. According to the literature, pain, anxiety, and depression were evaluated during the pandemic in a study performed with female patients diagnosed with FMS.[6] In another study, patients with FMS were analyzed with the fibromyalgia impact questionnaire (FIQ) administered via email to compare curfew with no curfew.[7] In another study conducted with female patients with FMS, the pain, FIQ, anxiety, depression, sleep quality, and neuropathic pain were compared between the prepandemic period and the period of curfew.[8] Moreover, a study that reached patients with FMS through an online questionnaire evaluated access to health services during the pandemic, strategies to combat the pandemic, as well as social support conditions, general pain, and symptom status of the patients.[9] Like FMS, CLBP is also a chronic pain syndrome, and data about the effects of the pandemic on patients with CLBP are limited. Licciardone[5] investigated the availability of noninvasive treatment methods for patients with CLBP during the COVID-19 pandemic. However, there are no multicenter nationwide studies including the healthy population as the control group and face- to-face survey methods to compare the prepandemic period with the pandemic period in terms of the two common chronic pain syndromes, FMS and CLBP. Hence, the present study aimed to evaluate the physical activity status, medical treatments, work status, pain levels, general health status, and stress levels during the pandemic and the prepandemic period, as well as the effects of the pandemic on disease activity in chronic pain patients with FMS and CLBP, and to compare these parameters with the healthy population.
Patients and Methods
This multicenter, analytic, controlled, and cross- sectional study was performed with 1,360 participants (332 males, 1,028 females; mean age: 42.3±12.5 years; range, 18 to 65 years) between September 2020 and February 2021. The patients were selected from 31 different physical medicine and rehabilitation (PMR) outpatient clinics in Türkiye. The patients were evaluated in three groups: the FMS group (n=465), the CLBP group (n=455), and the healthy control group (n=440). Patients who were admitted to PMR outpatient clinics were evaluated by PMR specialists. The diagnosis of FMS was based on the 2016 American College of Rheumatology diagnostic criteria.[10] Healthy volunteers without any health problems were included in the control group. The flow chart of the study is presented in Figure 1. Inclusion criteria were as follows: (i) healthy volunteers were selected from the ones who did not have widespread pain or low back pain during the prepandemic period; (ii) for the CLBP group, patients with pain for at least three months (Visual Analog Scale score of ≥4) at rest, at night, or during physical activity were included.[11] Exclusion criteria were as follows: (i) for the FMS group, the presence of major depression, history of chronic diseases, such as heart disease, chronic organ failure, diabetes mellitus, and rheumatic diseases, and history of malignancy; (ii) for the CLBP group, history of spinal surgery, rheumatic disease, radiculopathy, malignancy, spine fractures, and infection disease.
Figure 1. Study flow chart.
A face-to-face survey was applied to all patients to compare the prepandemic and pandemic periods. The survey mainly included demographic properties, COVID-19 history, drug history, physical activity level, pain intensity, general health status, stress/ anxiety, and quality of life parameters. The patients' sociodemographic data, age, sex, marital status, body mass index (BMI), and, if needed, hospital visits were recorded. The drugs were categorized as antidepressants, serotonin-norepinephrine reuptake inhibitors, gabapentin, myorelaxants, nonsteroidal anti-inflammatory drugs (NSAIDs), paracetamol, pregabalin, and tramadol.
Physical activity levels were categorized and recorded as “I do not engage in physical activity,” “I do daily chores at home,” “I walk daily,” and “I exercise regularly.” The change with the pandemic was questioned, and the job status was categorized as "I am a housewife," "I work from home," "I work part-time," "I quit my job," and "no change." Level of pain and general health status were categorized as “much worse,” “worse,” “a little worse,” “no change,” “a little better,” “better,” and “much better.”
