Abstract
Objectives
To assess the effects of thoracic paravertebral block and s-ketamine on postoperative pain and perioperative neurocognitive disorder (PND) in video-assisted thoracoscopic surgery.
Methods
Patients (n = 120) aged 45–65 undergoing video-assisted thoracoscopic surgery were allocated randomly into the following three groups: patients in the C group received general anaesthesia; patients in the thoracic paravertebral block group, i.e. the TP group, received general anaesthesia and ultrasound-guided paravertebral block; and patients in the s-ketamine combined with ultrasound-guided thoracic paravertebral nerve block group, i.e. the TS group, received combined anaesthesia, which was administered as follows: general anaesthesia + ultrasound-guided paravertebral block + perioperative s-ketamine (a bolus of 0.3 mg/kg, followed by an infusion of 0.2 mg/kg/h until 30 min before the end of the surgical procedure).
Results
Cognitive function was measured using the Mini-Mental State Examination 1 day preoperatively, 1 day postoperatively, and 3 months postoperatively. Z-score was used to determine the incidence of PND. Postoperative pain was assessed using the visual analogue scale at 0.5 and 24 h postoperatively. The use of opioid drugs, intraoperative vital signs, and other secondary outcomes were also recorded. The final analysis included a total of 110 patients. The intraoperative heart rate and mean arterial pressure in the TS and TP groups were lower than that for group C (P<0.05). After surgery, patients in the TS group exhibited significantly lower pain scores at 0.5 h and 24 h (P<0.001 and P = 0.004,respectively) as well as significantly lower rates of postoperative nausea, vomiting, and pulmonary complications (P<0.05). The incidence of PND in the TP and TS groups was lower than those who received general anaesthesia. However, there was no significant difference in the incidence of PND between the TP and TS groups (P>0.05).
Conclusions
Ultrasound-guided paravertebral nerve block combined with s-ketamine decreased acute postoperative pain and improved the quality of recovery. However, perioperative s-ketamine did not improve cognitive function in patients under general anaesthesia with thoracic paravertebral block.
Keywords: S-ketamine, Postoperative pain, Cognitive function, Perioperative neurocognitive disorder, Video-assisted thoracoscopic surgery
S-ketamine; Postoperative pain; Cognitive function; Perioperative neurocognitive disorder; Video-assisted thoracoscopic surgery.
1. Introduction
Video-assisted thoracoscopic surgery (VATS) has been widely used in recent years owing to its low invasiveness. However, surgical stimulation, intraoperative single-lung ventilation, and pneumatic injuries can increase stress and cause various postoperative complications, such as perioperative neurocognitive disorder, which can seriously affect patients' quality of life. The incidence of PND after thoracoscopic surgery can be as high as 32% [1]. Therefore, it is necessary to explore a more reasonable anaesthetic method to reduce the occurrence of PND.
Reducing acute postoperative pain reduces inflammation in the central nervous system (CNS), improves cognitive function, and reduces the incidence of PND [2, 3]. Thoracic paravertebral block (TPVB) applied in thoracoscopic surgery can block the intercostal nerves and sympathetic trunk, maintain perioperative haemodynamic stability, inhibit the release of inflammatory factors, and reduce the incidence of postoperative pain and PND [4, 5]. As a dextroform of the traditional analgesic-sedative ketamine, s-ketamine has a more substantial analgesic effect and fewer adverse effects. Subanaesthetic doses of s-ketamine can reduce acute opioid tolerance, inhibit nociceptive hypersensitivity, and relieve acute postoperative pain [6, 7]. The combined application of the two techniques is consistent with the Enhanced recovery after surgery (ERAS) multimodal analgesia concept; however, few studies have explored the combined application of these techniques and predominantly focused on changes in cognitive function following surgery. Therefore, this study investigated the effect of ultrasound-guided thoracic paravertebral nerve block combined with intraoperative infusion of s-ketamine on acute pain and changes in cognitive function after thoracoscopic surgery to provide a clinical reference.
2. Method
This was a single-centre randomised placebo-controlled clinical trial that included 120 patients undergoing elective thoracoscopic radical lung cancer surgery between April 2021 and February 2022. Ethical approval for this study was granted by the ethical committees of the First Hospital of Qinhuangdao (identifier: 2021Y090). All the patients signed an informed consent form. The study protocol was registered at ClinicalTrials.gov (NCT05341375). The inclusion criteria were as follows: patients aged 45–65, body mass index 18–24 kg/m2, American Society of Anaesthesiologists physical status (ASA) II or III, and preoperative Mini-Mental State Examination (MMSE) score≥24 points. Exclusion criteria were as follows: patients with cardiopulmonary and other vital organ dysfunction; MMSE score≤23 points; preoperative Parkinson's disease and/or Alzheimer's disease; anxiety, depression, and affective disorders; previous cerebrovascular accidents or long-term use of drugs affecting the neuropsychiatric system; and severe visual, hearing, or speech impairment or who were otherwise unable to communicate with the visitor and refused to sign the informed consent form. Rejection criteria were as follows: conversion to thoracotomy; patients who developed severe complications after enrolment and did not follow the study methodology; and those lost to follow-up 3 months after surgery.
