Abstract
Background
Acute kidney injury is a highly common and multifactorial renal disease resulting in significant morbidity and mortality, especially sepsis-induced acute kidney injury. There is no effective therapy available to treat or prevent sepsis-induced acute kidney injury. One of the specialized pro-resolving mediators, Resolvin D1 exhibits special anti-inflammatory effects in several inflammatory disease models, but there is little evidence about the effect and mechanism of Resolvin D1 in sepsis-induced acute kidney injury.
Methods
We conducted experiments to explore the effect and mechanism of Resolvin D1 in sepsis-induced acute kidney injury. In vitro, human proximal tubular epithelial cells were used to test the apoptosis ratio, cell viability and reactive oxygen species level. In vivo, C57BL/6 mice were injected with lipopolysaccharide to establish a sepsis-induced acute kidney injury model. Renal function and structure, apoptosis ratio of kidney cells, mitochondrial structure and function and related protein and gene levels were assessed.
Results
In vitro, the resolvin D1-treated group showed higher cell viability and lower reactive oxygen species levels and apoptosis ratios than the LPS group. In vivo, Resolvin D1 can not only improve renal function and mitochondrial function but also reduce the apoptosis ratio, while mediating mitochondrial dynamics and inhibiting NF-κB pathway.
Conclusions
Resolvin D1 has a good renoprotective effect by maintaining mitochondrial dynamics and inhibiting the NF-κB pathway.
Keywords: Sepsis-induced acute kidney injury, RvD1, Mitochondrial function, Apoptosis, NF-κB pathway
Sepsis-induced acute kidney injury; RvD1; Mitochondrial function; Apoptosis; NF-κB pathway.
1. Introduction
Acute kidney injury (AKI) is a highly common and multifactorial renal disease characterized by renal function decline and acid-base balance disorders [1, 2, 3]. In China, it is estimated that approximately 1.4–2.9 million people are hospitalized for AKI each year [4]. Furthermore, AKI is accompanied by serious mortality (approximately 50%–80%) in intensive care units (ICUs) [5, 6]. Even if patients survive after AKI, there is still the possibility of chronic kidney disease (CKD) and even end-stage renal disease (ESRD) if renal function does not recover completely [7, 8]. AKI is mainly caused by renal ischemia–reperfusion injury (IRI), endogenous or exogenous nephrotoxins, sepsis, etc., of which sepsis is the major cause in critical patients [9, 10]. Nearly 50% of AKI cases are related to sepsis, and the mortality rate is as high as 20% [11]. Although the pathophysiological mechanism of sepsis-induced acute kidney injury (SI-AKI) has been gradually revealed, the severity and complexity of SI-AKI bring great challenges to nephrologists in clinical treatment. At present, there is still no exact and effective drug for the treatment or prevention of SI-AKI [12].
Previous studies have suggested that oxidative stress, proinflammatory mediators, mitochondrial dysfunction, apoptosis and necroptosis of kidney tubule cells may contribute to AKI in sepsis [7, 13, 14]. SI-AKI is an inflammatory disease essentially, and it is the persistence of inflammation that leads to small vessel congestion and renal dysfunction [15]. The important role of inflammatory storms and mitochondrial dysfunction in SI-AKI make them potential therapeutic targets [16]. Therefore, it is promising to explore effective therapeutic methods targeting mitochondrial dysfunction and inflammatory storms to improve the prognosis and survival rate in SI-AKI [17, 18, 19, 20]. The ideal outcome of SI-AKI is the dissipation of inflammation and the return to steady state [21]. In the traditional concept, dissipation of inflammation is a passive process that depends on the dissipation or dilution of inflammatory mediators. However, increasing evidence has shown that inflammation dissipation is an active process that depends on complete mediators, receptors and biological pathways to complete the transmission of inflammation dissipation [22]. Therefore, the mediators that can actively promote the dissipation of inflammation may be potential drugs for the treatment of SI-AKI.
Recently, endogenous pro-resolving and anti-inflammatory lipid mediators have received tremendous attention in inflammatory diseases [23]. Resolvins, protectins, maresins and lipoxins, known as specialized pro-resolving mediators (SPMs), can downregulate infiltration, cytokine, and chemokine production and induce catabasis in acute or chronic inflammation [24]. The characteristic of SPMs is controlling inflammation and promoting tissue repair without compromising host defense [25]. Resolvins (Rvs), derived from ω-3 polyunsaturated fatty acids, exhibit pro-resolving and anti-inflammatory effects both in vitro and in vivo [26, 27, 28]. Studies have reported that RvD1 appears to limit excessive inflammation by reducing reactive oxygen species (ROS) production, inflammatory cytokine and adhesion molecule expression, leukocyte trafficking and leukocyte-endothelial interactions [29]. In our previous studies, we confirmed the renoprotection of RvD1 in sepsis-induced AKI and found that RvD1 could protect kidney function [30]. However, the mechanism of the protective effect of RvD1 in SI-AKI is not entirely clear.
In this study, we investigated the effect of RvD1 on SI-AKI and demonstrated a renoprotection mechanism targeting mitochondrial dysfunction and NF-κB signaling pathways in vivo and in vitro, which may serve as a scientific basis for the future research and development of new drugs for SI-AKI.
2. Materials and methods
2.1. Reagent and materials
Lipopolysaccharide (LPS) was purchased from Sigma–Aldrich (St. Louis, MO, USA). Resolvin D1 (RvD1) was obtained from Cayman Chemical Co. (Ann Arbor, Michigan, USA). SYBR Green Master Mix was purchased from Bio–Rad (Hercules, CA, USA). The following antibodies were obtained commercially: dynamin-related protein-1 (Drp-1) and optic atrophy-1 (Opa-1) antibodies were obtained from Novus Biologicals (A-8 Littleton, CO 80120 USA); anti-caspase3 antibody anti-cytochrome C antibody was obtained from Abcam plc (Cambridge, UK); and anti-GAPDH antibody was obtained from Biox Technology Co., Ltd. (Boston, MA, USA).
2.2. Cell culture and experimental protocol
The immortalized proximal tubule epithelial cell line from normal adult human kidneys (HK-2) was purchased from American Type Culture Collection (ATCC number: CRL-2190TM, Lot number: 70018643) and cultured in Keratinocyte-SFM (Gibco, Rockville, MD, USA) supplemented with recombinant epidermal growth factor (0.5 ng/ml) and bovine pituitary extract (25 μg/ml). These cells were incubated in a humid environment with 5% CO2 at 37 °C and sub cultured under 80% confluence. The cells were cultured in Keratinocyte-SFM (with supplement media) for 24 h and then exposed to the following conditions: (1) control group, (2) model group (100 μg/ml LPS), and (3) RvD1 group (100 μg/ml LPS +100 nM RvD1) for 24 h.
2.3. Cell viability assay
In this experiment, cell viability was identified by CCK-8 assay (Dojindo Molecular Technologies, Gaithersburg, MD). After 24 h of conventional cell culture, CCK-8 solution was added to 96-well plates containing 5000 cells/well according to the manufacturer's instructions. Then, the cells were incubated with CCK-8 for another 2 h under the same conditions. After that, the absorbance was measured at 450 nm by a microplate reader (Bio–Rad, Hercules, CA).