Patient stress was evaluated with the perceived stress scale (PSS), which was designed to measure how stressful some situations in a person's life were perceived. Evaluation statements of this 5-point Likert-type scale were classified as "never," "almost never," "sometimes," "fairly often," and "very often." The stress level perceived by the respondent was determined by adding the scores obtained from the items, and a high score indicates a high level of perceived stress.[12] The Turkish reliability and validity study of the scale has been conducted.[12]
The FIQ is a specific 10-item scale that evaluates the physical function and health status in fibromyalgia. In the first item, there are 11 questions about physical functionality. In items 2 and 3, the patient was asked about the number of days they felt well and the number of days they could not work (including housework) due to fibromyalgia symptoms. Items 4 to 10 assess the patient ʼs work difficulty, pain, fatigue, morning fatigue, stiffness, anxiety, and depression.[13] Only FMS patients were evaluated with the FIQ. The Turkish reliability and validity study of the scale was performed.[13]
Statistical analysis
Statistical analyses were performed with the IBM SPSS version 21.0 software (IBM Corp., Armonk, NY, USA). The distribution of the quantitative data was examined using the Shapiro-Wilk test for normality assumption and Levene’s test for homogeneity of variances, and they were expressed as mean±standard deviation (SD) or median and interquartile range (IQR). The Fisher Freeman-Halton test and the chi-square test were used to identify differences in proportions or association between categorical variables. The Wilcoxon signed-rank test was used to compare measurements of the same participant at different time points. Pearson correlation analysis was used to calculate the correlation between two numerical variables. The McNemar test was used to assess the dependence of matched categorical variables. The Kruskal-Wallis test was used for age comparisons. The Bonferroni post hoc correction method was used for multiple comparisons between groups. A p value <0.05 was considered statistically significant.
Results
The median (IQR) age in the FMS, CLBP, and healthy control groups was 45 (13), 45 (17), and 36 (15), respectively. Females were predominant in all three groups with 89% (n=414), 66.2% (n=301), 71.1% (n=313) in the FMS, CLP and healthy control groups, respectively (Table 1).
Table 1. Demographic features of the participants.
| FMS group | CLBP group | Healthy control group | ||||||||||||||
| Features | n | % | Mean±SD | Median | Q1-Q3 | n | % | Mean±SD | Median | Q1-Q3 | n | % | Mean±SD | Median | Q1-Q3 | p |
| Age (year) | 45.0±10.0** | 45 | 38-51 | 45.1±12.5** | 45 | 37-54 | 37.0±10.0 | 36 | 29-44 | |||||||
| Sex | <0.001* | |||||||||||||||
| Male | 51 | 11.0† | 154 | 33.8‡ | 127 | 28.9‡ | ||||||||||
| Female | 414 | 89.0‡ | 301 | 66.2† | 313 | 71.1† | ||||||||||
| Having a child | <0.001* | |||||||||||||||
| Yes | 376 | 80.9‡ | 335 | 73.8 | 269 | 61.6† | ||||||||||
| No | 89 | 19.1† | 119 | 26.2 | 168 | 38.4‡ | ||||||||||
| Education | <0.001* | |||||||||||||||
| Primary school | 211 | 45.4‡ | 158 | 34.8 | 43 | 9.8 | ||||||||||
| High school | 111 | 23.9 | 128 | 28.2 | 100 | 22.7 | ||||||||||
| University | 143 | 30.8† | 168 | 37.0† | 297 | 67.5‡ | ||||||||||
| Marital status | <0.001* | |||||||||||||||
| Single | 62 | 13.3† | 61 | 13.4† | 121 | 27.5‡ | ||||||||||
| Divorced | 31 | 6.7 | 30 | 6.6 | 31 | 7.0 | ||||||||||
| Married | 372 | 80.0‡ | 364 | 80.0† | 288 | 65.5† | ||||||||||
| FMS: Fibromyalgia syndrome; CLBP: Chronic low back pain; SD: Standard deviation; * Pearson chi-squared test; ** Kruskal Wallis test; Percentages with ‡ and † indicate higher and lower values than expected values considering the independence, respectively. | ||||||||||||||||
A significant increase in weight and BMI in all groups was noted during the pandemic compared to the prepandemic period (p<0.01, Table 2). Regarding visits to the outpatient clinic during the pandemic, proportion of the patients who admitted to outpatient clinic was highest in the patients with FMS (Table 3). Patients with CLBP were mostly admitted to a health center with the complaint of low back pain (n=103, 51.8%; p<0.01, Table 3).
Table 2. Weight and BMI measurements before and during the pandemic.
