Abstract
Background
The present study aimed to evaluate the effects of whole-body exposure to 150 kHz Intermediate-frequency electromagnetic radiation (IF EMR) on fertility indicators of male rats since human exposure to this frequency has increased in recent years. Fourteen adult male Sprague-Dawley rats were used in this study. The rats were randomly divided into a control and an EMR group (n = 7/group). The EMR group was continuously irradiated with 150 kHz EMR for 8 weeks. Male fertility indicators, body mass, testicular mass, rectal temperature, testicular histology, histometry, sperm analysis, and serum gonadotrophic hormone levels were evaluated.
Results
The study showed no negative effect on body mass (grams) (323.78 ± 37.09 to 305.09 ± 26.36; p = 0.72), rectal temperature (Control: 34.5 °C–35.8 °C; EMR: 34.4 °C–36.1 °C; p < 0.05), and testicular histology. There were significant reductions in left and right testicular mass (1.04 ± 0.10 to 0.96 ± 0.32: p = 0.03 and 1.02 ± 0.08 to 0.96 ± 0.35 p = 0.04, respectively), interstitial cell count/1000 μm2 (5.33 ± 0.56 to 4.47 ± 0.48; p = 0.01), sperm motility trajectories (p = 0.05) and sperm distal cytoplasmic droplet (%) (2.27 ± 2.28 to 6.84 ± 5.01; p = 0.05). A significant increase in follicle-stimulating hormone levels was observed (13.44 ± 6.38 IU/ml to 26.96 ± 8.07 IU/ml; p = 0.01).
Conclusions
Most male fertility parameters of rats in the present study were not affected by 8 weeks of whole-body exposure to 150 kHz EMR. However, significant decreases in testicular mass, interstitial cell count/1000 μm2, sperm motility trajectories, and distal cytoplasmic droplets were observed, as well as an increase in FSH level.
Keywords: Testis, Histology, Sperm analysis, Testosterone, Electromagnetic radiation, Intermediate frequency
Testis; Histology; Sperm analysis; Testosterone; Electromagnetic radiation; Intermediate frequency.
1. Introduction
Human exposure to electromagnetic fields, especially in the intermediate frequency (IF) and radio frequency (RF) ranges, has increased in recent years [1, 2]. The IF range extends from 300 Hz to 10 MHz, as classified by the World Health Organization (WHO) under the Electromagnetic Fields Project [3]. The intermediate frequency electromagnetic radiation (IF EMR) is categorized as non-ionizing radiation and is considered non-threatening because it does not contain enough energy to damage biological tissue [4]. Due to this fact, only a limited number of studies have been conducted on the biological effects or health risks of IF-EMR.
The development of new types of electrical appliances in recent decades has led to daily exposure in the IF-EMR. Sources in this range are found at home, at work, and in public places. These include induction stoves, electronic article surveillance (EAS) systems, wireless power transmission systems, power lines, electric train lines, energy-saving lamps, monitors, magnetic resonance imaging (MRI) scanners, and medical devices used to stimulate nerves and bones [5]. In recent years, public concern about the potential health hazards associated with this frequency from unavoidable daily and occupational exposure has increased dramatically. The WHO has emphasized that more research is needed on the potential health effects of exposure to this frequency [3] and suggested that more detailed parameters should be studied. Currently, several studies are addressing the biological effects of IF, including developmental and reproductive effects from maternal exposure [6, 7, 8], carcinogenic effects [9], and genotoxic effects [10]. However, most of these studies consider only a limited range of frequencies.
A variety of studies have been conducted to investigate the effects of IF on the testes, although the sample size was small [11, 12, 13, 14]. This area of research is important because the testes are known to be very sensitive to radiation, which can cause germ cell apoptosis [11] and affect male fertility. Studies have used different frequencies to assess the risks of different technologies. For example, magnetic fields (MF) of 7.5 kHz relevant to EAS technology used in supermarkets to protect goods from theft [12], 10 kHz MF, as used in high-frequency inductive power distribution [13], and 20 kHz and 60 kHz MF, relevant to induction stoves [14], have been studied.
Recently, IF has been successfully used for treatment purposes in the field of oncology and is called Tumor Treating Fields (TTFs). TTFs is an innovative and non-invasive cancer therapy that interrupts mitosis and selectively kills rapidly dividing cancer cells by continuously (over 18 h per day) applying low-intensity, intermediate frequency (100 kHz–300 kHz) alternating electric fields to the tumor [15]. The optimal frequency for the antimitotic effect varies by cancer type and can be adjusted for maximum anti-cancer effect. In the preclinical setting, 150-kHz TTFs are effective in treating pancreatic cancer, non-small cell lung carcinoma (NSCLC), brain metastases from NSCLC, and mesothelioma when combined with chemotherapy [16] and in reducing the number of cysts in polycystic ovary disease in rats [17, 18]. Since mammalian testicular germ cells proliferate clonally via mitotic rounds before differentiating into mature spermatozoa, the effect of IF on any reproductive risks in this TTF range (100 kHz–300 kHz) needs to be investigated. Therefore, the objective of this study was to investigate the effects of whole-body exposure of IF EMR at 150 kHz on male fertility indicators in Sprague-Dawley rats for eight weeks.
