Abstract
Objectives
This study aims to evaluate the efficacy of therapeutic pulsed ultrasound (US) applied to the occipital nerve distribution area in chronic migraine patients.
Patients and methods
This prospective, randomized, double-blind, placebo-controlled, study was conducted on 58 patients diagnosed with migraine at the University of Health Sciences Bursa Yüksek Ihtisas Training and Research Hospital, Department of Physical Medicine and Rehabilitation between January 2018 and October 2018. The patients were randomly divided into two groups as sham and pulsed US group. Pulsed US was applied in Group 1 (n=29), and sham US was used in Group 2 (n=29) for 5 min for a total of 10 sessions in each group. The evaluation was made at pre-treatment, post-treatment first month (week six) and third month (week 14) using the numeric rating scale, and the Migraine Disability Assessment Scale (MIDAS). The final analysis was conducted with 52 patients (7 males, 45 females; mean age: 38.3±7.9 years; range, 20 to 65 years) due to drop-outs, with 27 patients in Group 1 and 25 patients in Group 2.
Results
A statistically significant improvement was found in all parameters at week six and week 14 compared to pre-treatment values in Group 1. A statistically significant worsening was detected in MIDAS 1, 4, 5, total questions, and a significant improvement was found in MIDAS A and B at week six compared to pre-treatment values in Group 2. A statistically significant worsening was found only in MIDAS total score at week 14 compared to pre-treatment values. In the comparison of the difference scores of the two groups, all parameters in Group 1 showed significant improvement at week six and week 14.
Conclusion
The findings obtained in this study show that pulsed US applied to the occipital nerve distribution region in migraine patients can be effective on headache frequency, severity, and disability.
Keywords: Chronic migraine, disability, greater occipital nerve, pain, ultrasound therapy
Introduction
In the adult population, migraine affects approximately 11.5% of individuals and can result in significant disability.[1,2] A wide variety of methods are used to treat chronic migraine and headache; these include pharmacological therapies, physical therapy modalities involving the use of ultrasound (US) and exercise, greater occipital nerve block, Botulinum toxin injections, and occipital nerve stimulation (ONS).[3-8]
Occipital nerve stimulation is applied for the treatment of persistent neck and head pain syndromes.[8-11] Successful outcomes have been achieved with ONS through the subcutaneous insertion of leads into the greater occipital nerve at the cervical (C1) segment to treat cervical and occipital pain that developed after occipitocervical fusion.[8] It has been stated that ONS increases the mechanical threshold in animal models and provides pain relief in chronic migraine.[12,13]
Therapeutic US is commonly used for the treatment of musculoskeletal diseases. Therapeutic US waves produce vibration in all tissue compartments, including intracellular and extracellular fluids and cell membranes, while being transmitted across the tissues.[14] The changes that occur as a result of these vibrations are classified as thermal and nonthermal effects in nature.[15] Ultrasound waves in continuous mode produce thermal effects. It is assumed that thermal energy affects the functions of ion channels and results in rapid repolarization before action potentials can be triggered.[16-18] The nonthermal effects occur in pulsed US mode and include anti- inflammatory effects and changes in membrane potential.[16-18]
In literature, there are a wide range of studies investigating the use of physical therapy modalities for the treat of migraine, although these studies have often focused on the trigger points in the cervical region and trapezius muscle.[4,5,19] Occipital nerve stimulation techniques performed until recently had only been invasive.[8] In this study, our hypothesis was to relieve the migraine symptoms by stimulating the large occipital nerve with pulse US, a noninvasive method. Thus, the present study evaluates the efficacy of noninvasive therapeutic pulse US in patients with chronic migraine applied to the upper cervical region, innervated by the occipital nerve and the craniocervical junction from where the nerve is distributed.