| Before pandemic | During pandemic | Difference | ||||||||
| Measurements | Mean±SD | Median | Q1-Q3 | Mean±SD | Median | Q1-Q3 | Mean±SD | Median | Q1-Q3 | p |
| FMS group | ||||||||||
| Weight (kg) | 71.4±12.6 | 70 | 63-79 | 72.3±12.5 | 70 | 64-80 | 0.9±3.1 | 0 | 0-2 | <0.001 |
| BMI (kg/cm2) | 26.7±4.7 | 26.1 | 23.44-29 | 27.0±4.6 | 26.56 | 23.6-29.3 | 0.3±1.3 | 0 | 0-0.8 | <0.001 |
| CLBP group | ||||||||||
| Weight (kg) | 76.1±14.1 | 75 | 65.5-85 | 76.6±14.0 | 76 | 23.6-29.3 | 0.5±3.5 | 12 | 0-2 | 0.001 |
| BMI (kg/cm2) | 26.8±4.4 | 26.4 | 23.5-29.4 | 27.0±4.4 | 26.81 | 23.9-30 | 0.2±1.1 | 0 | 0-0.7 | <0.001 |
| Healthy control group | ||||||||||
| Weight (kg) | 68.5±13.2 | 66 | 58.5-78 | 69.0±13.6 | 67 | 59-78 | 0.5±2.9 | 20 | 0-1 | <0.001 |
| BMI (kg/cm2) | 24.6±4.1 | 24 | 21.6-27 | 24.8±4.3 | 24.03 | 21.9-27 | 0.2±1.0 | 0 | 0-0.48 | <0.001 |
| BMI: Body mass index; SD: Standard deviation; FMS: Fibromyalgia syndrome; CLBP: Chronic low back pain; Wilcoxon signed rank test. | ||||||||||
Table 3. Departments that the participants applied to before and during the pandemic period.
| Question | FMS group | CLBP group | Healthy control group | p | |||
| n | % | n | % | n | % | ||
| Have you ever admitted any outpatient clinic during pandemic? | <0.001* | ||||||
| Yes | 267 | 57.54 | 199 | 44.1 | 131 | 29.8t | |
| No | 197 | 42.5t | 252 | 55.9 | 309 | 70.24 | |
| For what reason? | <0.001** | ||||||
| COVID-19 | 11 | 4.2t | 20 | 10.1 | 48 | 40.34 | |
| Others | 60 | 23.1 | 58 | 29.1 | 46 | 38.7 | |
| Low back pain | 35 | 13.5t | 103 | 51.84 | 7 | 5.9 | |
| Chest pain | 6 | 2.3 | 5 | 2.5 | 0 | 0.0 | |
| For prescription | 15 | 5.84 | 0 | 0.0 | 0 | 0.0 | |
| Abdominal pain | 13 | 5.0 | 4 | 2.0 | 5 | 4.2 | |
| Diffuse pain | 120 | 46.24 | 9 | 4.5t | 13 | 10.9t | |
| Which department? | <0.001** | ||||||
| Emergency | 33 | 12.4 | 23 | 11.6 | 40 | 34.54 | |
| Internal medicine | 14 | 5.3 | 12 | 6.0 | 4 | 3.4 | |
| Infection disease | 5 | 1.9t | 8 | 4.0 | 21 | 18.14 | |
| Physical medicine and rehabilitation | 146 | 54.94 | 70 | 35.2 | 12 | 10.3t | |
| General surgery | 2 | 0.8 | 1 | 0.5 | 0 | 0.0 | |
| Chest disease | 2 | 0.8 | 2 | 1.0 | 4 | 3.4 | |
| Psychiatry | 3 | 1.1 | 3 | 1.5 | 2 | 1.7 | |
| Others | 67 | 25 | 80 | 40 | 48 | 36.6 | |
| FMS: Fibromyalgia syndrome; CLBP: Chronic low back pain; COVID-19: Coronavirus disease-2019; * Pearson chi-squared test; ** Fisher Freeman-Halton test; Percentages with ‡ and † indicate higher and lower values than expected values considering the independence, respectively. | |||||||
In all groups, drug usage habits during the pandemic showed no significant difference compared to the prepandemic period except in the CLBP group (Table 4), in which a significant increase in the use of amitriptyline, food supplements, and pregabalin (all p values <0.05). Furthermore, a significant decrease was observed in the use of duloxetine and oral muscle relaxants in the CLBP group (all p values <0.05).
Table 4. Medications used by the participants.