2.1. Intervention
According to the random number table method, patients were divided into the following: control group (Group C, no nerve block performed), ultrasound-guided TPVB group (TP group), and s-ketamine combined with ultrasound-guided thoracic paravertebral nerve block group (TS group), with 40 patients in each group. In addition, we included 40 healthy individuals of similar age and ability to the experimental group as normal controls (group N), most of whom were family members of the patients, to eliminate the learning effect that may result from repeated assessment. In group C, general anaesthesia was applied; in group TP, an ultrasound-guided thoracic paravertebral nerve block was performed in the lateral position (with the operated side on top) after intubation; in group TS, 0.3 mg/kg of s-ketamine was applied during induction of anaesthesia, followed by a continuous infusion of 0.2 mg/kg/h until 30 min before the end of the procedure, and the rest was the same as the TP group.
2.2. Thoracic paravertebral block
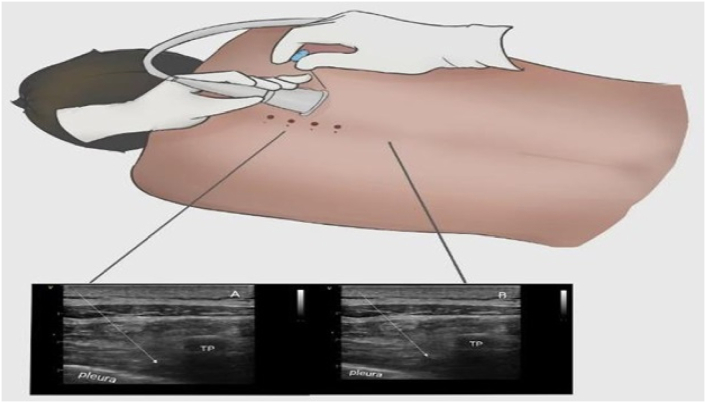
Following induction of anaesthesia by the same anaesthesiologist, an ultrasound-guided (GEViVid IQ Portable Ultrasound) TPVB was performed in the lateral position (with the operative side on top). To identify the hyperechoic image of the pleura between the shadows of successive transverse processes, a high-frequency linear probe was placed longitudinally 2.5 cm lateral to the tip of the T4 spinous process. Using an in-plane technique, the puncture needle position was adjusted under ultrasound visualisation to reach the paravertebral space through the skin and transverse costal ligament in sequence. After the syringe was free of blood and gas, 0.375% (20 ml) ropivacaine (AstraZeneca, lot H20140764, UK) was injected, and diffusion of the drug outside the pleura was noted; the appearance of the "Shanshui sign" indicated that the block was successful. Detailed images are shown in Figure 1.
Figure 1.
Ultrasound-guided thoracic paravertebral block. (A) Local anaesthetic before diffusion in the thoracic paravertebral space. (B) After diffusion of local anaesthetic in the thoracic paravertebral space. The white line represents the needle trajectory TP: transverse processes.
2.3. Anaesthesia and analgesia protocol
After entering the operating room, patients were monitored using pulse oximetry, electrocardiography, and invasive arterial blood pressure. The same anaesthetist performed the induction of anaesthesia: intravenous midazolam 0.02 mg/kg, sufentanil 0.4 μg/kg, etomidate 0.4 mg/kg, and cisatracurium 0.15 mg/kg. The patient was preoxygenated for 3 min and then intubated with a double-lumen bronchial tube. Fibrinoscopy was performed to determine good catheter position. Anaesthetic maintenance was performed as follows: propofol 4–6 mg/kg/h, remifentanil 0.1–0.2 μg/kg/min, cisatracurium 0.05 mg/kg/h, dexmedetomidine 0.4 μg/kg/h,and Sevoflurane 0.8–1.0 MAC. For single-lung ventilation, the lung-protective ventilation parameters were adjusted. Intraoperative temperature monitoring was routinely performed, and all patients were admitted to the PACU with atropine 0.01 mg/kg and neostigmine 0.02 mg/kg to antagonise muscle relaxants. All patients were administered postoperative intravenous patient-controlled analgesia (PCIA), and analgesic drugs were prepared as follows: sufentanil 0.04 μg/kg/h diluted to 100 ml with saline at a background dose of 2 ml/h, PCA 0.5 ml, with a lock time of 15 min, at a constant rate.
2.4. Observation indicators
The primary observation indicators were as follows:
The MMSE scale was applied to assess patients' cognitive function 1 day preoperatively, 1 day postoperatively, and 3 months postoperatively. In this study, patients with a preoperative MMSE score ≥24 were selected, and PND was determined using the Z-score method [8]. The following is a formula for the Z-score method, where B and A refer to the patient's postoperative and preoperative MMSE scores, respectively; XC is the mean of the change in the normal control group over the same period; SDXC represents the standard deviation of the change in the normal control group; and the final Z value ≥1.96 indicates that the patient had developed PND.
