Abstract
Autoantibodies targeting epitopes contained within the intracellular domain (IC) of the protein phosphatase-like islet antigen 2 (IA-2) are a common marker of autoimmune type 1 diabetes (T1D), however the isolation of genuine, serum derived anti-IA-2 autoantibodies has proven challenging due to a lack of suitable bioassays. In the current study, an ELISA format was developed for affinity purification of human anti-IA-2ic autoantibodies utilizing a fusion protein (FP) incorporating maltose binding protein and the full-length IA-2IC domain. Using a T1D patient cohort validated for anti-IA-2ic autoantibodies by commercial ELISA, we demonstrate the MBP-IA-2ic FP ELISA detects serum anti-IA-2IC autoantibodies from 3 of 9 IA-2 positive patients. Further to this, a multi-plate MBP-IA-2ic FP ELISA protocol specifically affinity purifies IgG enriched for anti-IA-2ic autoantibodies. Interestingly, serum derived autoantibodies immobilised on the MBP-IA-2ic FP ELISA demonstrate increased Kappa light chain usage when compared to the respective total IgG derived from donor patients, suggesting a clonally restricted repertoire of anti-IA-2ic autoantigen specific B plasma cells is responsible for autoantibodies detect by the MBP-IA-2ic FP ELISA. This study is the first to demonstrate the generation of specific, genuine human derived anti-IA-2ic autoantibodies, thereby facilitating further investigation into the origin and functional significance of IA-2 autoantibodies in T1D.
Keywords: Type 1 diabetes, IA-2, Autoantibodies, Affinity purification, Clonal restriction
Abbreviations: T1D, Type 1 Diabetes; β, beta cell; GAD65, glutamic acid decarboxylase 65; IA-2, islet antigen 2; IC, intracellular; FP, fusion protein; OD, optical density
Highlights
-
•
ELISA approach facilitates affinity purification of anti-IA-2 antibodies.
-
•
Affinity purification specifly enriches anti-IA-2 autoantibodies.
-
•
Anti-IA-2 antibodies show enrichment for kappa light chains relative to total immunoglobulin.
1. Introduction
Autoimmune targeting of pancreatic beta (β) cells is a key feature of type 1 diabetes (T1D) and a number of autoantigens expressed primarily by β cells have been identified [[1], [2], [3]]. While the aetiology driving autoimmune-mediated β cell loss remains under investigation, several studies support a central role for T cells [4]. Particularly convincing are observations that onset of symptoms is concurrent with predominantly CD8+ T cell-mediated insulitis [5,6], and that both CD8+ and CD4+ T cells specific for islet-derived epitopes are present in islet-derived isolates from patients [6,7].
To date, considerations of humoral responses in T1D have focused primarily on the role of antibodies as diagnostic and/or prognostic markers of disease, and a considerable body of evidence supports the predictive value of serotyping autoantibody responses in at risk individuals [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]]. While a correlation between the emergence of serum autoantibodies and conversion to clinical disease is suggestive of antibody-mediated pathogenesis, a direct relationship between the generation in individuals of autoantibodies to β cell targets and β cell loss has yet to be established [20]. This is perhaps unsurprising, given the major β cell targets in T1D, including glutamic acid decarboxylase 65 (GAD65), protein phosphatase-like islet antigen 2 (IA-2), and the zinc transporter, ZnT8, are intracellular [[21], [22], [23]]. However, the linking of anti-GAD65 antibodies to a number of central nervous system disorders such as stiff-person syndrome, cerebellar ataxia and epilepsy [24], and observations that monoclonal anti-GAD autoantibodies impair neurotransmission in experimental models [25] warrants further characterisation of the key autoantibodies in T1D.
IA-2 is a member of the protein tyrosine phosphatase (PTP) family, with expression largely confined to nerve and neuroendocrine cells [26,27]. Similar to GAD, IA-2 is a common target of the autoimmune process in T1D, with approximately 70% of patients testing positive for anti-IA-2 autoantibodies at diagnosis [19]. IA-2 is localised to the insulin secretory granules and appears to contribute to granule motility, insulin production and β cell division [28,29], suggesting that autoantibodies binding to IA-2 in vivo could exert deleterious effects on β cell function. Interestingly, stratification of autoimmune responses to the various domains of IA-2 has prognostic utility [30,31], potentially linking pathophysiological relevance to specific epitope recognition. Of the three major IA-2 domains, the intracellular (IC) domain spanning amino acids 601 to 979 is arguably the best characterised for antigenic determinants, with this PTP-containing domain hosting at least two conformational epitopes [19,32]. Previous studies characterising epitopes within the IC domain isolated several anti-IA-2 antibody-producing B cells from the peripheral blood of T1D patients [33]. However, there are no studies as yet describing the isolation of patient-derived circulating anti-IA-2 autoantibodies, an advancement that would facilitate more in depth interrogation of the physiological consequences of anti-IA-2 autoantibodies.
In the current study we have used a novel fusion protein incorporating maltose binding protein (MBP) and the IC domain of IA-2 in combination with a validated ELISA plate technique to affinity purify (AP) and enrich anti-IA-2 autoantibodies from the serum from two patients with T1D. The AP anti-IA-2 immunoglobulin (Ig) shows enriched Kappa light chain expression compared to Igs in the starting serum, suggesting that humoral responses to the IC domain of IA-2 are clonally restricted, a finding consistent with autoantibodies in a number of systemic autoimmune diseases [34]. This study therefore provides a methodology for purifying genuine, patient-derived anti-IA-2 antibodies that should facilitate investigations of both the origins and potential pathogenic consequences of antibodies directed against this major human autoantigen.
2. Materials and methods
2.1. Patient and control samples
Blood samples were obtained with informed consent from patients with T1D (n = 66; 35 males with an average age of 34.6, and 31 females with an average age of 38.9), and healthy individuals (n = 10; 5 females with an average age of 33.6, and 5 males with an average age of 27.8). Samples were collected from patients with established disease (disease duration 1–22 years with an average of 9.3 for male patients; and 2–20 years with an average of 13.2 for female patients). All procedures were reviewed and approved by the Southern Adelaide Clinical Human Research Ethics Committee (no 39.034).
2.2. Determination of anti-IA-2 and ZnT8 autoantibody status
Patient sera were screened for the presence of autoantibodies against the IC domain of IA-2 (aa604-979) and ZnT8 (aa275-369) using validated immunoassays (RSR Limited, Lianishen, Cardiff, United Kingdom). Assays were performed as per manufacturer's instructions. Briefly, 50 μL of patient sera (neat), negative controls and calibrators were transferred in duplicate to individual assay wells, along with reaction enhancer (25 μL). The plate was briefly agitated on an orbital shaker (5 s, 500 shakes per minute) and incubated overnight at 4 °C. Following the incubation period, well contents were discarded and the plate was washed (x3) with diluted wash solution. Reconstituted IA-2 Biotin (100 μL) was then added to the wells and the plate incubated for 1 h at 4 °C, followed by a further washing step (x3). Following this, streptavidin peroxidase (SA-POD, 100 μL) was transferred to the wells and the plate incubated for 20 min at room temperature on an ELISA plate shaker. Excess SA-POD was then removed by a further wash step (x3), and 3,3′,5,5′-tetramethylbenzidine (TMB, 100 μL) was added and incubated at room temperature in the dark for 20 min without shaking. Stop solution (100 μL) was then added to the wells, and absorbance determined at 450 nm using a microplate reader (BIO-RAD Model 680 XR, Hercules, California). Sera positivity for anti-IA-2, ZnT8 and GAD autoantibodies was determined using the positive controls and calibrators provided by the manufacturer.