| Variables | FMS group | CLBP group | Healthy control group | p | |||
| n | % | n | % | n | % | ||
| Drug using before pandemic | <0.001** | ||||||
| Amitriptyline | 12 | 2.6‡ | 1 | 0.2 | 0 | 0.0 | |
| Food supplement | 5 | 1.1† | 12 | 2.7 | 34 | 7.7‡ | |
| Duloxetine | 206 | 44.9‡ | 33 | 7.5† | 2 | 0.5† | |
| Gabapentin | 10 | 2.2 | 17 | 3.9‡ | 0 | 0.0† | |
| Muscle relaxants | 42 | 9.2 | 81 | 18.4‡ | 4 | 0.9† | |
| Muscle relaxants topical | 3 | 0.7 | 12 | 2.74 | 0 | 0.0 | |
| Not using | 34 | 7.4† | 126 | 28.6† | 385 | 87.5‡ | |
| NSAID oral | 34 | 7.4 | 96 | 21.8‡ | 2 | 0.5 | |
| NSAID topical | 0 | 0.0 | 17 | 3.9‡ | 1 | 0.2 | |
| Paracetamol | 10 | 2.2 | 19 | 4.3 | 11 | 2.5 | |
| Pregabalin | 63 | 13.7‡ | 20 | 4.5 | 0 | 0.0† | |
| Pregabalin-Duloxetine | 15 | 3.3‡ | 0 | 0.0 | 0 | 0.0 | |
| SSRI | 25 | 5.4‡ | 4 | 0.9 | 1 | 0.2† | |
| Tramadol | 0 | 0.0 | 3 | 0.7 | 0 | 0.0 | |
| Drug using after pandemic | <0.001** | ||||||
| Amitriptyline | 14 | 3.0 | 7 | 1.6 | 0 | 0.0 | |
| Food supplement | 9 | 2.0† | 20 | 4.5 | 35 | 8.0‡ | |
| Duloxetine | 209 | 45.4‡ | 25 | 5.7† | 2 | 0.5† | |
| Gabapentin | 12 | 2.6 | 12 | 2.7 | 0 | 0.0† | |
| Muscle relaxants | 40 | 8.7 | 64 | 14.5‡ | 5 | 1.1† | |
| Muscle relaxants topical | 3 | 0.7 | 14 | 3.2‡ | 1 | 0.2 | |
| Not using | 29 | 6.3† | 130 | 29.5† | 380 | 86.4‡ | |
| NSAID oral | 33 | 7.2 | 92 | 20.9‡ | 3 | 0.7† | |
| NSAID topical | 2 | 0.4 | 19 | 4.3‡ | 0 | 0.0 | |
| Paracetamol | 13 | 2.8 | 24 | 5.4 | 11 | 2.5 | |
| Pregabalin | 63 | 13.7‡ | 28 | 6.3 | 0 | 0.0† | |
| Pregabalin-Duloxetine | 11 | 2.4‡ | 0 | 0.0 | 0 | 0.0 | |
| SSRI | 22 | 4.8‡ | 3 | 0.7 | 2 | 0.5 | |
| Tramadol | 0 | 0.0 | 3 | 0.7 | 1 | 0.2 | |
| FMS: Fibromyalgia syndrome; CLBP: Chronic low back pain; NSAID: Nonsteroidal anti-inflammatory drug; SSRI: Selective serotonin reuptake inhibitors; * Pearson chi-squared test; ** Fisher Freeman-Halton test; Percentages with ‡ and † indicate higher and lower values than expected values considering the independence, respectively. | |||||||
Regular exercising was statistically significantly higher in the healthy control group compared to the other groups before and during the pandemic (p<0.001, Table 5). There was a significant decrease in regular exercise during the pandemic compared to the prepandemic, except in the CLBP group (p<0.05, Table 5).
Table 5. Physical activity of the participants.
| FMS group | CLBP group | Healthy control group | |||||
| Variables | n | % | n | % | n | % | p |
| Physical activity (Before pandemic) | <0.001* | ||||||
| Regular exercise | 133 | 29.1 | 161 | 37.0 | 213 | 49.0‡ | |
| Daily housework | 238 | 52.1‡ | 185 | 42.0 | 165 | 38.0† | |
| Nothing | 86 | 18.8 | 94 | 21.0 | 61 | 14.0 | |
| Physical activity (During pandemic) | <0.001* | ||||||
| Regular exercise | 101 | 21.8 | 143 | 31.0 | 176 | 41.0‡ | |
| Daily housework | 264 | 56.9‡ | 218 | 48.0 | 192 | 44.0† | |
| Nothing | 99 | 21.3 | 93 | 20.0 | 70 | 16.0 | |
| FMS: Fibromyalgia syndrome; CLBP: Chronic low back pain; * Pearson chi-squared test; ** Fisher Freeman-Halton test; Percentages with ‡ and † indicate higher and lower values than expected values considering the independence, respectively. | |||||||
The rate of individuals who did not change their job was significantly higher in the healthy control group compared to the other groups (n=274, 62.3%; p<0.001). It was found that some of them switched to part-time work (n=101, 23.0%; p<0.001, Table 6). Furthermore, the change in the general health status and pain levels with the pandemic was significantly higher in the FMS group than in the other groups (n=282, 60.6%; p<0.01, Table 6). Additionally, the health status was significantly worsened in the FMS group compared to the CLBP and healthy control groups. Assessment of the change in pain intensity between the prepandemic and pandemic showed a statistically significant increase in FMS patients compared to the CLBP group (p<0.001, Table 6).