The secondary observation indicators:
The visual analogue scale (VAS) score was measured after extubation and 1 day after surgery for each of the three groups, and the number of analgesic pump presses was also measured on the postoperative day. The VAS was used by patients to indicate their pain level on a ruler, with a score of 0–10 indicating no pain to unbearably severe pain. Other secondary outcomes include intraoperative vital signs (T1: pre-operation; T2: immediately after initiating the operation; T3: end of the operation; T4: after the extubation), dosage of anaesthetic drugs, Ramsay score, comparison of anaesthetic time, and the time of surgery. Ramsay score after extubation is an indicator of the responsiveness of the patient post surgery. Postoperative monitoring of rehabilitation indicators includes the incidence of postoperative nausea and vomiting (PONV), chest tube indwelling time, and the occurrence of pulmonary complications (atelectasis, pneumonia).
2.5. Statistical analysis
The sample size of this study was calculated using the PASS software. The incidence of PND in the general anaesthesia control group was 27.27%, which was determined by a literature review. Assuming α = 0.05 and β = 0.10, the formula was substituted to obtain 30 cases in each group of this study. Taking into account the 20% loss of the follow-up rate, a final determination of 40 cases in each group was made.
The SPSS 23.0 software was used for statistical analysis. The Shapiro–Wilk method was used to test the normality of the data, and the Levene method was used to test the homogeneity variance. Normally distributed measures were expressed as mean (standard deviation), one-way analysis of variance (ANOVA) was used for between-group comparisons, and ANOVA for repeated-measures data was used for within-group comparisons. The non-normally distributed measures were expressed as median (interquartile range), and the Kruskal–Wallis H test was used for comparison between groups. The Wilcoxon test was used for comparison between two groups, and count data were expressed as numbers [proportion,%] and compared with the χ2 test. Statistical significance was set at P < 0.05 (two-tailed).
3. Results
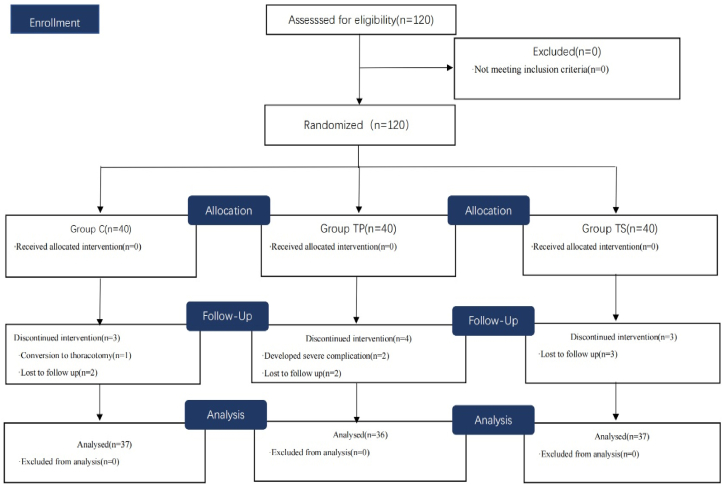
This study enrolled 120 patients, of which 10 were excluded because of conversion to open thoracotomy, serious postoperative complications, or failure to complete follow-up. Finally, 110 patients were selected and categorized in the following groups: 37 in C, 36 in TP, and 37 in TS (Figure 2). No statistical differences were observed between the demographic and surgical data (P>0.05) (Table 1).
Figure 2.
Flow diagram of the study.
Table 1.
Comparison of general conditions, duration of surgery and anaesthesia, and intraoperative cardiovascular active drug dosage.
| General analgesia (n = 37) | TPVB (n = 36) | TPVB + S-ketamine (n = 37) | Group N (n = 40) | P Value | |
|---|---|---|---|---|---|
| Age (y) | 56.46 ± 6.07 | 58.81 ± 5.58 | 57.14 ± 5.94 | 57.62 ± 4.78 | 0.527 |
| Sex (M/F) | 17/20 | 15/21 | 19/17 | 22/18 | 0.628 |
| Weight (kg) | 63.65 ± 7.04 | 63.86 ± 8.21 | 66.57 ± 7.24 | 69.13 ± 6.43 | 0.237 |
| BMI (kg/m2) | 22.56 ± 1.46 | 22.31 ± 1.86 | 22.81 ± 1.65 | 23.17 ± 1.96 | 0.453 |
| ASA (I/II/III) | 15/18/4 | 12/20/4 | 13/21/3 | 12/25/3 | 0.822 |
| Duration of analgesia | 183.78 ± 60.93 | 189.72 ± 60.01 | 182.97 ± 62.72 | … | 0.877 |
| Duration of surgery | 147.16 ± 54.18 | 155.14 ± 57.58 | 145.54 ± 56.23 | … | 0.737 |
| Atropine dosage | 0(0-0) | 0(0-0) | 0(0-0) | … | 0.477 |
| Ephedrine dosage | 0(0–1.5) | 0(0–6) | 0(0–6) | … | 0.497 |
ASA, American Society of Anaesthesiologists; BMI, body mass index; TPVB, thoracic paravertebral block.