2.4. Reactive oxygen species (ROS) assay
The ROS level of cells was assessed by 2,7-dichlorofluorescein diacetate (DCFH-DA, Solarbio). Cells were cultured in a 12-well plate. After treatment for 24 h, the cell medium was removed, and the cells were coincubated with FBS-free medium containing DCFH-DA (final concentration of 10 μM) at 37 °C for 30 min. Then, the cells were collected and rinsed. Finally, the fluorescence of resuspended cells was evaluated by flow cytometry (Beckman Coulter, Brea, CA).
2.5. Cell apoptosis assay
An Annexin V-FITC/PI apoptosis detection kit (Dojindo Molecular Technologies, Gaithersburg, MD) was used to measure the cell apoptotic rate. First, cells were seeded in a 6-well plate at a density of 2 × 105 cells/well and cultured under conventional conditions for 24 h. Then, the treated cells were collected and washed twice with 4 °C phosphate-buffered saline (PBS). Next, the cells were resuspended in 4 °C PBS and mixed with 5 μl Annexin V-FITC and 5 μl PI. Finally, after 15 min of incubation in the dark, the samples were detected by flow cytometry (Beckman Coulter, Brea, CA).
2.6. Animals and euthanasia
Eight-week-old C57BL/6 mice were purchased from Chengdu Dashuo Experimental Animal Co. LTD (Chengdu, China). Mice were housed at the Animal Experiment Center of West China Hospital, Sichuan University (Chengdu, China). All mice were maintained under specific pathogen-free conditions. This experiment was approved by the Institutional Animal Care and Use Committee at the Sichuan University of West China Hospital, China (Approval NO. 2018169A). All institutional and national guidelines for the care and use of laboratory animals were followed.
2.7. LPS-induced AKI and treatment with RvD1
Mice were injected with lipopolysaccharide (LPS) (5 mg/kg body weight) or normal saline intraperitoneally in combination with or without RvD1 (5 μg/kg body weight). At 6 h, 12 h, 24 h, and 48 h after injection, the mice were sacrificed by pentobarbital sodium overdose, and the kidneys were isolated for further analysis as needed.
2.8. Renal function analysis
Blood was collected from mouse eyes for biochemical analysis. Blood urea nitrogen (BUN) and creatinine concentrations in serum were measured using the corresponding detection kits (Changchun Huili, Changchun, China) according to the manufacturer's instructions.
2.9. ATP production assay
The ATP production level of kidney tissue was detected by an ATP Assay Kit (Beyotime, Shanghai, China). Kidney tissues were lysed by a glass homogenizer with lysate at 4 °C. After centrifugation at 12000 g for 5 min, the supernatant was collected for luminescence analysis by microplate reader. To eliminate the error caused by the difference in protein content in the sample preparation, the BCA protein assay kit (Biosharp, Guangzhou, China) was employed to measure the concentration of proteins in the sample. Then, we converted the concentration of ATP into nmol/mg protein.
2.10. TNF-α ELISA
The TNF-α level in the serum of mice was detected by a TNF alpha (mouse) ELISA Kit (Multisciences, Hangzhou, China) according to the standard manufacturer's instructions.
2.11. Western blot
Total protein was extracted from kidney tissues using RIPA lysis buffer (Beyotime, Guangzhou, China). Then, the total protein level was measured by a BCA protein assay kit (Biosharp, Guangzhou, China) based on the manufacturer's instructions. The detailed procedures of the Western blot assay can be referenced in a previous study [31]. The chemiluminescence detection system was used to assess the expression of designated proteins through the peroxidase reaction, and the images of the bands were recorded with the ChemiDoc XRS digital imaging system (Bio–Rad Laboratories, Inc.). GAPDH was used as the internal loading control. All experiments were repeated at least three times.
2.12. Real-time PCR analysis
Total RNA was isolated from whole kidney tissues using TRIzol reagent (Vazyme, Nanjing, China). For each sample, 1 μg of RNA was reverse transcribed using the PrimeScript RT Reagent Kit (Vazyme, Nanjing, China). For the purposes of RT–PCR, β-actin was used as the reference. Real-time PCR analysis was performed using the Power SYBR Green PCR master mix (Vazyme, Nangjing, China). The PCR conditions were as follows: preincubation at 95 °C for 5 min (1 cycle); 95 °C for 10 s, 60 °C for 30 s (40 cycles). The fluorescence emission data were collected during the annealing process. All treatments were performed in triplicate to calculate statistical significance, and the results were calculated using the 2−ΔΔCt method.
2.13. Renal histology immunohistochemistry and immunofluorescence
Kidney tissues were collected immediately after mice were sacrificed and then fixed with formalin. Kidney tissues were embedded in paraffin and cut into 5 μm slices for hematoxylin-eosin (H&E) staining, immunohistochemistry, immunofluorescence and TUNEL fluorescent staining. H&E staining, immunohistochemistry immunofluorescence staining and TUNEL staining were performed according to the standard manufacturer's instructions. Sections were deparaffinized with xylene and rehydrated in graded ethanol. Sections were microwaved in 10 mM sodium citrate and then incubated with DRP1 (1:300; Novus Biologicals), OPA1 (1:300; Novus Biologicals), p65 (1:600, CST), p-p65 (1:600, CST), TNF-α (1:200, Abcam), IL-6 (1:500, CST), IL-1β (1:500, CST), cytochrome C (1:200, Proteintech) and cleaved-caspase3 (1:100; Abcam) antibodies overnight at 4 °C and the secondary antibodies for 30 min at 37 °C.
2.14. Transmission electron microscopy (TEM)
Approximately 1 mm3 of fresh kidney tissue was removed quickly to electron microscopy fixative at 4 °C. Tissues were embedded, cut into 60–80 nm ultrathin sections, and then subjected to uranium lead double staining. The mitochondrial morphology of renal tubular epithelial cells and image acquisition were obtained by transmission electron microscopy.