Table 6. Work conditions, pain, and general health conditions of the participants.
| Parameters | FMS group | CLBP group | Healthy control group | p | |||
| n | % | n | % | n | % | ||
| Working condition during pandemic | <0.001* | ||||||
| Housewife | 249 | 53.5 | 137 | 30.1 | 33 | 7.5 | |
| Homeoffice | 42 | 9.0 | 39 | 8.6 | 15 | 3.4 | |
| Part time | 42 | 9.0 | 62 | 13.6 | 101 | 23.0 | |
| Left from work | 44 | 9.5 | 34 | 7.5 | 17 | 3.9 | |
| Not changed | 88 | 18.9 | 183 | 40.2 | 274 | 62.3 | |
| Change of health condition with pandemic | <0.001** | ||||||
| Yes | 282 | 60.6 | 189 | 41.5 | 102 | 23.2 | |
| No | 183 | 39.4 | 266 | 58.5 | 338 | 76.8 | |
| How change of health condition with pandemic | <0.001** | ||||||
| Much worse | 17 | 6.0 | 4 | 2.1 | 2 | 2.0 | |
| Worse | 77 | 27.3 | 40 | 21.2 | 2 | 2 | |
| A little bad | 166 | 58.9 | 124 | 65.6 | 58 | 56.9 | |
| A little good | 17 | 6.0 | 15 | 7.9 | 18 | 17.6 | |
| Better | 1 | 0.4 | 4 | 2.1 | 15 | 14.7 | |
| Much better | 4 | 1.4 | 2 | 1.1 | 7 | 6.9 | |
| Change of pain condition with pandemic | <0.001* | ||||||
| Yes | 309 | 66.5 | 208 | 45.7 | |||
| No | 156 | 33.5 | 247 | 54.3 | |||
| How change of pain condition with pandemic | <0.001** | ||||||
| Much worse | 16 | 5.2 | 4 | 1.9 | |||
| Worse | 107 | 34.6 | 61 | 29.3 | |||
| A little bad | 162 | 52.4 | 124 | 59.6 | |||
| A little good | 15 | 4.9 | 14 | 6.7 | |||
| Better | 6 | 1.9 | 3 | 1.4 | |||
| Much better | 3 | 1.0 | 2 | 1.0 | |||
| FMS: Fibromyalgia syndrome; CLBP: Chronic low back pain; * Pearson chi-squared test; ** Fisher Freeman-Halton test; Percentages with ‡ and † indicate higher and lower values than expected values considering the independence, respectively. | |||||||
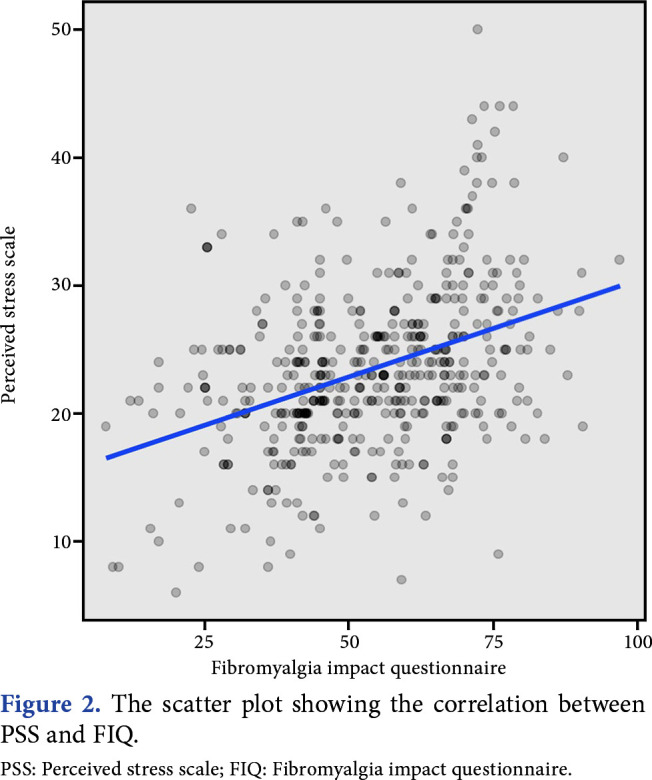
The median PSS (IQR) scores of the participants in the FMS, CLBP, and healthy control groups were 23 (7), 20 (6), and 18 (8.25), respectively. The median FIQ (IQR) score of the participants in the FMS group was 55 (25). The PSS score was highest in the FMS group, followed by the CLBP group (p<0.01). The mean FIQ score in the FMS group was 53.92±16.46, and the median (IQR) value was 55.1 (25). There was a weak- positive correlation between FIQ and PSS scores in patients with FMS (p<0.001, r=0.385, Figure 2).