Patients and Methods
This prospective, randomized, double-blind, placebo-controlled study was conducted at the University of Health Sciences Bursa Yüksek Ihtisas Training and Research Hospital, Department of Physical Medicine and Rehabilitation between January 2018 and October 2018. Eighty patients with chronic migraine who have been diagnosed according to the 2014 International Headache Society (IHS) criteria were evaluated by a neurologist for eligibility.[20] Fifty- eight eligible patients with chronic migraine diagnosed according to the IHS criteria, who were followed up in neurology outpatient clinics and received the routine medical therapy, including ergotamine, triptan, propranolol, flunarizine, topiramate, or their combination, for at least six months but did not respond adequately to treatment, were included in the study. Patients with cervical radiculopathy or neck pain, fibromyalgia, bleeding disorders, malignancies, uncontrolled hypertension, peripheral and central nervous system disorders, patients who declined to take part in the study, those who underwent surgery to the cervical vertebrae or posterior cranial region, and patients who were involved in any exercise program were excluded. The data of the demographic characteristics and pre-treatment assessment parameters of the patients were recorded. The patients included in the study were randomly divided into two groups by a random number table. In Group 1 (n=29), the patients underwent routine migraine therapy, includingergotamine,triptan,propranolol,flunarizine, topiramate, or their combination, and therapeutic pulsed US; in Group 2 (n=29), the patients underwent routine migraine therapy, including ergotamine, triptan, propranolol, flunarizine, topiramate, or their combination, and sham US therapy (Figure 1).
Figure 1. Flow chart of the study. US: Ultrasound.
During the therapeutic pulsed US therapy, the patients were placed in a prone position in a silent room. The patients in Group 1 bilaterally received pulsed US therapy to the craniocervical junction and the upper cervical paravertebral region for 5 min using a BTL 4625 US device (BTL Medical Technologies s.r.o, Praha 6-Vokovice, Czech Republic) in pulsed mode (1:1) at a frequency of 3 MHz and an intensity of 1.5 W/cm2. The headpiece area of the device was 5 cm2. The headpiece was positioned at 90o (perpendicular), and the application was at full contact using gel with continuous circular motions. The treatment comprised a total of 10 sessions for two weeks, five days a week.
During the sham US sessions, the ultrasound probe was bilaterally applied to the craniocervical junction and upper cervical paravertebral region for 5 min while the US device was switched off. The treatment comprised a total of 10 sessions for two weeks, five days a week. All US applications were performed by a physical therapy and rehabilitation specialist. The participants and the researcher who completed post-treatment first month (week 6) and post-treatment third month (week 14) evaluations were blind to the type of the treatment.
The numeric rating scale (NRS) was used to measure the pain severity experienced by the patient during the migraine attack. The NRS consists of a line with numbers from 0 (no pain) to 10 (most severe pain). The patient is asked to mark the number corresponding to the pain severity on this line.[21]
The Migraine Disability Assessment Scale (MIDAS) was used to assess the extent of disability in the workplace, home and social life in the last three months. There are a total of five questions in the MIDAS questionnaire. The first, third, and fifth questions were within the previous three months, at work or school due to headache; home stuff. These determine the lost days in leisure time. The second and fourth questions evaluate the number of additional days in the previous three months with productivity decline (defined as at least 50% reduction in productivity) at work and household chores. Each day is rated as 1 point, and the total score corresponds to the total number of days, which is noted as the disability score of the patients. Additional two questions (MIDAS A and B questions) assess the headache frequency and headache intensity but are not added to the score of total MIDAS value. The total score is evaluated in four grades. A score of 0-5 corresponds to Grade 1, indicating little or no disability; a score of 6-10 corresponds to Grade 2, indicating mild disability; a score of 11-20 corresponds to Grade 3, indicating moderate disability; and a score of 21 indicates severe disability.[22] The validity and reliability study of the MIDAS has been conducted.[23]
No additional analgesics were given to the patients during the study period. Two patients in Group 1 dropped out of the study due to personal reasons (one of them moved to another city, and the other patient could not come to the follow-up due to family issues). Four patients in Group 2 (routine migraine therapy + sham pulsed US) were excluded from the study as they could not attend the follow-up. Thus, the final analysis was conducted with 52 patients (7 males, 45 females; mean age: 38.3±7.9 years; range, 20 to 65 years) (Group 1, n= 27; Group 2, n= 25; Figure 1).