2.3. Generation of an MBP-IA-2 fusion protein incorporating the IC domain (ICD)
Soluble MBP fusion protein (FP) incorporating IA-2 ICD (IA-2 aa 601–969) were produced using BL21-Gold (DE3) bacteria (Stratagene, San Diego, California, USA) transformed by a pMAL-c5X vector (New England Biolabs, Ipswich, MA). Briefly, bacterial clones were generated by transformation with the MBP-IA-2 vector and antibiotic selection on agar plates, with Sanger sequencing of transformed cells used to confirm correct orientation of the MBP-IA-2 gene cassette. For FP expression, colonies were amplified overnight (37 °C with shaking at 220 rpm in Terrific media (12 g/L tryptone, 24 g/L yeast extract, 0.04 ml/L glycerol)) to an optical density (OD) of 1. Once achieved, Isopropyl B-D thiogalactopyranoside (IPTG, 0.1 mM) was added to the culture to induce expression of the fusion protein, and the bacteria was further cultured overnight at 30 °C on a shaking platform. Following this, cells were harvested by centrifugation at 4600 RPM for 20 min and washed with 0.9% NaCl and stored at −20 °C. MBP fusion proteins were isolated from cell lysates by liquid column chromatography. Firstly, defrosted cells were re-suspended in ∼30 mL column buffer and pulse sonicated (100 Amplitude, 30 Watts, 878 J) (Misonix Sonicator Ultrasonic Liquid Processor) for 2 min. Lysates were centrifuged at 9000×g for 30 min and the resulting supernatants were loaded on amylose resin columns. Weakly bound and unbound proteins were washed off the column with 12 column volumes of column buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA, pH 7.4). Bound fusion protein was eluted off the column with sequential addition of 5 column volumes at 10 mM and 20 mM Maltose in column buffer. The eluted proteins were pooled together and dialyzed against PBS using a 10K spin column (Ultra −15 Centrifugal filter, Amicon, Ireland). Purity and molecular size of the dialysed FP was confirmed by single dimension SDS gel electrophoresis, and protein concentrations determined by Bradford and EZQ assays as per manufacturers instructions, and immunohistochemical confirmation of the purified FP performed by Western blot using an anti-MBP antibody. Briefly, following SDS gel electrophoresis FP was immobilised on nitrocellulose paper using Trans-Blot Electrophoretic Transfer Cell (BioRad, Hercules, CA). Once protein transfer was complete, the nitrocellulose was blocked with 5% non-fat milk in TBS-T (20 mM Tris, 150 mM NaCl, 0.1% Tween-20, pH 7.5) for 1 h at room temperature on a stirrer, and washed three times with TBS-T. The nitrocellulose was then incubated with mouse monoclonal anti-MBP antibody (1:1000, New England BioLabs, Ipswich, MA) in 1% skim milk TBS-T for 1 h in room temperature, followed by incubation with an anti-mouse IgG antibody conjugated with horseradish peroxidase (1:1000, Sigma-Aldrich, St. Louis, Missouri, USA). Anti-MBP antibody binding was detected by incubation in chemiluminescent substrate and imaged using an LAS-4000 luminescent analyzer (Life Science, Fuji Film, Tokyo). Molecular mass of the proteins was determined using dual stained ladders (Biorad, USA).
2.4. Optimisation of the FP coating concentration for ELISA
To determine an optimized well coating concentration for establishing the anti-IA-2 autoantibody ELISA, a range of IA-2 MBP fusion protein coating concentrations was assessed by ELISA using 96 well Maxisorp plates (Nunc, Rockslide, Denmark). Briefly, wells were coated with MBP-IA-2 FP at 0.06, 0.1, 0.6, 1, 2, 4, 6, 10 and 16 μg/mL in 50 mM carbonate coating buffer (pH 9.6) incubated overnight at 4 °C. Following the coating step, the plates were washed and non-specific sites blocked with 1% BSA in PBS for 1 h at 37 °C and plates washed with 0.05% Tween-20 in PBS. Anti-MBP antibody at 1:1000 in 1% Skim milk in PBS was transferred to the wells and incubated at 37 °C for 1 h. Following washing, bound anti-MBP antibody was detected by alkaline phosphatase conjugated anti-mouse IgG secondary antibody (1:1000) for 1 h at 37 °C. Secondary antibody was detected using a p-nitrophenyl phosphate substrate (Sigma, St Louis, MO) in diethalamine buffer (1 mg/mL, 90 mM, pH 9.6). All samples were tested in duplicate. OD values were read at 405 nm.
2.5. Optimisation of the serum screening protocol for anti-IA-2 ELISAs
To establish background binding, sera from two healthy individuals were tested at several MBP-IA-2 FP protein coating concentrations ranging from 0.06 to 16 μg/mL. Sera were diluted at 1:10, 1:20, 1:50 and 1:100 in PBS containing 1% skim milk, and transferred to coated plates and incubated at 37 °C for 1 h. Following rigorous washing (3x PBS) wells were sequentially incubated with anti-human IgG conjugated with alkaline phosphatase (1 h at room temperature on a shaking platform) and p-nitrophenyl phosphate substrate. OD values were determined at 5, 10, 20 and 30 min, and normalised to control wells incubated with serum alone (uncoated) at equivalent serum dilutions. All samples were tested in duplicate. OD values were read at 405 nm. Specific reactivity to the IA-2 component of MBP-IA-2 was determined by comparison to reactivity of the serum with MBP alone, or other MBP-FPs, including MBP-ZnT8(A) and (W) variants (kind gift from Jens Lagerstedt, Lund University, Sweden).
2.6. Affinity purification of anti-IA-2 autoantibodies
Autoantibodies specific to the MBP-IA-2 FP were affinity purified from serum samples identified as having specific reactivity for IA-2 (Table 2) using a multiplate ELISA protocol [35]. Briefly, individual serum samples from selected patients and controls were incubated on ELISA plates coated with MBP-IA-2 FP (4 plates were used for each serum sample), as described above. Following incubation and washing (x6) anti-IA-2 autoantibodies were eluted off by addition of elution buffer (200 μL, 7.5 g/L Glycine, 29.2 g/L NaCl, pH 2.3) (5 min at room temperature on shaking platform), and collected supernatant balanced at pH 7 by addition of 1M-Tris (pH 8). The elution step was repeated 3 times. The samples were then concentrated from ∼250 mL down to ∼200 μL by centrifuging the supernatant in a 10K spin column (Merck Millipore, MA) for 8 min at 4600 rpm at room temperature. The concentrated antibody sample was washed with PBS/dH2O by centrifugation at 4600 rpm for 15 min. The antibody sample was further concentrated by centrifugation at 14 000 g for 30 min in a 0.5 mL spin column (Merck Millipore).
Table 2.
Establishment of cut off values for positivity by IA2/MBP FP and ZnT8/MBP FP ELISAs.
| CTR | Mean OD | SD |
|---|---|---|
| 1 | 0.0195 (0.0318) | 0.0212 (0.0025) |
| 2 | 0.0903 (0.0985) | 0.0470 (0.0608) |
| 3 | 0.08 (0.0255) | 0.0071 (0.0099) |
| 4 | 0.0238 (0.145) | 0.0166 (0.0219) |
| 5 | 0.1333 (0.0065) | 0.0124 (0.0085) |
| 6 | 0.0495 (0.1295) | 0.0113 (0.0191) |
| 7 | 0.0325 (0.0465) | 0.0127 (0.000) |
| 8 | 0.074 (0.0630) | 0.0453 (0.0127) |
| 9 | 0.109 (0.018) | 0.0523 (0.0106) |
| 10 | 0.0213 (0.0295) | 0.0301 (0.0347) |
| Pooled CTRs |
0.0634 (0.0594) |
0.045 (0.0486) |
| OD value cut off for positivity | ||
| IA-2 FP ELISA 0.198 | ||
| ZnT8 FP ELISA 0.194 | ||
*Values are mean and SD of n = 4 replicates for individual control (CTR 1–10) serums assessed on IA-2/MBP (unbracketed) and ZnT8/MBP (bracketed) FP ELISAs. Pooled values and SDs were used to generate positivity cut off scores representing the mean + 3 SDs.
2.7. Confirmation of anti-IA-2 autoantibody enrichment by reactivity ELISA
Affinity purified antibody was tested by ELISA to determine the specificity and enrichment using the FPs MBP-IA-2IC and MBP-ZnT8.
