Abstract
Bartonella henselae is known to cause central nervous system (CNS) disease in humans, and neurological signs have been observed in experimentally infected cats. However, the pathogenesis of CNS disease remains unclear. This study was undertaken to determine whether B. henselae infects feline fetal brain cells in vitro. Microglial-cell- and astrocyte-enriched cultures were inoculated with B. henselae. Giménez staining identified bacterial organisms within microglial cells by day 7 postinoculation. The viability of the intracellular bacteria was demonstrated by incubating cultures with gentamicin and plating cell lysate on agar. Electron microscopy identified intracellular organisms with characteristic Bartonella morphology but identified no ultrastructural abnormalities within infected microglial cells. No evidence of infection was seen in Bartonella-inoculated astrocyte cultures. These findings suggest a role for microglia in the pathogenesis of B. henselae-associated neurological disease.
Bartonella henselae, a bacterial pathogen first identified in 1990, is the cause of an expanding spectrum of clinical syndromes in human patients, including cat scratch disease, bacillary angiomatosis, bacillary peliosis hepatis, endocarditis, and relapsing bacteremia. Central nervous system (CNS) involvement can occur in conjunction with many of these disorders. Neurologic sequelae of cat scratch disease include encephalopathy, myelopathy, meningitis, cerebral arteritis, optic neuritis, and radiculopathy (2, 4, 21, 27, 32). Cerebral bacillary angiomatosis has been reported in human immunodeficiency virus (HIV)-infected patients (34), and aseptic meningitis has been described in association with relapsing B. henselae bacteremia (22). In addition, two recent studies suggest a role for B. henselae in the development of AIDS-related neurological disease: B. henselae-specific antibodies have been identified in the cerebrospinal fluid (CSF) of HIV patients presenting with neurological disease (30), and HIV-associated dementia has been associated with serum antibodies to B. henselae (31). Despite the growing number of reports implicating B. henselae in the development of neurological disease, the pathogenesis of CNS dysfunction remains unclear.
Epidemiological studies have established that cat exposure is a risk factor for B. henselae-associated disease in humans (35, 39) and that Bartonella infection in cats is widespread. Seroprevalence of B. henselae antibodies in cats has been reported to be as high as 54% (13). In regions of the world where fleas are endemic, the prevalence of bacteremia in cats is high and cats can remain infected for months to years (19). However, the clinical significance of B. henselae infection in cats remains controversial. Several studies have not identified disease manifestations in experimentally infected cats (1, 28). Others have documented clinical abnormalities, including fever, anorexia, lethargy, lymphadenopathy, muscle pain, and neurologic dysfunction, in cats following experimental infection (12, 18, 26). It has been suggested that these disparate results are due to differences in virulence among strains of B. henselae (26). The pathogenesis of B. henselae infection in cats and the determinants of organism virulence for this species require further elucidation.
In the present study, we utilized in vitro culture systems to examine B. henselae's ability to invade and persist in feline fetal brain cells. We investigated the relative susceptibilities of astrocytes and microglial cells to infection with B. henselae and assessed the ultrastructural effects of infection.
(This study was presented in part at the 18th Annual Forum of the American College of Veterinary Internal Medicine, 25 to 28 May 2000, Seattle, Wash.)
Fetuses with a gestational age of 40 to 60 days were obtained from random-source pregnant cats following routine ovariohysterectomy. Brains were aseptically removed in toto and placed in a petri dish containing Hanks' balanced salt solution (Mediatech, Herndon, Va.). The meninges were carefully removed, and the brains were washed three times in Hanks' balanced salt solution. Brain tissue was minced with scissors in Dulbecco's modified enriched medium (Mediatech) supplemented with 10% fetal bovine serum (Mediatech) and mechanically dissociated by passage through a 40-mesh tissue sieve (Bellco Glass, Vineland, N.J.). The dissociated tissue was centrifuged at 3,000 × g for 5 min and the supernatant was discarded. The pellet was resuspended in medium and seeded into 75-cm2 polystyrene tissue culture flasks (Fisher Scientific, Pittsburgh, Pa.). Cells were incubated at 37°C in 5% CO2. The cultures were left undisturbed for 5 days, at which time the medium was changed to remove cell debris. The medium was changed every 3 to 5 days thereafter.