2.15. Statistical analysis
Data are expressed as the means ± SD of the indicated number of independent experiments. Statistical significance between multiple groups was analyzed by one-way ANOVA. Statistical analysis was performed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. ROS generation and apoptosis of HK-2 cells stimulated by LPS were reduced by RvD1 in vitro
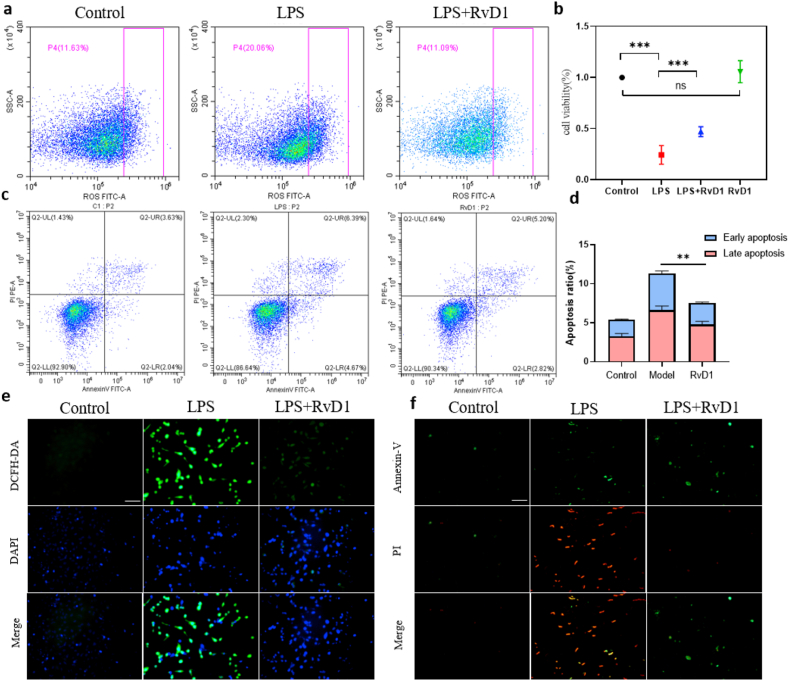
To evaluate the effect of RvD1 on ROS levels, apoptosis inhibition and cell proliferation at the cellular level, we conducted a series of experiments with HK-2 cells including CCK-8 assay, ROS detection and apoptosis detection. CCK-8 assay can detect the quantity of living cells. The more living cells, the darker the color. ROS detection kit uses DCFH-DA probe to evaluate the level of ROS including peroxide, superoxide, hydroxyl radical, etc. Annexin V-FITC/PI apoptosis detection kit distinguish apoptotic cells from normal cells. First, cell viability was assessed by a CCK-8 assay, and the results showed that HK-2 cells treated with RvD1 exhibited higher cell viability than those stimulated with LPS alone, indicating that RvD1 had a protective effect on LPS-treated HK-2 cells (Figure 1b). To explore the protective mechanism of RvD1 on LPS-induced HK-2 cells, the intracellular ROS level and apoptosis ratio were detected. We used a DCFH-DA probe to detect intracellular ROS in HK-2 cells at 24 h after exposure to LPS and RvD1. The results showed that intracellular ROS in the RvD1 group were lower than those in the model group through flow cytometry and fluorescence microscopy (Figure 1a, e). In addition, we employed Annexin V-FITC/PI staining to detect the apoptosis ratio in HK-2 cells exposed to LPS and RvD1 for 24 h. The flow cytometry and fluorescence microscopy results showed that LPS-treated cells showed a significant increase in early and late apoptosis, while RvD1-treated cells showed a significant decrease in the apoptosis ratio (Figure 1c, d, f). Therefore, we conclude that RvD1 can protect HK-2 cell viability while decreasing the production of ROS and the apoptosis ratio.
Figure 1.
(a) ROS detection by flow cytometry with HK-2 cells at 24 h (n = 5). (b) CCK-8 assay at 24 h (n = 5). (c) (d) The apoptosis ratio was assessed with HK-2 by flow cytometry statistical analysis at 24 h (n = 5). (e) ROS detection by fluorescence microscopy at 24 h (n = 5). Scale bars = 100 μm. (f) The apoptosis ratio was assessed with HK-2 by fluorescence microscopy at 24 h (n = 5). Scale bars = 100 μm. The data are presented as the mean ± SD ∗∗P < 0.01, ∗∗∗P < 0.001.
3.2. Renal function and resolution of inflammation in LPS-induced AKI were markedly improved by RvD1 in mice
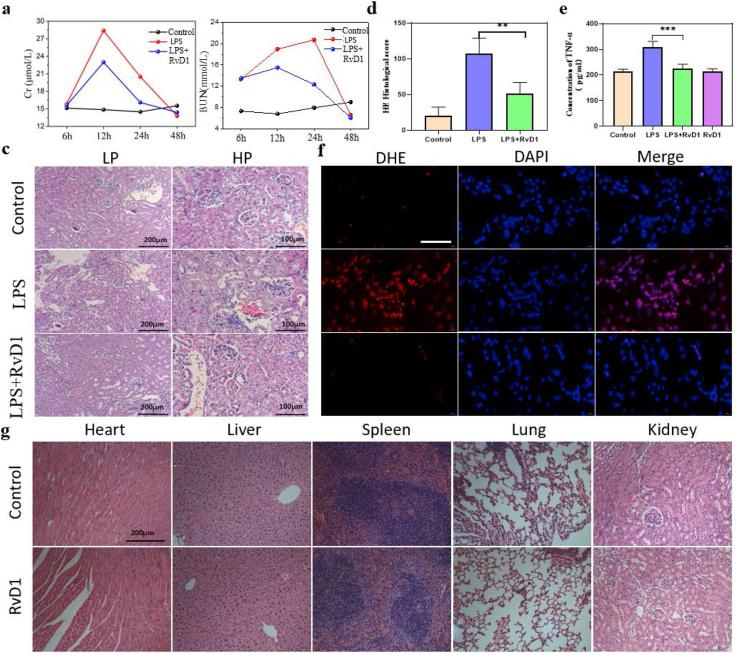
The SI-AKI model was induced by lipopolysaccharide (LPS) intraperitoneal injection in wild-type C57BL/6 mice, and the animals were sacrificed at 6 h, 12 h, 24 h, and 48 h after injection. The serum creatinine of the LPS group was higher than that of the control group from 6 h and peaked at 12 h. Then, the serum creatinine returned to normal at 48 h in live mice (Figure 2a). For BUN, the result was similar to the serum creatinine except for the peak time (24 h) (Figure 2b). Treatment with RvD1 by intraperitoneal injection significantly decreased the levels of serum creatinine at 12 h and 24 h compared with those in the LPS group, and the BUN level showed a similar tendency, which suggested that RvD1 can protect renal function by reducing the severity and accelerating the recovery of LPS-induced AKI in mice. To assess the histological injury of the kidney, we performed H&E staining, and the LPS group showed obvious tubular damage, including widespread tubular cell death, severe cell shedding, interstitial edema, and brush border disruption in the kidney cortex. Obviously, tubular damage was mitigated by RvD1. The H&E histological score showed the same trend (Figure 2c, d). In addition, to investigate the inflammation level, one of the most important proinflammatory cytokines, TNF-α, was assessed by ELISA, and the results showed that RvD1 decreased the TNF-α level (Figure 2e). ROS levels in kidney tissue were also detected by a DHE probe, and the results showed that RvD1 obviously decreased ROS levels in kidney tissue (Figure 2f). The above results indicated that RvD1 had proresolving and anti-inflammatory effects in LPS-induced AKI, which protected renal function and structure from damage. To explore the safety of RvD1, we observed H&E-stained tissues of the heart, liver, spleen, lung and kidney after injecting RvD1 alone, and we found that there was no obvious tissue toxicity (Figure 2g).
Figure 2.
