Abstract
Psoriasis is a chronic disabling complex inflammatory disorder prevalent worldwide with environmental and genetic components that involve predominantly skin in addition to nails and joints associated with various systemic comorbidities having periods of exacerbations and remissions. Psoriasis is characterized by hyper-proliferation as well as abnormal differentiation of epidermal keratinocytes and lymphocyte infiltration (mainly T cells) with resultant inflammatory cytokines and chemokines. Immunological and genetic studies over the last decade have identified genetic susceptibility risk alleles, molecular, cellular and immunological mechanisms involved in immunopathogenesis of psoriasis. The current disease model emphasizes the role of aberrant Th1 and Th17 responses regulated by a complex network of different cytokines, including TNF-α, IL-17 and IL-23; signal transduction pathways downstream to the cytokine receptors; and various activated transcription factors, including NF-κB, interferon regulatory factors and signal transducer and activator of transcriptions. Cytokines targeting biologics (IL-17, IL-23 and TNFα) therapies have revolutionized the management of severe skin disease having beneficial effects on joints and systemic inflammation of psoriasis as well. Further better understanding of immunopathogenesis of psoriasis will pave way for precision medicine based on specific immunopathogenic targets in a given phenotype of disease. Complex interplay of psoriasis with associated comorbidities is also a future area of research for overall better patient management and to improve their quality of life.
KEY WORDS: Immunopathogenesis, psoriasis, recent update
Introduction
Psoriasis, affecting ~100 million individuals worldwide, is a chronic immune-mediated multi-systemic inflammatory disorder. It represents a unique skin barrier defect caused by aberrant, dysregulated proliferation and altered differentiation of keratinocytes (KCs) associated with marked angiogenesis. Psoriasis is one of the common dermatoses, constituting a significant health burden to the community as it is associated with multiple morbidities and impaired quality of life for patients with no uniformly effective therapy available.[1] Psoriasis is now considered to be a systemic disease that is more than skin deep, involving joints (psoriatic arthritis [PsA]) as well as extra-articular sites such as the gastrointestinal tract and eye, etc.
Psoriatic disease is an umbrella term used to define a unique illness involving several different anatomical sites in the same patient either simultaneously or in different sequential stages.[1] It is a multi-factorial illness with a complex genetic basis showing an interplay of multiple genes situated on different loci, triggered by a variety of environmental factors including infections (Streptococcus pyogenes, Malassezia, Candida albicans, Staphylococcus aureus), trauma, stress, alcohol, smoking, stressful life events, dry weather conditions (causing stress on the skin barrier by indirectly affecting the internal immunological balance), drugs (lithium, β-blockers, antimalarials, non-steroidal anti-inflammatory drugs, tetracyclines) and obesity.[2,3,4,5,6]
The immunopathobiology of psoriatic disease is driven by monocyte and T-cell-derived cytokines which metamorphose into a devastating pathology culminating in cutaneous plaques or pustules due to self-catenating inflammatory loops.[7,8,9] Psoriasis and PsA have a lot in common, including their human leukocyte antigen (HLA) phenotypes, structure of immune pathways, T-cell phenotypic profiles in the target organs, namely epidermis, synovial fluid compartment and the enthesis, diverse cytokine spectrum, and T-cell receptor beta chain variable gene repertoires, thereby suggesting a common inciting antigen for both cutaneous and articular disease that switches on a common, precisely orchestrated axis of inflammatory networks by regulating immune cell recruitment and cytokine–chemokine production.[10,11,12,13]
An amazing aspect about the psoriatic cutaneous lesions is the fact that, while complex, the skin lesions heal completely with appropriate therapy, with little if any evidence of altered changes in the epidermis and dermis; in contrast, PsA is generally characterized by irreversible inflammatory joint pathology.[14] In PsA, the relevance of genetic predisposition appears to be greater than in psoriasis, as evidenced by additional occurrence of HLA-B38, HLA-B39 and with HLA-B27 in patients with spinal disease.[3,4,6]
In this review, we will touch upon some interesting pathophysiological aspects of psoriatic disease. In this review, established and emerging regulatory nodes and their interaction with inflammatory networks to gain further insight into the mechanistic pathways of psoriatic disease will be narrated. Improved understanding of the molecular mechanisms and pathogenic KC activation might facilitate improved therapeutic approaches targeting novel mediators.
