Abstract
There are several reports about the effect of gravity removal on some characteristics of microorganisms due to possible change in surface layer thickness and adherence properties. In this study, bioremoval efficiency of Lactobacillus acidophilus ATCC 4356 from water under simulated microgravity conditions was investigated. Furthermore, pretreatment effects (untreated, NaOH, and heat pretreated) of L. acidophilus ATCC 4356 on heavy metal removal was evaluated on microgravity, as our previous research showed impact of pretreatment on adherence properties of probiotics to environmental metals. The results showed that ability of L. acidophilus for arsenic adsorption enhanced following heat-pretreatment in simulated and normal gravity. Moreover, in both conditions of simulated microgravity and normal gravity NaOH-treated L. acidophilus increased the removal of cadmium and lead. In none of the conditions, pretreatment of lactobacillus affects mercury removal. Evaluation of stability of binding of L. acidophilus-heavy metal was investigated to check irreversibility of complex formation between microorganisms and metals in simulated gastrointestinal conditions. Data showed release of heavy metals from complex in normal gravity. Obtained results of this research show the favorable potential of simulated microgravity condition to increase bioremoval capacity of L. acidophilus for heavy metals.
Keywords: Complex stability, Heavy metals, L. acidophilus, Simulated microgravity
Complex stability; Heavy metals; L. acidophilus; Simulated microgravity.
1. Introduction
Gravity is one of the most important physical factors in the environment of inhabitants of the earth, which has a great impact on them. Microbiota, like other organisms, have been affected by this force and have adapted to that. Any alterations in gravity have led to changes in microbiota composition, as well as, growth rate, secondary metabolites production, biofilm formation, pathogenicity, and gene expression of them (Senatore et al., 2018). Recent studies have shown that gravity reduction can affect the cellular functions of microorganisms (Huang et al., 2018). Some of these studies about the bacteria mainly included, L. acidophilus (Shao et al., 2017; Stahl et al., 2016), Escherichia coli (Yim et al., 2020), Bacillus subtilis (Arotupin et al., 2019), Salmonella typhimurium (Wilson et al., 2002), Pseudomonas aeruginosa (Kim et al., 2013), Staphylococcus aureus (Rosado et al., 2010), Streptococcus pneumoniae (Allen et al., 2007), Streptomyces hygroscopicus (Fang et al., 2000), the fungi mainly included Saccharomyces cerevisiae (Van Mulders et al., 2011) and Candida albicans (Crabbé et al., 2013); and the archaea included Haloarchaea (Dornmayr-Pfaffenhuemer et al., 2011). One of the consequences of gravity changes on microorganisms can be related to human health (Benoit et al., 2006). Since biological studies in real space conditions are not cost-effective due to high costs and frequent missions, scientists are trying to simulate the space environment on earth, and without doing space travel to study the effects of space on biological microorganisms (Hajebrahimi, 2017). So that, most experiments with simulated microgravity (SMG) by ground-based simulators are performed. Microgravity simulators are devices that simulate weightlessness by rotating the sample around a horizontal axis. Microgravity refers to the conditions that the gravitational forces are not entirely equal to zero but are very close to zero (approximately 10−3 to 10−6 g) (Huang et al., 2018). Among the types of ground-based microgravity simulators, it could mention to 2-D clinostat, 3-D clinostats or random positioning machines, high-aspect rotating vessels, rotating wall vessels, rotating-wall bioreactor, rotary cell culture system, and diamagnetic levitation apparatus (Huang et al., 2018).
Environmental pollution entering the body through water, air, and food, has devastating effects on human health (Abdel-Megeed, 2021; Breton et al., 2013). Heavy metals such as arsenic, cadmium, lead, and mercury are some of these pollutants, and elimination of them from the body is on the research agenda (Ahmed et al., 2017; Feng et al., 2022). Several studies have proven that probiotic bacteria have the ability to absorb heavy metals and remove pollution caused by them (Massoud and Zoghi, 2022; Mirmahdi et al., 2022). The Lactobacillus bacteria can absorb and remove some extent of these heavy metals (Singh et al., 2022; Majlesi et al., 2017). Gut microbiota and its metabolites in addition to modifying absorption, metabolism of heavy metals, oxidative stress, and modulating the pH act as a physical barrier and regulate detoxification enzymes or protein expression (Breton et al., 2013). The detoxification mechanism of heavy metals by gut microbiota is carried out via the binding of metallic ions to the cell wall of bacteria. Further, probiotic bacteria transformed from a more toxic form to less toxic (Liu et al., 2009). Probiotics are widely used for bioremoval of toxins (Massoud et al., 2018; Zoghi et al., 2014, 2017, 2019) as well as heavy metals including As, Hg, Pd and Cd (Hadiani et al., 2018a, 2018b, 2019), heterocyclic aromatic amines (Khosravi-Darani et al., 2019), and even pesticides (Massoud et al., 2020).
The Lactobacillus species have a high adsorption capacity for heavy metals because of the large amounts of peptidoglycan and teichoic acids in their cell walls. Many studies have shown that Lactobacillus has a high tolerance and tendency to absorb heavy metals in water and food (Xu et al., 2019; Khosravi et al., 2020). Few studies had been reported to show changes in physiology, morphology, and even pathogenicity of some microorganisms stimulating impact of simulated microgravity which could be explained by change in surface layer thickness and agglomeration properties of microorganisms (Salavatifar et al., 2023). Although, there are some documents for increased virulence, antibiotic resistance, and gene expression during microgravity experience of some bacteria, there is no report on possible changes on probiotics capacity in biosorption of heavy metal. Also, based on our previous research, we assumed possible impact of pretreatment on adherence properties of probiotics.