Figure 2. The scatter plot showing the correlation between PSS and FIQ. PSS: Perceived stress scale; FIQ: Fibromyalgia impact questionnaire.
Discussion
In this study, various measurements, mainly general health status and pain, were analyzed in FMS and CLBP, which are common chronic pain diseases, and the prepandemic and pandemic periods were compared. The general health status and pain levels tended to worsen with the COVID-19 pandemic in patients with FMS and CLBP, and the stress level was higher among these patients than in the healthy control group. The high level of stress appeared to affect disease activity in patients with FMS.
It was previously reported that there was no change in pain levels and other somatic complaints of patients with FMS around the time of the World Trade Center bombing, indicating that most types of stress factors do not exacerbate FMS.[4] Conversely, stressors that affect each individual in society, such as earthquakes and epidemics, were shown to impact the course of FMS negatively.[4,14] From this point of view, it is crucial to document data during the pandemic concerning the fight against common diseases, such as FMS and CLBP.
In the literature, studies evaluating the effects of catastrophic events, such as a pandemic, on health discuss variables such as female sex, sedentary lifestyle, anxiety about the future, and staying away from work rather than the intensity of experienced stressors as a poor prognostic factor.[15] Accordingly, having a statistically higher proportion of females in the FMS group may have caused the perceived stress scale to be higher in the FMS group.
In the present study, an increase in weight and BMI with the pandemic was observed in all three groups. Working from home, curfews, and limiting daily social and business participation may have contributed to the increase.[16] The literature showed that increased BMI reduces functionality and quality of life in patients with chronic pain conditions such as FMS and CLBP.[17] It is possible to think that the increased BMI affected the worsening of pain and general health status in the participants of this study. In addition, studies have shown that there is a negative correlation between BMI and pain and functionality scales in FMS and CLBP patients.[18,19] It is thought that optimal body weight may be one of the main factors in the management of symptoms, particularly in FMS.
It was observed that almost half of the patients with FMS and CLBP were not admitted to an outpatient clinic. Although nearly two years have passed since the beginning of the pandemic, this high rate may be due to the fear of contracting COVID-19. However, we observed that FMS patients visited outpatient clinics more often than the healthy control group. Chronic pain conditions, such as FMS and CLBP, would likely be aggravated without regular examinations and follow-ups. One study stated that 43% of patients with FMS were no longer in contact with their physicians.[9] The fact that there was no significant change in the use of medical treatment compared to the prepandemic period can be related to the fact that the majority of patients receive their drugs with medical reports and that the health system in Türkiye offers the opportunity to obtain medication from the pharmacy without the need for a prescription during the pandemic.
More than half of the patients with FMS had a change in their work status during the pandemic. Some of these patients became home office employees, and some started working part-time. We understand that the patients with FMS, who have to be separated from their work environment and thus from their social environment, experience less intense networking. Although this may reduce the possibility of exposure to COVID-19, it can cause people to face economic problems and experience a setback in social relationships, resulting in exacerbation of stress and the disease. The scores of the FMS group in the PSS were statistically higher than in both other groups. One of the factors that most frequently cause anxiety in patients with FMS during the pandemic is economic difficulties. The government can support these patients in their financial struggles by establishing a fund.[6] One study reported that 75% of FMS patients worked from home during the curfew period.[8] In the present study, the rate of those who worked from home was around 8.6% in the CLBP group. In a survey by Šagát et al.[20] conducted on patients who had low back pain, the same rate was reported to be 3.9% before the curfew, which rose to 48.3% during the curfew period. This demonstrates how much the curfew affects work status during the pandemic. Since the curfew period was not separately evaluated in the present study, no data could be presented from this perspective.
Although the drug use status of the participants in the present study does not seem to be affected by the pandemic, it was understood from the data that there were setbacks in the use of medication under the supervision of a physician in parallel to the drop in the number of visits to the outpatient clinic. Aloush et al.[9] found that one third of the patients with FMS could not continue their medical treatment during the pandemic due to logistics challenges. In another study conducted during the pandemic, it was found that 3% of the patients with FMS used myorelaxants, 35% used NSAIDs, and 38% used opioids.[6] In that study gabapentinoids were excluded. In the present study, it was observed that 9.4% of the patients with FMS used myorelaxants, 7.6% used NSAIDs, 13.7% used pregabalin, and 45.4% used duloxetine during the pandemic. Licciardone et al.[5] reported a decrease in the use of NSAIDs and an increase in the use of opiates in patients with CLBP during the pandemic. Similarly, the use of oral NSAIDs also decreased in the present study. However, no increase was observed in opiate use.