(a) Serum creatinine assay (n = 5). (b) BUN assay (n = 5). (c) (d) Histological changes in the renal cortex by HE staining and tissue injury scores at 24 h (n = 5). LP, Low power field; HP, High power field. (e) ELISA analysis showing the expression of TNF-α in serum at 24 h (n = 5). (f) ROS level of kidney tissue by DHE fluorescent probe at 24 h (n = 5). (g) Organ toxicity detection of RvD1 at 7 days (n = 5). The data are presented as the mean ± SD ∗∗P < 0.01, ∗∗∗P < 0.001.
3.3. The mitochondrial function and mitochondrial dynamics balance in LPS-induced AKI were markedly improved by RvD1 in mice
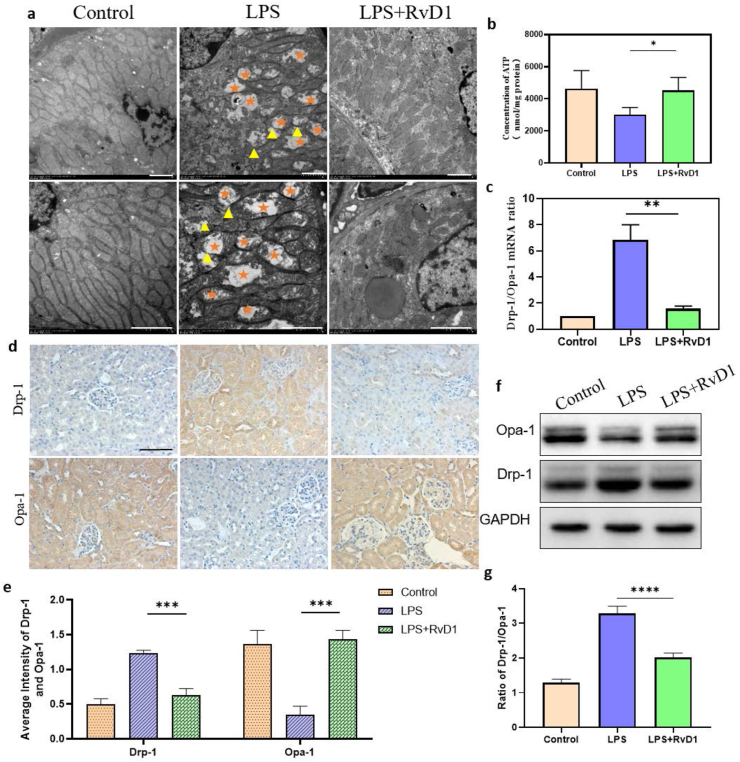
Mitochondria can change their metabolic processes and dynamic state during many stress conditions. Therefore, mitochondrial dysfunction and bioenergetic failure are the center of the pathophysiology of SI-AKI and could be potential therapeutic targets [16, 32]. To demonstrate the protective mechanism of RvD1 on LPS-induced AKI, we focused on mitochondrial function and mitochondrial dynamics balance. Normal mitochondrial morphology can be damaged in septic AKI. The ultrastructural characteristics of mitochondrial damage include swelling with rarefaction of the cristae and mitochondrial fragmentation. Thus, we performed transmission electron microscopy, which showed that kidney tissue of LPS-treated mice exhibited morphological changes, such as more swollen mitochondria and a reduced number of cristae with a more lamellar phenotype compared with the normal control. Expectedly, these changes were significantly alleviated by RvD1 treatment (Figure 3a).
Figure 3.
(a) Mitochondrial morphology of the kidney cortex was observed by TEM at 24 h (n = 5). Scale bar = 2 μm. The five-pointed star symbol represents swollen, ruptured and vacuolated mitochondria; the triangle symbol represents the disappearance of the double membrane structure of mitochondria; (b) ATP assay at 24 h (n = 5). (c) Detection of the mRNA levels of Drp-1/Opa-1 by quantitative real-time PCR (n = 5). (d) (e) The expression of Drp-1 and Opa-1 in renal tissue was determined by immunohistochemical staining, followed by semiquantitative statistical analysis of each group (n = 5). Scale bar = 100 μm. (f) (g) The expression of Drp-1 and Opa-1 in renal tissue was determined by western blotting, followed by semiquantitative statistical analysis of each group (n = 5). The data are presented as the mean ± SD ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. The uncropped images of (f) were referred to in Supplementary Full non-adjusted Figure.
To explore the mitochondrial function of kidney cells, we assessed the ATP production ability of kidney tissue and found that the ATP production ability of LPS mice was decreased compared with that of control mice, but RvD1 restored the ATP production ability of mitochondria (Figure 3b). The above results indicated that RvD1 can not only repair the damage to mitochondrial structure but also restore the ATP production capacity of mitochondria. In addition, mitochondrial dynamics are profoundly altered in SI-AKI, and the dynamics of mitochondria have garnered considerable attention. Mitochondrial fragmentation occurs before obvious renal tubular damage or cell death, and SI-AKI is associated with excessive mitochondrial fission compared with fusion [33]. The transmission electron microscopy results in our study showed a similar phenomenon to previous studies. The mitochondria of the LPS group presented more fission fragments, such as short rods or spheres, whereas the RvD1 group had more fusion mitochondria (Figure 3a). To demonstrate the change in mitochondrial dynamic balance, dynamic regulatory-related proteins were detected. Dynamin-related protein 1 (Drp-1), which mediates mitochondrial fission, and one of the fusion proteins, Opa-1, were detected by immunohistochemistry (IHC), western blotting and RT–PCR. The RT–PCR results showed that the mRNA expression level of opa-1 was upregulated and that of drp-1 was downregulated by RvD1 (Figure 3c), and the results were consistent with IHC staining (Figure 3d, e) and western blotting (Figure 3f, g), suggesting that mitochondrial dynamics transform to fusion rather than fission after RvD1 treatment. These data suggested that the renoprotective effect of RvD1 was achieved through regulating the balance of mitochondrial structure, function and dynamics.
3.4. The NF-κB signaling pathway and proinflammatory cytokines were inhibited by RvD1 in LPS-induced AKI mice
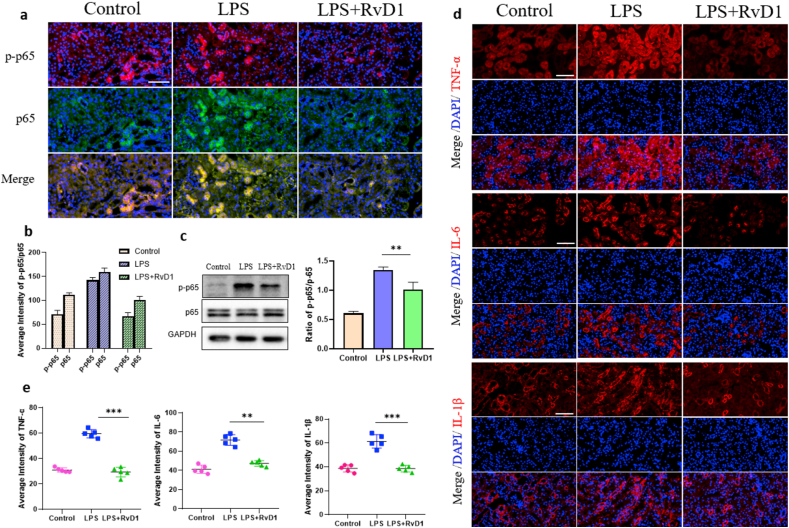
The NF-κB protein family is a central mediator that induces proinflammatory gene expression, which plays a pathogenic role in renal inflammatory diseases [34]. To assess activation of the NF-κB signaling pathway, renal cortex samples were taken for western blotting and immunofluorescence (IF) to detect the protein expression of NF-κB p65 and NF-κB p-p65. The results showed that the expression of NF-κB p65 in the three groups was basically the same; the expression of NF-κB p-p65 in the model group was significantly increased, and the expression of NF-κB p-p65 in the RvD1 group was significantly decreased compared with that in the model group, and the difference was statistically significant (Figure 4a, b, c). In conclusion, LPS stimulated the activation of the NF-κB signaling pathway, while RvD1 inhibited NF-κB pathway activation by inhibiting the phosphorylation of NF-κB p65. Renal cortex samples were also obtained to detect the expression levels of the proinflammatory cytokines TNF-α, IL-6 and IL-1β by IF. The expression levels of TNF-α, IL-6 and IL-1β in the RvD1 group were significantly decreased compared with those in the model group, and the differences were statistically significant (Figure 4d, e). Above all, we can conclude that RvD1 can inhibit the activation of the NF-κB signaling pathway and then reduce the release of proinflammatory cytokines to achieve anti-inflammatory and protective effects on the kidney.