So, in this research for the first time the impact of microgravity on bioremoval efficiency of heavy metals has been investigated. There are not enough reports about the effect of microgravity conditions on the biosorption of heavy metals by Lactobacillus bacteria. In this study, the effect of simulated microgravity conditions as well as pretreatments has been investigated on bioremoval of As, Pb, Hg, and Cd by L. acidophilus. Also, the stability of L. acidophilus-heavy metal complexes under simulated gastrointestinal tract (Yigit and Velioglu, 2020) conditions was evaluated.
2. Materials and method
2.1. Biomass and bacterial culture preparation
L. acidophilus ATCC 4356 was taken from Tak Gene Zist, Tehran, Iran. It was routinely aerobic cultured in MRS (provided from Merck (Darmstadt, Germany)) broth at 37 °C for 24 h seed culture was prepared with 5 ml of master culture to 50 ml MRS broth and incubated at 37 °C for 48 h (Vinderola and Reinheimer, 2000).
2.2. L. acidophilus pretreatments: bacteria cells
L. acidophilus cell autoclaved 20 min at 121 °C for Heat pretreatment. The NaOH pretreatment L. acidophilus cell at the first mixed 0.1 N NaOH at 37 °C for 1 h, then samples were centrifuged to remove supernatants. Triple time we washed bacterial cells with sterile distilled water and centrifuged them. These conditions were ready for to removal of multi-metals solution (Wang et al., 2015).
2.3. L. acidophilus biosorption aqueous solution of heavy metals
Analytical reagent chemicals were purchased from Merck, Darmstadt, Germany except for Standard solutions of As (1000 mg L−1 in 0.1 M HNO3), which were provided from Panreac (Panreac Quimica SA, Spain, Barcelona). At the first 700 μg of each metal; Pb (10 mg L−1 in HCl 10%), Cd (10 mg L−1 in HCl 10%), As (10 mg L−1 in HCl 10%), and Hg (10 mg L−1 in HCl 2%) were mixed with 6.2 mL of sterile deionized water and adjusting pH to 4 by HCl and NaOH 0.1 M, then added 1 ml L. acidophilus to a solution (2.6 × 1012 CFU mL−1). Finally, this mixed solution was incubated for 24 h at 37 °C under SMG (Afraz et al., 2020).
2.4. Preparation of simulated gastric and small intestinal juice
Patulin, pepsin, bile salts, and pancreatin were purchased from Sigma-Aldrich, Darmstadt, Germany. Simulated gastric juice was prepared by pepsin (3g L−1) in a sterile NaCl (0.5 % w/v) and adjusting the pH to 2 using HCl. Simulated small intestinal juice was prepared by pancreatin (1 g L−1) and bile salt (1.5 g L−1) in sterile NaCl (0.5 % w/v) and adjusting the pH to 8 with 0.1 mol L−1 NaOH. Both gastric and small intestinal juices were sterilized using 0.45-μm membranes filter (Nalge Co., Rochester, USA). These were freshly prepared. After 24 h of biosorption under SMG, 10 ml of each metal-bacteria solution was inserted to 40 ml of simulated gastric juice then vortexed (Vortex Genie 2, Scientific Industries, Bohemia, USA) for 10 s, and incubated at 37 °C for 2 h under SMG. After sampling for heavy metals analysis, 10 ml of gastric solution, was added to 50 ml of simulated small intestinal juice and incubated at 37 °C for 2 h under SMG, and repeated sampling for heavy metals analysis (Yin et al., 2018).
2.5. Simulated microgravity condition (SMG)
In the current project, a one-axis clinostat was used (UN00SA, USA) for SMG, which rotates samples perpendicular to the direction of the gravity vector (Figure 1). Clinostat was placed in an incubator at 37 °C. The falcons were filled with samples without any bubbles that disrupt microgravity. Then samples were well-fixed around the center, and rotational speed was adjusted to 15 rpm (Salavatifar, 2019).
Figure 1.
The 2-D clinostats which used to generate SMG conditions on the ground.
2.6. Analytical quality assurance
To ensure validity of obtained results of yeast biosorption, method was the same described before including determining of limit of detection (LOD), limit of quantification (LOQ), linearity range and accuracy (recovery level) (Hadiani et al., 2018a). The analyzer limit of quantitation (LOQ) and the analyzer limit of detection (LOD) for Pb and As were 1 μg L−1 and 3.3 μg L−1 respectively, also LOQ and LOD for Cd and Hg were 5 μg L−1 and 1.7 μg L−1 respectively.
2.7. Analysis of heavy metals by inductively coupled plasma-mass spectroscopy
In this study, the amount of heavy metals concentration was measured by ICP-MS (Perkin Elmer ELAN 6100 DRC-e). Device conditions for testing were included, Nebulizer Gas Flow: 0.69 L/min, ICP Radio Frequency (RF) generator power: 1100 W, Lenz Voltage: 6V, Analog Stage Voltage: -2300V, Pulse Stage Voltage: 1600 V. The ICP-MS experiments were carried out in triplicate for each sample.
2.8. Statistical analysis
In this study all experiments were carried out in triplicate and data were expressed as the mean ± standard deviation (X ± SD). Data processing was carried out using SPSS Software v.22.0 (SPSS, Chicago, USA). Results were used in one-way analysis of variance (ANOVA) to estimate p-values and confidence levels. For all data p-values < 0.05 were considered.
