Abstract
Vaccination of interleukin-4 (IL-4) receptor α (IL-4Rα) chain-deficient BALB/c mice with Helicobacter pylori urease and cholera toxin or with urease-expressing, live attenuated Salmonella enterica serovar Typhimurium cells revealed that protection against H. pylori infection is independent of IL-4- or IL-13-mediated signals. A comparison of male and female mice suggests a sexual dimorphism in the extent of bacterial colonization that is particularly evident in the absence of the IL-4Rα chain.
Vaccination against Helicobacter pylori infection is a desirable alternative to antibiotic treatment for the prevention of infection-associated severe gastritis, ulcus formation, and an increased risk of developing gastric cancer in patients. Effective vaccination is well documented in animal models (for a review, see reference 8). Understanding the mechanism of protection, however, is needed to eventually develop a vaccine for treating humans. Through the analysis of mice with defined genetic lesions, Helicobacter-specific CD4+ T cells were found to be sufficient for protection after vaccination with bacterial lysates or recombinant urease together with adjuvants such as cholera toxin (CT) or Escherichia coli heat-labile enterotoxin (5, 13). Infection with H. pylori also induces specific CD4+ T cells commonly classified as type 1 effector cells characterized based on their secretion of gamma interferon (IFN-γ) (9, 10, 15; for a review of clinical data see references 17 and 18). This response is not effective in reducing the bacterial burden. In contrast, results of vaccination studies with mice suggest a role for type 2 CD4+ T cells and the secretion of cytokines such as interleukin-4 (IL-4) and IL-10 by these cells in protection against Helicobacter infection (2, 7, 10, 15). In line with these findings, mice deficient in IFN-γ expression were successfully vaccinated with H. pylori antigens and CT (16), while vaccination in IL-4 gene-defective mice was quoted to be less efficient (14). These studies further supported the hypothesis that type 2 CD4+ T cells and IL-4 in particular play a role in protection but did not distinguish between its relevance for the induction of Helicobacter-specific, protective CD4+ T cells and their effector function. This distinction is important in view of the fact that antigen-specific immune responses stimulated by CT depend on IL-4 for full induction (12). In contrast, the amplitude of responses induced by recombinant Salmonella enterica serovar Typhimurium in IL-4-deficient mice was similar to that for wild-type mice (12). We aimed to assess the importance of IL-4 and also IL-13 (which overlap in function and use the same IL-4 receptor α (IL-4Rα) chain for signaling [11]) for induction of immunity and protection against H. pylori afforded by vaccination with either CT in combination with H. pylori urease or with recombinant urease-expressing, aroA attenuated live Salmonella enterica serovar Typhimurium SL3261/pYZ97 cells (6).
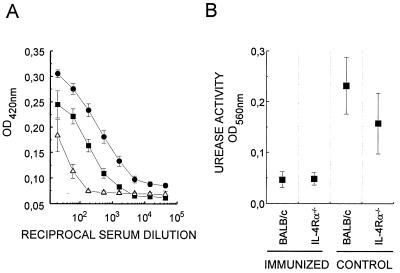
For this purpose, IL-4Rα chain-deficient BALB/c ES cell-derived mice (IL-4Rα−/−) (11), which are not able to respond to IL-4 or IL-13, and normal female BALB/c mice were vaccinated at weekly intervals with four intragastric applications of 10 μg of CT (Sigma) mixed with 5 μg of native urease purified from H. pylori strain 26695 as previously described (4). The animals were challenged intragastrically 1 week later with 109 CFU of mouse-adapted, streptomycin-resistant H. pylori P76 (a kind gift from H. Kleanthous; OraVax) grown to late log phase at 37°C with shaking at 200 rpm in an atmosphere of 5% O2, 85% N2, and 10% CO2 in brain heart infusion broth (Becton Dickinson) containing 10% fetal calf serum (Gibco), supplemented with 400 μg of streptomycin/ml. Three weeks later, mice were bled and killed, and their stomachs were removed. Stomachs were cut in half to determine in parallel tissue-associated urease activity and H. pylori burdens as described previously (6). In order to assess a general parameter of the immunogenicity of the vaccine, relative titers of urease-specific immunoglobulin (Ig) in serum were determined by enzyme-linked immunosorbent assay using plate-bound urease-positive or -negative H. pylori lysates (6). Immunized and challenged IL-4Rα−/− mice had fivefold-lower Ig titers specific for urease than BALB/c mice (P < 0.003 in a t test), confirming that IL-4 and IL-13 are important factors in the induction of this immune response by CT and urease (Fig. 1A). This was also reflected in lower proliferation by spleen cells from vaccinated IL-4Rα−/− mice than by those from wild-type animals in the presence of urease-containing H. pylori lysates (not shown). However, in our hands IL-4Rα−/− mice could still be protected from challenge infection with this treatment since no tissue-associated urease activity was detected in their stomachs (Fig. 1B).
FIG. 1.