Data are expressed as mean ± standard deviation or numbers (percentages).
3.1. Postoperative pain and recovery indicators
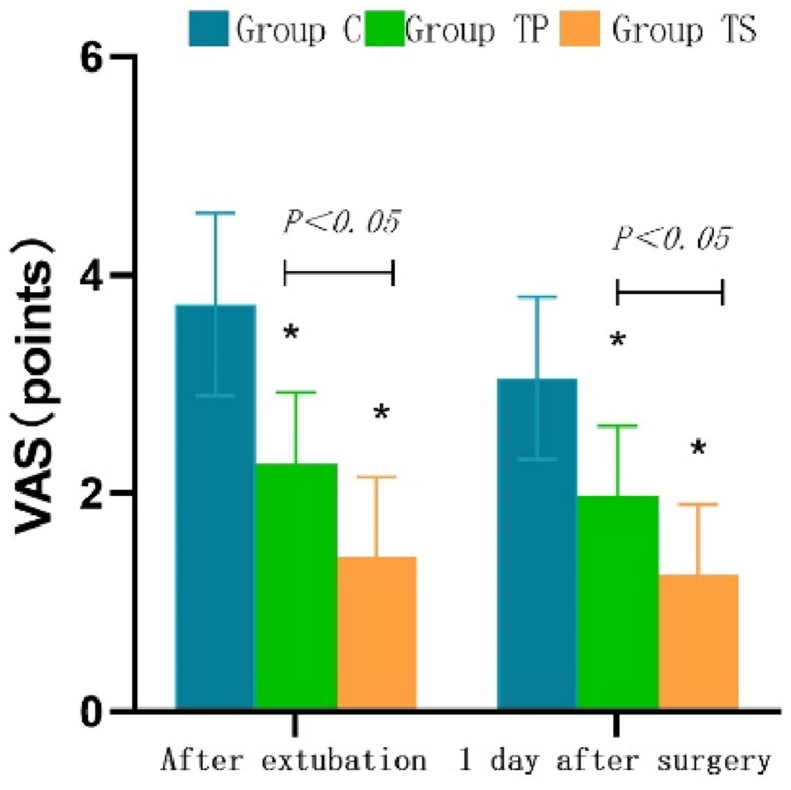
The comparison of VAS scores and number of PCIA presses in the three groups are detailed in Table 2. Compared with the TP group, VAS was significantly higher in the C group and lower in the TS group after extubation and 1 day after surgery (1.43 ± 0.73 vs. 2.28 ± 0.66, 3.73 ± 0.83 vs. 2.28 ± 0.66, P < 0.001). Detailed trends are shown in Figure 3. The number of PCIA presses was significantly higher in the C and TP groups on the postoperative day than in the TS group (P < 0.001). The incidence of postoperative PONV and pulmonary complications was significantly lower in the TS group than in the other two groups (P < 0.05). Compared to the TP group, the Ramsay score was significantly higher in the C group; however, the difference was not statistically significant in the TS group (P > 0.05).
Table 2.
Rehabilitation indicators.
| General analgesia (n = 37) | TPVB(n = 36) | TPVB + S-ketamine (n = 37) | P Value | |
|---|---|---|---|---|
| VAS(points) | ||||
| After extubation | 3.73 ± 0.83 | 2.28 ± 0.66 | 1.43 ± 0.73 | <0.001 |
| 1 day after surgery | 3.05 ± 0.74 | 1.97 ± 0.65 | 1.27 ± 0.65 | 0.004 |
| Remedial analgesia | 12(12–13) | 4(3–4) | 2(2–3) | <0.001 |
| Ramsay (points) | 4 (3–4) | 2 (2–2) | 2 (2–2) | <0.001 |
| PONV(%) | 14 (37.8) | 8 (22.2) | 5 (13.5) | 0.048 |
| Pulmonary complication (%) | 10 (27) | 4 (11.1) | 0 (0) | 0.002 |
| Chest tube indwelling time (days) | 4 (3–4) | 4 (3–5) | 4 (3–5) | 0.396 |
VAS, visual analogue score. Data are expressed as mean ± standard deviation or numbers (percentages).
Figue 3.
Box plot comparing VAS scores of three groups at. ∗:P<0.05, vs group C.