2.8. Assessment of kappa/Lambda light chain expression in anti-IA-2 autoantibodies
For determination of Kappa/Lambda light chain expression, anti-IA-2 autoantibodies in serum from selected patients were bound to MBP-IA-2 FP by ELISA as described above. Bound anti-IA-2 autoantibodies were then incubated with rabbit anti-human Kappa or Lambda antibodies (1 h at RT) (A0191, A0193, Dako, Agilent, CA, USA), followed by washing and were detected by using a Goat anti-rabbit alkaline phosphatase secondary antibody (Sigma, Merck, New Jersey, USA). All samples were tested in duplicates. To determine Kappa and Lambda expression in total IgG from the selected patients, IgG (prepared using caprylic acid precipitation [36]) from each individual patient was coated onto ELISA plates and incubated with rabbit anti-human Kappa or Lambda antibodies and detected as above.
2.9. Statistical analysis
Where appropriate, two-way Friedmann ANOVA (non-parametric) testing was used for comparison of patient verses control binding to the various FPs etc. Tukey's multiple comparisons ANOVA test was used to test any correlation between serum binding of IA-2 positive patients and healthy controls at 1/20 serum dilution.
3. Results
3.1. Determination of autoantibody status by RSR ELISA
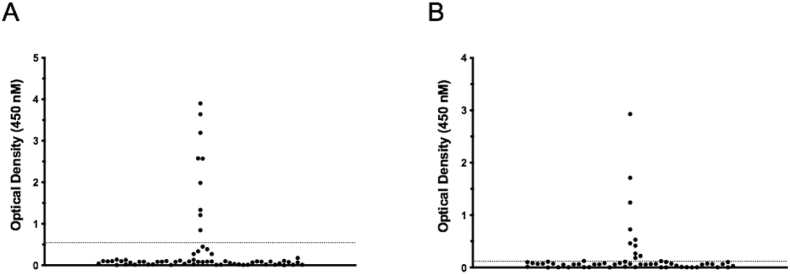
To identify appropriate positive and negative controls for the development of the MBP-IA-2 FP ELISA, the autoantibody status of patients was assessed by RSR ELISA. Nine of 66 T1D patient serum samples were identified as positive for anti-IA-2IC autoantibodies (Fig. 1A; Table 1), and 10/66 positive for anti-ZnT8 autoantibodies (Fig. 1B; Table 1). Of the IA-2 and ZnT8 autoantibody positive patients, 3 of 19 displayed positivity for both autoantibodies.
Fig. 1.
Prevalence of anti-IA-2 and ZnT8 autoantibodies in a cohort of 66 patients with established type 1 diabetes. Patients with confirmed type 1 diabetes were assessed for autoantibodies against the intracellular domain of IA-2 (A), and ZnT8 (B) using commercial ELISA assays (RSR Limited, Cardiff UK). Antibodies to IA-2 were present in individual serum samples from 9 of 66 patients, with individual serum samples from 10 patients containing anti-ZnT8 autoantibodies. Serum samples were assessed in triplicate. Assay cut offs for positivity were determined by standards and controls as per manufacturers instructions, and indicated on the figures by dotted lines. Individual points represent mean OD values for individual patient serum samples.
Table 1.
Demographics and antibody status of patients and controls.
| Autoantibody Status (RSR ELISA) |
||||||||
|---|---|---|---|---|---|---|---|---|
| (n = ) | Avg. age | IA-2 |
ZnT8 |
IA-2/ZnT8 Pos | ||||
| Pos | Neg | Pos | Neg | |||||
| T1D | Male | 35 | 34.6 | 5 | 30 | 5 | 30 | 2 |
| Female | 32 | 38.9 | 4 | 28 | 5 | 27 | 1 | |
| CTR | Male | 5 | 27.8 | - | - | - | - | - |
| Female | 5 | 33.6 | - | - | - | - | - | |
3.2. ELISA optimisation using an MBP-IA-2 FP: Plate coating optimisation and assessment of non-specific serum binding
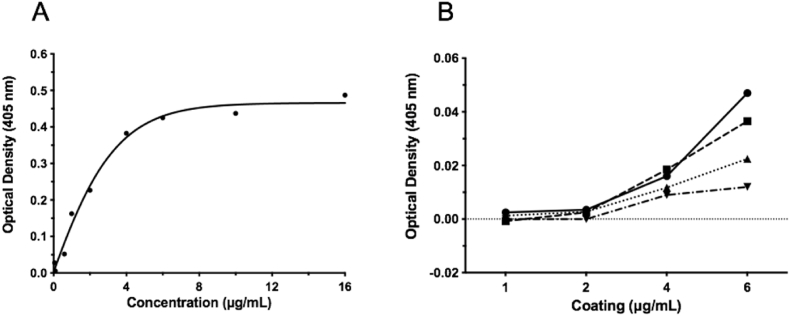
Following liquid chromatographic purification of the MBP-IA-2 FP, various concentrations of FP were assessed for coating efficiency by ELISA. Using an anti-MBP antibody, saturation of binding was observed at between 6 and 16 μg/ml of coating concentration, with linear binding observed at lower concentrations lower (Fig. 2A). To further confirm an optimal FP coating concentration, ELISAs were performed using between 1 and 6 μg/ml of plate coating concentrations in combination with various dilutions of serum from healthy donors (n = 2). As demonstrated in Fig. 2B, when adjusted for background, coating concentrations of 1 and 2 μg/ml of FP resulted in pooled serum OD values below 0.01, with no statistical difference observed across the serum dilutions (1:10, 1:20, 1:50 and 1:100). At a FP coating concentration of 4 μg/ml serum OD values ranged from 0.01 to 0.018, again with no statistical difference observed between the serum dilutions. However, at a 6 μg/ml FP coating concentration, considerable variation in OD values were observed, with serum dilutions of 1:100 maintaining low OD values, but significant increases observed at all lower serum dilutions, with a maximum OD of 0.05 observed at a serum dilution of 1:10. Given these data, a coating concentration of 4 μg/ml of FP and a serum dilution of 1:20 was deemed to represent a suitable combination for assessing the utility of the IA-2 FP ELISA to detect anti-IA-2 autoantibodies, and all subsequent ELISAs using the MBP-IA2 FP were conducted using these parameters.
Fig. 2.
Optimisation of coating concentration and serum dilution in an MBP-IA-2 fusion protein ELISA. A. Incubation of ELISA plates with between 0.06 and 16 μg/mL with MPB-IA-2 fusion protein resulted in saturation at 6 μg/mL as determined using an anti-MBP monoclonal antibody. B. Subsequently, fusion protein coated ELISA plates were incubated with serum from 2 healthy individuals diluted at 1:10 (solid line - circle), 1:20 (dashed line - square), 1:50 (dotted line - triangle) and 1:100 (dotted/dashed line - inverted triangle). Background binding was highest at a coating concentration of 6 μg/mL, followed by 4 μg/mL. At most coating concentrations, serum dilutions of 1:10 produced the highest background and 1:100 the lowest. To achieve maximum assay sensitivity and acceptable background serum binding, a coating concentration of 4 μg/mL with serum dilution of 1:20 was selected for all subsequent fusion proten ELISAs. Data points represent the mean of individual experimental conditions. All experimental conditions were performed in triplicate.
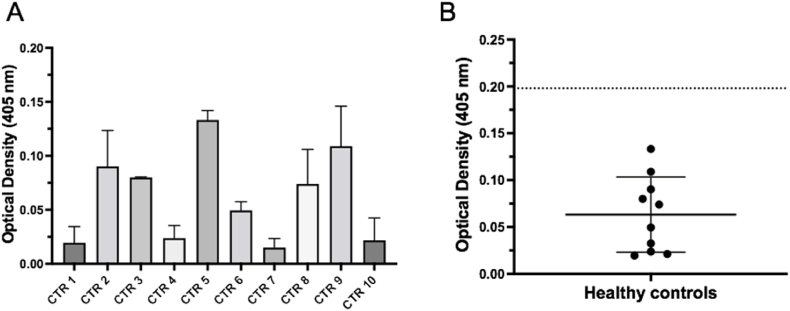
Following establishment of the ELISA screening conditions, mean OD values following MBP-IA-2 FP ELISA were determined for sera from 10 individual healthy controls to establish the background binding values for comparison to patient samples (Fig. 3A). These ELISAs generated a mean OD value of 0.0634, with a SD of 0.045. The criteria for positivity by the FP ELISA was set at greater than the mean + 3 SD (0.198) (Fig. 3B, Table 2). The control sera were also subjected to ELISA evaluation using the MBP-ZnT8 FP, resulting in a pooled mean OD of 0.058, with SD of 0.051, and a mean + 3 SD OD value of 0.212 for positivity (Table 2).