3. Results and discussion
3.1. Effects of pretreatments of L. acidophilus ATCC 4356 on heavy metal removal
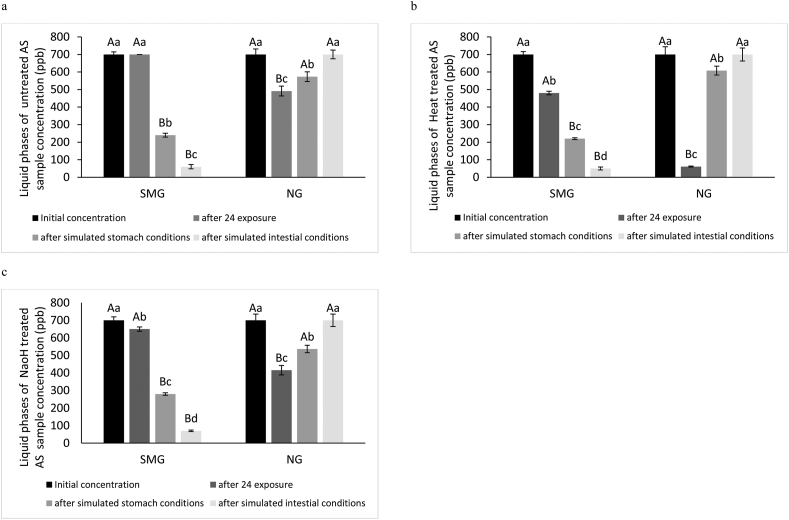
In the following figures (Figures 2, 3, 4, and 5) the biosorption of heavy metal by L. acidophilus in liquid phases after 24 h of exposure to untreated, NaOH and Heat pretreated of SMG and normal gravity (NG) conditions were reported. There were significant differences in biosorption of all conditions (P < 0.05).
Figure 2.
As concentration in Liquid phases of simulated microgravity (SMG) and normal gravity (NG) after 24h exposure and simulated gastrointestinal conditions to treated (a: untreated, b: Heat and c: NaOH) L. acidophilus. Error amount 5% different small letters among the same samples, differ significantly (P < 0.05). Capital letters between SMG and NG samples, differ significantly (P < 0.05).
Figure 3.
Cd concentration in Liquid phases of simulated microgravity (SMG) and normal gravity (NG) after 24h exposure and simulated gastrointestinal conditions to treated (a: untreated, b: Heat and c: NaOH) L. acidophilus. Error amount 5% different small letters among the same samples, differ significantly (P < 0.05). Capital letters between SMG and NG samples, differ significantly (P < 0.05).
Figure 4.
Pb concentration in Liquid phases of simulated microgravity (SMG) and normal gravity (NG) after 24h exposure and simulated gastrointestinal conditions to treated (a: untreated, b: Heat and c: NaOH) L. acidophilus. Error amount 5% different small letters among the same samples, differ significantly (P < 0.05). Capital letters between SMG and NG samples, differ significantly (P < 0.05).
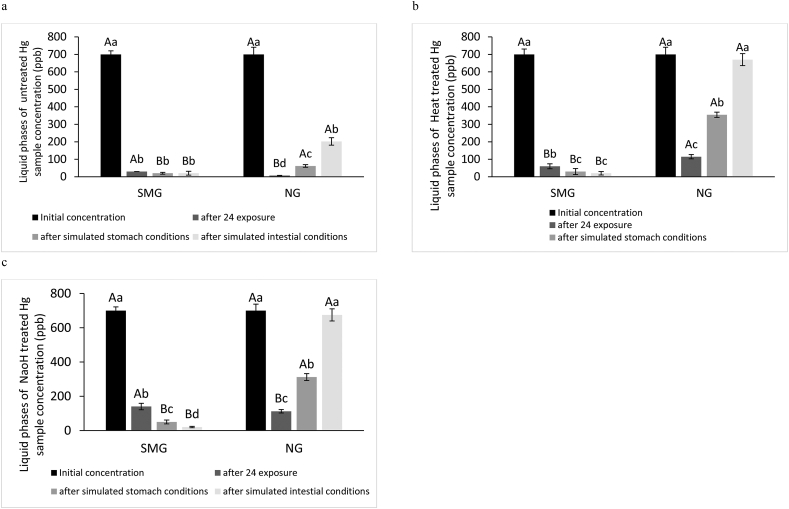
Figure 5.
Hg concentration in Liquid phases of simulated microgravity (SMG) and normal gravity (NG) after 24h exposure and simulated gastrointestinal conditions to treated (a: untreated, b: Heat and c: NaOH) L. acidophilus. Error amount 5% different small letters among the same samples, differ significantly (P < 0.05). Capital letters between SMG and NG samples, differ significantly (P < 0.05).
As shown in Figure 2, in untreated bacteria cells of SMG conditions, no significant differences between initial concentration and after 24hr exposure. Also, it reports that in both conditions (SMG and normal gravity), the lower liquid phases of As concentration was observed in Heat-treated bacteria cells. Heat-treated L. acidophilus for removing As in SMG and normal gravity conditions 31.4% and 91.28%, respectively. The reason for this was exposed more hydrophobic regions to bind to the toxins which are due to denaturation of the cell wall proteins in LAB strains and establishment of Maillard reaction products. Several studies have shown similar results (Pan et al., 2006; Teemu et al., 2008; Tunali et al., 2006). It has been reported that an iincrement in surface activity and kinetic energy of the solute with an increase in temperature causes the removal of heavy metals (Aryal and Liakopoulou-Kyriakides, 2015).