Pathology of Psoriasis
Psoriasis is regarded as a T-cell-mediated disease, whereby Th1 and Th17 T-cell pathways converge, overlap and cross-talk, generating a multicellular conspiracy, driving the characteristic pathological hyper-proliferation, amplification and aberrant differentiation of KCs, manifesting as thick, erythematous, scaly plaques in genetically susceptible individuals. The psoriatic disease has been recently modeled as a chronic debilitating autoimmune-mediated disease with a complex genetic determinism having heterogeneous subphenotypes, each subphenotype under the control of a different susceptibility-associated HLA allele reflecting a distinct adaptive autoimmune response against a different target molecule.[15] As substantiated by twin studies, linkage analysis and genome-wide association studies (GWAS), psoriasis results from an interplay of multiple genes situated on different loci affecting complex signalingpathways and resulting in immune dysregulation. Many psoriasis susceptibility (PSORS) loci have been identified with their number approaching 80 in the latest meta-analyses of GWAS. The MHC (HLA-Cw6 and other MHC variance) is the major locus involved in immunological diseases and has consistently been linked to psoriasis, both in previous linkages and recent GWAS.[16,17,18,19] Mutations in CARD-14 (a family encompassing scaffolding proteins that activate nuclear factor of kappa light chain enhancer in B-cells, NF-kB) gene resulting in aberrant NF-kB activation have been reported in psoriasis and PsA,[20,21] while a systematic review of single nucleotide polymorphisms analyses of several major studies demonstrated an association with various immune system nodes, including Th17 and Th2 pathways, innate immune signaling pathways and skin barrier function (LCE3B, LCE3C).[22] A diverse array of immune responses resulting in activation of different immune effector pathways might be, therefore, responsible for differential responses to various specific biologic agents.
Hyper-proliferation and abnormal differentiation of KCs are the two critical outcomes of the underlying immunological dysregulation in psoriasis. Histologically, psoriatic plaques are characterized by marked thickening of the epidermis, parakeratosis, prominent and uniform elongation of epidermal rete pegs, loss of granular cell layer, supra-papillary thinning [Figure 1], microabscess of Munro (collection of neutrophils in the stratum corneum), spongiform pustule of Kojog (epidermal spongiotic pustule with neutrophilic infiltration) [Figure 2] and marked dermal vascularity.[23,24,25,26,27] These histological changes are accompanied by a chronic inflammatory cell infiltrate chiefly comprising CD4+ lymphocytes, localized primarily to the dermal papillae in the skin and sublining layer stroma in the joints. In contrast, the epidermis, synovial tissue and enthesis are populated by CD8+ T cells indicating these cytotoxic T cells as the main drivers of psoriatic disease pathophysiology, as also supported by an association of psoriasis with HLA class I.[11,28,29] The classical histopathological dermal and epidermal features of psoriasis are similar across various psoriatic subtypes except for a few distinct histological characteristics.[30,31,32]
Figure 1.
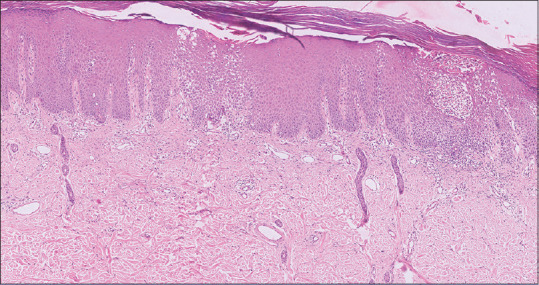
Photomicrograph of psoriatic skin biopsy showing parakeratosis, regular acanthosis and broadening of rete ridges (H&E, ×10)
Figure 2.