2. Materials and methods
2.1. Animals and experimental design
A total of fourteen (14) sexually mature (8–12 weeks old) male Sprague-Dawley rats weighing 250–300 g were obtained for the study from the Laboratory Animal Facility at the School of Veterinary Medicine. Animals were housed in ventilated metal cages measuring 40 × 24 × 14 cm. Rats were housed on sawdust bedding in a pathogen-free room at a temperature of 24 ± 2 °C, a humidity of 50–60%, and a 12-hour light-dark cycle. Rats were fed a standard pellet diet and received water ad libitum. They were acclimatized to laboratory conditions for 14 days prior to the experiment. The rats were divided into a control group and an electromagnetic radiation (EMR) group (n = 7/group) and 3 cages per control group (2 rats/cage in 2 cages and 3 rats/cage) and 4 cages for the EMR group (2 rats/cage in 3 cages and one rat in one cage). In this experiment, the minimum number of animals was used to obtain a result before repeating it with a larger number. The EMR group was continuously irradiated with 150 kHz EMR for eight weeks (56 days) (except for approximately 1 h per week when the cages were cleaned). The duration of irradiation was chosen to correspond to the duration of a complete spermatogenic cycle (54 days) [19]. The control group was kept under similar conditions as the EMR group but without EMR device exposure. The rectal temperature of each rat was recorded weekly with a digital thermometer. The final body mass was weighed using a top-loading scale, and the right and left testicular masses were weighed using an analytical balance. The protocols for the animal experiments were approved by the University of the West Indies Campus Research Ethics Committee (No. CEC 454/02/18).
2.2. EMR-inducing system
Rats were maintained in an EMR field with a frequency of 150 kHz and an amplitude of 12 V. Electrical potentials were generated at the maximum possible result intensity using a Kenwood AG-203A oscillator (10 Hz–1 MHz) (Trio-Kenwood Electronic; Komagane, Japan). The uniform EMR field was generated by two flat electrodes (40 cm high and 2 m long) made of aluminum foil-covered cardboard placed vertically at opposite ends of the cage. The distance between the vertically arranged parallel electrodes was 40 cm. The EMR field intensity was measured as 0.3 V/cm by using an Airmed Narda NBM-550 field radiation meter (100 kHz–6 GHz) (Narda Safety Test Solutions GmbH, Pfullingen, Germany). The control animals were housed in the same room as the experimental group. The cages of the EMR group were surrounded on 4 sides with cardboard boxes covered with aluminum foil to prevent radiation leakage. The intensity of the EMR field was 50–80 W/cm2 in the EMR cages and 20–50 nW/cm2 in the control cages. The variability of the intensities was related to the spatial variability of the field in the cages of the irradiated animals and the diurnal variability in the control cages. The total exposure in the experimental room was measured as 0–100 nW/cm2. Thus, the intensity of EMR in the cages of the EMR group was 1000 times higher than in the cages of the control group. The room was located in a building shielded from noise and vibration. The specific absorption rate (SAR) for all groups was in accordance with the occupational exposure limits set by the International Commission on Non-ionizing Radiation Protection (INCIRP) (1998) in the spectrum from 1 Hz to 300 GHz. The animals were not kept in a Faraday cage because exposure to low-intensity EMR fields cannot be avoided in our daily life. To ensure consistent exposure for each group and cage, EMR levels were measured weekly. During the electromagnetic field strength measurements, all cell phones were put away. The device was the only source of emission of the desired EMR frequency. Since the lamps and sockets in the experimental room did not fall within the desired frequency range, they were not considered. The geometry and positions of the cages, electrodes, and oscillator were not changed during the experiment. To exclude the influence of temperature due to possible heating by the EM radiation, an independent study was performed with the same setup used for the experiment. The Testo 905-T1 compact penetration thermometer (Testo, Hampshire, United Kingdom) was used to measure the temperature in 300 ml of water (the mass of our rats). The step size of the digital thermometer was 0.1 °C. The measured temperature difference between EM irradiated water and the control sample was 0.3 °C ± 0.3 °C. Therefore, the temperature should not affect the results of the experiments, especially in animals that can keep their body temperature constant.
2.3. Hormonal analysis
Animals were weighed at the end of the experiment and sedated intraperitoneally (i.p) with an injection of ketamine hydrochloride 10% solution (Dutch Farm Veterinary Pharmaceuticals, Dutch Farm International BV, Holland) at a dose of 80 mg/kg body weight. After sedation, deep anesthesia was induced by injecting pentobarbital sodium 20% solution (Kela NV, St. Lenaartseweg, Belgium) at a dose of 40 mg/kg intraperitoneally. After the animal reached deep anesthesia, 5 ml of blood was obtained from the heart by the standard procedure of terminal cardiac puncture. Then the animals were euthanized by an overdose of pentobarbital sodium 20% solution at a dose of 120 mg/kg by intraperitoneal injection. Serum was separated by centrifuging the blood samples at 1500 rpm for 10 min at 4 °C and stored at −80 °C until assayed. A testosterone enzyme-linked immunosorbent assay (ELISA) kit (Cat. No. ab 108666, Abcam, Cambridge, United Kingdom), which is sensitive to 0.07 ng/ml. ELISA assays for luteinizing hormone (LH) (Cat. No. ENZ-KIT 107, Enzo Life Sciences, Farmingdale, NY) and follicle-stimulating hormone (FSH) (Cat. No. LS-F38636, Lifespan Biosciences, Seattle, WA) were used to determine the levels of LH and FSH. Assays were performed with serum according to the manufacturer's instructions. Absorbance values were measured in a Thermo Scientific Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific OY, Vantaa, Finland), and hormone concentrations in samples were extrapolated from the corresponding absorbance values of a calibration curve and then calculated according to the instructions in the manuals of each ELISA kit.
2.4. Sperm analysis
The epididymis was quickly removed by dissection in each animal, and adherent fat, blood vessels, and connective tissue were excised. The epididymis was then clamped with a hemostat at both ends, a small incision was made in the plugged tubules of the distal cauda of the epididymis with a scalpel blade, and placed in a Petri dish (55 mm) containing 2 ml of phosphate-buffered saline (PBS) (pH 7.4). After the sperm were dispersed in the PBS for approximately 5 min, the epididymis was removed and the sample was swirled to mix. An aliquot of the PBS/sperm mixture was then removed for analysis. Next, 10 μl of the sperm mixture was dropped into the centre of the preheated slide chamber and a coverslip was placed over the sample. The slide was placed in the thermostat and sperm parameters were determined using the Computer-Assisted Sperm Analyser (CASA) (Hamilton Thorne, Beverly, MA) and the software CEROS II at 100× magnification. For each analysis, sperm parameters were acquired and recorded by taking at least 5 non-consecutive fields (with a total of at least 500 sperm) within 30 s. The spermatozoa in each field were selected by adjusting the grayscale threshold, and the selected debris and round cells were manually removed before analysis.