As shown in Figure 3, NaOH-treated L. acidophilus demonstrated the highest Cd concentration decrease in SMG and normal gravity (NG) conditions (Figure 3c). NaOH-treated L. acidophilus demonstrated the highest remove 57.14% and 61.9% Cd in SMG and NG conditions, respectively (Figure 3c). Also, Figure 4 demonstrated that concentrations of Pb after 24 h of exposure to NaOH-pretreated L. acidophilus ATCC were decreased in SMG conditions (Figure 4c). Also, Figure 4 demonstrated that remove of Pb after 24 h of exposure to NaOH-pretreated L. acidophilus ATCC were 42.85% in SMG conditions (Figure 4c). NaOH-treatment of lactic acid bacteria cells by altering the availability of various latent sites, rupturing cell membranes, neutralizing acidic chemical groups, and improving cell surface negative electrons can eliminate components porous surface coatings. Therefore, the electrophilic properties of heavy metals can increase the absorption of heavy metals in the lactic acid bacteria strains treated with NaOH treatment. This result is consistent with the results of Guo et al., (2013) and Wang et al., (2015) that reported pretreatment L. acidophilus had a high ability to increase patulin absorption (Guo et al., 2013; Wang et al., 2015). On the other hand, a report by Göksungur et al., (2005) states that Heat treatment increases the removal of Cd and Pb by bacterial lactic acid, which increases access to metal-binding sites on the bacterial surface.
Based on Figure 5, The most adsorption of Hg for SMG and NG conditions was shown in untreated bacteria cells. Based on Figure 5, showed that best biosorption of Hg untreated bacteria for SMG (95.71%) and NG (99.01%) conditions. As a result, no significant differences between SMG and NG conditions by untreated bacteria cells. Lactic acid bacteria (Toolabi et al., 2018) are generally known as probiotic microorganisms for decreasing aflatoxins (Hotel and Cordoba, 2001; Zoghi et al., 2014). The main structural component of the LAB cell wall is peptidoglycan, teichoic, lipoteichoic acid, proteinaceous S-layer, and some neutral polysaccharides (Zoghi et al., 2014). According to previous studies, negatively charged functional groups (such as O–H, C–H, C=O, and C–O–C) can adsorb cations of heavy metals (Chen et al., 2016; Xing et al., 2017). Moreover, S-layer proteins in the L. acidophilus cell wall cause the filling of the cell surface with anionic compounds to absorb the cationic metal ions (Gerbino et al., 2011). The rapid removal of Hg by aminated L. acidophilus indicates that the binding occurs at the bacterial surface and the peptidoglycan layer of the L. acidophilus cell wall plays main role in adsorption of heavy metal (Tripathi et al., 2012; Zoghi et al., 2014).
3.2. Stability assessment of L. acidophilus–heavy metal complex through simulated gastrointestinal conditions
Changes in heavy metal concentration following exposure to pretreated (heat and NaOH) L. acidophilus ATCC 4356 (2 h in simulated gastric juice, 2 h of exposure to the simulated small intestinal condition) are shown in Figures.
As illustrated in Figure 2, considering that Heat-treated L. acidophilus was mighty to reduce the concentration of As in normal gravity (NG) conditions, but after simulated stomach conditions more bonds were released and it could not maintain any As binding. Also, no significant differences between initial concentration and intestinal conditions in all treated L. acidophilus (untreated, heat, and NaOH treated) normal gravity conditions. These results were similar to the Petruzzi et al. results (Petruzzi et al., 2016). However, in the SMG conditions, the best ability of As bioremediation belongs to heat-treated L. acidophilus were after simulated stomach conditions (68.57%) and after simulated intestinal conditions (92.85%). As you can see in Figure 3, pretreatment of L. acidophilus cells using all treated led to the highest Cd concentration absorption (97.14%) after simulated intestinal conditions in SMG conditions. Also, for normal gravity conditions, biosorption of Cd increased after simulated stomach conditions by untreated L. acidophilus (37.71%). The best removed in normal gravity conditions is related to NaOH-treated L. acidophilus (71.57%) after simulated intestinal conditions.
Regarding absorption of Pb in normal gravity conditions, after exposure to simulated stomach conditions release of Pb increased but after exposure to simulated intestinal conditions biosorption of Pb was increased. In connection with SMG conditions, no significant differences between treated bacteria cells (untreated, heat and NaOH treated) were observed after simulated intestinal conditions. In connection with SMG conditions, NaOH treated L. acidophilus could remove 80% after simulated gastric conditions. Also, heat and NaOH treated L. acidophilus could remove 97.14% after simulated intestinal conditions. Consequently, high concentrations of Pb were removal after exposure to under simulated GIT conditions in SMG conditions (Figure 4).
According to Figure 5, in all NG conditions, some Hg ions were released after exposure to simulated stomach conditions and this action continued after simulated intestinal conditions. Thus, stable L. acidophilus-Hg complexes did not occur in simulated GIT conditions. Even, there was no significant difference between the initial concentration and after exposure to simulated intestinal conditions (except untreated bacteria). In SMG conditions, the trend of increasing adsorption simulated GIT conditions of all treatments was ascendant, so that in gastrointestinal conditions, Hg concentration reached its lowest value, and the highest stability related to L. acidophilus-Hg complexes in intestinal conditions was observed for all treatments. The NaOH-treated L. acidophilus-Cd and L. acidophilus-Pb complexes showed the best stability in simulated GIT conditions compared to other complexes for both conditions in Figures 2 and 3. Generally, after exposure to simulated intestinal conditions biosorption of all metals in this study for SMG conditions was enhanced by treated and untreated cells.
According to previous studies, factors affecting the metal removal of microorganisms include microbial strains, environmental conditions, pH, temperature, cell wall structure and surface charge (Afraz et al., 2020; Banwo et al., 2021; Zoghi et al., 2014).