The mixed cell culture reached confluence after a minimum of 14 to 21 days, with the astrocytes forming a monolayer that tightly adhered to the flask. The microglia were located more superficially and remained loosely adhered to the underlying astrocyte monolayer. Microglia were removed by mechanical shaking. Flasks were placed on an orbital shaker at 150 rpm for 1 h, after which the culture supernatant containing microglial cells was aspirated and centrifuged at 300 × g for 5 min. The pellet was resuspended in medium and seeded onto single-chamber slides (Nunc, Naperville, Ill.) and into 25-cm2 flasks (Fisher Scientific) to yield cultures enriched (>95%) in microglia. Cells were confirmed to be microglia based on characteristic morphology, uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine perchlorate-labeled acetylated low-density lipoprotein (Biomedical Technologies, Stoughton, Mass.), and positive staining with nonspecific esterase (Sigma Diagnostic, St. Louis, Mo.) (8).
The astrocyte monolayer remaining in the flasks was briefly incubated in 0.25% trypsin (Mediatech) and reseeded onto single-chamber slides and into 25-cm2 flasks to yield cultures enriched (90 to 95%) in astrocytes. Cells were confirmed to be astrocytes by characteristic morphology and positive immunohistochemical staining with glial fibrillary acidic protein (Dako, Santa Barbara, Calif.) utilizing an ABC immunoperoxidase technique (Vector Laboratories, Burlingame, Calif.).
Microglia- and astrocyte-enriched cell cultures were infected 5 days after being established with isolate NCSU 93-F029 of B. henselae. The organism had originally been isolated from the blood of a cat that displayed signs of transient neurological dysfunction subsequent to its owner developing cat scratch disease. The B. henselae isolate was passaged three times in Vero cell culture. Quantification of CFU on trypticase soy agar with 5% rabbit blood (BBL, Becton Dickinson and Co., Cockeysville, Md.) yielded a count of 1.5 × 1010 CFU/ml. One-milliliter aliquots of the harvest were stored frozen at −70°C. Each aliquot was diluted in 10 ml of medium, and 1 ml of the suspension (1.5 × 109 CFU) was added to coat the bottom of each vessel. The cultures were rocked at room temperature for 4 h, after which the inoculum was removed and replaced with fresh medium. Parallel-inoculated Vero cell cultures served as the positive control and uninfected brain cell cultures derived from the same fetal tissue served as the negative control for each experiment. Following inoculation, all cell cultures were incubated at 37°C with 5% CO2.
Giménez stains.
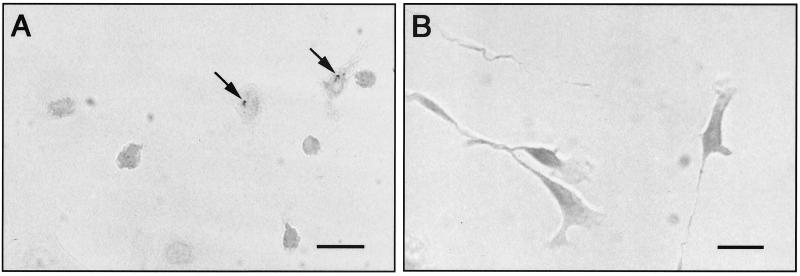
Chamber slides were harvested at 1, 4, 7, 11, and 14 days after inoculation and were stained by the Giménez method (11). Slides were rinsed in phosphate-buffered saline (PBS), fixed in methanol for 15 min, and air dried. Fixed slides were covered with a solution of 1% basic fuchsin and phenol (Sigma-Aldrich Chemical, St. Louis, Mo.) for 90 s and then rinsed in tap water for 30 s. The slides were counterstained with 1% malachite green oxalate (Sigma-Aldrich Chemical) for 9 s and washed in tap water for 10 s, and then the counterstaining sequence was repeated. The air-dried slides were observed with light microscopy. No organisms were observed in microglial cells at 1 or 4 days postinoculation. However, at 7 days postinoculation, organisms were observed within the cytoplasm of the microglial cell. The bacterial staining imparted a pink color to the cell cytoplasm, and aggregates of organisms could be visualized in a perinuclear location (Fig. 1A). Positive staining persisted in microglial cells at 11 and 14 days postinoculation. There was no evidence of cellular pathology; i.e., there were no changes in cellular morphology or evidence of cell loss in infected cultures. Bacterial organisms were never identified within astrocytes at any time throughout the course of the experiment (Fig. 1B).