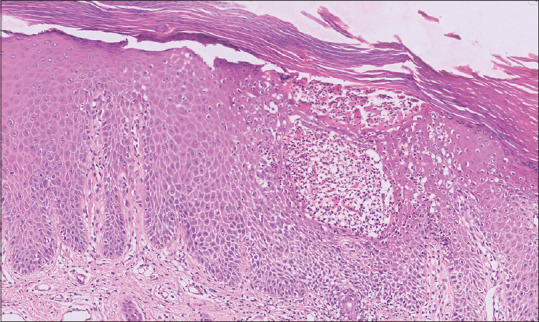
Photomicrograph of psoriatic skin biopsy showing evidence of parakeratosis, hypogranulosis, mild spongiosis, Munro's micro-abscess and pustule of Kogoj (H&E, ×40)
Psoriatic plaques represent a special, if not unique, tissue response, paralleling 'regenerative maturation', an alternative cell differentiation program transiently expressed during wound healing.[33] Skin biologists often consider the immunobiology of psoriasis as a 'persistent and aberrant wound response', given the histological and clinical parallels. Similar to damaged KCs, suprabasal KCs in psoriatic lesions exhibit elevated expression of keratins (K) 6, 16 and 17, which is considered to be a hallmark of psoriasis.[34] Recent studies performed in psoriatic patients have demonstrated deleterious mutations, mainly clustered in the rod domain of the keratins K14, K10, K16 and K17, severely affecting their stability and expression patterns.[35]
Immunopathogenesis of Psoriasis
Keratinocyte Proliferation and Differentiation
In the last two decades, an enormous amount of work has been done to decode the invisible immunological mechanisms underlying the epidermal hyperplasia and neoangiogenesis seen in psoriatic disease. The intersection of various immune-regulatory nodes generates a confederacy of both soluble mediators like Th1, Th17 and Th22-associated cytokines, chemokines, adhesion molecules, growth factors like nerve growth factor (NGF), neuropeptides and specific cellular constituents ultimately evolving into a unique inflammatory and proliferative process typical for psoriasiform inflammation.
Psoriasis has been characterized as a T-cell-mediated autoimmune disease of the skin and joints as supported by various experiments in severe combined immune-deficiency (SCID) mouse-psoriasis xenograft model as well as human placebo-controlled pilot studies.[36,37,38,39,40,41,42] It is currently classified primarily as an IL-23/IL-17-mediated disorder with this axis representing the core of psoriasis immunobiology.[43] The cross-talk among dendritic cells (DCs), T cells, KCs, neutrophils, mast cells, innate lymphoid cells (ILCs), fibroblasts and endothelial cells creates auto-inflammatory and pro-proliferative circuits mediated by upstream cytokines (IFN-α, IFN-γ and TNF-α), synergizing cytokines (IL-22 and TNF-α) and downstream mediators (IL-8, IL-1F9 and CCL20) that generate feed-forward loops fuelling inflammation.
The activation of IFN-α-producing plasmacytoid dendritic cells (pDC) s and/or autoreactive T cells producing psoriasis-signature cytokines IL-17 and IFN-γ is one of the preliminary steps in pathogenic cascade that indicates an autoimmune hypothesis for psoriatic disease. A variety of auto-antigens trigger the inflammatory cascade by way of molecular mimicry. These auto-antigens include type-I Ks (K16 and K17); ezrin, maspin, peroxiredoxin 2 and heat shock protein 27, exhibiting homologies to Streptococcal antigens; LL-37; thrombospondin type-1 motif-like 5 (ADAMTSL5), an HLA-C*06:02-restricted auto-antigen; lipid neoantigens generated by phospholipase A2 (PLA2) group IV D (PLA2G4D); and HNRNPA1.[43,44,45,46,47,48,49,50]
The innate immune cells including both pDCs (expressing Toll-like receptors [TLR] 7/9) and myeloid DCs (expressing TLR3/8) are activated by an array of molecules including:
TLR agonists, namely self-nucleic acids (both deoxyribonucleic acid [DNA] and ribonucleic acid [RNA] derived from damaged cells) – complexes with anti-microbial peptides (AMPs) including LL-37, DEFB4, hBD3, lysozyme 9 and IL-26 (Th17-derived cytokine).
Chemerin (produced by fibroblasts, mast cells and endothelial cells).
Thymic stromal lymphopoietin (TSLP, produced by KCs).