Based on the results, the adsorption of heavy metals using L. acidophilus ATCC 4356 was somewhat reversible under simulated GIT conditions in normal gravity conditions. It might be due to the simultaneous chemical and physical adsorption in the heavy metal adsorption process. Zoghi et al. stated reversibility of binding indicating the significance of non-covalent electrostatic bonds (hydrogen and Van der Waals bonds) (Zoghi et al., 2021). In another study, potential probiotic capacity against Cd toxicity was screened with 33 strains of lactic acid bacteria (Toolabi et al., 2018). The results noted that Lactobacillus plantarum CCFM8610 showed significantly better binding ability and Cd tolerance than the other tested strains (Zhai et al., 2015). Henceforth, Elsanhoty et al. reported that the complex of M1-LAB (L. rhamnosus TISTR 541 and L. Plantarum EMCC) was stable under simulated GIT conditions (Elsanhoty et al., 2014).
The results of SMG conditions showed significant performance under GIT conditions. So that in the simulated intestinal conditions had the highest absorption of heavy metals compared to the simulated stomach conditions, which could be due to the effect of pH on the removal of heavy metals. According to studies, the lowest Heavy metal removal occurs at pHs below 2–3 and the highest Heavy metal removal occurs at pHs above 3.
Also, maximum absorption is often achieved at pH 4–6 (Aryal and Liakopoulou-Kyriakides, 2015). In addition, it has been inferred that one of the obstacles to metal ion adsorption is the high aggregation of H+ ions. However, high pH leads to an increase in the number of negatively charged ligands that adsorb metal cations (Afraz et al., 2020).
Changes in gravity can lead to changes in cell types. Thus, microgravity is a specially environmental condition for cell types that some of the significant effects of this environment include reduction or elimination of shear stress and low-turbulence environment. Such conditions lead to changes in microorganisms, such as changes in growth rate, changes in the production of secondary metabolism, changes in pathogenicity, changes in resistance to environmental stresses, including antibiotics, changes in genetic, and changes in morphology and physiology are some of these (Nickerson et al., 2004; Senatore et al., 2018). In the human body, environments with low shear stress and low turbulence due to weightlessness were found (Senatore et al., 2018).
Differences in movement properties of the strains are one of the characteristics of microbial growth that was SMG, were investigated. In a study by Baker, two bacteria were isolated, Sphingobacterium thalpophilium that was motile and flagellated, and Ralstonia pickettii that was non-motile and not flagellated, were inoculated into the water, high and low concentrations of nutrient broth and subjected to SMG. The results showed no significant differences between SMG and the normal gravity control regardless of the method of enumeration and medium for Sphingobacterium thalpophilium. While Ralstonia pickettii had significantly higher numbers in high nutrient broth under SMG compared to normal gravity. According to the experiments performed, microgravity depended on the strain used (differences in motility), enumeration method, and nutrient concentration (Baker and Leff, 2004).
4. Conclusion and future perspective
Water and food heavy metal contamination is the major problems in maintaining public health. One of the safe strategy to minimize and even completely eliminate these types of contamination is biosorbtion of them by bacteria. Exopolymeric substances in bacterial outer membrane are able to effectively bind to positively charged heavy metals (Gupta and Diwan, 2017). Any agent that increases the thickness of the bacterial exopolymeric substances will improve the removal capacity of heavy metals. Due to the increasement of exopolymeric substances of some bacteria under microgravity condition compared to normal gravity (Mauclaire and Egli, 2010), in the current study, potency absorption of heavy metals metals (As, Cd, Pb, and Hg) from water by L. acidophilus ATCC 4356 was investigated. The results showed that biosorption of As increased by Heat-pretreated L. acidophilus for both SMG (31.42%) and NG (91.28%) conditions. Also, we observed that Cd (57.14% and 61.9%) and Pb (42.85% and 83%) absorption were increased by NaOH-pretreated L. acidophilus respectively in SMG and NG conditions, but according to the results, biosorption of Hg by untreated bacterial cells was more effective for both conditions SMG (95.71%) and NG (99.01%). Regarding absorption of Hg and As concentration in normal gravity conditions, the highest release was observed after exposure to simulated intestinal conditions in all treated. While the biosorption of Cd and Pb were increased after exposure to simulated intestinal conditions compared to exposure to simulated stomach conditions. For SMG conditions, the highest stability related to L. acidophilus-Heavy metals complexes in GIT conditions were observed for all treatments. Taken together, these experiments indicate that in SMG conditions desired Heavy metals decrease levels by pretreated (NaOH and Heat pretreated) L. acidophilus ATCC 4356 occurred Due to the incensement of heavy metals biosorption by this bacteria in under simulated microgravity conditions, the results of this project can be used in the food industry to elimination of these contaminations. According to that the adsorption of the heavy metals by L. acidophilus ATCC 4356 is controlled by many functional factors, our study is not perfect enough, needs more assessment in this field. So for preciseness, future studies should take another important aspect into attention: bacterial strains, assess the possible binding sites and functional groups with FTIR, different concentrations of bacteria cells, type of clinostat (3-D clinostats, high-aspect rotating vessels, rotating wall vessels, rotating-wall bioreactor, rotary cell culture system, diamagnetic levitation apparatus), and rotation speeds. Obtained results of this research show the favorable potential of simulated microgravity condition to increase bioremoval capacity of L. acidophilus for heavy metals. Future research about investigation of in vivo bioremoval of heavy metals by probiotics could be recommended and applicable for astronauts and life on earth.
Declarations
Author contribution statement
Zahra Afsharian: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Maryam Salavatifar: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kianoush KHosravi-Darani: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Prof. Kianoush KHosravi-Darani was supported by National Nutrition and Food Technology Research Institute [28292].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran, and Aerospace Research Institute, Ministry of Science, Research and Technology, Tehran, Iran.