In chronic diseases, physical activity and exercise directly affect both mental and physical health.[21,22] In the present study, the number of patients who regularly exercised and walked daily was decreased in both the FMS and CLBP groups during the pandemic. Although the tendency to live a sedentary life during the pandemic has a negative effect even on healthy people, it is likely to cause some physical and mental problems in the population with chronic pain. According to one study, 57% of FMS patients stopped exercising during the pandemic. In our study, there was a decrease in the rate of those who regularly engaged in exercise and walking in FMS patients. According to Šagát et al.,[20] the rate of not exercising in patients with low back pain rose from 7.3% to 20% due to the curfew. In the present study, the rate of not exercising during the pandemic decreased from 21% to 20% compared to the prepandemic period. In the study by Šagát et al.,[20] it was found that regular physical activity had a negative correlation with the intensity of low back pain during the pandemic. Since the level of pain and exercise activities were not evaluated with a numeric rating scale, a relevant correlation analysis could not be presented in this study.
A study comparing the periods with and without curfew in patients with FMS during the pandemic showed that decreased physical activity and increased anxiety caused an increase in FIQ scores. In contrast, regular exercise and smart working variables decreased FIQ scores.[7] Therefore, it has been concluded that a mixed effect can be observed on FMS activity during the pandemic. Although FIQ was not evaluated for the prepandemic period in the present study, a positive correlation was found between FIQ and PSS scores.
Limitations of this study were as follows: obtaining prepandemic data with surveys instead of documented information, nonsimilarity of age and sex distribution between groups, and the lack of analysis with Visual Analog Scale and quality of life scales in the CLBP group. In addition, PSS and FIQ evaluations were not compared with the prepandemic period, and the period with a curfew was not separately evaluated. Sleep quality, an essential stressor in patients with FMS, was also not documented, and the functionality of the patients was not questioned.
In conclusion, the PSS score was higher in patients with FMS than in the healthy control group during the pandemic. Restriction of the multidisciplinary approach to chronic pain conditions, such as FMS and CLBP, during the pandemic and physical, mental, emotional, and financial stressors had a direct and negative effect on the patient groups. Comunication seems to have beneficial effects on preventing them from becoming isolated by using telemedicine and telerehabilitation techniques under appropriate circumstances. There is a need for studies that compare the effects of the pandemic on the functionality measurements in these patients with the prepandemic period and the healthy population, with the participation of a more extensive patient population as a result of cooperation between different countries.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Biruni University, Faculty of Medicine Ethics Committee (date: 16.09.2020, no: 2020/43-01). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Conceptualization: F.B., S.K., D.E., A.K., D.Ş., B.S.T., Design, supervision, data analysis/data interpretetation, critical reading: F.B., S.K., D.E., A.K., D.Ş., B.S.T., Z.Ü., C.T., D.E., A.A., S.A., B.Y., D.G.K., M.K., F.G.K.N., K.N.K.O., M.K.A., G.F., F.E., A.A., O.V.Y., F.A.K., E.E.K., Y.Ö., Ş.K.D., T.E.S., B.O., E.K.U., E.D., H.Ç.Y., Ö.B., N.T., S.S., S.A., M.S.A., Literature review, writing: F.B., S.K., D.E.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from each patient.