FIG. 1.
(A) Microglial-cell-enriched cultures, 14 days after inoculation with B. henselae, display evidence of infection based on positive Giménez staining for bacteria. Bacterial aggregates are identified in the perinuclear region of the cells (arrows). (B) Astrocyte-enriched cultures, 14 days after inoculation with B. henselae, reveal no evidence of cellular infection when stained by the Giménez method. Bars, 25 μm.
Isolation of organisms from cell culture.
At 14 and 28 days after inoculation, gentamicin sulfate (Mediatech) was added to the flasks at a concentration of 250 μg/ml and the cultures were allowed to incubate for 3 h at 37°C. This was done to selectively remove any extracellular bacteria from the culture system. Gentamicin is not taken up by mammalian cells, and therefore bacteria that have entered cells are presumably protected from its antimicrobial effects. Cultures were then washed with sterile PBS, and cells were scraped from the flasks and spun gently to form a pellet. The pellet was washed twice and resuspended in 1 ml of sterile PBS. The cell suspension was lysed by freezing at −70°C for 3 min followed by a thaw at room temperature. The freeze-thaw sequence was repeated and the lysate was streaked onto trypticase soy plates with 5% rabbit blood (BBL, Becton Dickinson and Co.). The agar plates were incubated at 37°C with 5% CO2. Bacterial colonies were isolated 7 days after plating lysate from microglial cells harvested at 14 and 28 days postinoculation. Bacterial culture of the cell lysate derived from Bartonella-inoculated astrocyte cultures yielded no growth after 14 days of incubation.
DNA was extracted from multiple bacterial colonies isolated on the blood agar plates and from 200 μl of the inoculum utilized to infect the microglial cells using a commercially available kit (QIA DNA minikit; Qiagen, Valencia, Calif.) according to the protocol for extraction from tissue culture cells. PCR amplification was performed utilizing Bartonella-specific primers Bart ITS1 and Bart ITS2 (Heska, Fort Collins, Colo.) (14). As a positive control, Bartonella elizabethae DNA was simultaneously amplified. Water was extracted and amplified along with the above samples as a negative control. A blank control lacking template DNA was included in each experiment to test for DNA contamination. Reactions were set up using Taq polymerase (Boehringer-Mannheim, Indianapolis, Ind.) and the following cycling conditions: denaturation at 94°C for 1 min, annealing at 60°C for 45 s, and chain extension at 72°C for 1 min. This was repeated for 60 cycles and was followed by a final 10-min chain extension at 72°C. All PCR products were electrophoresed through a 2% agarose gel in Tris-boric acid-EDTA buffer, and the DNA fragments were visualized by ethidium bromide staining under UV fluorescence. Bacterial DNA isolated from the inoculated microglial cells yielded the predicted-size product of 147 bp. PCR of the B. henselae inoculum produced an identical product.
One amplicon derived from the bacterial colonies isolated on blood agar and one amplicon from the inoculum were prepared for sequencing using a commercially available kit (QIAquick PCR purification kit; Qiagen). The purified samples were sequenced using an automated DNA sequencer (Prism 377 apparatus; Perkin-Elmer, Norwalk, Conn.) at an on-site facility. The nucleotide sequences obtained were compared with those in the GenBank nucleotide sequence database using the BLAST comparison program, and their identities were confirmed as B. henselae.