Fig. 3.
Determination of background absorption of serum from healthy controls in a MBP-IA-2 fusion protein ELISA. A. Absorption values of serum (1:20 dilution) from 10 individual healthy donors (CTR) ranged from approximately 0.03 to 0.13 at a wavelength of 405 nM at a MBP-IA-2 fusion protein concentration of 4 μg/mL. B. Following the ELISA, the means from the 10 individual CTR serum samples were plotted and overall CTR binding means and SD assessed. As demonstrated by the Scatter Plot, the combined mean of the 10 CTR samples (solid long bar) was 0.063, with a SD ( ± solid short bars) of 0.045. Using the CTR data to set the value for positivity for anti-IA-2 autoantibodies, a value equal to the combined mean plus 3 SDs (dotted line) was generated, which provided a cut off absorbance of 0.198. This value was used in subsequent ELISAs as a cut off for the presence of anti-IA-2 autoantibodies in serum samples from patients with Type 1 diabetes. Data for panel A represents normalised means and SEM from at least n = 6 individual replicates from 2 separate experiments, with the means (solid circles) used to generate the scatter plot in panel B.
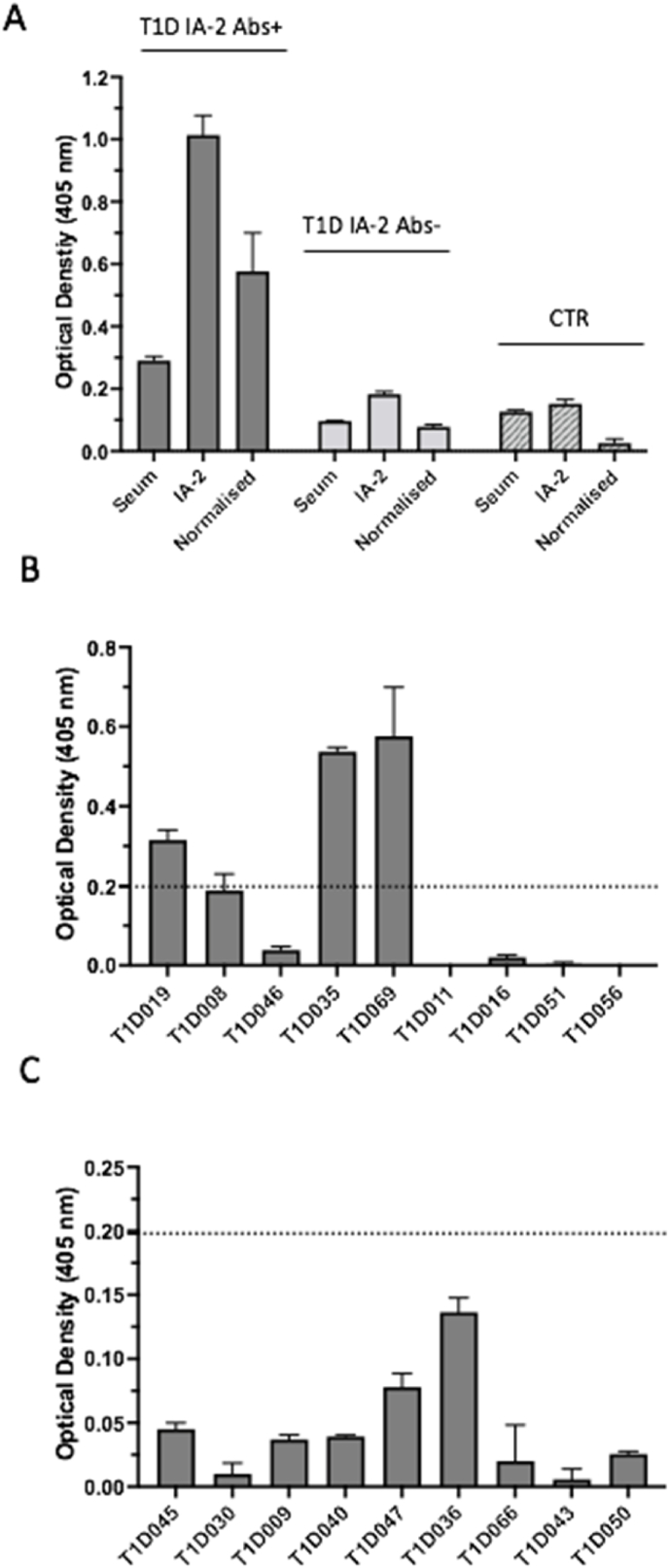
3.3. The MBP-IA-2 FP ELISA discriminates a subset of IA-2 autoantibody positive patients
To determine whether the MBP-IA-2 FP ELISA was able to detect the presence in sera of anti-IA-2 autoantibodies, individual patient sera either positive (n = 9) or negative (n = 9) for anti-IA-2 autoantibodies by RSR ELISA were assessed for binding to the MBP-IA-2 FP (Fig. 4). As demonstrated in Fig. 3, Fig. 4 (patients T1D019, T1D035 and T1D069) of 9 IA-2 positive sera gave mean OD values greater that the mean and 3 SD from pooled healthy controls. By contrast, none of the OD values from the 9 IA-2 negative patients reached the threshold for positivity, and did not differ significantly from pooled control values.
Fig. 4.
Detection of anti-IA-2 autoantibodies in the serum of a subset of IA-2 positive type 1 diabetes patients using an MBP-IA-2 fusion protein (FP) ELISA. A. Representative MBP-IA-2 fusion protein ELISA binding using patient and control serum. Serum from a patient positive (T1D IA-2+) and negative (T1D IA-2-) for IA-2 autoantibodies by RSR commercial ELISA were assessed by MBP-IA-2 FP ELISA. Normalised OD values for the anti-IA-2 antibody positive patient of 0.58 exceeded the 0.198 cut off for positivity by MBP-IA-2 FP ELISA, as determined in Fig. 3. By comparison, the normalised OD of 0.078 for the T1D IA-2 – patient was below the cut off, as was value (0.026) for the healthy control serum (CTR). B. Nine individual sera from T1D patients positive for anti-IA-2 autoantibodies by RSR ELISA were then screened using the MPB-IA-2 FP ELISA. Of these, 3 (T1D019, T1D035 and T1D069) resulted in OD values above the assay cut off for positivity (dotted line). C. By comparison to the RSR positive patients, all 9 sera from RSR negative patients produced OD values by MBP-IA-2 ELISA below the assay cut off for positivity (dotted line). Values represent mean +SEM of at least n = 6 individual replicates. Each serum was assessed by MBP-IA-2 ELISA on at least 2 separate occasions.
To further assess the specificity of the binding obtained from the 3 sera positive by MBP-IA-2 FP ELISA, these sera were also assessed for binding using an MBP-ZnT8 FP. In the MBP-ZnT8 ELISAs, mean OD values from patients T1D019 and T1D069 did not differ significantly from healthy controls, whereas the mean OD value of patient T1D035 was above the mean + 3 SD of pooled healthy control values. These results mirrored the anti-ZnT8 autoantibody status of these patients by RSR ELISA, where only sera from patient T1D035 showed positivity for anti-ZnT8 autoantibodies. These data suggest that for the 3 patients positive by MBP-IA-2 ELISA, the increased OD values are due to specific binding of anti-IA-2 autoantibodies to the IA-2 protein present in the FP.