This is followed by their maturation to become highly inflammatory dermal DCs and start secreting a variety of pro-inflammatory cytokines (IFN-α by pDCs; IL-23, TNF-α, nitric oxide, IL-20 by mDCs) that initiate the pathogenic inflammatory cascade, setting the stage for the development of early psoriatic disease.[51,52,53] IL-23-producing DCs interact with T cells within the dermis, priming their differentiation towards a dominant IL-17+ T-cell phenotype (Th17, Tc17), the 'signature' pathologic cell underlying psoriatic pathophysiology. They also activate other IL-17-producing cells [Figure 3], including γδ T cells, ILC3, mast cells, macrophages, DCs and neutrophils.[54,55,56,57,58]
Figure 3.
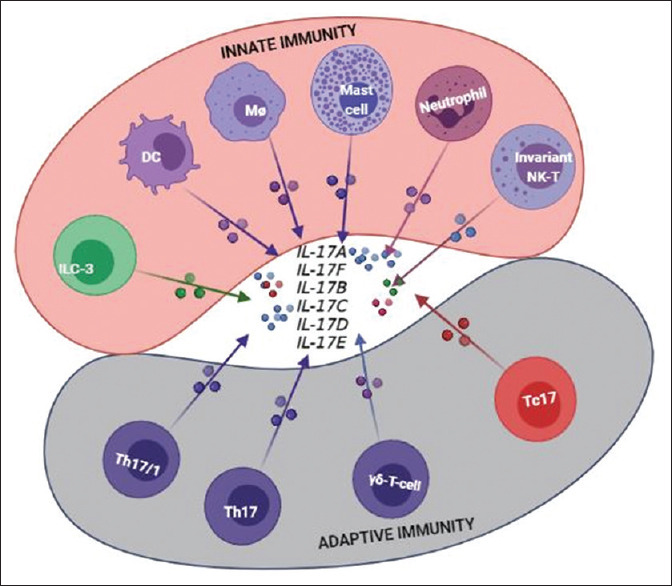
Schematic listing a wider and expanded array of interleukin-17 (IL-17)-producing cells in psoriasis
IL-17, in synergism with other cytokines like TNF-α and IL-22, induces the development of the psoriasis phenotype through tissue cell (KC) activation. Gradually, the amplification phase ensues, marked by a wider and large-scale activation of T-cell subsets, amplifying the typical psoriatic tissue cell response, manifested by proliferation of KCs the 'key responding' tissue cell in psoriasis which bear receptors for psoriasis-signature cytokines. Activated KCs secrete a variety of cytokines (i.e., IL-1F9, (IL-36γ), TNFα, IL-17C, IL-19, TSLP, IL-1β), chemokines (CCL20, CXCL5, CXCL8, CXCL9, CXCL10), proliferation-stimulating factors (epidermal growth factor [EGF], vascular endothelial growth factor [VEGF] and heparin-binding EGF) and other pro-inflammatory products, such as AMPs [Figure 4], each, in turn, modulating distinct KC-response pathways leading to sustained, amplified skin inflammation.[59,60]
Figure 4.
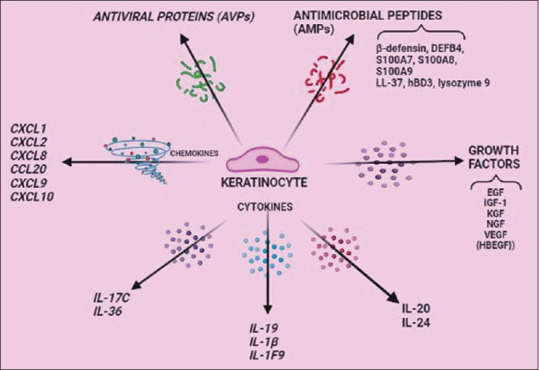
Tissue cell/Target cell (keratinocyte) response in psoriatic inflammation. Keratinocytes (innate cell) secrete a variety of soluble mediators/factors amplifying psoriatic inflammation
Regulatory Role of the Th17 Cells: Effect of IL-17/23
Psoriasis is an 'IL-17-centric' pathogenic cutaneous disease where Th17 cells are the predominant players driving disease pathology. Psoriasis is characterized as a disease with a vicious loop where IL-17 signal reverberates within the lesional site. Increased numbers of Th17 cells have been detected in the lesional skin as well as in the peripheral blood of psoriatic patients.[61,62]
IL-17A, the most relevant cytokine of the Th17 cytokine family (IL-17A to IL-17F), with the highest pro-inflammatory biological activity, is the key effector cytokine mediating psoriasis pathogenesis and has been shown to be critical for sustaining inflammation in psoriatic plaques. Increased IL-17 mRNA expression levels and/or protein concentrations have been detected in lesional skin, uninvolved skin, serum, synovium and tear fluid of psoriatic patients compared to healthy controls.[17,63,64,65,66,67,68]
IL-17A, produced by a variety of cells in the psoriatic inflammatory milieu, plays the most crucial and fundamental role as the amplifying effector arm in manifesting the characteristic cutaneous tissue response in genetically predisposed individuals. The lead contributions of this central cytokine in psoriasis pathogenesis include:
Promotion of neutrophil recruitment to the lesional epidermis, their survival and activation by inducing neutrophil-specific chemokines (CXCL-1, -3, -8 (IL-8) or AMPs).[69,70]
Induction of TNF-α release from DCs and macrophages.[70]
Stimulation of auto-antigen production directly or indirectly by inducing KCs to produce LL-37 or melanocyte-stimulating factor-alpha.