Figure 4.
(a) (b) Protein expression and location of p-p65 and p65 detected by immunofluorescence and semiquantitative analysis (n = 5). Scale bar = 100 μm. (c) Protein expression and location of p-p65 and p65 detected by Western blot and semiquantitative analysis (n = 5). (d) (e) Protein expression and location of TNF-α, IL-6 and IL-1β detected by immunofluorescence and semiquantitative analysis (n = 5). Scale bar = 100 μm. The data are presented as the mean ± SD ∗∗P < 0.01, ∗∗∗P < 0.001. The uncropped images of (c) were referred to in Supplementary Full non-adjusted Figure.
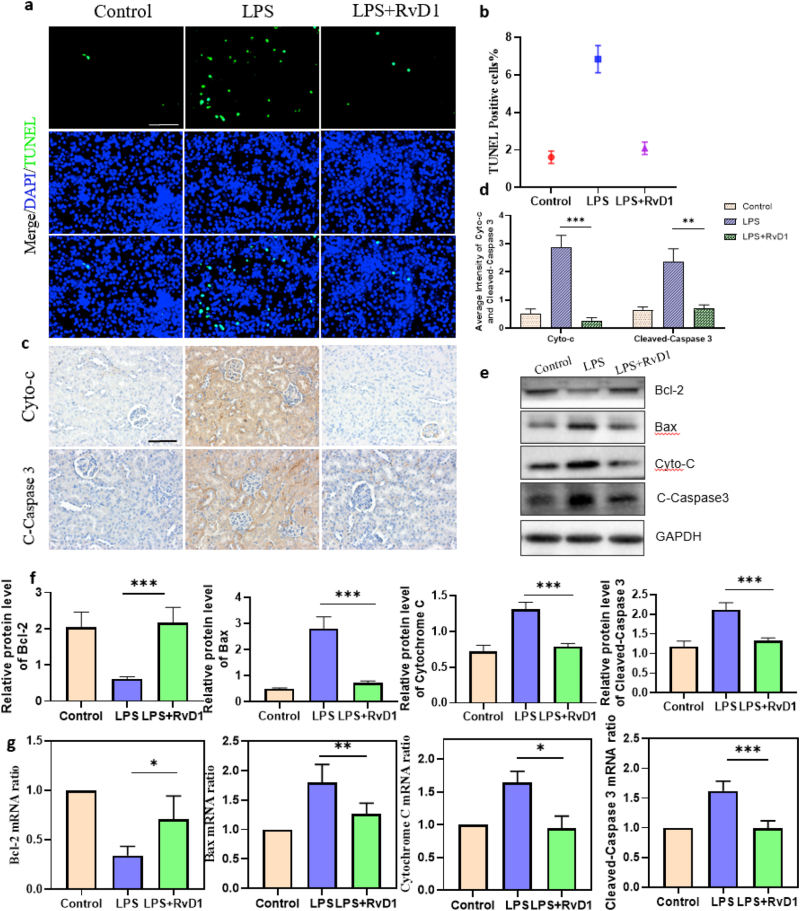
3.5. The apoptosis ratio of kidney cells was markedly reduced in LPS-induced AKI by RvD1 in mice
Apoptosis, which is closely related to mitochondria, is an important pathological process in LPS-induced AKI. To determine the apoptosis ratio of kidney cells in different mouse groups, we performed a terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick–end labeling (TUNEL) assay. As expected, we found that the apoptosis ratio of kidney cells in the RvD1 group was obviously lower than that in the LPS group and was similar to that in control mice, showing that RvD1 remarkably reduced the apoptosis ratio of kidney cells induced by LPS (Figure 5a, b). To explore the underlying antiapoptotic mechanism of RvD1, we detected the protein and mRNA expression levels of apoptotic regulatory proteins and bcl-2 family proteins by western blotting and RT–PCR. The results showed that in the RvD1 group, bax, a pro-apoptotic protein, was downregulated, while bcl-2, an anti-apoptotic protein, was upregulated compared with the LPS group at both the protein and mRNA levels (Figure 5e, f, g). Cytochrome C is an apoptogenic factor leaked by mitochondria that can activate the apoptotic executive protein cleaved-caspase3 [16]. The protein and mRNA levels of Cytochrome C and cleaved-caspase3 in the RvD1 group were downregulated compared with those in the LPS group (Figure 5e, f, g). As expected, the IHC results were consistent with the RT–PCR and Western blot results (Figure 5c, d). The above results showed that RvD1 can reduce the apoptosis ratio of kidney cells by regulating bcl-2 family proteins and then influencing the expression of cytochrome C and cleaved caspase-3.
Figure 5.
(a) (b) TUNEL fluorescence staining of proximal tubular epithelial cells in the kidney cortex and statistical analysis of cell death rates in each group (n = 5). Green fluorescence in the pictures represents positive signals (cell death), while blue fluorescence represents nuclear staining. Scale bars = 100 μm. (c) (d) Expression and location of Cytochrome C and Cleaved Caspase 3 by immunohistochemistry, followed by semiquantitative statistical analysis of each group (n = 5). Scale bar = 100 μm. (e) (f) Protein expression levels of Bax, Bcl-2, Cytochrome C, and Cleaved-Caspase 3 by Western blot, followed by semiquantitative statistical analysis of each group (n = 5). (g) Detection of mRNA levels of Bax, Bcl-2, Cytochrome C, and Cleaved-Caspase 3 by quantitative real-time PCR (n = 5). The data are presented as the mean ± SD ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Cyto-C, Cytochrome C; C- Caspase 3, Cleaved Caspase 3. The uncropped images of (e) were referred to in Supplementary Full non-adjusted Figure.