3.4. The MBP-IA-2 FP facilitates affinity purification of anti-IA-2 autoantibodies
ELISA-based affinity purification of autoantibodies from patient serum has been reported previously, using a multiple ELISA plate capture approach [35]. In the current study a similar approach utilizing multiple MBP-IA-2 FP ELISAs was used in an attempt to affinity purify anti-IA-2 autoantibodies from 2 (T1D0019 and T1D069) of 3 patient serums demonstrating positivity on the ELISA. As described in the methods, this approach uses 4 MBP-IA-2 FP coated ELISA plates for each individual serum sample, with serum incubation followed by elution of bound antibodies.
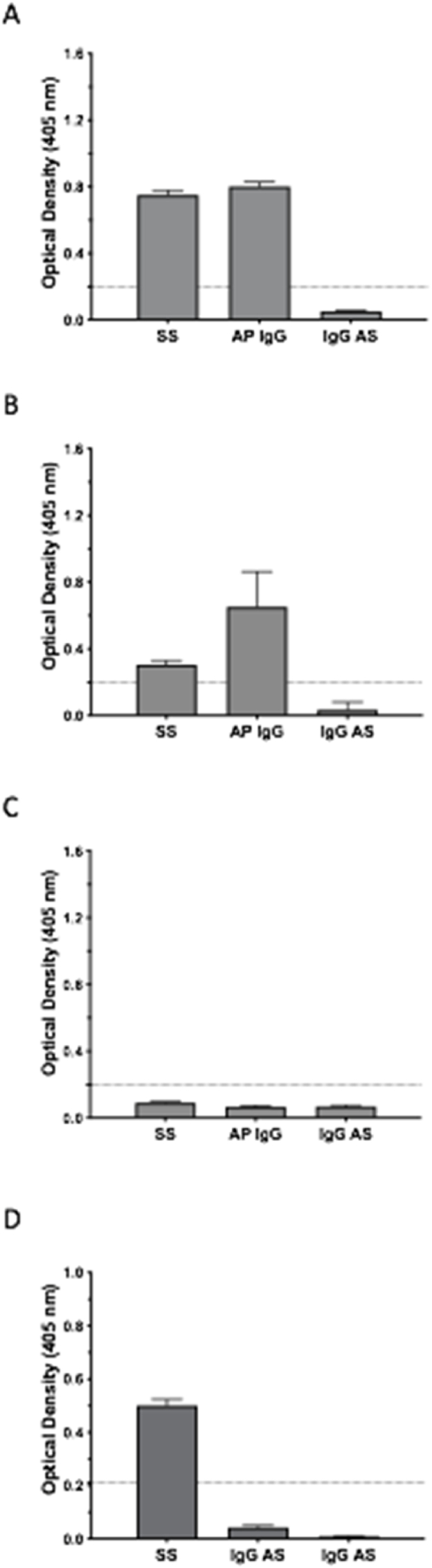
In the current study, the eluted fractions were first concentrated and then assessed for enriched binding by using the MBP-IA-2 FP ELISA. In these experiments, the OD values of concentrated affinity purified antibodies from patient T1D069 (Fig. 5A) and T1D019 (Fig. 5B) achieved positivity for anti-IA-2 antibodies by ELISA, as did starting serum samples from both patients. By contrast, when the starting serum was adjusted to match the IgG concentration of the respective affinity purified samples, neither patients’ adjusted serum sample retained positivity for anti-IA-2 by MBP-IA-2 FP ELISA (Fig. 5). These data therefore support enrichment of anti-IA-2 autoantibodies. By comparison, where serum from a healthy donor was assessed, neither serum or the eluted antibodies following the affinity purification step yielded positive results on the MBP-IA-2 FP ELISA (Fig. 5C). To further demonstrate specificity of the IgG enrichment, the starting serum and affinity purified samples from patient T1D069, whose serum tested positive for both anti-IA-2 and ZnT8 autoantibodies by RSR ELISA, were further analysed by MBP-ZnT8 ELISA (Fig. 5D). As expected, the starting serum for this patient demonstrated positivity on both the MBP-ZnT8 FP and MBP-IA-2 ELISAs (Fig. 5A and D). However, while the affinity purified sample retained positivity by MBP-IA-2 FP ELISA, positive reactivity was not maintained on the MBP-ZnT8 FP ELISA (Fig. 5D). Hence, IgG enrichment following affinity purification was restricted to anti-IA-2 autoantibodies, confirming the specificity of the ELISA affinity purification method.
Fig. 5.
Confirmation of reactivity and specificity in anti-IA-2 autoantibodies affinity purified using the MBP-IA-2 fusion protein ELISA. A. Multi-plate ELISA capture and subsequent elution of anti-IA-2 immunoglobulin (AP IgG) from patient T1D069 achieved OD values above the cut off for anti-IA-2 positivity (dotted line) on the MBP-IA-2 FP ELISA. Similar positive OD binding was achieved with the starting serum (SS) from the same patient. By contrast, when the starting serum was diluted to match the IgG concentration of the AP IgG, positivity on the ELISA was lost (IgG AS). B. Similar to T1D069, both SS and AP IgG samples from patient T1D019 achieved positivity on the MBP-IA-2 FP ELISA, with positivity lost in the IgG AS sample. C. No positivity was achieved on the ELISA by SS, AP IgG or IgG AS samples from a healthy control. D. To assess specificity of the affinity purified anti-MBP-IA-2 FP autoantibodies, SS, IgG AS and IgG AS samples from patient T1D069 were subjected to MBP-ZnT8 fusion protein ELISA. As expected, the starting serum of this patient achieved positivity on the MBP-ZnT8 fusion protein ELISA (this patient tested positive for both anti-IA-2 and ZnT8 antibodies by RSR ELISA). However, consistent with specific purification of anti-IA-2 antibodies, positivity on the MBP-ZnT8 fusion protein ELISA was lost in the AP IgG sample generated by multi-plate MBP-IA-2 fusion protein ELISA capture. As expected, the IgG AS sample from this patient was also negative on the MBP-ZnT8 FP ELISA. Data represents normalised means and SEM from at least n = 6 individual replicates from 2 separate experiments.