Upregulation of signature AMPs (β-defensin, S100A7, S100A8, S100A9) that provide pro-inflammatory niche.
Downregulation of filaggrin expression in KCs, thereby disrupting the skin barrier.[70,71,72]
Recruitment of CCR6+ T cells, that is, additional pathogenic Th17 cells (Th17, Tc17, γδ T cells) and mature mDCs by regulating CCL20 production from KCs.[70]
Destruction of bone and cartilage in inflammatory arthritis and significant upregulation of IL-6, IL-8 and matrix metalloproteinases (MMP) in fibroblast-like synovial (FLS) cells.[67,73,74,75]
IL-17A directly impacts DC distribution and activation and it is the synchronicity between IL-17A and TNF-α that modulates KC gene expression in psoriatic plaques.[76,77]
IL-25 (IL-17E), another IL-17 family cytokine, constitutes another KC autoregulatory circuit, sustaining chronic psoriasiform inflammation.[78] Figure 5 summarizes sequential immunopathogenic events in psoriatic inflammation.
Figure 5.
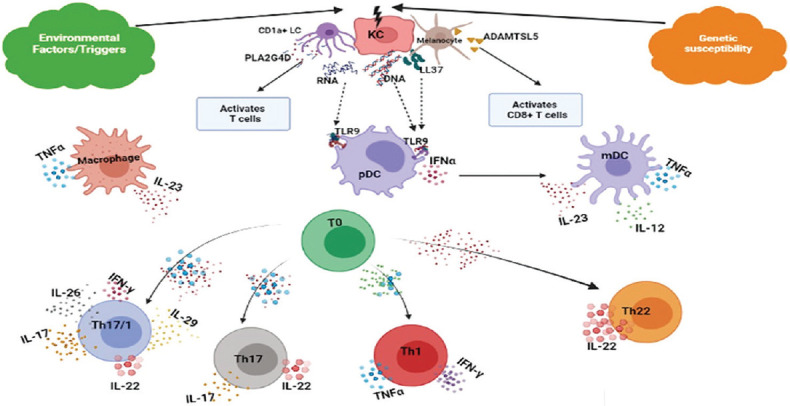
Immunopathogenic cascade in psoriatic inflammation. KC: Keratinocyte, pDC: plasmacytoid dendritic cell, mDC: myeloid dendritic cell, LC: Langerhans cell
Recently a new concept of 'immunometabolism', that is, a fusion between metabolism and immunity, has emerged that includes two linked concepts: metabolite-facilitated regulation of immune cells resulting in their proliferation/differentiation/activation and the influence of immune response/inflammation on systemic metabolic regulation.[79,79,80,81,82,83] Psoriasis has long been considered as a metabolism-associated disease. The metabolism-modulating activity of IL-17, orchestrating the choreography of cellular and organismal metabolism, is a relatively recently appreciated key function of this cytokine. IL-17-mediated metabolic regulation during psoriasis is manifested by reprogramming of the urea cycle resulting in excessive polyamine generation that facilitates self-RNA sensing by immune cells independent of RNA-binding proteins (RBPs) LL37 and HNRNPA1 (the proven auto-antigens) ultimately leading to amplification of inflammatory circuits.[84,85] It has also been observed that IL-17 induces intracellular cholesterol accumulation that causes upregulation of CCL20, IL-8 and S100A7 expression in KCs via activation of NF-κB, further exacerbating IL-17A-induced psoriatic inflammation.[86] Collectively, IL-17-mediated effect on metabolic adaptations in KCs, fuelled by KC-intrinsic metabolic fluxes, is a pivotal component of the eventual immune response. Hence, in future studies, it will be quite intriguing to define the relationships between IL-17-driven metabolic reprogramming and epigenetics/chromatin remodeling that are responsible for chronic, sustained transcriptional responses seen in psoriasis, which in turn modulate the activity of IL-17-related proinflammatory cytokine programs.