4. Discussion
AKI is the most common complication of sepsis in the intensive care unit (ICU). However, there is currently no effective therapy to treat or prevent human septic AKI in the clinic [16]. Encouragingly, RvD1 plays an important protective role in several acute and chronic inflammatory diseases. In our previous studies, we found renoprotection of RvD1 in SI-AKI and found that RvD1 could downregulate the NF-κB signaling pathway (24). However, because of the complexity of septic AKI, the mechanism of RvD1 in sepsis-induced AKI is not yet completely clear. In this study, we found that RvD1 could decrease not only the ROS production but also the apoptosis ratio of HK-2 cells caused by LPS stimulation. Additionally, RvD1 can decrease the severity and accelerate renal function recovery after LPS-induced AKI by regulating mitochondrial function and inhibiting the NF-κB signaling pathway. These results indicate that RvD1 has great potential in the treatment of AKI caused by sepsis, which is worthy of further development and research. In the experiment, we found that cell death measured by the CCK-8 assay was much higher than the percentage of apoptotic cells quantified using Annexin V-FITC/PI staining. We think there may be two reasons. On the one hand, the substantial difference between the CCK-8 assay and Annexin V-FITC/PI staining may be attributed to experimental error between different assays. On the other hand, other types of cell death may be involved in SI-AKI, such as necrosis and ferroptosis. Therefore, there are other mechanisms by which RvD1 reduces cell death in addition to reducing apoptosis, which warrants further exploration.
Mitochondria, the center of energy metabolism and the primary utilizers of oxygen, are crucial for cells. Epithelial cells of the nephron, especially proximal tubular cells, are rich in mitochondria to move sodium and other solutes against strong electrochemical gradients [35]. Mitochondrial function has been shown to be dramatically altered when abnormalities occur with mitochondrial structure and function during SI-AKI. Mitochondria can change their metabolic processes and dynamic state during many stress conditions. Therefore, mitochondrial dysfunction and bioenergetic failure are the center of the pathophysiology of SI-AKI and could be potential therapeutic targets [16, 32]. Thus, restoring healthy mitochondrial function and dynamic balance may be critical to the repair of renal cells [32]. Providing energy (ATP) is the key function of mitochondria. ATP can be used by renal cells to clear waste products from the blood and regulate the balance of fluids and electrolytes. Hernando, G et al. reported that primary tubular metabolism is altered in SI-AKI, which could secondarily affect partial metabolism by decreasing ATP levels [36]. Thus, the ATP level is an important marker of mitochondrial function. Our results showed that RvD1 treatment damaged the ATP generation capacity and mitochondrial function induced by LPS. Therefore, we demonstrate that RvD1 can protect the mitochondrial function of kidney cells. Several studies have shown that the mitochondrion is a highly dynamic organelle [4, 37]. Under physiological conditions, the balance of fusion and fission is essential for maintaining mitochondrial homeostasis and controlling the quality of mitochondria [38]. Specifically, the fusion of mitochondria promotes the filamentous network, while fission breaks the mitochondria into short rods or pellets. Fusion can maintain ATP production and redistribute mitochondrial proteins. Fission can isolate impaired mitochondria from the mitochondrial network. Dysfunctional mitochondria, unbalanced and depolarized, which are toxic to renal tubular cells, are targets of mitophagy [39]. Functional speaking, fusion is a heathier condition and is more critical to the recovery of kidney function than fission. To examine the dynamic changes in mitochondria, Opa-1 and Drp-1 were detected. The results were consistent with the mitochondrial morphology observed by transmission electron microscopy. In the LPS group, mitochondria presented short rods or spheres, Drp-1 was upregulated, and Opa-1 was downregulated. However, the RvD1 group presented fusion rather than fission, and the Drp-1/Opa-1 ratio was obviously lower than that in the LPS group. The damage caused by mitochondrial fragmentation may be related to the overproduction of ROS, the increased sensitivity to stimulation that initiates the insertion of the proapoptotic protein Bax, or other processes [40]. These results revealed that RvD1 can restore healthy mitochondrial function and prevent mitochondrial fission and apoptosis.
As a nuclear transcription factor, NF-κB plays an important role in regulating the production of inflammatory factors and participates in different processes of immune and inflammatory responses. The NF-κB protein family is composed of five structurally related members: NF-κB1, NF-κB2, rela, RelB and c-Rel. They mediate the transcription of target genes by binding to specific DNA element κB enhancers as heterodimers or homodimers [41, 42]. Under normal circumstances, inflammation is beneficial to the host and can be eliminated in time. However, uncontrolled inflammation can lead to severe or persistent tissue damage, leading to acute or chronic inflammatory diseases [43]. NF-κB signaling plays a pathogenic role in a variety of inflammatory diseases. Studies have proven that inhibiting the phosphorylation and activation of key signaling molecules of the NF-κB pathway can downregulate the production of inflammatory factors, and blocking the development of inflammation is an important treatment strategy for inflammatory diseases. The protein and gene expression levels of NF-κB p65, NF-κB p-p65, TNF-α, IL-6 and IL-1β were detected. The results showed that RvD1 inhibited the downstream signal of the NF-κB pathway by reducing the phosphorylation of NF-κB p65 induced by LPS, thus reducing the expression of the proinflammatory factors TNF-α, IL-6 and IL-1β. Marchese et al. found that RvD1 can reduce the activation of the NF-κB pathway mediated by TNF-α in cells rich in the ALX/FPR2 or GPR32 receptor [44]. In our previous study, we verified the distribution of the RvD1-specific antibody ALX on renal tubular epithelium and proposed that pretreatment with RvD1 prior to LPS stimulation could effectively protect the kidneys of septic mice [45], In the current research, we further confirmed a similar renoprotective effect of RvD1 when RvD1 was administered to mice/cells after LPS challenge. In addition to confirming the inhibitory effect of RvD1 on the NF-κB pathway, the study further focused on mitochondrial dynamic balance and the mitochondrial apoptosis pathway. Otherwise, the relationship between NF-κB pathway and mitochondrial dynamic balance is an interesting topic, which provides insights for future related research. Our experimental results are consistent with the conclusions of the above studies, which confirmed that RvD1 can inhibit the NF-κB pathway and reduce the expression of inflammatory factors in AI-AKI mice. In addition to confirming the inhibitory effect of RvD1 on NF-κB pathway, the advantage of this study is to focus on mitochondrial dynamic balance and mitochondrial apoptosis pathway. Otherwise, the relationship between NF-κB pathway and mitochondrial dynamic balance is an interesting topic, which can provide new ideas for future related research.
The pathological characteristic of SI-AKI is sublethal and lethal damage to renal tubules. In essence, segmental renal tubular cell death may mainly occur through apoptosis. Recently, many studies have demonstrated the important role of tubular cell apoptosis in ischemia reperfusion injury models and some clinical samples in patients with AKI [46]. In particular, injury and death of tubular cells are thought to be the precipitating factors of SI-AKI. Although sublethal damage is reversible, the death of renal tubular cells is accompanied by the inevitable loss of function in affected cells. For the past few years, it has been increasingly believed that apoptosis is an essential pathological process and potential target in SI-AKI.