3.5. Anti-IA-2 autoantibodies binding the MBP-IA-2 FP are clonally restricted
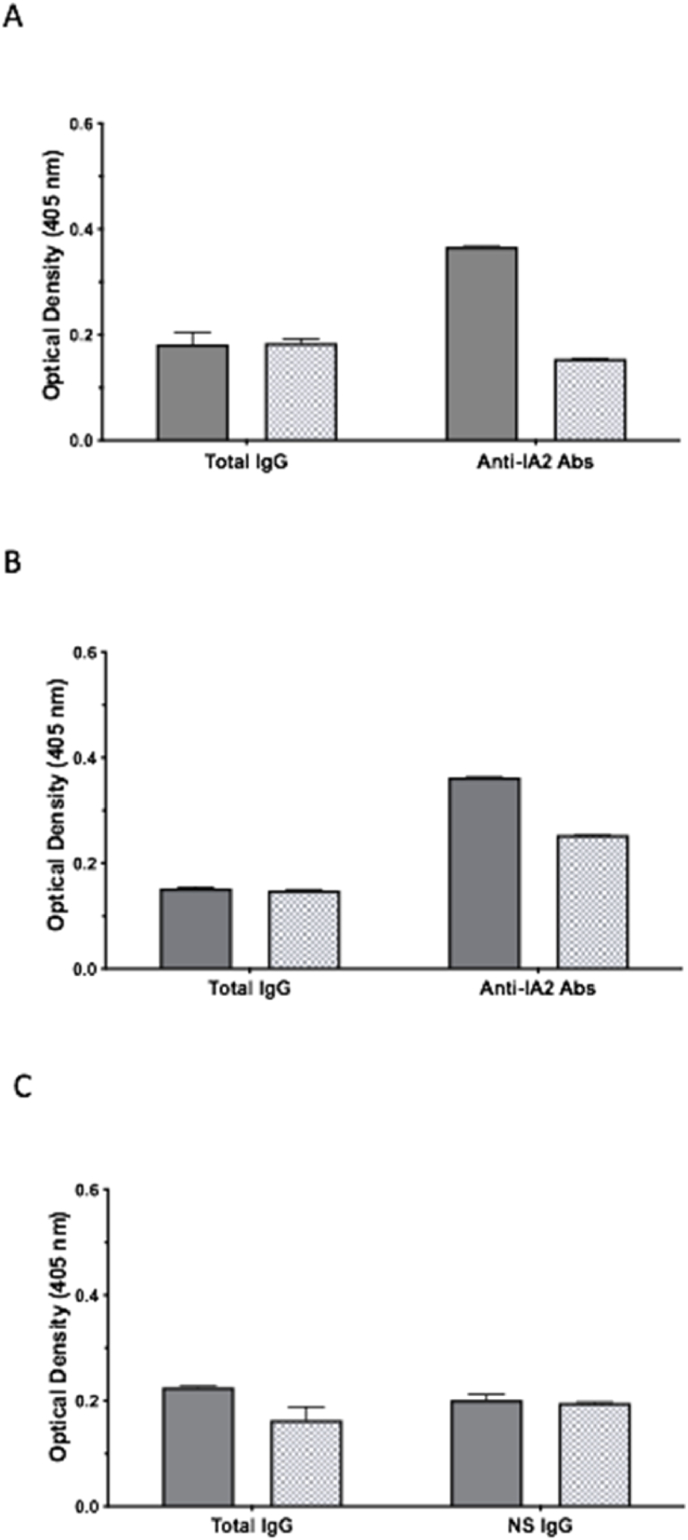
Clonal restriction of humoral autoimmunity to specific autoantigens has been reported recently [37,38]. In an attempt to determine if the specific anti-IA-2 autoantibodies binding the MBP-IA-2 FP are similarly clonally restricted, ELISAs were performed using patient serum and Ig preparations in combination with anti-human Kappa and Lambda antibodies for the detection of immobilised Ig light chains. For these experiments, the Kappa and Lambda ratios of total IgG from individual patients and controls were compared to the ratios of antibodies bound to the MBP-IA2 FP (Fig. 6). For T1D patient T1D069, a Kappa and Lambda ratio of approximately 1:1 was observed for light chains present in total IgG, as determined by optical density. By comparison, when antibodies captured by the MBP-IA-2 FP ELISA were assessed, a Kappa and Lambda ratio of approximately 2:1 was observed, indicating the predominance of Kappa expressing light chains in patient anti-MBP-IA-2 antibodies (Fig. 6A). Similarly, assessment of total IgG from patient T1D019 indicated a Kappa and Lambda ratio of approximately 1:1, with anti-MBP-IA-2 antibodies from this patient displaying a skewing to predominantly Kappa antibodies (Kappa and Lambda ratio of 1.44:1) (Fig. 6B). By contrast, assessment of the Kappa and Lambda ratio of total IgG from healthy control sera (CTR068) resulted in a ratio of 1.37:1. While the control sera did not show specific binding to the MBP-IA-2 FP ELISA, assessment of background IgG binding resulted in a Kappa and Lambda ratio of 1.03:1 (Fig. 6C).
Fig. 6.
Predominance of Kappa light chains in anti-IA-2 autoantibodies suggest clonal restriction of autoreactive B cells. A. Assessment of clonality in anti-IA-2 autoantibodies was achieved by determination of the Kappa/Lambda light chain ratio in immobilised total IgG (Total IgG) verses anti-MPB-IA-2 fusion protein specific IgG (Anti-IA-2 Abs) from a patient (T1D069) positive for anti-IA-2 autoantibodies. While the Kappa/Lambda ratio of approximately 1:1 in total IgG suggests near equal light chain representation in total serum IgG, for antibodies recognising IA-2 epitopes present in the MBP-IA-2 fusion protein, a ratio of approximately 2:1 indicates predominantly Kappa chains, indicative of clonal restriction in anti-IA-2 autoreactive B plasma cells. B. A predominance of Kappa chains in anti-IA-2 autoantibodies (Kappa/Lambda ratio 1.44:1) relative to total IgG (Kappa/Lambda ratio 1:1) in a second patient (T1D019) positive by MPB-IA-2 fusion protein ELISA confirms clonal restriction of anti-IA-2 autoreactive B plasma cells. C. In contrast to patients with T1D, the Kappa/Lambda ratio of IgG from a healthy control (CTR068) did not display evidence of increased Kappa expression in antibodies immobilised by fusion protein ELISA. The Kappa/Lambda ratio of 1.36:1 was observed in total IgG from this individual, with a post MBP-IA-2 ELISA (NS IgG) ratio of 1.03:1. Data represents normalised means and SEM from at least n = 6 individual replicates from 2 separate experiments.
4. Discussion
The current study describes a method for the AP of patient derived anti-IA-2 IgG autoantibodies by ELISA. The described methodology utilises an MBP FP incorporating the IC domain of the diabetic autoantigen IA-2 and results in selective enrichment of anti-IA-2 autoantibodies. Further, the anti-IA-2 autoantibodies captured by the FP demonstrate clonal restriction in light chain usage, with increased representation of Kappa light chains relative to total IgG. To our knowledge we are the first to report the AP of genuine, patient derived, circulating anti-IA-2 autoantibodies in T1D.
The described methodology is a novel yet simple process for isolating patient derived circulating autoantibodies against a classic autoantigen in T1D. The isolation and interrogation of circulating autoantibodies has proved crucial in elucidating humoral contributions to immunopathology in a number of autoimmune diseases. For example, AP of the ribonuclear complex targeting anti-Ro (SSa) and La (SSb) autoantibodies from patients with systemic autoimmunity has lead to advancements including solid phase diagnostics [39,40], experimental demonstration of pathogenicity [41,42], and more recently, the identification of clonally restricted B cell repertoires giving rise to anti-Ro and La autoantibodies [37,43,44]. While these studies describe the journey of discovery for a subset of the major autoantibodies identified in systemic autoimmunity, the impact on our understanding of the origin and role of autoantibodies has been substantial, and provides a road map for other autoimmune diseases such as T1D. Indeed, while considerable advancements in antibody diagnostic platforms have been achieved for T1D, an understanding of the pathogenic relevance and origins of the key autoantibodies associated with the disease remains largely unexplored. Given the key role AP has played in advancements in systemic autoimmunity, it is therefore hoped that the method described in this manuscript facilitates substantial advances in our understanding of the provenance and pathogenic significance of humoral autoimmunity in T1D.
Bacterially-expressed IA-2 has been used previously to detect anti-IA-2 antibodies, with a variety of assay platforms described, including ELISA, radioimmunoassay and immunoprecipitation [[45], [46], [47], [48]]. However, to our knowledge the current study is the first to describe combining IA-2 expression with MBP. The incorporation of MBP tags in FPs confers a number of benefits, including improved yields and solubility, facilitating FP recovery, and decreased degradation of the protein of interest [49]. In the current study the MBP tag was utilised to purify the FP on maltose beads prior to its use in the ELISAs. Compared to the RSR ELISA, the MBP-IA-2 FP successfully detected 3 of 8 IA-2 autoantibody positive sera, with the 3 FP positive sera recording OD values at the higher range of the RSR ELISA. This disparity is not unexpected, given the commercial RSR ELISA uses a sandwich approach to first capture anti-IA-2 autoantibodies using recombinant IA-2, with detection of monovalent-bound antibody by subsequent capture of biotin-labelled IA-2. Additionally, the relative size of the MBP component of the FP (being approximately 50%) potentially reduces the sensitivity of the FP ELISA, given the available IA-2 would be significantly reduced in the FP compared to recombinant IA-2 alone. As such, it is acknowledged that the FP assay described appears limited to the detection and affinity purification of high titre anti-IA-2 responses. Alternatively, the described ELISA may present a restricted number of epitopes compared to the commercial assay, with further work required to confirm what autoantigenic targets are successfully expressed in the FP used for this study.