3.2. Cognitive function
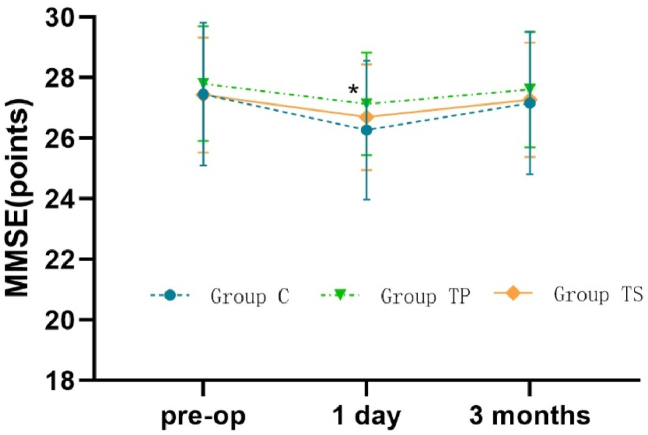
Compared with the baseline, the MMSE score was significantly lower in group C at 1 day after operation (27.43 ± 2.36 vs. 26.27 ± 2.29, P = 0.025), and the difference between the changes in the TP and TS groups was not statistically significant (P > 0.05). At the 1-day postoperative time point, the MMSE score in the TP group was significantly higher than that in the C group (P < 0.05). At the 3-month postoperative time point, the MMSE scores were similar among the three groups (P > 0.05). Detailed trends are shown in Figure 4. There was a statistically significant difference in the incidence of PND among the three groups at different time points. The incidence of PND was significantly lower in the TS and TP groups than in the C group at the 1-day and 3-month postoperative time points (P < 0.05); however, the difference between the TP and TS groups was not statistically significant (Table 3).
Figue 4.
Trend graph comparing MMSE scores of the three groups different time points ∗:P<0.05, vs group C at three different time points; ∗: P<0.05, group TP vs group C.
Table 3.
Cognitive function.
| General analgesia (n = 37) | TPVB (n = 36) | TPVB + S-ketamine (n = 37) | Group N (n = 40) | P Value | |
|---|---|---|---|---|---|
| MMSE (points) | |||||
| Before operation | 27.43 ± 2.36 | 27.81 ± 1.89 | 27.52 ± 1.89 | 28.03 ± 1.27 | 0.581 |
| After operation | |||||
| 1 day | 26.27 ± 2.29 | 27.13 ± 1.70 | 26.70 ± 1.72 | 28.77 ± 1.16 | 0.041 |
| 3 months | 27.16 ± 2.31 | 27.61 ± 1.90 | 27.27 ± 1.85 | 28.41 ± 1.19 | 0.625 |
| Incidence of PND,n (%) | |||||
| 1 day | 8 (21.6) | 4 (11.1) | 4 (10.8) | … | 0.043 |
| 3 months | 4 (10.8) | 1 (2.7) | 2 (5.4) | … | 0.031 |
MMSE, Mini-Mental State Examination; PND, Perioperative Neurocognitive Disorder.
Data are expressed as mean ± standard deviation or numbers (percentages).
3.3. Hemodynamics
The differences in mean arterial pressure (MAP) and heart rate (HR) among the three groups were statistically significant (Table 4). Fluctuations in MAP and HR were significantly higher in group C than in groups TP and TS at T2 and T4 time points (P < 0.05). Similarly, remifentanil dosage was significantly less in the TS group (1139.73 ± 337.59 vs. 987.22 ± 299.64 vs. 914.05 ± 294.27, P = 0.003).
Table 4.
Intraoperative data.
| General analgesia (n = 37) | TPVB (n = 36) | TPVB + S-ketamine (n = 37) | P Value | |
|---|---|---|---|---|
| MAP (mmHg) | ||||
| Pre-operation | 82.08 ± 11.04 | 80.72 ± 14.10 | 80.41 ± 10.16 | 0.813 |
| During operation | ||||
| Immediately | 106.89 ± 17.37 | 88.56 ± 13.79 | 88.84 ± 13.99 | <0.001 |
| End | 95.05 ± 14.39 | 92.47 ± 12.19 | 91.89 ± 9.92 | 0.504 |
| After extubation | 109.51 ± 17.18 | 107.17 ± 12.41 | 104.14 ± 12.39 | 0.047 |
| HR (beat) | ||||
| Pre-operation | 62.51 ± 9.03 | 60.25 ± 7.83 | 60.19 ± 8.14 | 0.407 |
| During operation | ||||
| Immediately | 69.46 ± 11.13 | 64.61 ± 8.03 | 63.11 ± 8.15 | 0.010 |
| End | 66.03 ± 7.85 | 65.97 ± 9.49 | 64.86 ± 8.35 | 0.337 |
| After extubation | 75.92 ± 11.07 | 70.64 ± 8.91 | 70.43 ± 8.75 | 0.025 |
| Dose of remifentanil (μg) | 1139.73 ± 337.59 | 987.22 ± 299.64 | 914.05 ± 294.27 | 0.003 |
| Dose of propofol (mg) | 492.43 ± 208.10 | 416.64 ± 199.10 | 422.16 ± 151.48 | 0.073 |
MAP, mean arterial pressure; HR, heart rate.
Data are expressed as mean ± standard deviation or numbers (percentages).