Statistical analysis
Statistical analysis was performed using the IBM SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). In the comparison of the categorical variables, Pearson’s chi-square test or the Yates test were used. The Shapiro-Wilk test was used to test for normality. The independent samples t-test was used for between-groups comparisons in normally distributed data. For intragroup comparisons, the paired sample test was used. The Wilcoxon test was used in intragroup comparisons, and the Mann-Whitney U test was used in between-group comparisons for non-normally distributed data. The Bonferroni correction was made for multiple time comparisons. A p value of <0.05 was accepted as statistically significant.
A decrease of 25% in the NRS score at the one-month control visit compared to baseline was designated as the primary endpoint of the study.[6] Considering a power of 85% (α=0.05), the calculated sample size was a total of 58 patients, with each group comprising 29 patients.
Results
The demographic characteristics of the patients are presented in Table 1. The pre-treatment evaluation parameters in both groups are presented in Table 2. No statistically significant difference was detected between the groups for demographic characteristics and pre-treatment evaluation parameters (p>0.05).
Table 1. Comparison of Group 1 and Group 2 at the demographic characteristics of the patients.
| Group 1 (n=27) | Group 2 (n=25) | p | |||||
| n | % | Mean±SD | n | % | Mean±SD | ||
| Age (year) | 37.5±7.9 | 39.0±7.9 | 0.492 | ||||
| Body mass index (kg/m2) | 27.1±4.2 | 26.5±4.3 | 0.617 | ||||
| Sex | 0.772 | ||||||
| Female | 23 | 85.2 | 22 | 88.0 | |||
| Number of drugs used for migraine | 1.3±0.5 | 1.4±0.7 | 0.532 | ||||
| SD: Standard deviation; Independent samples t-test was used for the inter-group comparisons. | |||||||
Table 2. Comparison of the baseline, week 6 and week 14 values between the groups.
| Week 0 | Week 6 | Week 14 | ||||||
| Median | Min-Max | Median | Min-Max | Median | Min-Max | p(W6-W0) | p(W14-W0) | |
| NRS | ||||||||
| Group 1 | 10 | 5-10 | 5 | 2-10 | 4 | 0-8 | <0.001 | <0.001 |
| Group 2 | 9 | 5-10 | 9 | 5-10 | 9 | 5-10 | 0.241 | 0.523 |
| MIDAS 1st question Group 1 |
0 | 0-9 | 0 | 0-2 | 0 | 0-2 | 0.027 | 0.027 |
| Group 2 | 0 | 0-15 | 0 | 0-20 | 0 | 0-10 | 0.027 | 0.206 |
| MIDAS 2nd question Group 1 |
0 | 0-18 | 0 | 0-10 | 0 | 0-7 | 0.003 | 0.002 |
| Group 2 | 0 | 0-24 | 0 | 0-25 | 0 | 0-30 | 0.085 | 0.167 |
| MIDAS 3rd question Group 1 |
10 | 0-45 | 3 | 0-25 | 1 | 0-16 | <0.001 | <0.001 |
| Group 2 | 10 | 0-45 | 12 | 0-35 | 14 | 0-40 | 0.433 | 0.255 |
| MIDAS 4th question Group 1 |
10 | 0-50 | 6 | 0-35 | 0 | 0-25 | <0.001 | <0.001 |
| Group 2 | 10 | 0-45 | 15 | 0-45 | 10 | 0-45 | 0.050 | 0.962 |
| MIDAS 5th question Group 1 |
9 | 0-50 | 5 | 0-41 | 1 | 0-20 | <0.001 | <0.001 |
| Group 2 | 10 | 0-50 | 10 | 0-50 | 12 | 0-45 | 0.027 | 0.114 |
| MIDAS total | ||||||||
| Group 1 | 40 | 6-129 | 21 | 0-81 | 6 | 0-56 | <0.001 | <0.001 |
| Group 2 | 39 | 4-130 | 46 | 2-142 | 45 | 4-130 | 0.008 | 0.038 |
| MIDAS A | ||||||||
| Group 1 | 20 | 6-65 | 15 | 1-51 | 3 | 0-30 | <0.001 | <0.001 |
| Group 2 | 24 | 5-65 | 20 | 0-60 | 20 | 0-60 | 0.005 | 0.210 |
| MIDAS B | ||||||||
| Group 1 | 8 | 5-10 | 5 | 2-10 | 4 | 0-9 | <0.001 | <0.001 |
| Group 2 | 9 | 5-10 | 8 | 5-10 | 8 | 5-10 | 0.032 | 0.300 |
| NRS: Numeric rating scale; MIDAS: Migraine Disability Assessment Scale; Wilcoxon test was used for intragroup comparisons. Bonferroni correction was made. | ||||||||
A statistically significant improvement was detected in all parameters at the post-treatment first month (week 6) and third month (week 14) values compared to pre-treatment values in Group 1 (p<0.05; Table 2).