References
- Abdel-Megeed R.M. Probiotics: a promising generation of heavy metal detoxification. Biol. Trace Elem. Res. 2021;199:2406–2413. doi: 10.1007/s12011-020-02350-1. [DOI] [PubMed] [Google Scholar]
- Afraz V., Younesi H., Bolandi M., Hadiani M.R. Optimization of lead and cadmium biosorption by Lactobacillus acidophilus using response surface methodology. Biocatal. Agric. Biotechnol. 2020;29 [Google Scholar]
- Ahmed S., Islam M.R., Ferdousi J., Iqbal T.S. Probiotic Lactobacillus sp. with bioremediation potential of toxic heavy metals. Bangladesh J. Microbiol. 2017;34:43–46. [Google Scholar]
- Allen C., Galindo C., Pandya U., Watson D., Chopra A., Niesel D. Transcription profiles of Streptococcus pneumoniae grown under different conditions of normal gravitation. Acta Astronaut. 2007;60:433–444. [Google Scholar]
- Arotupin D.J., Adegoke T.V., Awojobi K.O., Aderanti T.S. 2019. Effect of Microgravity on Most Frequently Isolated Microorganisms from Cosmetics. [Google Scholar]
- Aryal M., Liakopoulou-Kyriakides M. Bioremoval of heavy metals by bacterial biomass. Environ. Monit. Assess. 2015;187:1–26. doi: 10.1007/s10661-014-4173-z. [DOI] [PubMed] [Google Scholar]
- Baker P.W., Leff L. The effect of simulated microgravity on bacteria from the Mir space station. Microgravity Sci. Technol. 2004;15:35–41. doi: 10.1007/BF02870950. [DOI] [PubMed] [Google Scholar]
- Banwo K., Alonge Z., Sanni A.I. Binding capacities and antioxidant activities of Lactobacillus plantarum and Pichia kudriavzevii against cadmium and lead toxicities. Biol. Trace Elem. Res. 2021;199:779–791. doi: 10.1007/s12011-020-02164-1. [DOI] [PubMed] [Google Scholar]
- Benoit M., Li W., Stodieck L., Lam K., Winther C., Roane T., Klaus D. Microbial antibiotic production aboard the international space station. Appl. Microbiol. Biotechnol. 2006;70:403–411. doi: 10.1007/s00253-005-0098-3. [DOI] [PubMed] [Google Scholar]
- Breton J., Daniel C., Dewulf J., Pothion S., Froux N., Sauty M., Thomas P., Pot B., Foligné B. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol. Lett. 2013;222:132–138. doi: 10.1016/j.toxlet.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Chen Z., Pan X., Chen H., Guan X., Lin Z. Biomineralization of Pb (II) into Pb-hydroxyapatite induced by Bacillus cereus 12-2 isolated from Lead–Zinc mine tailings. J. Hazard Mater. 2016;301:531–537. doi: 10.1016/j.jhazmat.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Crabbé A., Nielsen-Preiss S.M., Woolley C.M., Barrila J., Buchanan K., McCracken J., Inglis D.O., Searles S.C., Nelman-Gonzalez M.A., Ott C.M. Spaceflight enhances cell aggregation and random budding in Candida albicans. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornmayr-Pfaffenhuemer M., Legat A., Schwimbersky K., Fendrihan S., Stan-Lotter H. Responses of haloarchaea to simulated microgravity. Astrobiology. 2011;11:199–205. doi: 10.1089/ast.2010.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsanhoty R.M., Salam S.A., Ramadan M.F., Badr F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria. Food Control. 2014;43:129–134. [Google Scholar]
- Fang A., Pierson D., Mishra S., Demain A. Growth of Streptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits rapamycin production. Appl. Microbiol. Biotechnol. 2000;54:33–36. doi: 10.1007/s002539900303. [DOI] [PubMed] [Google Scholar]
- Feng P., Yang J., Zhao S., Ling Z., Han R., Wu Y., Salama E.-S., Kakade A., Khan A., Jin W. Human supplementation with Pediococcus acidilactici GR-1 decreases heavy metals levels through modifying the gut microbiota and metabolome. npj Biofilms Microbiomes. 2022;8:1–18. doi: 10.1038/s41522-022-00326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbino E., Mobili P., Tymczyszyn E., Fausto R., Gómez-Zavaglia A. FTIR spectroscopy structural analysis of the interaction between Lactobacillus kefir S-layers and metal ions. J. Mol. Struct. 2011;987:186–192. [Google Scholar]
- Göksungur Y., Üren S., Güvenç U. Biosorption of cadmium and lead ions by ethanol treated waste baker's yeast biomass. Bioresour. Technol. 2005;96:103–109. doi: 10.1016/j.biortech.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Guo C., Yue T., Yuan Y., Wang Z., Guo Y., Wang L., Li Z. Biosorption of patulin from apple juice by caustic treated waste cider yeast biomass. Food Control. 2013;32:99–104. [Google Scholar]
- Gupta P., Diwan B. Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017;13:58–71. doi: 10.1016/j.btre.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadiani M.R., Darani K.K., Rahimifard N., Younesi H. Biosorption of low concentration levels of Lead (II) and Cadmium (II) from aqueous solution by Saccharomyces cerevisiae: response surface methodology. Biocatal. Agric. Biotechnol. 2018;15:25–34. [Google Scholar]
- Hadiani M.R., Khosravi-Darani K., Rahimifard N., Younesi H. Assessment of mercury biosorption by Saccharomyces cerevisiae: response surface methodology for optimization of low Hg (II) concentrations. J. Environ. Chem. Eng. 2018;6:4980–4987. [Google Scholar]
- Hadiani M.R., Khosravi-Darani K., Rahimifard N. Optimization of as (III) and as (V) removal by Saccharomyces cerevisiae biomass for biosorption of critical levels in the food and water resources. J. Environ. Chem. Eng. 2019;7 [Google Scholar]