Electron microscopy.
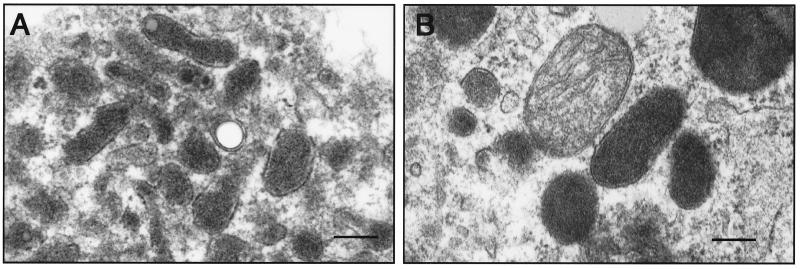
Cells were harvested at 7 and 14 days after inoculation, and cultures were fixed with McDowell and Trump's solution (4% formaldehyde and 1% glutaraldehyde in phosphate buffer) and processed according to standard techniques for transmission electron microscopy (9). Examination of microglial cells in culture revealed intracellular bacillary organisms with morphologic characteristics similar to those previously reported for Bartonella species (3, 6, 17) (Fig. 2). The organisms were short, pleomorphic rods measuring 0.1 to 0.4 μm in width and 0.5 to 1.0 μm in length. They were often seen in clusters within the cell cytoplasm. A double membrane was identified surrounding the bacteria in some sections, and vacuoles were present within a small number of the organisms. No morphologic changes were apparent in infected cells compared to cells in uninoculated cultures.
FIG. 2.
Electron photomicrographs of bacterial organisms within microglial cells 14 days after inoculation. (A) Intracellular aggregate of bacteria with morphologic characteristics similar to those previously reported for Bartonella species; (B) a group of bacteria adjacent to a mitochondrion. Bars, 0.09 μm.
In this study, we demonstrated that B. henselae can infect feline microglial cells in culture and survive intracellularly for up to 4 weeks after inoculation without inducing ultrastructural damage to the host cell. These results may facilitate increased understanding of the neuropathogenesis of B. henselae infection in cats, and they suggest that prolonged CNS infection with B. henselae can occur.
Bartonella species have been isolated from the blood of up to 41% of healthy cats, and studies have demonstrated that cats can remain bacteremic for periods of at least 18 months (16, 20). Bartonella bacteremia is often intermittent, presumably due to periodic sequestration of the organism in tissues with cyclic release into the bloodstream (18). However, the mechanisms that enable the organism to establish persistent infection in cats is not fully understood. Previous work by Kordick et al. with experimentally infected cats suggests that although the organism initially disseminates through the bloodstream to multiple organs, infection of brain tissue may contribute to the development of a latent infection (19). Pathologic examination of chronically infected cats revealed inflammatory foci in the lymph nodes, spleen, liver, heart, and kidneys. Although visualization of the organism was questionable, Bartonella DNA was amplified from many of these tissues. Interestingly, no histologic lesions were identified in the brains of these cats, and yet Bartonella DNA was amplified from brain tissue in 9 of 13 cats studied. In addition, there was a significant correlation between negative blood culture results at the time of necropsy and the ability to amplify DNA from brain tissue. The brain was the only tissue for which this correlation was demonstrated, leading the investigators to conclude that the organism may persist in brain tissue during periods of abacteremia. This conclusion is further supported by our finding that feline brain cells in vitro can be persistently infected with B. henselae without any evidence of a cytopathic effect. Hence, localization to the brain may enable the organism to establish a latent infection and thereby facilitate survival for prolonged periods within the host.