A statistically significant deterioration was found in MIDAS 1, 4, 5, and total questions, and a significant improvement was found in MIDAS A and B at the post-treatment first month compared to pre-treatment values in Group 2. However, there was no statistically significant difference in other parameters (p>0.05; Table 2). A statistically significant worsening was detected only in the MIDAS total score at the post- treatment third month compared to pre-treatment values (p<0.05). There was no statistically significant difference in other parameters (p>0.05; Table 2).
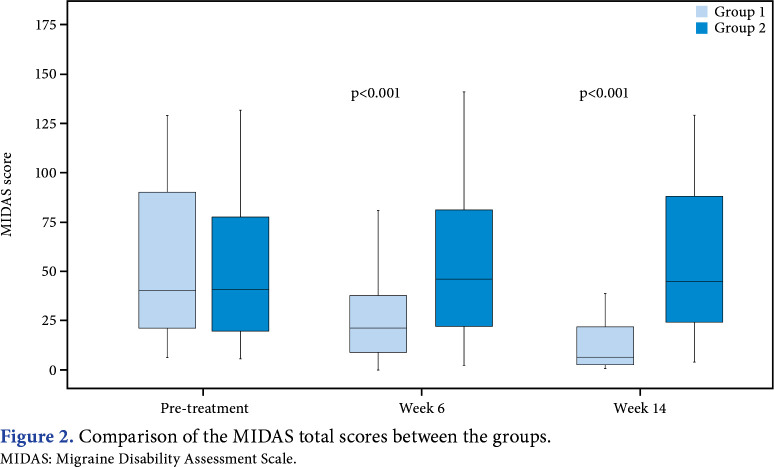
Statistically significant differences at 6 and 14 weeks were detected in the between-group comparison of MIDAS total scores (p<0.05; Figure 2). The comparison of the difference in scores between the groups showed significantly superior improvement in Group 1 for all parameters at the post-treatment first month and post- treatment third month (p<0.05; Table 3). No adverse effects were detected.
Figure 2. Comparison of the MIDAS total scores between the groups. MIDAS: Migraine Disability Assessment Scale.
Table 3. Comparison of the difference in scores between the groups.
| Week 6-Week 0 | Week 14-Week 0 | |||
| Median | Min-Max | Median | Min-Max | |
| Group 1 | -3 | -6 to 0 | -4 | -8 to -2 |
| Group 2 | 0 | -2 to 2 | 0 | -2 to 3 |
| p | <0.001 | <0.001 | ||
| MIDAS 1st question | ||||
| Group 1 | 0 | -7 to 0 | 0 | -8 to 0 |
| Group 2 | 0 | 0 to 5 | 0 | -5 to 6 |
| p | 0.003 | 0.024 | ||
| MIDAS 2nd question | ||||
| Group 1 | 0 | -8 to 0 | 0 | -12 to 0 |
| Group 2 | 0 | -1 to 15 | 0 | -5 to 15 |
| p | 0.002 | 0.001 | ||
| MIDAS 3rd question | ||||
| Group 1 | -9 | -30 to 0 | -9 | -44 to 0 |
| Group 2 | 0 | -15 to 8 | 0 | -15 to 13 |
| p | <0.001 | <0.001 | ||
| MIDAS 4th question | ||||
| Group 1 | -6 | -22 to 0 | -10 | -34 to 4 |
| Group 2 | 0 | -5 to 5 | 0 | -15 to 15 |
| p | <0.001 | <0.001 | ||
| MIDAS 5th question | ||||
| Group 1 | -4 | -21 to 4 | -7 | -35 to 5 |
| Group 2 | 0 | -3 to 12 | 0 | -10 to 15 |
| p | <0.001 | <0.001 | ||
| MIDAS total | ||||
| Group 1 | -25 | -65 to - 6 | -27 | -103 to -6 |
| Group 2 | 3 | -12 to 18 | 5 | -20 to 20 |
| p | <0.001 | <0.001 | ||
| MIDAS A | ||||
| Group 1 | -6 | -20 to -2 | -18 | -44 to -3 |
| Group 2 | -5 | -36 to 10 | -4 | -32 to 15 |
| p | 0.017 | <0.001 | ||
| MIDAS B | ||||
| Group 1 | -3 | -5 to 1 | -4 | -7 to 1 |
| Group 2 | 0 | -4 to 1 | 0 | -3 to 3 |
| p | <0.001 | <0.001 | ||
| NRS: Numeric rating scale; MIDAS: Migraine Disability Assessment Scale; Mann-Whitney U test was used for intergroup comparisons. Bonferroni correction was made. | ||||
Discussion
The results of the present study have shown the efficacy of therapeutic pulsed US therapy in addition to routine medical therapy in reducing the headache frequency, intensity, and disability at one and three months in patients with migraine.
Although preventive therapies show efficacy in some migraine patients, the majority of patients still suffer severe, intolerable headaches, among other symptoms related to migraine.[24] Current migraine medications sometimes fail or may not be sufficient to treat migraine, which has led to the development of new therapeutic approaches. There are various studies investigating the effectiveness of peripheral nerve blocks and radiofrequency therapies in the treatment of headaches and migraine. The most common target in this regard is the greater occipital nerve, although occipital, supratrochlear and supraorbital, auriculotemporal nerves, the cervical spinal roots, the sphenopalatine ganglion, and the facet joints of the upper cervical spine have also been investigated to a lesser extent.[6,25,26]