4. Discussion
PND are common neurological complications that include preoperative cognitive dysfunction, postoperative delirium, delayed neurological recovery, and postoperative cognitive dysfunction [9]. The patients’ quality of life is seriously affected owing to lack of response and decreased memory function. PND is generally believed to be caused by surgical stress and acute pain, which leads to an inflammatory cascade, a series of inflammatory factors that cross the blood-brain barrier (BBB) and accelerate neuronal degeneration. Cheon et al. [10] concluded that the expansion of peripheral immune cells was accompanied by high levels of NF-kB expression after surgical stimulation. Disruption of the BBB leads to central immune recruitment, and a series of peripheral inflammatory factors such as IL-6, IL-1β, and TNF-α enter the centre, causing hippocampal-inducing PND. Xiao et al. [11] also showed that acute postoperative pain triggers severe CNS inflammation, leading to the development of PND. Clinical studies have revealed that perioperative anaesthetic pain management is crucial for thoracoscopic surgery. Intraoperative surgical stimulation and imbalance of the ventilation-to-blood flow ratio during one-lung ventilation can aggravate perioperative inflammatory factor outbreaks. Patients may suffer from postoperative incisional pain and neuralgia, which can affect the respiratory depth and prevent coughing and expelling sputum smoothly, resulting in multiple complications and increased stress levels. Ewaga et al. [12] showed that the incidence of postoperative PND after thoracic surgery was 22.2%, which is roughly similar to the incidence of PND in the general anaesthesia control group in this study (21.6 %). The reason for this slightly lower incidence is that the existing literature suggests that dexmedetomidine improves neurocognitive function [13]; therefore, to ensure homogeneity in the present study, 0.4 μg/kg/h continuous infusion was applied in all groups to remove the influence of this factor.
Several ultrasound visualization techniques have been developed in recent years, and TPVB are increasingly used in thoracoscopic surgery. Ultrasound-guided TPVB can be used in thoracoscopic surgery to reduce the transmission of nociceptive signals to the centre and achieve analgesia by blocking the unilateral dorsal-ventral branch of the spinal nerve and sympathetic nerve trunk. The results of this study showed that MAP and HR in group C were significantly higher than those in TP and TS groups at T2 and T4 time points, and the postoperative VAS scores were also significantly higher (P < 0.05), which is similar to the findings of Zhang et al [14]. To some extent, this indicates that the TPVB may be beneficial in the prevention of surgery stress. Thoracoscopic surgery mainly involves the lateral and anterolateral cortical branches of the 4th and 7th intercostal nerves, respectively. The TPVB can block 2–3 paravertebral spaces by injecting 20 ml of the local anaesthetic drug ropivacaine into the T5 paravertebral space, which can diffuse upwards and downwards to achieve a good analgesic effect. Ropivacaine is a common local anaesthetic drug used for nerve blocks, with low neurotoxicity. Jeong [15] compared the drug safety of 0.75% vs 0.375% ropivacaine and found that high concentrations were prone to haemodynamic fluctuations; hence, 0.375% ropivacaine was used for nerve block in this study, and the puncture procedure was performed smoothly without a single case of nerve injury.
In addition, we observed that VAS scores (calculated after extubation and 1 day after surgery) were significantly reduced in the TS group than in the TP group; however, there was no statistically significant difference in Ramsay scores. It appears that s-ketamine, as an NMDAR antagonist, has an analgesic and sedative effect without impairing patient responsiveness when awake post operation. Previous studies have demonstrated that NMDAR functions as a glutamate receptor involved in pain signalling and that s-ketamine inhibits injurious signalling by binding to the phencyclidine site of NMDAR and antagonising it noncompetitively. Similarly, Joly et al. [16] found that remifentanil induced ectopic nociception and nociceptive sensitisation around the incision, and the application of subanaesthetic doses of s-ketamine antagonised the central nociceptive sensitisation (opioid-induced hyperalgesia) caused by the activation of the NR2B subunit of NMDAR, prophylactically reducing nociceptive sensitisation and relieving acute postoperative pain. Furthermore, the results of this study indicated that the amount of remifentanil used in the TS group was significantly lower than that in the C and TP groups. Thus, s-ketamine could also act on opioid receptors and produce synergistic analgesic effects with opioids, enhancing their analgesic effects and reducing their dosage [17]. This improved analgesia produced by a different analgesic mechanism and is in line with the modern concept of opioid-less multimodal analgesia under ERAS management.
The effectiveness of perioperative analgesia in improving cognition has also been demonstrated in previous studies [18]. The MMSE is the most widely used cognitive function assessment scale. However, the learning effect of repeated pre-and postoperative assessments may have a significant impact on true clinical cognitive function changes. Johnson et al. [8] compared three different methods for assessing the MMSE scale and concluded that the Z-score method takes into account the learning effect of neuropsychological retesting (this learning effect may result in a higher second neuropsychological score than the first). Cognitive function was assessed by selecting 40 healthy people of similar age and ability (experimental group) as of the normal control group during the same period. The mean of their postoperative and preoperative change values was considered as a learning effect, which was eliminated by substituting the formula. The Z value is marked with "+" or "-" to represent the direction of the patient's cognitive bias. The International Multicentre POCD Study Group (IS-POCD) also recommends the use of the Z-score method rather than the difference method to determine the incidence of PND.