Although it has been established that B. henselae can infect feline microglial cells, the organism's role as a neuropathogen in cats remains controversial. Some studies, which have used culture-derived bacteria as the inoculum, have failed to identify any evidence of disease in infected cats (1, 28). Two other reports describe the development of clinical signs, including neurological abnormalities, following experimental infection using blood- or culture-derived B. henselae organisms as inocula (18, 26). O'Reilly et al. inoculated 17 cats with B. henselae and observed signs of fever, depression, and anorexia in all of them (26). In addition, CNS disease signs, including exaggerated startle responses, staring into space, and reduced responsiveness to environmental stimuli, were evident in three cats. Neurological signs were first observed 7 to 10 days after intradermal B. henselae inoculation and persisted for up to 14 days. B. henselae was not isolated from CSF during periods of neurologic dysfunction. Kordick and Breitschwerdt identified transient neurological abnormalities in 2 of 18 experimentally infected cats (18). Cats stared into space, were poorly responsive to environmental stimuli, and had postural reaction deficits of 1 to 2 days' duration. Analysis of CSF collected from one cat manifesting neurological deficits was unremarkable, and antibodies to B. henselae were not detected in the fluid. One cat had a second episode of transient neurological signs during the chronic stages of infection (252 days postinfection) that included focal motor seizures, nystagmus, and intermittent rigidity (19). CSF collected during this episode revealed mild mononuclear inflammation with a normal protein concentration, and the sample was positive for B. henselae by PCR. Based on these reports, it appears that B. henselae has the potential to induce neurological disease in cats but that this occurs relatively infrequently and signs are transient.
Relman has postulated that strains of B. henselae differ with respect to virulence and that these differences determine whether a given infection becomes symptomatic and, if so, the clinical manifestations of disease (29). Strains of B. henselae may also differ in their pathogenicity in cats (26), including their potential to induce neurological disease. However, individual characteristics of the host must also play a role, as not all cats inoculated with similar strains displayed neurologic abnormalities. Other factors that influence the neurovirulence of a microorganism include the age and immunocompetence of the host, the presence of concurrent infection, and the microorganism burden within the CNS.
Currently, B. henselae has not been implicated as a cause of CNS dysfunction in naturally infected cats. However, the organism appears to have neuropathogenic potential. If naturally infected cats develop mild transient neurological signs as have been reported for experimental cats, the disease may go unnoticed and the owner may not seek medical care. The cause of many feline encephalopathy cases is never determined, and therefore it would seem prudent to test for Bartonella DNA in the CSF of suspected cases. Furthermore, based on the data from experimentally infected cats, it does not appear that documentation of normal CSF can rule out the presence of Bartonella CNS infection.
Neurological dysfunction in humans due to B. henselae infection has been most frequently reported in patients with cat scratch disease. Characteristics of cat scratch disease-associated encephalopathy include a long and variable period of time between inoculation with the organism and the subsequent development of CNS signs, a rapid onset of profound neurological dysfunction, and an eventual, and at times equally rapid, resolution of signs with no residual neurologic deficits. Behavioral abnormalities, depression of consciousness, and seizures are common manifestations of disease (4). Results of analysis of CSF are typically normal or reveal only mild inflammation. The human disease has several characteristics in common with the neurological disease in experimental cats, namely, the transient nature of the disturbance, the presence of behavioral abnormalities, depression of consciousness or seizures, and the lack of significant CSF abnormalities. It may be that B. henselae induces similar pathophysiologic mechanisms in the two species.
Findings of the present study indicate that B. henselae selectively infects microglial cells in the brain. Microglia are derived from bone marrow precursor cells and serve as the resident tissue macrophages of the nervous system. Previous studies have demonstrated that macrophages can become infected with B. henselae; the organism has been shown to infect murine macrophage-like cells in culture (25), and Warthin-Starry silver staining of lymph node samples from humans with cat scratch disease have identified numerous organisms within tissue macrophages (37). Research into the immune response to B. henselae infection using a rodent model also suggests that macrophages play a role in the pathogenesis of infection. Karem et al. isolated B. henselae from the liver and lymph nodes of BALB/c mice 6 h after intraperitoneal inoculation and theorized that this rapid distribution was due to uptake of the organism by local phagocytic cells with subsequent dissemination to tissues (15). Mice were not bacteremic from 6 h to 7 days after inoculation, which was also attributed to rapid uptake by phagocytic cells following infection. Dissemination via macrophages would provide a means for the organism to enter the CNS, as peripheral blood phagocytes are capable of traversing the blood-brain barrier. Once the organism is inside the brain, infection of local microglial cells may facilitate the development of persistent infections.