The greater occipital nerve originates from the fibers of the dorsal primary ramus of the second cervical nerve (medial division), and the third cervical nerve is also involved to a lesser extent. The greater occipital nerve is a sensory nerve that innervates the region from the medial part of the posterior scalp to the vertex. In addition, the dorsal root ganglion innervates the C1-C2 joint and zygapophyseal C2-C3 joints. It has been suggested that the reason for the effect of ONS on pain in different regions is the convergence of the C2 dorsal horn with the trigeminal nerve in the “trigeminal-cervical nucleus” extending from the trigeminal nucleus caudals to the C3 level.[27] Considering the localization of the greater occipital nerve, the present study preferred the use of pulsed US therapy for the stimulation of the upper cervical region and the craniocervical junction.
There have been many studies in the literature focusing on the greater occipital nerve block, the use of local anesthetics, and studies reporting successes with ONS, which is a new therapeutic approach in the treatment of migraine.[6,8,12,13] These studies have reported that ONS could be of benefit to patients with long-term, persistent chronic migraine.[8,28] Case-control and open-label studies using Botulinum toxin injections and ONS have achieved a significant decrease in the frequency and severity of migraine episodes in approximately 40% of patients.[29,30]
Although the mechanisms have not been clearly described, ONS is believed to be beneficial through its effects on the peripheral and central mechanisms. It has been reported that peripheral stimulation of sensory afferents can decrease pain by inhibiting nociceptive activity in C and A-delta fibers. With regard to the central mechanism of ONS, hypermetabolic activity has been previously detected in ONS on positron emission tomography scans in several brain regions that are responsible for processing pain, and the activity in these regions has been shown to normalize after ONS.[31]
Although ONS is a safe procedure, almost all studies have reported side effects in as many as 70% of patients in the long term, such as paresthesia (most common), lead migration, hospitalization (8.6%), repeat surgical intervention (40.7%), battery depletion (64%), and material infection (20%).[30,32-35]
It has been suggested that physical therapy modalities may be an alternative treatment approach in the treatment of migraine,[4,5,19] having been distinguished as noninvasive methods that have tried to find a place within different therapeutic applications. Studies in the literature on the use of physical therapy modalities in migraine treatment have focused on trigger points, muscle strengthening, and muscle relaxation.[4,5,19]
The therapeutic use of US on trigger points was described in a case report by Gonçalves et al.,[4] who treated 25 female patients with refractory migraine with a total of 20 sessions of physical therapy two days a week. The initially applied cervical stretching and traction therapies were supplemented with therapeutic US beginning from the sixth session. Ultrasound therapy was applied at a frequency of 1 MHz and an intensity of 1.5 W/cm2, delivering therapy for 1.5 min to each trigger point (maximum two points). At the end of the therapy, improvement was noted in the duration and frequency of the migraine episodes, along with a decrease in the pressure pain thresholds. The authors reported employing nonthermal effects rather than thermal effects of US, delivering US therapy with a low heating rate and for short application periods. They further reported that they were able to provide relief to the muscle hyperalgesia via nonthermal effects by decreasing the nociceptive input to the central nervous system, which presumably decreased central and peripheral sensitization. Using this approach, the authors suggested that US therapy could be valuable in the treatment of resistant migraine.