The following parameters were analyzed using the CASA system: (1) total sperm concentration, (2) sperm motion parameters: Straight-line velocity (VSL), curvilinear velocity (VCL), average path velocity (VAP), length of straight-Line path (DSL), length of average path (DAP), amplitude of lateral head displacement (ALH), beat cross frequency (BCF), linearity (LIN), straightness of trajectory (STR), wobble (WOB) (3) sperm motility parameters: total, motile, progressive, slow and static (4) sperm morphology parameters: proximal droplet, distal droplet, bent tail and coiled tail.
2.5. Histological and histometric analysis
The testes were removed by dissection and weighed. The tissue samples were trimmed and fixed in 10% buffered neutral formalin for histological examination. After fixation, the tissues were subjected to routine histological processing. Tissue sections were cut with a rotary microtome at 5 μm and stained with hematoxylin and eosin (H&E) according to standard procedures. Histological observations, measurements and photomicrographs were performed using an Olympus BX51 system microscope (Olympus, Tokyo, Japan) with cellSens Imaging Software (version 1.12). Testicular tissue was scored based on histomorphology using a Johnsen testicular biopsy score, as indicated in Table 1 [20]. The testicular tissues stained with H&E were also used for histometry. The diameter of the seminiferous tubule (ST), the diameter of the ST lumen, the diameter of the germinal epithelium (GE), and the number of interstitial cells were measured on 10 circular cross-sections of different testicular regions [21]. The mean ST diameter (D) was calculated as the average of two diameters taken at right angles from ST.
Table 1.
Evaluation of testicular biopsy score count by Johnsen's scoring method (Johnsen, 1970).
| Score | Description |
|---|---|
| 10 | Complete spermatogenesis with many spermatozoa |
| 9 | Much spermatogenesis, but germinal epithelium disorganized with marked sloughing or obliteration of the lumen |
| 8 | Only a few spermatozoa present (<5 to 10) |
| 7 | No spermatozoa but many spermatids present |
| 6 | No spermatozoa and only a few spermatids present (<5 to 10) |
| 5 | No spermatozoa, no spermatids but several and many spermatocytes present |
| 4 | Only a few spermatocytes were present (<5) but no spermatids or spermatozoa present |
| 3 | Spermatogonia are the only germ cells present |
| 2 | No germ cells, but Sertoli cells present |
| 1 | No cells in the tubular section. |
2.6. Statistical analysis
The program IBM SPSS Statistics V21 (Armonk, New York, USA) was used to perform the statistical analysis. Descriptive statistics were calculated for each group in the experiment. Normality of the data was examined and a nonparametric Kruskal-Wallis test was also performed. p < 0.05 was considered significant.
3. Results
3.1. Body and testicular mass
There was no significant difference in final body mass between the two groups (p > 0.05) (Table 2). A significant decrease in left and right testicular mass was observed in the EMR group (p = 0.03 and p = 0.04, respectively).
Table 2.
Effects of 150 kHz IF EMR on the mass of the male rats' testicles and body (n = 7).
| Parameters | Control group (Mean ± SD) | EMR group (Mean ± SD) | p-value |
|---|---|---|---|
| Body Mass/g | 323.78 ± 37.09 | 305.09 ± 26.36 | 0.72 |
| Left Testis mass/g | 1.04 ± 0.10 | 0.96 ± 0.32 | 0.03∗ |
| Right Testis mass/g | 1.02 ± 0.08 | 0.96 ± 0.35 | 0.04∗ |
∗p < 0.05 is statistical significance at 95% confidence interval.
3.2. Rectal temperature
The lowest mean temperature ranged from 34.5 °C to 35.8 °C in the control groups and from 34.4 °C to 36.1 °C in the EMR group, and all values were within the normal range for rats. No significant difference was found between the two groups (Figure 1).
Figure 1.
Effect of 150 kHz IF EMR on the rectal temperature of male rats.
3.3. Sperm analysis
The results of epididymal sperm analysis of rats in both groups are summarised in Table 3. The sperm concentration was 5.53 ± 8.31 and 3.26 ± 2.20 million per milliliter (M/ml) for the control and EMR groups, respectively, with no significant difference (p = 0.16). The sperm motility parameters (static, progressive, motile, and slow) also showed no significant difference (p > 0.05). However, the mean values of sperm concentration and all sperm motility parameters were lower in the EMR group. A borderline significant decrease was observed in all motility parameters VSL, VCL, VAP, DSL, DAP, ALH, BCF, LIN, STR, WOB in the EMR group at p = 0.05. The sperm morphology parameters, the bent tail, coiled tail and proximal droplet, were not significantly different, but the distal droplet showed a significant increase (p = 0.05).
Table 3.