IL-23
IL-23, a member of IL-6/IL-12 cytokine family sharing homology with IL-12 in having the p40 subunit, is instrumental in differentiation, expansion, and survival of Th17 cells.[87] An increased expression of IL-23 p19 (along with marked infiltration of CD11c+ myeloid DCs as a chief source of IL-23) in psoriatic lesions as well as increased IL-23 serum levels have been noted in psoriatic patients, indicating an important role of IL-23 signalling pathway in psoriasis.[88,89,90,91] An equally important role is played by IL-12 as well, evidenced by increased levels of IL12p40 messenger RNA (mRNA) in psoriatic lesions.[92,93,94]
Other Relevant Cytokines in the pathogenesis of psoriatic disease
IL-22
Among the chief psoriasis-signature cytokines (TNF-α, IFN-γ, IL-17, IL-22), IL-22 is the main driver for pathognomonic epidermal changes in psoriasis as its effects are mainly directed at KCs.[95,96] It is a member of IL-20 cytokine family, chiefly produced by CD4+ (Th22) and CD8+ T cells (Tc22) and causes basal KC proliferation and hypogranulosis; increased epidermal thickness; downregulation of KC differentiation pathways; enhanced KC migration; increased expression of K16, and AMPs such as defensin proteins (DEFβ-2,-3), S100A protein family (S100A7, S100A7A, S100A8, S100A9, S100A12) and Stat3 (hallmark biological feature of psoriatic KCs); and upregulation of expression of neutrophil chemoattractants (CXCL1, CXCL2, CXCL8), chemokines (CCL20) platelet-derived growth factor (PDGF) A and MMP3.[97,98,99,100,101,102] IL-22-mediated KC responsiveness and pro-inflammatory actions are boosted by its synergism with other cytokines like IFN-α, IL-17, TNF-α and IL-20.[103,104]
TNF-α
TNF-α was the first cytokine to be successfully targeted by therapeutic monoclonal antibodies. In psoriatic patients, the levels of TNF-α and its receptors (TNFR1/p55, constitutive; TNFR2/p75, inducible) are markedly elevated in both the cutaneous lesions and the serum as compared to uninvolved skin.[105,106,107] A variety of cells including activated T cells, KCs, DCs, macrophages, and mast cells in the immunological micro-environment, produce TNF-α.[54,108,109,110,111,112] As a pro-inflammatory cytokine, it acts synergistically with other mediators including IL-17 to induce diverse immune and inflammatory responses orchestrated by KCs, namely tissue remodeling, cell motility, cell cycling and apoptosis: playing an important role in both the initial and chronic phases of psoriasis;[113] this explains the efficacy of TNF-α neutralization strategies in psoriasis. TNF-α and its attendant exaggerated signaling pathways play an important part in the pathogenesis of PsA as well, possibly due to genetic polymorphism in predisposed individuals, leading to the induction of new players such as IL-19, IL-20, IL-22 and IL-24, enhancing angiogenesis, cell trafficking, proliferation of FLS and activation of osteoclasts.[104,114,115,116] CD137, a member of the TNF receptor family and a potent co-stimulator of CD8+ T cells, also has an undeniable role in regulating the immune responses as documented by increased percentage of the CD137-positive inflammatory cells in psoriatic skin biopsies, introducing CD137 as a novel target for psoriasis treatment.[117]
IFN-γ
A profound, meticulous, systematic revision of the pathogenic mechanisms has redefined the role of IFN-γ as an upstream cytokine in the IL-23/IL-17 axis in the pathogenic cascade associated with psoriasis, chiefly characterizing the early phase of the disease as evidenced by its ability to activate antigen-presenting cells. IFN-γ upregulates the expression of approximately 400 genes encoding for inflammatory mediators typical of psoriasis, including TNF-α, inducible nitric oxide synthase, IL-23p19, CCL19, intacellular adhesion molecule-1, vascular cell adhesion molecule-1 and TNF-related apoptosis-inducing ligand.[118,119,120] Thereby, it favors Th-17 cell recruitment and activation and also synergizes with these cells in inducing human β-defensin production.[71]