As with autoantibody AP described by Al Kindi et al. [35], the multi plate ELISA approach resulted in enrichment for antibodies against the target autoantigen. Indeed, in the current study, when starting serum was adjusted to contain a comparable Ig concentration as that present in the AP sample, positivity for IA-2 by FP ELISA was lost. Further to this, the confirmation of specificity for anti-IA-2 antibodies (as demonstrated by loss of positivity to MBP-ZnT8 in the AP sample generated from the IA-2 and ZnT8 positive patient, T1D0069) is an important advance. A major attraction of this approach is the purification of genuine, serum-derived human autoantibodies, which presumably represent the final secreted product resulting from a number of complex pathophysiological mechanisms. By comparison, generation of anti-IA-2 autoantibodies from isolated peripheral B cells, as described by Kolm-Litty et al. [33], may be more representative of potential autoimmunity, with in vitro differentiation to plasma cells and limited epitope concordance with circulating autoantibodies. This is an important distinction, as isolation of genuine circulating autoantibodies may hold the key to successful investigation of humoral autoimmune-mediated pathology in T1D.
The recent use of mass spectrometry to interrogate the secreted proteome in systemic autoimmune diseases (Reviewed in Ref. [37]) indicates that antigen-specific circulating autoantibodies appear to arise from B cells displaying restricted immunoglobulin variable region gene repertoires, often accompanied by public sharing of autoreactive B cell clones. As remarkable as it may seem, these findings suggest that systemic humoral autoimmunity results from common, near identical pathophysiological mechanisms and drivers of adaptive immune cell selection. In the current study, we demonstrated a predominance of Kappa light chains in anti-IA-2 autoantibodies recognising epitopes within the MBP-IA-2 FP, and noted this represented a skewing to Kappa of the light chain ratio observed in total IgG isolated from the same patient. While these findings need confirmation in a broader cohort of anti-IA-2 positive serum, the enrichment for Kappa light chains was observed in both patient serum samples analysed, and suggests that as with autoantibodies present in systemic autoimmunity, anti-IA-2 autoantibodies are similarly clonally restricted. While confirmation of restricted heavy and/or light chain variable region usage awaits more sophisticated analysis, these findings are the first to suggest that the origins of the humoral response to IA-2 in T1D arise from a discrete set of light chain expressing B cells. It is hoped that the affinity purification method described in this report facilitates a more extensive investigation of B cell clonality in T1D, and a deeper understanding of the pathophysiological mechanisms resulting in circulating autoantibodies in this organ confined autoimmune disease.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by a Grant from the Flinders Foundation (RPF21/534), and the generous support of the Whitehead Family, Tasmania.
References
- 1.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michels A., Zhang L., Khadra A., Kushner J.A., Redondo M.J., Pietropaolo M. Prediction and prevention of type 1 diabetes: update on success of prediction and struggles at prevention. Pediatr. Diabetes. 2015;16:465–484. doi: 10.1111/pedi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regnell S.E., Lernmark A. Early prediction of autoimmune (type 1) diabetes. Diabetologia. 2017;60:1370–1381. doi: 10.1007/s00125-017-4308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannering S.I., Pathiraja V., Kay T.W. The case for an autoimmune aetiology of type 1 diabetes. Clin. Exp. Immunol. 2016;183:8–15. doi: 10.1111/cei.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willcox A., Richardson S.J., Bone A.J., Foulis A.K., Morgan N.G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppieters K.T., Dotta F., Amirian N., Campbell P.D., Kay T.W., Atkinson M.A., Roep B.O., von Herrath M.G. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathiraja V., Kuehlich J.P., Campbell P.D., Krishnamurthy B., Loudovaris T., Coates P.T., Brodnicki T.C., O'Connell P.J., Kedzierska K., Rodda C., Bergman P., Hill E., Purcell A.W., Dudek N.L., Thomas H.E., Kay T.W., Mannering S.I. Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes. 2015;64:172–182. doi: 10.2337/db14-0858. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler A.G., Hummel M., Schenker M., Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–468. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 9.Steck A.K., Johnson K., Barriga K.J., Miao D., Yu L., Hutton J.C., Eisenbarth G.S., Rewers M.J. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34:1397–1399. doi: 10.2337/dc10-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikka V., Nanto-Salonen K., Saarinen M., Simell T., Ilonen J., Hyoty H., Veijola R., Knip M., Simell O. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia. 2012;55:1926–1936. doi: 10.1007/s00125-012-2523-3. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler A.G., Bonifacio E., Group B.-B.S. Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55:1937–1943. doi: 10.1007/s00125-012-2472-x. [DOI] [PubMed] [Google Scholar]
- 12.Ilonen J., Hammais A., Laine A.P., Lempainen J., Vaarala O., Veijola R., Simell O., Knip M. Patterns of beta-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62:3636–3640. doi: 10.2337/db13-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler A.G., Rewers M., Simell O., Simell T., Lempainen J., Steck A., Winkler C., Ilonen J., Veijola R., Knip M., Bonifacio E., Eisenbarth G.S. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krischer J.P., Lynch K.F., Schatz D.A., Ilonen J., Lernmark A., Hagopian W.A., Rewers M.J., She J.X., Simell O.G., Toppari J., Ziegler A.G., Akolkar B., Bonifacio E., Group T.S. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–987. doi: 10.1007/s00125-015-3514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonifacio E., Bingley P.J., Shattock M., Dean B.M., Dunger D., Gale E.A., Bottazzo G.F. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990;335:147–149. doi: 10.1016/0140-6736(90)90013-u. [DOI] [PubMed] [Google Scholar]
- 16.Schatz D., Krischer J., Horne G., Riley W., Spillar R., Silverstein J., Winter W., Muir A., Derovanesian D., Shah S., et al. Islet cell antibodies predict insulin-dependent diabetes in United States school age children as powerfully as in unaffected relatives. J. Clin. Invest. 1994;93:2403–2407. doi: 10.1172/JCI117247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verge C.F., Gianani R., Kawasaki E., Yu L., Pietropaolo M., Chase H.P., Eisenbarth G.S. Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J. Autoimmun. 1996;9:379–383. doi: 10.1006/jaut.1996.0051. [DOI] [PubMed] [Google Scholar]
- 18.Verge C.F., Gianani R., Kawasaki E., Yu L., Pietropaolo M., Jackson R.A., Chase H.P., Eisenbarth G.S. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 19.Lampasona V., Liberati D. Islet autoantibodies. Curr. Diabetes Rep. 2016;16:53. doi: 10.1007/s11892-016-0738-2. [DOI] [PubMed] [Google Scholar]
- 20.Bloem S.J., Roep B.O. The elusive role of B lymphocytes and islet autoantibodies in (human) type 1 diabetes. Diabetologia. 2017;60:1185–1189. doi: 10.1007/s00125-017-4284-5. [DOI] [PubMed] [Google Scholar]
- 21.Baekkeskov S., Aanstoot H.J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 22.Lampasona V., Bearzatto M., Genovese S., Bosi E., Ferrari M., Bonifacio E. Autoantibodies in insulin-dependent diabetes recognize distinct cytoplasmic domains of the protein tyrosine phosphatase-like IA-2 autoantigen. J. Immunol. 1996;157:2707–2711. [PubMed] [Google Scholar]