Effect of 150 kHz IF EMR on sperm concentration, motility, motion parameters, and morphology of male rats (n = 7).
| Parameters | Control group (Mean ± SD) | EMR group (Mean ± SD) | p-value |
|---|---|---|---|
| Total Sperm Concentration (M/ml) | 5.53 ± 8.31 | 3.26 ± 2.20 | 0.16 |
| Sperm Motility parameters | |||
| Static (M/ml) | 4.45 ± 6.00 | 2.74 ± 1.77 | 0.19 |
| Progressive (M/ml) | 0.46 ± 0.98 | 0.28 ± 0.31 | 0.19 |
| Motile (M/ml) | 1.02 ± 2.32 | 0.52 ± 0.45 | 0.11 |
| Slow (M/ml) | 0.14 ± 0.31 | 0.05 ± 0.05 | 0.09 |
| Motion parameters | |||
| Straight-line velocity (VSL) (μm/s) | 18.60 ± 5.74 | 10.85 ± 5.74 | 0.05∗ |
| Curvilinear velocity (VCL) (μm/s) | 28.12 ± 21.79 | 16.40 ± 8.67 | 0.05∗ |
| Average Path Velocity (VAP) (μm/s) | 20.83 ± 16.14 | 12.15 ± 6.43 | 0.05∗ |
| Length of Straight-Line Path (DSL) (μm) | 2.80 ± 2.17 | 1.63 ± 0.86 | 0.05∗ |
| Length of Average Path (DAP) (μm) | 3.13 ± 2.43 | 1.83 ± 0.97 | 0.05∗ |
| Amplitude of Lateral Head Displacement (ALH) (μm) | 0.98 ± 0.76 | 0.57 ± 0.30 | 0.05∗ |
| Beat Cross Frequency (BCF) (Hz) | 7.45 ± 5.77 | 4.35 ± 2.30 | 0.05∗ |
| Linearity (LIN) (%) | 37.06 ± 28.72 | 21.62 ± 11.44 | 0.05∗ |
| Straightness of Trajectory (STR) (%) | 50.05 ± 38.78 | 29.20 ± 15.44 | 0.05∗ |
| Wobble (WOB) (%) | 41.50 ± 32.16 | 24.21 ± 12.81 | 0.05∗ |
| Morphology | |||
| Bent tail (%) | 1.20 ± 1.12 | 2.93 ± 4.04 | 0.30 |
| Coiled tail (%) | 9.22 ± 10.95 | 5.61 ± 7.41 | 0.48 |
| Distal droplet (%) | 2.27 ± 2.28 | 6.84 ± 5.01 | 0.05∗ |
| Proximal Droplet (%) | 19.36 ± 18.28 | 25.04 ± 15.84 | 0.56 |
∗p = 0.05 is statistical significance at 95% confidence interval.
3.4. Histological and histometric analysis
Morphological examination revealed no significant structural changes in testicular morphology in either group (Figure 2). In both groups, the seminiferous tubules had a normal shape with a typical arrangement of spermatogonia and Sertoli cells resting on an intact basement membrane and lamina propria. The GE was intact and exhibited the usual morphologic features with an orderly development of spermatogonia to spermatocytes, with groups of spermatids and mature spermatozoa. The histometric parameters, the diameter of ST (p = 0.17), the diameter of the lumen of ST (p = 0.41) and the diameter of GE (p = 0.41), did not differ significantly between the groups. However, there was a significant decrease in the number of interstitial cells/1000 μm2/(p = 0.01) in the EMR group (Table 4). In addition, testicular biopsy scores by the Johnson method [19] were very high, ranging from 9 to 10, with no significant structural difference between the two groups (Table 5).
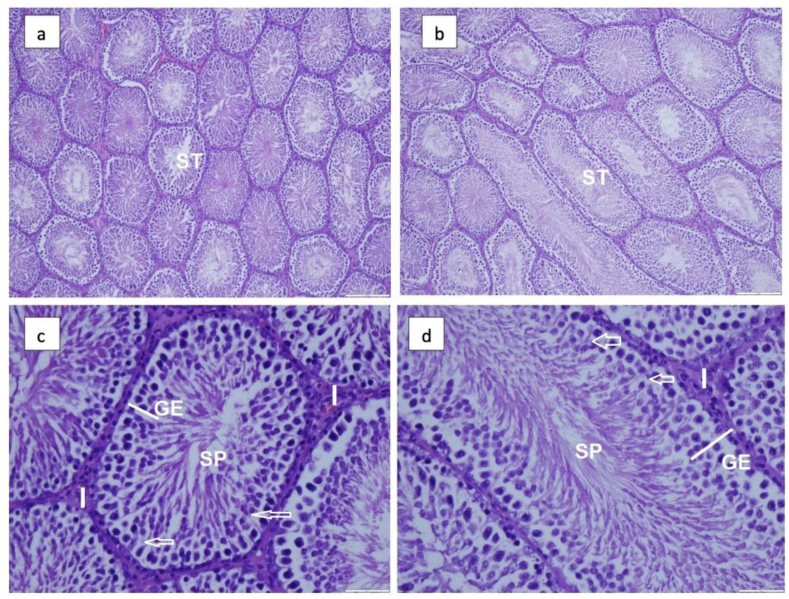
Figure 2.
Effect of 150 kHz IF EMR on testicular histomorphology of male rats. Photomicrograph showing seminiferous tubules (ST) with flattened cells that contract to move sperm (a) control and (b) IF EMR-exposed group of rats. Both groups show normal spermatogenesis (Haematoxylin and Eosin stain; magnification, ×100). The Sertoli cells (arrows) and interstitial cells (I) seminiferous tubule (ST) of the control (c) and exposed (d) group showed a typical with the usual arrangement of germinal epithelium (GE) and spermatozoa (SP) (Haematoxylin and Eosin stain; magnification, ×200).
Table 4.
Effects of 150 kHz IF EMR on the testicular morphometry of male rats (n = 7).
| Parameter | Control group Mean ± SD | EMR group Mean ± SD | p-Value |
|---|---|---|---|
| Mean diameter of seminiferous tubule (ST) (μm) | 27.52 ± 2.39 | 25.38 ± 3.07 | 0.17 |
| Mean diameter of ST lumen (μm) | 61.48 ± 24.42 | 70.86 ± 15.86 | 0.41 |
| Mean diameter of Germinal Epithelium (μm2) | 25.23 ± 4.65 | 31.36 ± 3.41 | 0.35 |
| No. of interstitial cells/1000/μm2 | 5.33 ± 0.56 | 4.47 ± 0.48 | 0.01∗ |
∗p < 0.05 is statistical significance at 95% confidence interval.