Vaccination with purified urease in combination with CT is less immunogenic but still protective against H. pylori infection in IL-4Rα−/− BALB/c mice. Groups of eight BALB/c and IL-4Rα−/− mice were immunized with purified native urease in combination with CT and challenged with H. pylori. (A) Urease-specific Ig titers in serially diluted serum were determined by enzyme-linked immunosorbent assay (6) in immunized and challenged BALB/c (●) and IL-4Rα−/− (■) mice. Serum of nonimmunized, infected controls (▵) was used for comparison. (B) Urease activity associated with gastric tissue was determined by colorimetry after 24 h of incubation in 330 mM urea–1 mM phosphate buffer (pH 6.9)–phenol red (0.001% [wt/vol]). Values represent means ± standard errors of the means (n = 8). OD420nm, optical density at 420 nm.
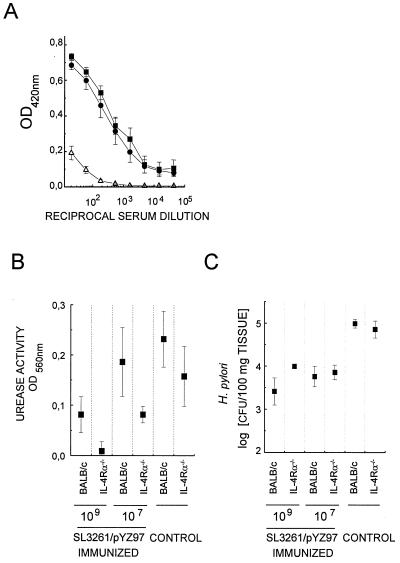
Next, IL-4Rα−/− and BALB/c female mice were vaccinated orally with 107 or 109 CFU of SL3261/pYZ97 as described previously (6). Five weeks later vaccinated and control animals were challenged intragastrically with 109 CFU of H. pylori P76. In contrast to immunization with CT, vaccination with SL3261/pYZ97 induced similar levels of urease-specific serum Ig in wild-type and IL-4Rα−/− mice (Fig. 2A). While the total IgG levels were equivalent, no urease-specific IgG1 was detectable in IL-4Rα−/− mice in contrast to what was found for wild-type BALB/c mice (data not shown). Immunization of BALB/c mice with 107 CFU instead of 109 CFU of SL3261/pYZ97 is not protective in every individual. A difference in vaccine efficacy at these doses between BALB/c and IL-4Rα−/− mice, in particular at the lower dose, should reveal a role if any for IL-4 and IL-13 signaling in protection. However, the reductions of bacterial burdens in BALB/c and IL-4Rα−/− animals were similar (Fig. 2B and C), confirming the results shown in Fig. 1B.
FIG. 2.
Vaccination with urease-expressing, recombinant Salmonella SL3261/pYZ97 is equally immunogenic in IL-4Rα−/− and BALB/c mice and protects against H. pylori infection. Mice were immunized with the indicated numbers of CFU of SL3261/pYZ97 and challenged with H. pylori. (A) Urease-specific Ig levels in serially diluted serum of BALB/c (●), IL-4Rα−/− (■), and control (▵) mice (A) and urease activity (B) were measured as described for Fig. 1. OD420nm, optical density at 420 nm. (C) The numbers of H. pylori cells colonizing gastric tissue were estimated by quantitative culture of serial dilutions of bacteria suspended by vortexing from weighed tissue fragments in brain heart infusion medium. The values shown are geometric means of CFUs determined from five mice per group ± standard errors of the means and are representative of three experiments with similar results.
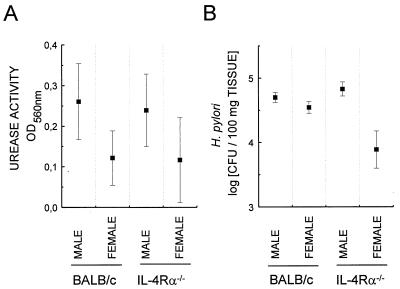
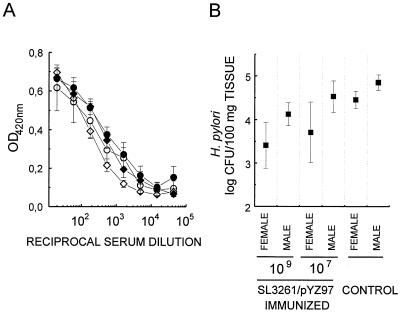
Control infected IL-4Rα−/− female mice tended to have lower levels of H. pylori colonization than infected female BALB/c mice (Fig. 1 to 3); however, this difference was not statistically significant (P > 0.1). This result is consistent with the recent reporting of similar burdens in IL-4 deficient and wild-type C57BL/6 mice (1) but is at odds with data of an earlier study showing increased bacterial colonization in IL-4-deficient mice (10). It is of note that female mice were infected here and in the former study while male animals were used in the latter. Interestingly, based on urease activity and CFU determinations male mice on average suffered from a higher H. pylori burden than age-matched female mice (Fig. 3). This was particularly pronounced in IL-4Rα−/− mice (P = 0.018 by t test [probability that this difference is due to chance]) and may account for the observed discrepancies between the aforementioned studies. A difference between genders with respect to the extent of H. pylori colonization was also found when comparing the efficiencies of SL3261/pYZ97 vaccination in male and female BALB/c mice (Fig. 4). Vaccination with 107 or 109 CFU of SL3261/pYZ97 against H. pylori challenge was less efficient in males (Fig. 4B). This is unlikely to reflect lower immunogenicity of the vaccine in males since levels of urease-specific antibodies were similar in both sexes (Fig. 4A).