There is no study in the literature using physical therapy applications to the occipital nerve to treat patients with migraine. While the present study primarily focused on evaluating the efficacy of pulsed US applied to the occipital nerve, Walling et al.[36] recently investigated the efficacy of pulsed focus US applied to the occipital nerve for the treatment of cutaneous allodynia in rats with chronic migraine. In the study evaluating sumatriptan and ablative lesioning therapies in a rat model with chronic migraine, it was reported that focus US combined with sumatriptan provided greater improvement to the mechanical thresholds in the periorbital area than ablative lesioning, and it was suggested that focus US could be an important therapeutic option in patients with chronic migraine. The authors concluded that their study was the first step toward the development of a long-standing, noninvasive therapy for the treatment of chronic refractory migraine that alleviated the need for an implantable device. As a limitation of the study, however, the authors reported burns in some rats, as they could not adjust the optimal dose of focus US. In the present study, no burns were detected, as US therapy was applied in a circular motion rather than through pulsed focus US to the occipital nerve.
The nonthermal effects of US include the stimulation of phagocytic activity in inflammatory cells such as macrophages and neutrophils, the activation of degranulation of macrophages and mast cells, an increase in the permeability of biological membranes, the exertion of antiedema effects through anti-inflammatory pathways, and changes in membrane potential.[16-18] We believe that the action mechanism of therapeutic pulsed US used in the present study resembles that of ONS. It can be further suggested that US therapy increases membrane stabilization and ion channel interaction as a result of the nonthermal and, to a lesser extent, thermal effects, although there are various effects on the tissue.[16-18] This in turn reduces inputs to the CNS and contributes to a decrease in peripheral sensitization.
The limitations of this study are the small sample size and the lack of long-term follow-up.
In conclusion, pulsed US applied to the craniocervical and upper cervical region, in addition to the routine medical treatment used in migraine patients, is effective on headache frequency, intensity, and disability. We consider that the effects of therapeutic pulsed US on the greater occipital nerve and its use in the treatment of migraine justify the conducting of further studies involving larger patient groups.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Uludağ University Faculty of Medicine Clinical Research Ethics Committee (date: 11.01.2018, no: 2011-KAEK-2018-1/25).
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Contributed all parts of the study: B.M.Ö.; Contributed all parts of the study except for literature research: A.G.; Lale Altan contributed all parts of study except resources, materials, data collection and literature search: L.A.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from each patient.