References
- 1.Xiong J, Lipsitz O, Nasri F, Lui LMW, Gill H, Phan L, et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J Affect Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrey LB, Roberts WO, Wichser L. Exercise-related mental health problems and solutions during the COVID- 19 pandemic. Curr Sports Med Rep. 2020;19:194–195. doi: 10.1249/JSR.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein J, Visco CJ, Barbuto S. Rehabilitation medicine response to the COVID-19 pandemic. Am J Phys Med Rehabil. 2020;99:573–579. doi: 10.1097/PHM.0000000000001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clauw DJ, Häuser W, Cohen SP, Fitzcharles MA. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. 2020;161:1694–1697. doi: 10.1097/j.pain.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Licciardone JC. Demographic characteristics associated with utilization of noninvasive treatments for chronic low back pain and related clinical outcomes during the COVID- 19 pandemic in the United States. S77-S84J Am Board Fam Med. 2021;34(Suppl) doi: 10.3122/jabfm.2021.S1.200352. [DOI] [PubMed] [Google Scholar]
- 6.Kharko AY, Hansford KJ, Furlong PL, Hall SD, Roser ME. The anxiety and pain of fibromyalgia patients during the COVID-19 pandemic. medRxiv. 2020 Available at: https://www.medrxiv.org/content/10.1101/2020.11.24.20188011v1 . [Google Scholar]
- 7.Cavalli G, Cariddi A, Ferrari J, Suzzi B, Tomelleri A, Campochiaro C, et al. Living with fibromyalgia during the COVID-19 pandemic: Mixed effects of prolonged lockdown on the well-being of patients. Rheumatology (Oxford) 2021;60:465–467. doi: 10.1093/rheumatology/keaa738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batres-Marroquín AB, Medina-García AC, Vargas Guerrero A, Barrera-Villalpando MI, Martínez-Lavín M, Martínez-Martínez LA. Effect of COVID-19 pandemic lockdown on fibromyalgia symptoms. e289-e291J Clin Rheumatol. 2022;28 doi: 10.1097/RHU.0000000000001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aloush V, Gurevich-Shapiro A, Hazan E, Furer V, Elkayam O, Ablin JN. Relationship between religiosity, spirituality and physical and mental outcomes in fibromyalgia patients. Clin Exp Rheumatol. 2021;39 Suppl 130:48–53. doi: 10.55563/clinexprheumatol/fcxigf. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46:319–329. doi: 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kibar S, Konak HE, Ay Sarıoglu S, Doğanay Erdogan B, Evcik FD. The effectiveness of combined transcutaneous electrical nerve stimulation and ınterferential current therapy on chronic low back pain: A randomized, double- blind, sham-controlled study. J PMR Sci. 2020;23:32–40. [Google Scholar]
- 12.Erci B. Reliability and validity of the Turkish version of perceived stress scale. Atatürk Üniversitesi Hemşirelik Yüksekokulu Dergisi. 2006;9:57–62. [Google Scholar]
- 13.Sarmer S, Ergin S, Yavuzer G. The validity and reliability of the Turkish version of the Fibromyalgia Impact Questionnaire. Rheumatol Int. 2000;20:9–12. doi: 10.1007/s002960000077. [DOI] [PubMed] [Google Scholar]
- 14.Zajacova A, Jehn A, Stackhouse M, Choi KH, Denice P, Haan M, et al. Mental health and economic concerns from March to May during the COVID-19 pandemic in Canada: Insights from an analysis of repeated cross- sectional surveys. SSM Popul Health. 2020;12:100704–100704. doi: 10.1016/j.ssmph.2020.100704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean SA, Clauw DJ. Predicting chronic symptoms after an acute “stressor”--lessons learned from 3 medical conditions. Med Hypotheses. 2004;63:653–658. doi: 10.1016/j.mehy.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Katsoulis M, Pasea L, Lai AG, Dobson RJB, Denaxas S, Hemingway H, et al. Obesity during the COVID-19 pandemic: Cause of high risk or an effect of lockdown? A population-based electronic health record analysis in 1 958 184 individuals. medRxiv. 2020 doi: 10.1016/j.puhe.2020.12.003. Available at: https://www.medrxiv.org/content/10.1101/2020.06.22.20137182v1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arranz LI, Rafecas M, Alegre C. Effects of obesity on function and quality of life in chronic pain conditions. Curr Rheumatol Rep. 2014;16:390–390. doi: 10.1007/s11926-013-0390-7. [DOI] [PubMed] [Google Scholar]
- 18.Neumann L, Lerner E, Glazer Y, Bolotin A, Shefer A, Buskila D. A cross-sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin Rheumatol. 2008;27:1543–1547. doi: 10.1007/s10067-008-0966-1. [DOI] [PubMed] [Google Scholar]
- 19.Chou L, Brady SRE, Urquhart DM, Teichtahl AJ, Cicuttini FM, Pasco JA, et al. The association between obesity and low back pain and disability is affected by mood disorders: A population-based, cross-sectional study of men. e3367Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Šagát P, Bartík P, Prieto González P, Tohănean DI, Knjaz D. Impact of COVID-19 quarantine on low back pain intensity, prevalence, and associated risk factors among adult citizens residing in Riyadh (Saudi Arabia): A cross-sectional study. Int J Environ Res Public Health. 2020;17:7302–7302. doi: 10.3390/ijerph17197302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biddle S. Physical activity and mental health: Evidence is growing. World Psychiatry. 2016;15:176–177. doi: 10.1002/wps.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silveira MP, da Silva Fagundes KK, Bizuti MR, Starck É, Rossi RC, de Resende E Silva DT. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin Exp Med. 2021;21:15–28. doi: 10.1007/s10238-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]