- 23.Wenzlau J.M., Juhl K., Yu L., Moua O., Sarkar S.A., Gottlieb P., Rewers M., Eisenbarth G.S., Jensen J., Davidson H.W., Hutton J.C. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graus F., Saiz A., Dalmau J. GAD antibodies in neurological disorders - insights and challenges. Nat. Rev. Neurol. 2020;16:353–365. doi: 10.1038/s41582-020-0359-x. [DOI] [PubMed] [Google Scholar]
- 25.Manto M., Honnorat J., Hampe C.S., Guerra-Narbona R., Lopez-Ramos J.C., Delgado-Garcia J.M., Saitow F., Suzuki H., Yanagawa Y., Mizusawa H., Mitoma H. Disease-specific monoclonal antibodies targeting glutamate decarboxylase impair GABAergic neurotransmission and affect motor learning and behavioral functions. Front. Behav. Neurosci. 2015;9:78. doi: 10.3389/fnbeh.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solimena M., Dirkx R., Jr., Hermel J.M., Pleasic-Williams S., Shapiro J.A., Caron L., Rabin D.U. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 1996;15:2102–2114. [PMC free article] [PubMed] [Google Scholar]
- 27.Wasmeier C., Hutton J.C. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J. Biol. Chem. 1996;271:18161–18170. doi: 10.1074/jbc.271.30.18161. [DOI] [PubMed] [Google Scholar]
- 28.Mziaut H., Kersting S., Knoch K.P., Fan W.H., Trajkovski M., Erdmann K., Bergert H., Ehehalt F., Saeger H.D., Solimena M. ICA512 signaling enhances pancreatic beta-cell proliferation by regulating cyclins D through STATs. Proc. Natl. Acad. Sci. U. S. A. 2008;105:674–679. doi: 10.1073/pnas.0710931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trajkovski M., Mziaut H., Schubert S., Kalaidzidis Y., Altkruger A., Solimena M. Regulation of insulin granule turnover in pancreatic beta-cells by cleaved ICA512. J. Biol. Chem. 2008;283:33719–33729. doi: 10.1074/jbc.M804928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morran M.P., Casu A., Arena V.C., Pietropaolo S., Zhang Y.J., Satin L.S., Nelson P., Omenn G.S., Trucco M., Becker D.J., Pietropaolo M. Humoral autoimmunity against the extracellular domain of the neuroendocrine autoantigen IA-2 heightens the risk of type 1 diabetes. Endocrinology. 2010;151:2528–2537. doi: 10.1210/en.2009-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acevedo-Calado M., James E.A., Morran M.P., Pietropaolo S.L., Ouyang Q., Arribas-Layton D., Songini M., Liguori M., Casu A., Auchus R.J., Huang S., Yu L., Michels A., Gianani R., Pietropaolo M. Identification of unique antigenic determinants in the amino terminus of IA-2 (ICA512) in childhood and adult autoimmune diabetes: New biomarker development. Diabetes Care. 2017;40:561–568. doi: 10.2337/dc16-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bearzatto M., Lampasona V., Belloni C., Bonifacio E. Fine mapping of diabetes-associated IA-2 specific autoantibodies. J. Autoimmun. 2003;21:377–382. doi: 10.1016/j.jaut.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Kolm-Litty V., Berlo S., Bonifacio E., Bearzatto M., Engel A.M., Christie M., Ziegler A.G., Wild T., Endl J. Human monoclonal antibodies isolated from type I diabetes patients define multiple epitopes in the protein tyrosine phosphatase-like IA-2 antigen. J. Immunol. 2000;165:4676–4684. doi: 10.4049/jimmunol.165.8.4676. [DOI] [PubMed] [Google Scholar]
- 34.Lee A.Y.S., Chataway T., Colella A.D., Gordon T.P., Wang J.J. Quantitative mass spectrometric analysis of autoantibodies as a paradigm shift in autoimmune serology. Front. Immunol. 2019;10:2845. doi: 10.3389/fimmu.2019.02845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al Kindi M.A., Chataway T.K., Gilada G.A., Jackson M.W., Goldblatt F.M., Walker J.G., Colella A.D., Gordon T.P. Serum SmD autoantibody proteomes are clonally restricted and share variable-region peptides. J. Autoimmun. 2015;57:77–81. doi: 10.1016/j.jaut.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Steinbuch M., Audran R. [Isolation of IgG immunoglobulin from human plasma using caprylic acid] Rev. Fr. Etud. Clin. Biol. 1969;14:1054–1058. [PubMed] [Google Scholar]
- 37.Al Kindi M.A., Colella A.D., Chataway T.K., Jackson M.W., Wang J.J., Gordon T.P. Secreted autoantibody repertoires in Sjogren's syndrome and systemic lupus erythematosus: a proteomic approach. Autoimmun. Rev. 2016;15:405–410. doi: 10.1016/j.autrev.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Wang J.J., Colella A.D., Beroukas D., Chataway T.K., Gordon T.P. Precipitating anti-dsDNA peptide repertoires in lupus. Clin. Exp. Immunol. 2018;194:273–282. doi: 10.1111/cei.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamagata H., Harley J.B., Reichlin M. Molecular properties of the Ro/SSA antigen and enzyme-linked immunosorbent assay for quantitation of antibody. J. Clin. Invest. 1984;74:625–633. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaither K.K., Fox O.F., Yamagata H., Mamula M.J., Reichlin M., Harley J.B. Implications of anti-Ro/Sjogren's syndrome A antigen autoantibody in normal sera for autoimmunity. J. Clin. Invest. 1987;79:841–846. doi: 10.1172/JCI112892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander E.L., Buyon J.P., Lane J., Lafond-Walker A., Provost T.T., Guarnieri T. Anti-SS-A/Ro SS-B/La antibodies bind to neonatal rabbit cardiac cells and preferentially inhibit in vitro cardiac repolarization. J. Autoimmun. 1989;2:463–469. doi: 10.1016/0896-8411(89)90176-5. [DOI] [PubMed] [Google Scholar]
- 42.Garcia S., Nascimento J.H., Bonfa E., Levy R., Oliveira S.F., Tavares A.V., de Carvalho A.C. Cellular mechanism of the conduction abnormalities induced by serum from anti-Ro/SSA-positive patients in rabbit hearts. J. Clin. Invest. 1994;93:718–724. doi: 10.1172/JCI117025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arentz G., Thurgood L.A., Lindop R., Chataway T.K., Gordon T.P. Secreted human Ro52 autoantibody proteomes express a restricted set of public clonotypes. J. Autoimmun. 2012;39:466–470. doi: 10.1016/j.jaut.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Thurgood L.A., Arentz G., Lindop R., Jackson M.W., Whyte A.F., Colella A.D., Chataway T.K., Gordon T.P. An immunodominant La/SSB autoantibody proteome derives from public clonotypes. Clin. Exp. Immunol. 2013;174:237–244. doi: 10.1111/cei.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgenthaler N.G., Lobner K., Morgenthaler U.Y., Christie M.R., Seissler J., Scherbaum W.A. Recombinant IA-2 expressed in E. coli can be used for the routine detection of autoantibodies in Type-I diabetes. Horm. Metab. Res. 1998;30:559–564. doi: 10.1055/s-2007-978933. [DOI] [PubMed] [Google Scholar]
- 46.Lobner K., Khoo-Morgenthaler U.Y., Seissler J., Morgenthaler N.G., Scherbaum W.A. Detection of autoantibodies to the diabetes-associated antigen IA-2 by a sensitive enzyme-linked immunosorbent assay. Horm. Metab. Res. 1999;31:686–691. doi: 10.1055/s-2007-978821. [DOI] [PubMed] [Google Scholar]
- 47.Sica M.P., Primo M.E., Ermacora M.R., Poskus E. High-yield expression of properly folded insulinoma-associated protein intracellular domain (IA-2ic) in Escherichia coli. Biotechnol. Appl. Biochem. 2003;37:301–309. doi: 10.1042/BA20020090. [DOI] [PubMed] [Google Scholar]
- 48.Guerra L.L., Faccinetti N.I., Trabucchi A., Rovitto B.D., Sabljic A.V., Poskus E., Iacono R.F., Valdez S.N. Novel prokaryotic expression of thioredoxin-fused insulinoma associated protein tyrosine phosphatase 2 (IA-2), its characterization and immunodiagnostic application. BMC Biotechnol. 2016;16:84. doi: 10.1186/s12896-016-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun P., Tropea J.E., Waugh D.S. Enhancing the solubility of recombinant proteins in Escherichia coli by using hexahistidine-tagged maltose-binding protein as a fusion partner. Methods Mol. Biol. 2011;705:259–274. doi: 10.1007/978-1-61737-967-3_16. [DOI] [PubMed] [Google Scholar]