References
- 1.Merikangas KR. Contributions of epidemiology to our understanding of migraine. Headache. 2013;53:230–246. doi: 10.1111/head.12038. [DOI] [PubMed] [Google Scholar]
- 2.Steiner TJ, Stovner LJ, Birbeck GL. Migraine: The seventh disabler. Cephalalgia. 2013;33:289–290. doi: 10.1177/0333102412473843. [DOI] [PubMed] [Google Scholar]
- 3.Weatherall MW. The diagnosis and treatment of chronic migraine. Ther Adv Chronic Dis. 2015;6:115–123. doi: 10.1177/2040622315579627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonçalves MC, Teixeira da Silva ER, Chaves TC, Dach F, Speciali JG, Roberto de Jesus Guirro R. Static ultrasound and manual therapy in refractory migraine. Case report. Rev Dor. 2012;13:80–84. [Google Scholar]
- 5.Tali D, Menahem I, Vered E, Kalichman L. Upper cervical mobility, posture and myofascial trigger points in subjects with episodic migraine: Case-control study. J Bodyw Mov Ther. 2014;18:569–575. doi: 10.1016/j.jbmt.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Okmen K, Dagistan Y, Dagistan E, Kaplan N, Cancan E. Efficacy of the greater occipital nerve block in recurrent migraine type headaches. Neurol Neurochir Pol. 2016;50:151–154. doi: 10.1016/j.pjnns.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 8.Guzzi G, Della Torre A, Gabriele D, Volpentesta G, Chirchiglia D, Stroscio CA, et al. Occipital nerve stimulation for refractory pain after occipitocervical fusion. Acta Neurochir Suppl. 2019;125:365–367. doi: 10.1007/978-3-319-62515-7_52. [DOI] [PubMed] [Google Scholar]
- 9.Vallejo R, Benyamin R, Kramer J. Neuromodulation of the occipital nerve in pain management. Techniques in Regional Anesthesia and Pain Management. 2006;10:12–15. [Google Scholar]
- 10.Paemeleire K, Bartsch T. Occipital nerve stimulation for headache disorders. Neurotherapeutics. 2010;7:213–219. doi: 10.1016/j.nurt.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goadsby PJ. Neuromodulatory approaches to the treatment of trigeminal autonomic cephalalgias. Acta Neurochir Suppl. 2007;97:99–110. doi: 10.1007/978-3-211-33081-4_12. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Song M, Fan Y, Ma K. Occipital nerve stimulation for migraine: A systematic review. Pain Pract. 2016;16:509–517. doi: 10.1111/papr.12303. [DOI] [PubMed] [Google Scholar]
- 13.De La Cruz P, Gee L, Walling I, Morris B, Chen N, Kumar V, et al. Treatment of allodynia by occipital nerve stimulation in chronic migraine rodent. Neurosurgery. 2015;77:479–485. doi: 10.1227/NEU.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 14.Schortinghuis J, Stegenga B, Raghoebar GM, de Bont LG. Ultrasound stimulation of maxillofacial bone healing. Crit Rev Oral Biol Med. 2003;14:63–74. doi: 10.1177/154411130301400106. [DOI] [PubMed] [Google Scholar]
- 15.Dyson M, Brookes M. Stimulation of bone repair by ultrasound. Ultrasound Med Biol. 1983;Suppl 2:61–66. [PubMed] [Google Scholar]
- 16.Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol. 2009;35:1737–1747. doi: 10.1016/j.ultrasmedbio.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan JC. 2nd ed. Philadelphia: Mosby; 2006. Practical manual of physical medicine and rehabilitation; pp. 141–166. [Google Scholar]
- 18.Fu SC, Shum WT, Hung LK, Wong MW, Qin L, Chan KM. Low-intensity pulsed ultrasound on tendon healing: A study of the effect of treatment duration and treatment initiation. Am J Sports Med. 2008;36:1742–1749. doi: 10.1177/0363546508318193. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-de-Las-Peñas C, Cuadrado ML. Physical therapy for headaches. Cephalalgia. 2016;36:1134–1142. doi: 10.1177/0333102415596445. [DOI] [PubMed] [Google Scholar]
- 20.Gladstone JP, Dodick DW. Revised 2004 International Classification of Headache Disorders: New headache types. Can J Neurol Sci. 2004;31:304–314. doi: 10.1017/s031716710000336x. [DOI] [PubMed] [Google Scholar]