- Hajebrahimi Z. 3-D clinostat for microgravity simulation in cellular and molecular studies. J. Technol. Aerosp. Eng. 2017;1:27–33. [Google Scholar]
- Hotel A.C.P., Cordoba A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention. 2001;5:1–10. [Google Scholar]
- Huang B., Li D.-G., Huang Y., Liu C.-T. Effects of spaceflight and simulated microgravity on microbial growth and secondary metabolism. Military Med. Res. 2018;5:1–14. doi: 10.1186/s40779-018-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Darani K., Barzegar F., Baghdadi M. Detoxification of heterocyclic aromatic amines by probiotic to inhibit medical hazards. Mini Rev. Med. Chem. 2019;19:1196–1203. doi: 10.2174/1389557519666190318102201. [DOI] [PubMed] [Google Scholar]
- Khosravi-Darani K., Sharifan A., Asadi G., Zoghi A. Lead and cadmium biosorption from milk by Lactobacillus acidophilus ATCC 4356. Food Sci. Nutr. 2020;8:5284–5291. doi: 10.1002/fsn3.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Tengra F.K., Shong J., Marchand N., Chan H.K., Young Z., Pangule R.C., Parra M., Dordick J.S., Plawsky J.L. Effect of spaceflight on Pseudomonas aeruginosa final cell density is modulated by nutrient and oxygen availability. BMC Microbiol. 2013;13:1–10. doi: 10.1186/1471-2180-13-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Qu W., Kadiiska M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majlesi M., Shekarforoush S.S., Ghaisari H.R., Nazifi S., Sajedianfard J., Eskandari M.H. Effect of probiotic Bacillus coagulans and Lactobacillus plantarum on alleviation of mercury toxicity in rat. Probiot. Antimicrob. Proteins. 2017;9:300–309. doi: 10.1007/s12602-016-9250-x. [DOI] [PubMed] [Google Scholar]
- Massoud R., Zoghi A. Potential probiotic strains with heavy metals and mycotoxins bioremoval capacity for application in foodstuffs. J. Appl. Microbiol. 2022;133(3):1288–1307. doi: 10.1111/jam.15685. [DOI] [PubMed] [Google Scholar]
- Massoud R., Cruz A., Darani K.K. Ochratoxin A: from safety aspects to prevention and remediation strategies. Curr. Nutr. Food Sci. 2018;14:11–16. [Google Scholar]
- Mauclaire L., Egli M. Effect of simulated microgravity on growth and production of exopolymeric substances of Micrococcus luteus space and earth isolates. FEMS Immunol. Med. Microbiol. 2010;59(3):350–356. doi: 10.1111/j.1574-695X.2010.00683.x. [DOI] [PubMed] [Google Scholar]
- Mirmahdi R.S., Mofid V., Zoghi A., Khosravi_Darani K., Mortazavian A.M. Risk of Low Stability Saccharomyces cerevisiae ATCC 9763-heavy Metals Complex in Gastrointestinal Simulated Conditions. Heliyon. 2022 doi: 10.1016/j.heliyon.2022.e09452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson C.A., Ott C.M., Wilson J.W., Ramamurthy R., Pierson D.L. Microbial responses to microgravity and other low-shear environments. Microbiol. Mol. Biol. Rev. 2004;68:345–361. doi: 10.1128/MMBR.68.2.345-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Ge X., Liu R., Tang H. Characteristic features of Bacillus cereus cell surfaces with biosorption of Pb (II) ions by AFM and FT-IR. Colloids Surf. B Biointerfaces. 2006;52:89–95. doi: 10.1016/j.colsurfb.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Petruzzi L., Corbo M.R., Sinigaglia M., Bevilacqua A. Ochratoxin A removal by yeasts after exposure to simulated human gastrointestinal conditions. J. Food Sci. 2016;81:M2756–M2760. doi: 10.1111/1750-3841.13518. [DOI] [PubMed] [Google Scholar]
- Rosado H., Doyle M., Hinds J., Taylor P.W. Low-shear modelled microgravity alters expression of virulence determinants of Staphylococcus aureus. Acta Astronaut. 2010;66:408–413. [Google Scholar]
- Salavatifar M. Evaluation of RKIP gene expression changes of MDA-MB-231 breast cancer cell line after microgravity treatment. J. Space Sci. Technol. 2019;12:69–76. [Google Scholar]
- Salavatifar M., Ahmadi S.M., Todorov S.D., Khosravi-Darani K., Tripathy A. Impact of microgravity on virulence, antibiotic resistance, and gene expression in beneficial and pathogenic microorganisms. Mini Rev. Med. Chem. 2023 doi: 10.2174/1389557523666230109160620. accepted, in press. [DOI] [PubMed] [Google Scholar]
- Senatore G., Mastroleo F., Leys N., Mauriello G. Effect of microgravity & space radiation on microbes. Future Microbiol. 2018;13:831–847. doi: 10.2217/fmb-2017-0251. [DOI] [PubMed] [Google Scholar]
- Shao D., Yao L., Zhu J., Shi J., Jin M., Huang Q., Yang H. Simulated microgravity affects some biological characteristics of Lactobacillus acidophilus. Appl. Microbiol. Biotechnol. 2017;101:3439–3449. doi: 10.1007/s00253-016-8059-6. [DOI] [PubMed] [Google Scholar]
- Singh H., Verma S., Jaswal A., Rani S., Ram C. In-vitro evaluation of indigenous probiotic lactobacilli for lead bio-adsorption potential, its tolerance and complex stability. J. Funct.Foods. 2022;95 [Google Scholar]
- Stahl, S., Voorhies, A., Lorenzi, H., Castro-Wallace, S., Douglas, G., 2016. Effect of low shear modeled microgravity (LSMMG) on the probiotic Lactobacillus acidophilus ATCC 4356. In: Meeting of"Human Research Program Investigators’ Workshop (No. JSC-CN-34809)". Galveston, TX, December 2015.