Table 5.
Effect of 150 kHz IF EMR on the testicular biopsy score in male rats (Johnsen, 1970) (n = 7).
| Group | Biopsy Score |
||
|---|---|---|---|
| 9 | 9–10 | 10 | |
| Control (NA) | 2 | 3 | 2 |
| EMR group (NA) | 2 | 1 | 4 |
NA-number of animals.
3.5. Follicle-stimulating hormone, luteinizing hormone, and total testosterone levels
Serum levels of FSH were significantly higher in the EMR group (26.96 ± 8.07) international units per milliliter (IU/ml) than in the control group (13.44 ± 6.38 IU/ml) at p = 0.01. There was no significant difference in LH (p = 0.32) and total testosterone concentration (p = 0.18) between the groups. However, the mean concentration of LH (9.17 ± 4.59 IU/ml; control vs. 12.58 ± 7.30 IU/ml; EMR group) and total testosterone (1.44 ± 0.77 IU/ml; control vs. 2.30 ± 1.38 IU/ml; EMR group) was slightly higher in the EMR group (Figure 3).
Figure 3.
Effect of 150 kHz EMR on Testosterone, Follicle Stimulating Hormone (FSH) and Luteinising hormone (LH) level of male rats.
4. Discussion
Male reproductive functions may be affected by EMR through three mechanisms: an EMR-specific effect, a thermal molecular effect, or a combination of both effects [22].
In this study, no differences in body mass were observed between the two groups of rats, but the testicular mass was reduced in the EMR group. Similar studies showed no changes in body weight in rats [14] and both body and testicular weight in mice [12]. However, a reduction in testicular weights was reported in rats exposed to a low frequency (LF) at 50 Hz horizontal electric field [23]. The reduction in testicular weights observed in the present study could be caused by thermal effects due to oxidative stress or by induced germ cell apoptosis due to IF EMR exposure.
The heat generated by IF EMR exposure can increase body temperature by 1 °C [24], which may affect the testes and lead to decreased sperm production. However, no significant changes in rectal temperature were observed in this study. A significant increase in FSH with rapid regression of germ cells was reported when male rats were exposed to heat [25].
In this study, exposure to IF EMR was performed on sexually mature animals (8–12 weeks old), and sperm analysis was performed after 8 consecutive weeks (56 days) of exposure. Since the duration of spermatogenesis in rats is 54 days [19], it can be assumed that all the evaluated spermatozoa were exposed to IF EMR for the entire duration of spermatogenesis. However, sampling was performed once at the end of the study, so the study can only cover one complete spermatogenesis cycle within the study duration, which is considered a limitation of this study.
Rodents are considered the best model to study environmental effects on reproduction due to their high fertility rates and short gestation periods [26]. Spermatogenesis is a very complex process and represents one of the most dramatic examples of cellular proliferation and differentiation in any mammalian organ system [27].
Successful fertilization depends on the male animal having a sufficient number of sperm [28]. This study showed that mean sperm concentration was lower in the EMR group was than in the control group but not statistically significant. This result is also supported by the normal histology observed in the EMR group.
Sperm motility is an important element of male fertility [29]. Increased sperm motility was reported in mice to 7.5 kHz MFs at 120 μT exposure [12]. In another report in humans with exposure to 50 Hz MFs at 5 mT, increased sperm motility was reported after 2 h of exposure, and the increased motility persisted for 21 h after the end of the 3 h exposure period [29]. However, in the present study, the mean values of sperm motility parameters were lower but not statistically significant. This could be due to the higher frequency used in the present study than in other previously reported studies. The mechanism behind the regulation of sperm motility is not well understood, but motion requires energy and published evidence suggests that mitochondrial oxidative phosphorylation plays a key role in mediating the effect of 50-Hz square wavelength EMF on sperm motility [29, 30].
The decrease in sperm motility parameters has been demonstrated in various experiments with radiofrequency electromagnetic radiation (RF-EMR) exposure. As RF EMR is known to have the ability to induce oxidative stress, characterized by excessive generation of reactive oxygen species (ROS) that overwhelm the intrinsic cellular antioxidant capacity, in a variety of tissue types. In the current study, a borderline decrease in sperm motility parameters like VSL, VCL, VAP, DSL, DAP, ALH, BCF, LIN, STR, and WOB was observed in the EMR group (p = 0.05). could be due to oxidative stress but IF EMR does not contain enough energy to cause major changes other than the heating effect. There is a possibility that the heating effect triggers chain reaction effects, which could be the possible reason for the decrease in sperm motility parameters.
The frequency of spontaneous changes in sperm morphology is generally low and the percentage of normal sperm ranges from 78% to 85% [31]. An increase in the frequency of sperm abnormalities is considered to indicate germ cell damage. In the present study, no adverse effects on sperm morphology and abnormal sperm count were observed between the groups. However, a borderline significant increase in the distal cytoplasmic droplet (p = 0.05) was observed in the EMR group. Spermatozoa mature and acquire the ability to fertilize ova during their transit through the epididymis. The cytoplasmic droplets migrate from the proximal position down the tail to the midpiece or distal position where they mature and are released. This usually occurs just before the spermatozoa enter the cauda epididymis. Ejaculates showing high numbers of spermatozoa with distal cytoplasmic droplets usually indicate that the time spent by the spermatozoa in the epididymis has decreased or, conversely, the time required for their normal maturation has increased. In the present study, the sperm was collected from the epididymis and not from ejaculation. Therefore, the increased distal droplets in the present study may be due to the normal ongoing maturation process in the epididymis and may be considered less severe as we could not determine the time spent by the spermatozoa in the epididymis.