- 21.Williamson A, Hoggart B. Pain: A review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 22.Bigal ME, Rapoport AM, Lipton RB, Tepper SJ, Sheftell FD. Assessment of migraine disability using the migraine disability assessment (MIDAS) questionnaire: A comparison of chronic migraine with episodic migraine. Headache. 2003;43:336–342. doi: 10.1046/j.1526-4610.2003.03068.x. [DOI] [PubMed] [Google Scholar]
- 23.Ertaş M, Siva A, Dalkara T, Uzuner N, Dora B, Inan L, et al. Validity and reliability of the Turkish Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2004;44:786–793. doi: 10.1111/j.1526-4610.2004.04146.x. [DOI] [PubMed] [Google Scholar]
- 24.Blumenfeld AM, Bloudek LM, Becker WJ, Buse DC, Varon SF, Maglinte GA, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: Results from the second international burden of migraine study (IBMS-II) Headache. 2013;53:644–655. doi: 10.1111/head.12055. [DOI] [PubMed] [Google Scholar]
- 25.Ho KWD, Przkora R, Kumar S. Sphenopalatine ganglion: Block, radiofrequency ablation and neurostimulation - a systematic review. J Headache Pain. 2017;18:118–118. doi: 10.1186/s10194-017-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carcamo CR. Pulsed radiofrequency of superior cervical sympathetic ganglion for treatment of refractory migraine. Pain Med. 2017;18:1598–1600. doi: 10.1093/pm/pnw301. [DOI] [PubMed] [Google Scholar]
- 27.Lee P, Huh BK. Peripheral nerve stimulation for the treatment of primary headache. Curr Pain Headache Rep. 2013;17:319–319. doi: 10.1007/s11916-012-0319-2. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigo D, Acin P, Bermejo P. Occipital nerve stimulation for refractory chronic migraine: Results of a long-term prospective study. E151-E159Pain Physician. 2017;20 [PubMed] [Google Scholar]
- 29.Conway S, Delplanche C, Crowder J, Rothrock J. Botox therapy for refractory chronic migraine. Headache. 2005;45:355–357. doi: 10.1111/j.1526-4610.2005.05073a.x. [DOI] [PubMed] [Google Scholar]
- 30.Saper JR, Dodick DW, Silberstein SD, McCarville S, Sun M, Goadsby PJ, ONSTIM Investigators Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia. 2011;31:271–285. doi: 10.1177/0333102410381142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vukovic Cvetkovic V, Jensen RH. Neurostimulation for the treatment of chronic migraine and cluster headache. Acta Neurol Scand. 2019;139:4–17. doi: 10.1111/ane.13034. [DOI] [PubMed] [Google Scholar]
- 32.Dodick DW, Silberstein SD, Reed KL, Deer TR, Slavin KV, Huh B, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Long-term results from a randomized, multicenter, double- blinded, controlled study. Cephalalgia. 2015;35:344–358. doi: 10.1177/0333102414543331. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen JP, Nizard J, Kuhn E, Carduner F, Penverne F, Verleysen-Robin MC, et al. A good preoperative response to transcutaneous electrical nerve stimulation predicts a better therapeutic effect of implanted occipital nerve stimulation in pharmacologically intractable headaches. Neurophysiol Clin. 2016;46:69–75. doi: 10.1016/j.neucli.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Brewer AC, Trentman TL, Ivancic MG, Vargas BB, Rebecca AM, Zimmerman RS, et al. Long-term outcome in occipital nerve stimulation patients with medically intractable primary headache disorders. Neuromodulation. 2013;16:557–562. doi: 10.1111/j.1525-1403.2012.00490.x. [DOI] [PubMed] [Google Scholar]
- 35.Hann S, Sharan A. Dual occipital and supraorbital nerve stimulation for chronic migraine: A single-center experience, review of literature, and surgical considerations. E9Neurosurg Focus. 2013;35 doi: 10.3171/2013.6.FOCUS13233. [DOI] [PubMed] [Google Scholar]
- 36.Walling I, Panse D, Gee L, Maietta T, Kaszuba B, Kumar V, et al. The use of focused ultrasound for the treatment of cutaneous allodynia associated with chronic migraine. Brain Res. 2018;1699:135–141. doi: 10.1016/j.brainres.2018.08.004. [DOI] [PubMed] [Google Scholar]