IL-29
IL-29 (interferon (IFN)-λ1), a newly recognized member of the IFN-λ (type III IFN) family, has been shown to enhance the mRNA expression of TNF-α, IL-6 and IL-17 in peripheral blood mononuclear cells and KCs, thereby further expanding the inflammatory loops in psoriasis.[121] It also regulates massive epidermal expression of antiviral proteins, which protects the damaged psoriatic skin from viral infections.[122]
IL-36
IL-36, member of IL-1 family of cytokines (IL-36α, IL-36β, IL-36λ and IL-36 R antagonist, IL-36RN), chiefly secreted by KCs, is particularly implicated in causation of generalized pustular psoriasis. This cytokine has a significant contribution in maintaining feed-forward amplification loops in psoriasis, thereby enhancing inflammatory activity leading to influx of neutrophils to the psoriatic lesions.[123]
Role of Growth Factors
Experimental studies conducted in the SCID mouse model have shown a role of non-classical inflammatory mediators in T-cell activation.[124] Overexpression of various growth factor receptors and cytosolic proteins exhibiting tyrosine kinase activity has been demonstrated in psoriatic KCs.[125,126,127,128,129,130,131,132] These include the epidermal growth factor-receptor and its ligands (members of the EGF family, TGF-α, amphiregulin and betacellulin), insulin growth factor-1 receptor and proteins of the Src family; these factors play a signal role in sustaining autocrine hyper-proliferation of psoriatic KCs.[125,128,133] The KC growth factor receptor overexpressed in psoriatic basal and suprabasal KCs; and its ligand KGF secreted by stromal cells, together augment secretion of TGF-α leading to KC proliferation. NGF secreted by epidermal KCs and its high-affinity receptors such as TrkA and p75 are overexpressed in skin and synovial tissues affected by psoriatic disease and have been shown to regulate almost all the key events of psoriatic pathology, namely KC proliferation, angiogenesis, T-cell activation, adhesion molecules expression, proliferation/survival of FLS and upregulation of inflammatory mediators.[134,135,136,137,138]
Other Important Cellular Elements in Psoriasis
Although T cells and DCs primarily drive psoriasis, interesting roles for macrophages, neutrophils and others cell lineages have recently been elucidated. Psoriasis pathogenesis involves polymorphonuclear neutrophil (PMN) infiltration and high levels of the PMN-associated AMP, LL37, an important RBP.[139,140] Using transcriptome profiling approaches and a pre-established set of transcriptional modules as a common framework, and relying on extensive annotations and expression patterns of purified leukocyte populations, Rawat et al.[141] compared the blood transcriptome signatures of independent psoriasis datasets and observed a neutrophil-driven inflammatory signature as a hallmark of the blood transcriptome signature of psoriasis. Due to their abundance in psoriatic skin and tremendous functional versatility, metabolic plasticity, phenotypic heterogeneity, differential hierarchical cytoplasmic granule mobilization and their ability to form NETs (neutrophil extracellular traps), these short-lived cells of the immune system are emerging as important linkers between psoriasis and associated co-morbidities.[142,143] A recent study has implicated NET-associated RNA-LL37 complexes in psoriasis, independent of the canonical NET component DNA,[144] which highlighted a hitherto unappreciated potential axis that involves neutrophil–LL37 (RBP-like)–RNA which are surprisingly abundant in psoriatic as opposed to healthy skin; this suggests a novel role for NET-derived RNA–LL37 complexes in self-propagating inflammatory circuits.