The present study showed no significant change in testicular structure and histometric parameters, which was also confirmed by Johnsen's testicular biopsy score. However, a significant decrease in the number of interstitial cells was noted, which could be due to apoptosis and cellular depletion. This decrease may be correlated with the decrease in testicular weights, but the mean total testosterone produced by the interstitial cells did not vary significantly between the groups. There are no previous reports on the effect of IF EMR exposure on testicular histology and histometry. However, variable results ranging from no change to major structural changes in testicular histology have been reported for LF exposure [22] and various RF exposures (depending on frequency and exposure time) in rats [32, 33].
There is no previous report on the effects of IF exposure on serum gonadotrophic hormones. The production of FSH is dependent on the pulsatile release of gonadotropins controlled by the feedback loop of the hypothalamic-pituitary-gonadal (HPG) axis. Stimulation is determined by the negative feedback of testicular inhibin B secreted by Sertoli cells [34], and alteration of the testicular feedback signal leads to changes in the production of FSH and LH in males. The significant increase in FSH in the present study may be due to changes in negative testicular feedback due to a decreased spermatogenic activity in the EMR group, which could lead to accelerated release of gonadotropin-releasing hormone (GnRH) [35]. Another possible reason for increased FSH in the present study may be increased gonadotropin production at the hypothalamic level due to EMR exposure. However, a previous study showed that exposure to low-frequency EMR did not affect the regulation of the HPG axis in rats [36], but high FSH levels are often associated with high-dose radiation [37]. In another study, a significant increase in FSH levels with rapid germ cell regression was observed in male rats exposed to heat [25]. However, we could not conclude the reason for the increased FSH level because there were no changes in rectal temperature and the statistically insignificant low mean sperm concentration, and no significant change histometric data of the germinal epithelium in this study. In addition, we did not investigate the effects of EMR radiation on the HPG axis at this frequency. Thus, further studies are needed to find an explanation for the increased FSH.
The FSH fluctuations could directly affect Sertoli cells, which in turn affects spermatogenesis because Sertoli cells regulate spermatogenesis. In this study, a slight decrease was observed only in interstitial cells, i.e., stromal cells, and not in germ cells. Therefore, FSH may not be the cause of the decrease in interstitial cells. However, FSH may indirectly affect the number of interstitial cells, which is mediated via interstitial androgen receptors through paracrine factors from Sertoli cells, as observed in mice [38]. We were unable to establish the relationship between the significant increase in FSH and the concomitant decrease in interstitial cells, which requires further investigation at the receptor level. Furthermore, the decrease in interstitial cells in the present study did not affect testosterone production, which in turn may be due to the increased LH that stimulates interstitial cells to produce testosterone. Increased levels of gonadotrophic hormones result in improved sexual maturation and improved sperm production and libido in males. Exposure of male mice to an electric field has been shown to improve copulation rates with superovulated females, although plasma concentrations of testosterone and luteinizing hormone do not increase [39]. In the current study, sperm production did not change significantly; however, the sexual behavior of the rats was not assessed in the study.
Few previous studies have addressed the possible negative effects of IF on male fertility. Rats exposed to 20 kHz, 0.2 mT and 60 kHz, 0.1 mT MF for 22 h/d for 14 days showed no exposure-related changes in body weight, reproductive tissue weights, epididymal sperm count, motility, or sperm morphology [14]. There were no exposure-related effects on spermatogenesis or other signs of reproductive toxicity when male rats were exposed to 10 kHz IF MF at 0.095, 0.24, and 0.95 mT for 20–23.5 h/d for 45 days [13]. Male mice exposed to 7.5 kHz MF at 12 and 120 μT for 5 weeks showed no adverse effects on indicators of male fertility [12]. The current study is also in agreement with these three studies in that no adverse effects were observed on most parameters of male fertility. However, in the present study, in which rodents were exposed to the highest IF EMR levels reported to date, increased gonadotrophin hormone levels and reduced testicular weights were observed. The results of these studies suggest that if fertility is affected by IFs, such effects do not occur at wavelengths from ≥7.5 kHz to 60Khz. But 150kHz IF EMR shows some changes in the parameters such as testicular weights, FSH hormone levels, number of interstitial cells and sperm movement trajectories. These changes could be wavelength-dependent, but further work is needed to confirm this. Therefore, including the current study, five different IF MF frequencies (7.5, 10, 20, 60 and 150 kHz) and magnetic flux densities ranging from 12 to 950 μT have now been tested in rodents to measure effects on male fertility parameters.
5. Conclusion
Whole-body exposure of IF EMR to 150 kHz for 8 weeks had no adverse effects on body weight, rectal temperature, and testicular histology of male rats. However, there was a significant decrease in testicular mass and interstitial cell count/1000 μm2. There was a significant increase in follicle-stimulating hormone and a slight increase in testosterone and luteinizing hormone concentrations in the EMR group compared with the control group. There was a borderline significant decrease in sperm motility (p = 0.5), and the percentage of distal and cytoplasmic droplets (p = 0.5). However, more detailed studies should be performed to build on this study to understand the increased hormonal levels.
Declarations
Author contribution statement
Venkatesan Sundaram: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Stephanie Mohammed: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nikolay Zyuzikov: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by School of Gradute Studies and Research, The University of the West Indies, St.Augustine Campus, Trinidad and Tobago.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2022.e12228.