FIG. 3.
Male mice suffer from higher levels of H. pylori colonization. IL-4Rα−/− and BALB/c mice were infected with H. pylori and colonization was assessed 3 weeks after challenge by determining urease activity (A) and estimating bacterial burden (B) as described in the legends to Fig. 1 and 2. Values represent arithmetic (A) or geometric (B) means from individual determinations of six mice per group ± standard errors of the means. OD560nm, optical density at 560 nm.
FIG. 4.
Vaccination against H. pylori with urease-expressing, recombinant Salmonella SL3261/pYZ97 is less protective in male than in female mice. (A) Mean ± standard errors of the means of urease-specific Ig levels in serially diluted serum prepared from male (diamonds) and female (circles) mice (n < 4) immunized with 107 (open symbols) or 109 CFU (solid symbols) of SL3261/pYZ97 3 weeks after infection with H. pylori. OD420nm, optical density at 420 nm. (B) H. pylori burden in vaccinated and control mice estimated as described for Fig. 2. Values correspond to means from individual determinations for four to six mice per group ± standard errors of the means. Statistical analysis of the difference between male and female mice by t tests yielded P values of 0.051 and 0.029 at the high- and low-dose inocula, respectively.
In summary, our results show that IL-4 and IL-13 are not critical for the CD4 T-cell-mediated mechanism responsible for H. pylori clearance in vaccinated animals. Differences noted between normal and IL-4Rα−/− mice in vaccination experiments likely reflect the dependence of the immune reaction on IL-4 during the induction phase when CT is used (12). Furthermore a sexual dimorphism in the degree of colonization by H. pylori, which may represent a novel factor contributing eventually to the higher prevalence of gastric cancer seen in male patients, is described (3).
Acknowledgments
We thank Anna Walduck and Ana Mißlitz for helpful comments on the manuscript.
REFERENCES
- 1.Chen W, Shu D, Chadwick V S. Helicobacter pylori infection in interleukin-4-deficient and transgenic mice. Scand J Gastroenterol. 1999;34:987–992. doi: 10.1080/003655299750025084. [DOI] [PubMed] [Google Scholar]
- 2.Corthesy-Theulaz I E, Hopkins S, Bachmann D, Saldinger P F, Porta N, Haas R, Zheng-Xin Y, Meyer T, Bouzourène H, Blum A L, Kraehenbuhl J-P. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–586. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crespi M, Citarda F. Helicobacter pylori and gastric cancer: what is the real risk? Gastroenterologist. 1998;6:16–20. [PubMed] [Google Scholar]
- 4.Dunn B E, Campbell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 5.Ermak T H, Giannasca P J, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Duarte O G, Lucas B, Yan Z X, Panthel K, Haas R, Meyer T F. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine. 1998;16:460–471. doi: 10.1016/s0264-410x(97)00247-8. [DOI] [PubMed] [Google Scholar]
- 7.Ikewaki J, Nishizono A, Goto T, Fujioka T, Mifune K. Therapeutic oral vaccination induces mucosal immune response sufficient to eliminate long-term Helicobacter pylori infection. Microbiol Immunol. 2000;44:29–39. doi: 10.1111/j.1348-0421.2000.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 8.Kleanthous H, Lee C K, Monath T P. Vaccine development against infection with Helicobacter pylori. Br Med Bull. 1998;54:229–241. doi: 10.1093/oxfordjournals.bmb.a011673. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 10.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 11.Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–7308. [PubMed] [Google Scholar]
- 12.Okahashi N, Yamamoto M, VanCott J L, Chatfield S N, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee J R. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappo J, Torrey D, Castriotta L, Savinainen A, Kabok Z, Ibraghimov A. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect Immun. 1999;67:337–341. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saldinger P F, Blum A L, Corthesy-Theulaz I E. Perspectives of anti-H. pylori vaccination. J Physiol Pharmacol. 1997;48(Suppl. 4):59–65. [PubMed] [Google Scholar]
- 15.Saldinger P F, Porta N, Launois P, Louis J A, Waanders G A, Bouzourene, Michetti P, Blum A L, Corthesy-Theulaz I E. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology. 1998;115:891–897. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 16.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y, Iwakura Y, Imanishi J. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–285. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommer F, Faller G, Konturek P, Kirchner T, Hahn E G, Zeus J, Rollinghoff M, Lohoff M. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–5546. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zevering Y, Jacob L, Meyer T F. Naturally acquired human immune responses against Helicobacter pylori and implications for vaccine development. Gut. 1999;453:465–474. doi: 10.1136/gut.45.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]