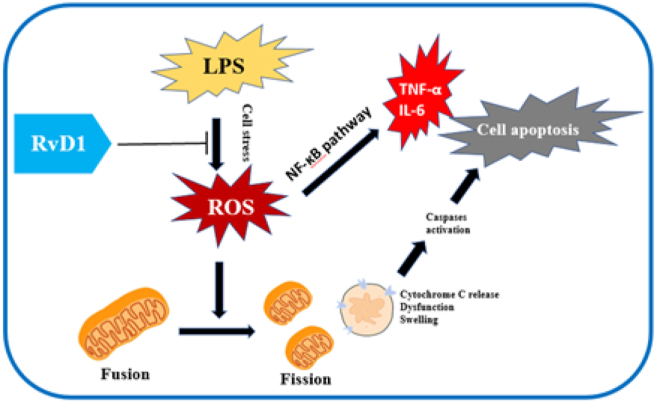
Mitochondria and proinflammatory cytokines play an essential role in regulating apoptosis and cell death. On the one hand, mitochondria become a key mediator of cell death by decreasing the production of ATP. On the other hand, mitochondria can affect apoptosis by several mechanisms. First, mitochondrial damage becomes the main source of excess ROS, and cell stress leads to mitochondrial outer membrane permeabilization (MOMP) directly. In addition to the mitochondrial pathway, bcl-2 family proteins are important regulators of apoptosis. Bcl-2 family proteins can be antiapoptotic or proapoptotic according to the presence of Bcl-2 homology (BH) domains. In addition, apoptosis can also be mediated by TNF-α in SI-AKI. Furthermore, the mitochondrial population may undergo a process from fusion to fission, followed by mitochondrial fragmentation, Bax/Bak activation, cytochrome-c release, caspase activation, and apoptosis. Thus, mitochondrial damage characterized by ATP, ROS, MOMP and fission are considered central control points of apoptosis. The results showed that renoprotection against LPS of RvD1 is associated with the suppression of tubular cell apoptosis through regulating mitochondria, bcl-2 family proteins and TNF-α expression. Through our experimental study, we proposed that RvD1 protects the kidney by stabilizing proximal renal tubule cells and reducing cell stress, thus reducing the production of ROS. Renal mitochondrial damage occurs in the early stage of sepsis, and the production of ROS is related to cell damage. In the LPS-induced AKI model, the mitochondrial dynamic balance shifts to mitochondrial fission. Under excessive ROS production, the mitochondria become fragmented, characterized by mitochondrial swelling and cristae deformation, resulting in changes in mitochondrial outer membrane permeability and loss of function [47]. The disruption of mitochondrial dynamics plays a crucial role in mitochondrial dysfunction as well as tubular cell damage and death. MOMP can promote the release of proapoptotic factors into the cytoplasm. Disabled mitochondria can not only generate enough ATP to supply the physiological functions of proximal renal tubule cells but also release cytochrome C from the inner mitochondrial membrane, which can further activate caspase 9 and caspase 3, ultimately leading to apoptosis of renal tubular cells. On the one hand, proinflammatory factors such as TNF-α, IL-6 and IL-1β were downregulated by inhibiting the NF-κB pathway. On the other hand, RvD1 reduces mitochondrial damage and the release of cytochrome C by inhibiting ROS production and ultimately reduces the apoptosis of renal tubule cells, thus protecting renal tubular function and shortening the disease process of AKI (Figure 6).
Figure 6.
The integrated NF-κB signaling pathway, mitochondrial changes and apoptosis in renal tubular cells.
There are some limits in this study. First, we didn't inhibit and up-regulate of the key molecule and signaling pathway to confirm the mechanism for the reno-protective effect of RvD1. Second, we found that RvD1 could protect against sepsis induced AKI by inhibiting NF-κB pathway and maintaining mitochondria dynamic at the same time, the relationship between NF-κB pathway and mitochondria dynamic was not explored. We plan to confirm the mechanism for the reno-protective effect of RvD1 by regulation key molecule and explore the relationship between NF-κB pathway and mitochondria dynamic in the future.
5. Conclusions
In summary, we demonstrate that RvD1 has a renoprotective effect by decreasing ROS production, sustaining mitochondrial function, inhibiting the NF-κB signaling pathway and decreasing apoptosis in LPS-induced AKI. Our work suggests that RvD1 may provide a novel therapeutic approach for the treatment of patients with SI-AKI. Further research is warranted to identify the possibility of translation into successful clinical trials with the aim of improving the prognosis of SI-AKI patients.
Declarations
Author contribution statement
Baihai Su, Ping Fu and Yuliang Zhao: Conceived and designed the experiments.
Liya Wang and Jiameng Li: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ruoxi Liao, Yupei Li and Luojia Jiang: Contributed reagents, materials, analysis tools or data.
Zhuyun, Zhang and Jiwen Geng: Analyzed and interpreted the data.
Funding statement
Yuliang Zhao was supported by Science and Technology Department of Sichuan Province [2021YJ0423].
Professor Baihai Su was supported by Science and Technology achievement transformation fund of West China Hospital of Sichuan University [CGZH19006], 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University [ZYJC21010].
Data availability statement
The authors do not have permission to share data.
Declaration of interest’s statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
We acknowledge the support of the Laboratory of Pathology, West China Hospital of Sichuan University, Chengdu, 610041, China.
Contributor Information
Baihai Su, Email: subaihai@scu.edu.cn.