Acknowledgements
The authors gratefully acknowledge financial support for this research project from the School of Graduate Studies and Research at the University of the West Indies, St. Augustine, Trinidad and Tobago. The authors thank Dr. Jenelle Johnson of the School of Veterinary Medicine's Laboratory Animal Facility for her assistance with the animal experiments. The authors also thank Mr. Reeza Ramgattie, Senior Instructor in Bio-Sciences, Department of Agriculture and Food Technology, University of Trinidad and Tobago for performing CASA sperm analysis and Prof. Andanappa Gadad of the School of Pharmacy for his helpful suggestions during the preliminary phase of the study. The authors would also like to thank Dr. Samuel Rampersad, Mr. Lester Gilkes, and Mr. Gerald Chandoo for their assistance with specimen collection, dissection, and histological processing.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Picture -1 EMR exposed Group 300dpi_V2.jpg.
Picture -2 Control Group 300dpi_V2.jpg.
References
- 1.Calin M.D., Ursachi C., Helerea E. Proceedings of the 4th International Symposium on Electrical and Electronics Engineering (ISEEE 2013) Romania; Galati: 2013. Electromagnetic environment characteristics in an urban area. [Google Scholar]
- 2.Scientific Committee on Emerging and Newly Identified Health Risks, Potential health effects of exposure to electromagnetic fields (EMF). European Commission.https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_041.pdf. Accessed on April 16, 2021.
- 3.World Health Organization. Electromagnetic fields & public health: intermediate frequencies (IF). WHO Information Sheet. https://www.who.int/peh-emf/publications/facts/intermediatefrequencies_infosheet.pdf?ua=1” Accessed on April 16, 2021.
- 4.Zamanian A., Hardman C. Electromagnetic radiation and human health: a review of sources and effects. High Freq. Electron. 2005;4:16. [Google Scholar]
- 5.Aerts S., Calderon C., Valič B., Maslanyj M., Addison D., Mee T., Goiceanu C., Verloock L., Bossche M.V., Gajsek P., Vermeulen R., Roosli M., Cardis E., Martens Joseph W. Measurements of intermediate-frequency electric and magnetic fields in households. Environ. Res. 2017;154:160–170. doi: 10.1016/j.envres.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Frölén H., Svedenstal B.M., Paulsson L.E. Effects of pulsed magnetic fields on the developing mouse embryo. Bioelectromagnetics. 1993;14:197–204. doi: 10.1002/bem.2250140303. [DOI] [PubMed] [Google Scholar]
- 7.Huuskonen H., Juutilainen J., Julkunen A., Maeki-Paakkanen J., Komulainen H. Effects of gestational exposure to a video display terminal-like magnetic field (20-kHz) on CBA/S mice. Teratology. 1998;58 doi: 10.1002/(SICI)1096-9926(199811)58:5<190::AID-TERA5>3.0.CO;2-I. 190–196.-I. [DOI] [PubMed] [Google Scholar]
- 8.Kim S.H., Song J.E., Kim S.R., Oh H., Gimm Y.M., Yoo D.S., Lee Y.S. Teratological studies of prenatal exposure of mice to a 20 kHz sawtooth magnetic field. Bioelectromagnetics. 2004;25:114–117. doi: 10.1002/bem.10164. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura I., Doi Y., Imai N., Kawabe M., Mera Y., Shiina T. Carcinogenicity of intermediate frequency magnetic field in Tg. rasH2 mice. Bioelectromagnetics. 2019;40(3):160–169. doi: 10.1002/bem.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrala M., Kumari K., Koivisto K., Juutilainen K. Genotoxicity of intermediate frequency magnetic fields in vitro and in vivo. Environ. Res. 2018;167:759–769. doi: 10.1016/j.envres.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Linster C.L., Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2006;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 12.Kumari K., Capstick M., Cassara A.M., Herrala M., Koivisto H., Naarala J., Tanila H., Viluksela M., Juutilainen J. Effects of intermediate frequency magnetic fields on male fertility indicators in mice. Environ. Res. 2017;157:64–70. doi: 10.1016/j.envres.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson B.V., Robertson I.G.C., Wilson W.R., Zwi L.J., Boys JT., Green A.W. Evaluation of potential health effects of 10 kHz magnetic fields: a rodent reproductive study. Bioelectromagnetics. 1998;19:162–171. doi: 10.1002/(sici)1521-186x(1998)19:3<162::aid-bem4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura I., Oshima A., Shibuya K., Mitani T., Negishi T. Absence of reproductive and developmental toxicity in rats following exposure to a 20-kHz or 60-kHz magnetic field. Regul. Toxicol. Pharmacol. 2012;64:394–401. doi: 10.1016/j.yrtph.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Stupp R., Taphoorn M., Driven L., Taillibert S. Tumour treating fields (TTfields) – a novel cancer treatment modality: translating preclinical evidence and engineering into a survival benefit with delayed decline in quality of Life. Int. J. Radiat. Oncol. 2017;99:1–9. 2017. [Google Scholar]
- 16.Mun J.E., Babiker H.M., Weinberg U., Kirson E.D., Von Hoff D.D. Tumor-treating fields: a fourth modality in cancer treatment. Clin. Cancer Res. 2018;24(2):266–275. doi: 10.1158/1078-0432.CCR-17-1117. [DOI] [PubMed] [Google Scholar]