Infection of microglial cells may result from either trophism of the organism for microglial cells or host cell-mediated phagocytosis. Bacterially mediated invasion of endothelial cells by B. henselae has been demonstrated in culture (6). Further studies are needed to determine whether a similar mechanism is involved in infection of microglia.
Despite the number of reports describing CNS disease associated with B. henselae, invasion of the brain parenchyma in immunocompetent human or feline hosts has not been demonstrated. The transient and profound nature of clinical signs in humans with cat scratch disease-associated encephalopathy have led investigators to suggest that an indirect pathophysiologic mechanism is responsible. B. henselae may incite an immune-mediated reaction or may induce the secretion of a neurotoxin (38). The lack of any observable cytopathic effect in infected cells supports an indirect mechanism of disease. Microglia are likely targets for involvement in such mechanisms. The cells play an important role in the maintenance of homeostasis within the CNS and are involved in the local regulation of immune and inflammatory reactions. Activated microglia express the inflammatory cytokines interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α) (7). The expression of IL-1 and TNF-α by microglia has been shown to attract inflammatory cells into the CNS and to stimulate endothelial cells and astrocytes to proliferate and produce other soluble factors (36). An increase in local cytokine production can be toxic to cellular elements within the CNS, as demonstrated by the effects of TNF-α on myelin and myelin-forming cells (33). Both IL-1 and TNF-α are known to induce fever and mediate inflammation and acute-phase responses, and they are cytotoxic to certain neural cell types in vitro, all of which may play a role in the development of encephalopathy (36).
Infection may also induce alteration of the normal structural associations that exist between neural cells in vivo. Microglia develop extensive branching of cell processes around neurons, and the close physical proximity between microglia and neurons suggests a functional significance to this association (7). Alterations in infected microglia may induce profound functional effects on neurons by disrupting the normal microglial-neuronal interactions. These types of structural alterations would not be appreciated in the cell culture system utilized for the present study.
In conclusion, we have determined that feline microglial cells in culture support infection of B. henselae. It appears likely that the organism may remain sequestered in these cells, allowing for the development of persistent infections. Microglia may therefore be involved in the pathogenesis of CNS disease associated with the organism, as this cell type plays an important role in the regulation of local immune responses. Additional study into the cell-specific response to infection is necessary to further elucidate the role of microglia in the development of CNS disease due to B. henselae infection.
Acknowledgments
This work was supported by grants from the North Carolina State University College of Veterinary Medicine State Research Funds and the North Carolina State University Faculty Research and Professional Development Fund.
We acknowledge the Electron Microscopy Laboratory at North Carolina State University College of Veterinary Medicine for the preparation of the electron micrographs.