Yuliang Zhao, Email: Dr.zhaoyuliang@gmail.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Solini A., Usuelli V., Fiorina P. The dark side of extracellular ATP in kidney diseases. J. Am. Soc. Nephrol. 2015;26(5):1007–1016. doi: 10.1681/ASN.2014070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan D.C. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 5.Wiersema R., Jukarainen S., Eck R.J., Kaufmann T., Koeze J., Keus F., Pettila V., van der Horst I.C.C., Vaara S.T. Different applications of the KDIGO criteria for AKI lead to different incidences in critically ill patients: a post hoc analysis from the prospective observational SICS-II study. Crit. Care. 2020;24(1):164. doi: 10.1186/s13054-020-02886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu C.Y. Yes, AKI truly leads to CKD. J. Am. Soc. Nephrol. 2012;23(6):967–969. doi: 10.1681/ASN.2012030222. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Feng Y.W., Yao Y.M. Potential therapy strategy: targeting mitochondrial dysfunction in sepsis. Mil Med Res. 2018;5(1):41. doi: 10.1186/s40779-018-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi H., Looker H.C., Satake E., D'Addio F., Wilson J.M., Saulnier P.J., Md Dom Z.I., O'Neil K., Ihara K., Krolewski B., Badger H.S., Petrazzuolo A., Corradi D., Galecki A., Wilson P.C., Najafian B., Mauer M., Niewczas M.A., Doria A., Humphreys B.D., Duffin K.L., Fiorina P., Nelson R.G., Krolewski A.S. Neuroblastoma suppressor of tumorigenicity 1 is a circulating protein associated with progression to end-stage kidney disease in diabetes. Sci. Transl. Med. 2022;14(657) doi: 10.1126/scitranslmed.abj2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang W., Wang Z., Li Q., Wang X., Zhang Y., Sun Y., Tang W., Ma C., Sun J., Li N., Yi F. Gpr97 exacerbates AKI by mediating Sema3A signaling. J. Am. Soc. Nephrol. 2018;29(5):1475–1489. doi: 10.1681/ASN.2017080932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellum J.A., Prowle J.R. Paradigms of acute kidney injury in the intensive care setting. Nat. Rev. Nephrol. 2018;14(4):217–230. doi: 10.1038/nrneph.2017.184. [DOI] [PubMed] [Google Scholar]
- 11.Uchino S., Kellum J., Bellomo R., Doig G.S., Morimatsu H., Morgera S. The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators, Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:313–318. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 12.Rice T.W., Wheeler A.P., Bernard G.R., Vincent J.L., Angus D.C., Aikawa N., Demeyer I., Sainati S., Amlot N., Cao C., Ii M., Matsuda H., Mouri K., Cohen J. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 2010;38(8):1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 13.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z., Zhang H., Yi B., Yang S., Liu J., Hu J., Wang J., Cao K., Zhang W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020;11(1):73. doi: 10.1038/s41419-020-2256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabb H., Griffin M.D., McKay D.B., Swaminathan S., Pickkers P., Rosner M.H., Kellum J.A., Ronco C. Acute dialysis quality initiative consensus, inflammation in AKI: current understanding, key questions, and knowledge gaps. J. Am. Soc. Nephrol. 2016;27(2):371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J., Zhang J., Tian J., Virzi G.M., Digvijay K., Cueto L., Yin Y., Rosner M.H., Ronco C. Mitochondria in sepsis-induced AKI. J. Am. Soc. Nephrol. 2019;30(7):1151–1161. doi: 10.1681/ASN.2018111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankar-Hari M., Phillips G.S., Levy M.L., Seymour C.W., Liu V.X., Deutschman C.S., Angus D.C., Rubenfeld G.D., Singer M., Sepsis Definitions Task F. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y.L., Zhai J.H., Chai Y.F. Recent advances in the molecular mechanisms underlying pyroptosis in sepsis. Mediat. Inflamm. 2018 doi: 10.1155/2018/5823823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez H., Kellum J.A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care. 2016;22(6):546–553. doi: 10.1097/MCC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez H., Ince C., De Backer D., Pickkers P., Payen D., Hotchkiss J., Kellum J.A. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv W., Booz G.W., Wang Y., Fan F., Roman R.J. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 2018;820:65–76. doi: 10.1016/j.ejphar.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Hong S., Lu Y. Omega-3 fatty acid-derived resolvins and protectins in inflammation resolution and leukocyte functions: targeting novel lipid mediator pathways in mitigation of acute kidney injury. Front. Immunol. 2013;4:13. doi: 10.3389/fimmu.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basil M.C., Levy B.D. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016;16(1):51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang N., Fredman G., Backhed F., Oh S.F., Vickery T., Schmidt B.A., Serhan C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484(7395):524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L., Wang Y., Wang L., Yao B., Chen T., Li Q., Liu Z., Liu R., Niu Y., Song T., Liu Q., Tu K. Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP. J. Exp. Clin. Cancer Res. 2019;38(1):170. doi: 10.1186/s13046-019-1163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M.J., Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu. Rev. Nutr. 2012;32:203–227. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- 29.Spite M., Norling L.V., Summers L., Yang R., Cooper D., Petasis N.A., Flower R.J., Perretti M., Serhan C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y.L., Zhang L., Yang Y.Y., Tang Y., Zhou J.J., Feng Y.Y., Cui T.L., Liu F., Fu P. Resolvin D1 protects lipopolysaccharide-induced acute kidney injury by down-regulating nuclear factor-kappa B signal and inhibiting apoptosis. Chin. Med. J. 2016;129(9):1100–1107. doi: 10.4103/0366-6999.180517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong R., Rifai A., Dworkin L.D. Hepatocyte growth factor suppresses acute renal inflammation by inhibition of endothelial E-selectin. Kidney Int. 2006;69(7):1166–1174. doi: 10.1038/sj.ki.5000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh S.M., Yang Y., He L., Tang C., Zhan M., Dong Z. Mitochondrial function and disturbances in the septic kidney. Semin. Nephrol. 2015;35(1):108–119. doi: 10.1016/j.semnephrol.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A., Dong Z., Harris R., Murray P., Parikh S.M., Rosner M.H., Kellum J.A., Ronco C. Acute dialysis quality initiative, cellular and molecular mechanisms of AKI. J. Am. Soc. Nephrol. 2016;27(5):1288–1299. doi: 10.1681/ASN.2015070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanz A.B., Sanchez-Nino M.D., Ramos A.M., Moreno J.A., Santamaria B., Ruiz-Ortega M., Egido J., Ortiz A. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 2010;21(8):1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 35.Pagliarini D.J., Calvo S.E., Chang B., Sheth S.A., Vafai S.B., Ong S.E., Walford G.A., Sugiana C., Boneh A., Chen W.K., Hill D.E., Vidal M., Evans J.G., Thorburn D.R., Carr S.A., Mootha V.K. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez H., Kellum J.A., Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat. Rev. Nephrol. 2017;13(3):143–151. doi: 10.1038/nrneph.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youle R.J., van der Bliek A.M. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatsuta T., Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27(2):306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Twig G., Hyde B., Shirihai O.S. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim. Biophys. Acta. 2008;1777(9):1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks C., Wei Q., Cho S.G., Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 2009;119(5):1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell J.P., Carmody R.J. NF-kappaB and the transcriptional control of inflammation. Int Rev Cell Mol Biol. 2018;335:41–84. doi: 10.1016/bs.ircmb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi K., Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 2018;18(5):309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 43.Kunnumakkara A.B., Shabnam B., Girisa S., Harsha C., Banik K., Devi T.B., Choudhury R., Sahu H., Parama D., Sailo B.L., Thakur K.K., Gupta S.C., Aggarwal B.B. Inflammation, NF-kappaB, and chronic diseases: how are they linked? Crit. Rev. Immunol. 2020;40(1):1–39. doi: 10.1615/CritRevImmunol.2020033210. [DOI] [PubMed] [Google Scholar]
- 44.Marchese A., Nguyen T., Malik P., Xu S., Cheng R., Xie Z., Heng H.H., George S.R., Kolakowski L.F., Jr., O'Dowd B.F. Cloning genes encoding receptors related to chemoattractant receptors. Genomics. 1998;50(2):281–286. doi: 10.1006/geno.1998.5297. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y.-L., Zhang L., Yang Y.-Y., Tang Y., Zhou J.-J., Feng Y.-Y., Cui T.-L., Liu F., Fu P. Resolvin D1 protects lipopolysaccharide-induced acute kidney injury by down-regulating nuclear factor-kappa B signal and inhibiting apoptosis. Chinese Med J. 2016;129(9):1100–1107. doi: 10.4103/0366-6999.180517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Q., Dong G., Chen J.K., Ramesh G., Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int. 2013;84(1):138–148. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8(11):870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.