- 17.Mohammed S., Sundaram V., Zyuzikov N. Effect of 150 kHz electromagnetic radiation on the development of polycystic ovaries induced by Estradiol Valerate in Sprague Dawley rats. BMC J. Ovarian Res. 2021;14:26. doi: 10.1186/s13048-021-00774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed S., Sundaram V., Adidam Venkata C.R., et al. Polycystic ovary rat model exposure to 150 kHz intermediate frequency: hypothalamic-pituitary-ovarian axis at the receptor, cellular, tissue, and hormone levels. BMC J. Ovarian Res. 2021;14:173. doi: 10.1186/s13048-021-00914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrard M., Sereni N., Schluth-Bolard C., Blondet A., d′Estaing S.G., Plotton I., Morel-Journel N., Lejeune H., David L., Durand P. Complete human and rat ex vivo spermatogenesis from fresh or frozen testicular tissue. Biol. Reprod. 2016;95(4):1–10. doi: 10.1095/biolreprod.116.142802. 89. [DOI] [PubMed] [Google Scholar]
- 20.Johnsen S.G. Testicular biopsy score count-a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 21.Panahi M., Keshavarz S., Rahmanifar F., Tamadon A., Mehrabani, Karimagha N., Sepehrimanesh M., Aqababa H. Busulfan induced azoospermia: stereological evaluation of testes in rat. Vet. Res. Forum. 2015;6(4):273–278. [PMC free article] [PubMed] [Google Scholar]
- 22.La Vignera S., Condorelli R.A., Vicari E., D’Agata R., Calogero A.E. Effects of the exposure to mobile phones on male reproduction: a review of the literature. J. Androl. 2012;33:350–356. doi: 10.2164/jandrol.111.014373. [DOI] [PubMed] [Google Scholar]
- 23.Erpek S., Bilgin M.D., Dikicioglu E., Karul A. The effects of low frequency electric field in rat testis. RMV (Rev. Med. Vet.) 2007;158:206–212. [Google Scholar]
- 24.Yamazaki K., Taki M., Ohkubo C. Safety assessment of human exposure to intermediate frequency electromagnetic fields. Denki Gakkai Ronbunshi. 2015;135A(9):500–506. [Google Scholar]
- 25.Collins P.M., Collins W.P., McNeilly A.S., Tsang W.N. Plasma FSH, LH and Testosterone levels in male rat during degeneration of the germinal epithelium caused by severe heat treatment or ligation of the vasa efferential. J. Reprod. Fertil. 1978;54:285–291. doi: 10.1530/jrf.0.0540285. [DOI] [PubMed] [Google Scholar]
- 26.Okano T., Ishiniwa H., Onuma M., Shindo J., Yokohata Y., Tamaoki M. Effects of environmental radiation on testes and spermatogenesis in wild large Japanese field mice (Apodemus speciosus) from Fukushima. Sci. Rep. 2016;6 doi: 10.1038/srep23601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evenson D.P., Higgins P.J., Grueneberg D., Ballachey B.E. Flow cytometric analysis of mouse spermatogenic function following exposure to ethylnitrosourea. Cytometry. 1985;6:238–253. doi: 10.1002/cyto.990060311. [DOI] [PubMed] [Google Scholar]
- 28.Nikolettos N., Kupker W., Demirel C., Schopper B., Blasig C., Sturm R., Al-Hasani S. Fertilization potential of spermatozoa with abnormal morphology. Hum. Reprod. 1999;14:47–70. doi: 10.1093/humrep/14.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- 29.Iorio R., Delle Monache S., Bennato F., Di Bartolomeo C., Scrimaglio R., Cinque B., Colonna R.C. Involvement of mitochondrial activity in mediating ELF-EMF stimulatory effect on human sperm motility. Bioelectromagnetics. 2011;32:15–27. doi: 10.1002/bem.20602. [DOI] [PubMed] [Google Scholar]
- 30.Turner R.M. Moving to the beat: a review of mammalian sperm motility regulation. Reprod. Fertil. Dev. 2006;18:25–38. doi: 10.1071/rd05120. [DOI] [PubMed] [Google Scholar]
- 31.Wyrobek A.J., Bruce W.R. Chemical induction of sperm abnormalities in mice. Proc. Natl. Acad. Sci. U. S. A. 1975;72:4425–4429. doi: 10.1073/pnas.72.11.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trosic I., Matausic-Pisl M., Pavicic I., Marjanovic A.M. Histological and cytological examination of rat reproductive tissue after short-time intermittent radiofrequency exposure. Arch. Ind. Hyg. Toxicol. 2013;64:513–519. doi: 10.2478/10004-1254-64-2013-2394. [DOI] [PubMed] [Google Scholar]
- 33.Bahaodini A., Owjfard M., Tamadon A., Jafari S.M. Low-frequency electromagnetic fields long-term exposure effects on testicular histology, sperm quality and testosterone levels of male rats. Asian Pac. J. Reprod. 2015;4:195–200. [Google Scholar]
- 34.Plant M.T., Marshall G.R. The functional significance of FSH in Spermatogenesis and the control of its secretion in male primates. Endocr. Rev. 2001;6:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 35.Lee S.J., Ahn S.S., Jung K.C. Effects of 60Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J. Androl. 2004;6:29–34. [PubMed] [Google Scholar]
- 36.McGivern R.F., Sokol R.Z., Adey W.R. Prenatal exposure to a low-frequency electromagnetic field demasculinizes adult scent-marking behavior and increases accessory sex organ weights in rats. Teratology. 1990;41:1–8. doi: 10.1002/tera.1420410102. [DOI] [PubMed] [Google Scholar]
- 37.Grajewski B., Cox C., Schrader S.M., Murray W.E., Edwards R.M., Turner T.W., Smith J.M., Shekar S.S., Evenson D.P., Simon S.D., Conover D.L. Semen quality and hormone levels among radiofrequency heater operators. J. Occup. Environ. Med. 2000;42(10):993–1005. doi: 10.1097/00043764-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 38.O'Shaughnessy P.J., Monteiro A., Verhoeven G., De Gendt K., Abel M.H. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reproduction. 2010;139(1):177–184. doi: 10.1530/REP-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hori T., Yamsaard T., Ueta Y.Y., Harakawa S., Kaneko E., Miyamoto A., Xuan X., Toyoda Y., Suzuki H. Exposure of C57BL/6J male mice to an electric field improves copulation rates with superovulated females. J. Reprod. Dev. 2005;51(3):393–397. doi: 10.1262/jrd.16088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.