REFERENCES
- 1.Abbott R C, Chomel B B, Kasten R W, Floyd-Hawkins K A, Kikuchi Y, Koehler J E, Pedersen N C. Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immunol Microbiol Infect Dis. 1997;20:41–51. doi: 10.1016/s0147-9571(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 2.Brazis P W, Stokes H R, Ervin F R. Optic neuritis in cat scratch disease. J Clin Neurol Opththalmol. 1986;6:172–174. [PubMed] [Google Scholar]
- 3.Breitschwerdt E B, Kordick D L, Malarkey D E, Keene B, Hadfield T L, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154–160. doi: 10.1128/jcm.33.1.154-160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carithers H A, Margileth A M. Cat scratch disease. Acute encephalopathy and other neurologic manifestations. Am J Dis Child. 1991;145:98–101. doi: 10.1001/archpedi.1991.02160010104026. [DOI] [PubMed] [Google Scholar]
- 5.Conley T L, Slater L, Hamilton K. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J Lab Clin Med. 1994;124:521–528. [PubMed] [Google Scholar]
- 6.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci. 1997;110:2141–2154. doi: 10.1242/jcs.110.18.2141. [DOI] [PubMed] [Google Scholar]
- 7.Dickson D W, Mattiace L A, Kure K, Hutchins K, Lyman W D, Brosnan C F. Microglia in human disease, with an emphasis on acquired immunodeficiency syndrome. Lab Investig. 1991;64:135–156. [PubMed] [Google Scholar]
- 8.Dow S W, Dreitz M J, Hoover E A. Feline immunodeficiency virus neurotropism: evidence that astrocytes and microglia are the primary target cells. Vet Immunol Immunopathol. 1992;35:23–35. doi: 10.1016/0165-2427(92)90118-a. [DOI] [PubMed] [Google Scholar]
- 9.Dykstra M J. A manual of applied techniques for biological electron microscopy. New York, N.Y: Plenum Publishing Corp.; 1985. [Google Scholar]
- 10.Freyer D, Manz R, Ziegenhorn A, Weih M, Angstwurm K, Docke W D, Meisel A, Schumann R R, Schonfelder G, Dirnagl U, Weber J R. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J Immunol. 1999;163:4308–4314. [PubMed] [Google Scholar]
- 11.Giménez D F. Staining rickettsiae in yolk sac cultures. Stain Technol. 1964;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- 12.Guptill L, Slater L, Wu C, Lin T, Glickman L, Welch D, HogenEsch H. Experimental infection of young cats with the zoonotic organism Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 13.Jameson P, Greene C, Regnery R, Dryden M, Marks A, Brown J, Cooper J, Glaus B, Greene R. Prevalence of Bartonella henselae antibodies in pet cats throughout regions of North America. J Infect Dis. 1995;172:1145–1149. doi: 10.1093/infdis/172.4.1145. [DOI] [PubMed] [Google Scholar]
- 14.Jensen W A, Fall M Z, Rooney J, Kordick D L, Breitschwerdt E B. Rapid identification and differentiation of Bartonella species using a single-step PCR assay. J Clin Microbiol. 2000;38:1717–1722. doi: 10.1128/jcm.38.5.1717-1722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karem K L, Dubois K A, McGill S L, Regnery R L. Characterization of Bartonella henselae-specific immunity in BALB/c mice. Immunology. 1999;97:352–358. doi: 10.1046/j.1365-2567.1999.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koehler J E, Glaser C A, Tappero J W. Rochalimaea henselae infection: a new zoonosis with the domestic cat as a reservoir. JAMA. 1994;271:531–535. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 17.Kordick D L, Breitschwerdt E B. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol. 1995;33:1655–1656. doi: 10.1128/jcm.33.6.1655-1656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kordick D L, Breitschwerdt E B. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res. 1997;58:492–497. [PubMed] [Google Scholar]
- 19.Kordick D L, Brown T T, Shin K, Breitschwerdt E B. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J Clin Microbiol. 1999;37:1536–1547. doi: 10.1128/jcm.37.5.1536-1547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kordick D L, Wilson K H, Sexton D J, Hadfield T L, Berkhoff H A, Breitschwerdt E B. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol. 1995;33:3245–3251. doi: 10.1128/jcm.33.12.3245-3251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis D W, Tucker S H. Central nervous system involvement in cat scratch disease. Pediatrics. 1986;77:714–721. [PubMed] [Google Scholar]