- Teemu H., Seppo S., Jussi M., Raija T., Kalle L. Reversible surface binding of cadmium and lead by lactic acid and bifidobacteria. Int. J. Food Microbiol. 2008;125:170–175. doi: 10.1016/j.ijfoodmicro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Toolabi A., Malakootian M., Ghaneian M.T., Esrafili A., Ehrampoush M.H., AskarShahi M., Tabatabaei M. Modeling photocatalytic degradation of diazinon from aqueous solutions and effluent toxicity risk assessment using Escherichia coli LMG 15862. Amb. Express. 2018;8:1–13. doi: 10.1186/s13568-018-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P., Beaussart A., Andre G., Rolain T., Lebeer S., Vanderleyden J., Hols P., Dufrêne Y.F. Towards a nanoscale view of lactic acid bacteria. Micron. 2012;43:1323–1330. doi: 10.1016/j.micron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Tunali S., Akar T., Özcan A.S., Kiran I., Özcan A. Equilibrium and kinetics of biosorption of lead (II) from aqueous solutions by Cephalosporium aphidicola. Separ. Purif. Technol. 2006;47:105–112. [Google Scholar]
- Van Mulders S.E., Stassen C., Daenen L., Devreese B., Siewers V., van Eijsden R.G., Nielsen J., Delvaux F.R., Willaert R. The influence of microgravity on invasive growth in Saccharomyces cerevisiae. Astrobiology. 2011;11:45–55. doi: 10.1089/ast.2010.0518. [DOI] [PubMed] [Google Scholar]
- Vinderola C.G., Reinheimer J.A. Enumeration of Lactobacillus casei in the presence of L. acidophilus, bifidobacteria and lactic starter bacteria in fermented dairy products. Int. Dairy J. 2000;10:271–275. [Google Scholar]
- Wang L., Wang Z., Yuan Y., Cai R., Niu C., Yue T. Identification of key factors involved in the biosorption of patulin by inactivated lactic acid bacteria (LAB) cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.W., Ott C.M., Ramamurthy R., Porwollik S., McClelland M., Pierson D.L., Nickerson C.A. Low-shear modeled microgravity alters the Salmonella enterica serovar Typhimurium stress response in an RpoS-independent manner. Appl. Environ. Microbiol. 2002;68:5408–5416. doi: 10.1128/AEM.68.11.5408-5416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S., Wang J., Liang J.B., Jahromi M.F., Zhu C., Shokryazdan P., Laudadio V., Tufarelli V., Liao X. Lead biosorption of probiotic bacteria: effects of the intestinal content from laying hens. Environ. Sci. Pollut. Control Ser. 2017;24:13528–13535. doi: 10.1007/s11356-017-8896-6. [DOI] [PubMed] [Google Scholar]
- Xu S., Xu R., Nan Z., Chen P. Bioadsorption of arsenic from aqueous solution by the extremophilic bacterium Acidithiobacillus ferrooxidans DLC-5. Biocatal. Biotransform. 2019;37:35–43. [Google Scholar]
- Yigit N., Velioglu Y.S. Effects of processing and storage on pesticide residues in foods. Crit. Rev. Food Sci. Nutr. 2020;60:3622–3641. doi: 10.1080/10408398.2019.1702501. [DOI] [PubMed] [Google Scholar]
- Yim J., Cho S.W., Kim B., Park S., Han Y.H., Seo S.W. Transcriptional profiling of the probiotic Escherichia coli Nissle 1917 strain under simulated microgravity. Int. J. Mol. Sci. 2020;21:2666. doi: 10.3390/ijms21082666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Wu B., Liu S., Hu S., Gong T., Cherr G.N., Zhang X.-X., Ren H., Xian Q. Rapid and complete dehalogenation of halonitromethanes in simulated gastrointestinal tract and its influence on toxicity. Chemosphere. 2018;211:1147–1155. doi: 10.1016/j.chemosphere.2018.08.039. [DOI] [PubMed] [Google Scholar]
- Zhai Q., Yin R., Yu L., Wang G., Tian F., Yu R., Zhao J., Liu X., Chen Y.Q., Zhang H. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control. 2015;54:23–30. [Google Scholar]
- Zoghi A., Khosravi-Darani K., Sohrabvandi S. Surface binding of toxins and heavy metals by probiotics. Mini Rev. Med. Chem. 2014;14:84–98. doi: 10.2174/1389557513666131211105554. [DOI] [PubMed] [Google Scholar]
- Zoghi A., Khosravi-Darani K., Sohrabvandi S., Attar H., Alavi S.A. Effect of probiotics on patulin removal from synbiotic apple juice. J. Sci. Food Agric. 2017;97:2601–2609. doi: 10.1002/jsfa.8082. [DOI] [PubMed] [Google Scholar]
- Zoghi A., Khosravi-Darani K., Sohrabvandi S., Attar H. Patulin removal from synbiotic apple juice using Lactobacillus plantarum ATCC 8014. J. Appl. Microbiol. 2019;126:1149–1160. doi: 10.1111/jam.14172. [DOI] [PubMed] [Google Scholar]
- Zoghi A., Khosravi-Darani K., Hekmatdoost A. Effects of pretreatments on patulin removal from apple juices using lactobacilli: binding stability in simulated gastrointestinal condition and modeling. Probiot. Antimicrob. Proteins. 2021;13:135–145. doi: 10.1007/s12602-020-09666-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.