Most previous studies have demonstrated that ultrasound-guided paravertebral nerve blocks can reduce stress, provide effective analgesia, and improve neurocognitive function during thoracoscopic surgery. Similarly, the results of this study found that the MMSE scores were significantly lower (P < 0.05) in the general anaesthesia control group 1 day after operation compared to that of baseline, and the difference between the changes in the TP and TS groups was not statistically significant. This may be because both clinical and basic studies have shown that general anaesthetic drug exposure increases brain remodelling and induces neuronal apoptosis [19, 20]. TPVB can improve cognition by reducing the stress response and decreasing the amount of intraoperative general anaesthetic drugs; besides, surgical stimulation decreases the central pain domain, leading to acute postoperative pain and inducing PND [21]. Ultrasound-guided paravertebral nerve blocks performed before surgery can reduce the transmission of surgical stimuli to the CNS, relieve patients' pain, and boost near and distant cognitive functions; moreover, Ayda [22] found no statistically significant difference between intraoperative and preoperative baseline rSO2-C values in patients with thoracic paravertebral nerve blocks, suggesting that thoracic paravertebral nerve blocks used in thoracoscopic surgery can maintain intraoperative hemodynamic stability, ensure perioperative cerebral perfusion, and reduce cerebral neuronal injury.
While most of the literature has long focused on the occurrence of cognitive dysfunction 1 day or 7 days after VATS [23, 24], this study examined the immediate and long-term cognitive function changes in patients after thoracoscopic surgery. We found that ultrasound-guided thoracic paravertebral nerve block with s-ketamine reduced PONV and pulmonary-related complications compared to that of other experimental groups, which is similar to the results of a large-sample study [25]. We conclude that the combination of TPVB and s-ketamine is an effective method for managing postoperative pain and promoting postoperative recovery. Interestingly, we found that although thoracic paravertebral nerve block combined with s-ketamine could further reduce opioid dosage and acute postoperative pain compared to that of the C and TP groups, the incidence of PND was not significantly lower in the TS group than in the TP group at 1 day and 3 months postoperatively (P > 0.05). The comparison of change rates between the two groups, although clinically significant, was not statistically significant, indicating that subanaesthetic doses of s-ketamine did not further improve the incidence of PND in patients who had undergone TPVB. Tan et al. [26] found that the ratio of brain-derived neurotrophic factor to phosphorylated TrKB was reduced in the hippocampus of the PCP-induced cognitive impairment model in mice, and pre-treatment with s-ketamine did not improve neurocognitive function, which is similar to the results of the present study. Some scholars believe that s-ketamine can alter the synaptic plasticity of the hippocampus and prefrontal cortex to affect neurocognitive function [27], and the related mechanism needs to be further investigated, which is also a limitation of this study.
5. Conclusion
Ultrasound-guided thoracic paravertebral nerve block combined with S-ketamine can maintain intraoperative haemodynamic stability, reduce acute postoperative pain, and promote rapid postoperative recovery. However, continuous intraoperative application of s-ketamine did not improve neurocognitive function in patients with TPVB.
Declarations
Author contribution statement
Xiaodan Chen: Performed the experiments; Wrote the paper.
Qinshuang Liu:Conceived and designed the experiments; Analyzed and interpreted the data.
Long Fan: Contributed reagents, materials, analysis tools or data.
Funding statement
This research was supported by Medical-Industrial Crossover Special Incubation Project of Yanshan University and The First Hospital of Qinhuangdao [UY202201].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
References
- 1.Ran J., Bai X., Wang R., et al. Role of dexmedetomidine in early POCD in patients undergoing thoracic surgery. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/8652028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheon S.Y., Kim J.M., Kam E.H., et al. Cell-penetrating interactomic inhibition of nuclear factor-kappa B in a mouse model of postoperative cognitive dysfunction. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-14027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawal N. Current issues in postoperative pain management. Eur. J. Anaesthesiol. 2016;33(3):160–171. doi: 10.1097/EJA.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 4.Xie H., Zhou J., Du W., et al. Impact of thoracic paravertebral block combined with general anesthesia on postoperative cognitive function and serum adiponectin levels in elderly patients undergoing lobectomy. Wideochir. Inn. Tech. Maloinwaz. 2019;14(4):538–544. doi: 10.5114/wiitm.2019.84742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argiriadou H., Papagiannopoulou P., Foroulis C.N., et al. Intraoperative infusion of S(+)-ketamine enhances post-thoracotomy pain control compared with perioperative parecoxib when used in conjunction with thoracic paravertebral ropivacaine infusion. J. Cardiothorac. Vasc. Anesth. 2011;25(3):455–461. doi: 10.1053/j.jvca.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Colvin L.A., Bull F., Hales T.G. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393(10180):1558–1568. doi: 10.1016/S0140-6736(19)30430-1. [DOI] [PubMed] [Google Scholar]