- 22.Lucey D, Dolan M J, Moss C W, Garcia M, Holis D G, Wegner S, Morgan G, Almeida R, Leong D, Greisen K S, et al. Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations. Clin Infect Dis. 1992;14:683–688. doi: 10.1093/clinids/14.3.683. [DOI] [PubMed] [Google Scholar]
- 23.Mehock J R, Greene C E, Gherardini F C, Hahn T W, Krause D C. Bartonella henselae invasion of feline erythrocytes in vitro. Infect Immun. 1998;66:3462–3466. doi: 10.1128/iai.66.7.3462-3466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakawa G J. Pathogenesis of Bartonella henselae in cutaneous and systemic disease. J Am Acad Dermatol. 1997;37:775–776. [PubMed] [Google Scholar]
- 25.Musso D, Drancourt M, Raoult D. Lack of bactericidal effects of antibiotics except aminoglycosides on Bartonella (Rochalimaea) henselae. J Antimicrob Chemother. 1995;36:101–108. doi: 10.1093/jac/36.1.101. [DOI] [PubMed] [Google Scholar]
- 26.O'Reilly K L, Bauer R W, Freeland R L, Foil L D, Hughes K J, Rohde K R, Roy A F, Stout R W, Triche P C. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU16) Infect Immun. 1999;67:3066–3072. doi: 10.1128/iai.67.6.3066-3072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickerill R G, Milder J E. Transverse myelitis associated with cat scratch disease in an adult. JAMA. 1986;246:2840–2841. [PubMed] [Google Scholar]
- 28.Regnery R L, Rooney J A, Johnson A M, Nesby S L, Manzewitsch P, Beaver K, Olson J G. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996;57:1714–1719. [PubMed] [Google Scholar]
- 29.Relman D A. Are all Bartonella henselae strains created equal? Clin Infect Dis. 1998;26:1300–1301. doi: 10.1086/516347. [DOI] [PubMed] [Google Scholar]
- 30.Schwartzmann W A, Patnaik M, Barka N E, Peter J B. Rochalimaea antibodies in HIV-associated neurologic disease. Neurology. 1994;44:1312–1316. doi: 10.1212/wnl.44.7.1312. [DOI] [PubMed] [Google Scholar]
- 31.Schwartzmann W A, Patnaik M, Angulo F J, Vusscher B R, Miller E N, Peter J B. Bartonella (Rochalimaea) antibodies, dementia and cat ownership among men infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:954–959. doi: 10.1093/clinids/21.4.954. [DOI] [PubMed] [Google Scholar]
- 32.Selby G, Walker G L. Cerebral arteritis in cat scratch disease. Neurology. 1979;29:1413–1418. doi: 10.1212/wnl.29.10.1413. [DOI] [PubMed] [Google Scholar]
- 33.Selmaj J W, Raine C S. Tumor necrosis factor mediated myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- 34.Spach D H, Panther L A, Thorning D R, Dunn J E, Plorde J J, Miller R A. Intracerebral bacillary angiomatosis in a patient infected with human immunodeficiency virus. Ann Intern Med. 1992;116:740–742. doi: 10.7326/0003-4819-116-9-740. [DOI] [PubMed] [Google Scholar]
- 35.Tappero J W, Mohle-Boetani J, Koehler J E, Swaiminathan B, Berger T G, LeBoit P E, Smith L L, Wenger J D, Pinner R W, Kemper C A, Rinegold A L. The epidemiology of bacillary angiomatosis and bacillary peliosis. JAMA. 1993;269:770–775. [PubMed] [Google Scholar]
- 36.Tyor W R, Glass J D, Griffin J W, Becker P S, McArthur J C, Bezman L, Griffin D E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 37.Wear D J, Margileth A M, Fisher G W, Schlagel C J, King F M. Cat scratch disease: a bacterial infection. Science. 1983;221:1403–1405. doi: 10.1126/science.6612349. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler S W, Wolf S M, Steinberg E A. Cat-scratch encephalopathy. Neurology. 1997;49:876–878. doi: 10.1212/wnl.49.3.876. [DOI] [PubMed] [Google Scholar]
- 39.Zangwill K M, Hamilton D H, Perkins B A, Regnery R L, Plikaytis B D, Hadler J L, Carter M L, Wenger J D. Cat scratch disease in Connecticut: epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993;329:8–13. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]