- 7.Kose E.A., Bakar B., Ayva S.K., et al. Neuroprotective effects of racemic ketamine and (S)-ketamine on spinal cord injury in rat. Injury. 2012;43(7):1124–1130. doi: 10.1016/j.injury.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Johnson T., Monk T., Rasmussen L.S., et al. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96(6):1351–1357. doi: 10.1097/00000542-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Rengel K.F., Pandharipande P.P., Hughes C.G. Special considerations for the aging brain and perioperative neurocognitive dysfunction. Anesthesiol. Clin. 2019;37(3):521–536. doi: 10.1016/j.anclin.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Cheon S.Y., Kim J.M., Kam E.H., et al. Cell-penetrating interactomic inhibition of nuclear factor-kappa B in a mouse model of postoperative cognitive dysfunction. Sci. Rep. 2017;7(1):13482. doi: 10.1038/s41598-017-14027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Q.X., Liu Q., Deng R., et al. Postoperative cognitive dysfunction in elderly patients undergoing hip arthroplasty. Psychogeriatrics. 2020;20(4):501–509. doi: 10.1111/psyg.12516. [DOI] [PubMed] [Google Scholar]
- 12.Egawa J., Inoue S., Nishiwada T., et al. Effects of anesthetics on early postoperative cognitive outcome and intraoperative cerebral oxygen balance in patients undergoing lung surgery: a randomized clinical trial. Can. J. Anaesth. 2016;63(10):1161–1169. doi: 10.1007/s12630-016-0700-4. [DOI] [PubMed] [Google Scholar]
- 13.Qian X.L., Zhang W., Liu M.Z., et al. Dexmedetomidine improves early postoperative cognitive dysfunction in aged mice. Eur. J. Pharmacol. 2015;746:206–212. doi: 10.1016/j.ejphar.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J.W., Feng X.Y., Yang J., et al. Ultrasound-guided single thoracic paravertebral nerve block and erector spinae plane block for perioperative analgesia in thoracoscopic pulmonary lobectomy: a randomized controlled trial. Insights Imag. 2022;13(1):16. doi: 10.1186/s13244-021-01151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong J.M., Lee H.J., Oh Y.J., et al. Observations on significant hemodynamic changes caused by a high concentration of epidurally administered ropivacaine: correlation and prediction study of stroke volume variation and central venous pressure in thoracic epidural anesthesia. BMC Anesthesiol. 2017;17(1):153. doi: 10.1186/s12871-017-0444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joly V., Richebe P., Guignard B., et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103(1):147–155. doi: 10.1097/00000542-200507000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Pacheco Dda F., Romero T.R., Duarte I.D. Central antinociception induced by ketamine is mediated by endogenous opioids and μ- and δ-opioid receptors. Brain Res. 2014;1562:69–75. doi: 10.1016/j.brainres.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Kristek G., RadoŠ I., Kristek D., et al. Influence of postoperative analgesia on systemic inflammatory response and postoperative cognitive dysfunction after femoral fractures surgery: a randomized controlled trial. Reg. Anesth. Pain Med. 2019;44(1):59–68. doi: 10.1136/rapm-2018-000023. [DOI] [PubMed] [Google Scholar]
- 19.Muller-Lissner S., Bassotti G., Coffin B., et al. Opioid-induced constipation and bowel dysfunction: a clinical guideline. Pain Med. 2017;18(10):1837–1863. doi: 10.1093/pm/pnw255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Shen F., Xu D., et al. A lasting effect of postnatal sevoflurane anesthesia on the composition of NMDA receptor subunits in rat prefrontal cortex. Int. J. Dev. Neurosci. 2016;54:62–69. doi: 10.1016/j.ijdevneu.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi K., Ikeda R., Shoji H., et al. Aging attenuates glucocorticoid negative feedback in rat brain. Neuroscience. 2009;159(1):259–270. doi: 10.1016/j.neuroscience.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Türköz A., Balcı S.T., Can Güner M., et al. Anesthesia management with single injection paravertebral block for aorta coarctation in infant. Paediatr. Anaesth. 2013;23(11):1078–1083. doi: 10.1111/pan.12252. [DOI] [PubMed] [Google Scholar]
- 23.Lei Y., Liu H., Xia F., et al. Effects of esketamine on acute and chronic pain after thoracoscopy pulmonary surgery under general anesthesia: a multicenter-prospective, randomized, double-blind, and controlled trial. Front. Med. 2021;8 doi: 10.3389/fmed.2021.693594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X., Li D., et al. Effect of epidural block anesthesia combined with general anesthesia on postoperative cognitive ability of elderly patients undergoing thoracoscopic surgery. Am. J. Transl. Res. 2020;13(10):2447–2454. [PMC free article] [PubMed] [Google Scholar]
- 25.Brinck E.C., Tiippana E., Heesen M., et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2018;12:CD012033. doi: 10.1002/14651858.CD012033.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Y., Fujita Y., Qu Y., et al. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent repeated intermittent administration of (R)-ketamine, but not (S)-ketamine: role of BDNF-TrkB signaling. Pharmacol. Biochem. Behav. 2020;188 doi: 10.1016/j.pbb.2019.172839. [DOI] [PubMed] [Google Scholar]
- 27.Morrison R.L., Fedgchin M., Singh J., et al. Effect of intranasal esketamine on cognitive functioning in healthy participants: a randomized, double-blind, placebo-controlled study. Psychopharmacology (Berl) 2018;235(4):1107–1119. doi: 10.1007/s00213-018-4828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.