CD56+CD16+ natural killer (NK) cells and particularly NKT cells (features from both T cells and NK cells), although playing a more subtle role, have also been implicated in psoriasiform inflammation by virtue of their cytotoxic properties, their capacity to secrete pathogenic cytokines such as IFN-γ, IL-17, TNF-α and IL-22, and to express chemokine receptors, such as CXCR3, CCR5 and CCR6.[57,145] Diverse and unique lymphocyte lineages belonging to both innate and adaptive immune arms including ILCs and γδ T cells, bestowed with 'innate memory', are gaining interest as contributors to different stages of psoriasis by exacerbating inflammatory pathology.[146,147,148]
Skin-resident regulatory T cells (Tregs) are quite heterogeneous due to their continuous exposure to localized antigenic challenges. Altered Treg frequency/phenotype/function and disturbed Treg/Th17 balance have been recently suggested as factors leading to disease perturbation.[149] Regulatory B-cells (Bregs), IL-10 secreting cells with an immunosuppressive phenotype, representing a B-cell differentiated stage rather than a distinct B-cell lineage, are the new frontiers that are beginning to gain attention in the development and maintenance of psoriasis in the last couple of years. The importance of Bregs is suggested by the improvement in disease symptoms noted with recombinant IL-10 therapy.[59,150,151]
Angiogenesis
One of the myriad manifestations of psoriasis is vascular remodeling/pathological angiogenesis, evaluated clinicopathologically by different techniques including histopathology, dermoscopy, videocapillaroscopy, fluorescence angiography, reflectance confocal microscopy, high-frequency ultrasound, laser Doppler imaging, optical coherence tomography, optical microangiography and multiphoton tomography.[152,153] Neovascularization/Angiogenesis is an important element of psoriasis pathobiology as demonstrated by the conspicuous presence of dilated, elongated, tortuous, hyperpermeable, abnormally oriented capillary loops with higher mean values of micromorphological parameters, namely area, perimeter and Ferret's diameter, increased number of capillary vessels per lesional dermal papilla (an approximate average of 50 capillaries/mm) and increased blood flow in psoriatic skin biopsy specimens.[154,155] Disruption of the oxidative stress–inflammation–angiogenesis axis with activation of the attendant MAPK signaling pathway resulting in the release of variety of angiogenic factors promoting the expansion of vascular networks completes the picture of full-blown clinical psoriasis.[156,157] Angiogenesis in psoriasis displays a closely knit, complex, reciprocal molecular cross-talk between diverse pathological networks including vascular endothelium activation, angiogenesis, inflammation and immunity.[157]
Compared to their normal counterparts, psoriatic KCs are bestowed with an extraordinary angiogenic capacity potentiated by IL-8 overproduction and a deficiency of angiogenesis inhibitor thrombospondin-1.[158] KCs, working along with pro-angiogenic cytokines such as IL-17, TNF-α, IL-6 and IL-1β, have been recognized as the main factors responsible for secreting pro-angiogenic molecules, namely VEGF, endothelial cell stimulating angiogenesis factor and PDGFs, NGF and von Willebrand factor, angiopoietin-1, hypoxia-inducible factor-1α. VEGF not only promotes pathological vasculogenesis in patients with psoriasis but also regulates epidermal hyperplasia and keratin imbalance.[159,160,161]
Psoriasis has been previously assumed to be solely fuelled by KC-intrinsic metabolic fluxes with unbalanced and altered sphingolipid metabolism leading to qualitative and quantitative alteration in epidermal sphingolipids including ceramide and sphingosine-1-phosphate.[162,163] Both these molecules exert antagonistic effects on vascular endothelium, so their quantitative imbalance is one of the inherent cues driving pathological angiogenesis and contributing to the severity and/or progression of psoriasis.[164]
Extracellular Matrix-Immune Cell Cross-talk in Psoriasis
Psoriasis has been considered a phenomenon resembling abnormal wound response, characterized by a unique form of 'scarless or hyper-regenerative' wound healing characterized by nonscarring, inflammatory and hyperproliferative tissue repair. Psoriatic plaques are an exciting conundrum: they do not manifest fibrosis despite rampant, multiple auto-inflammatory loops.[33] An interesting common mediator: oncofoetal fibronectin extra-domain B (Fn-EDB) has been reported to be present at significant levels in psoriatic lesions and is also involved in fetal tissue wound healing.[165] Despite considerable long-standing immune-mediated chronic inflammation with resultant aberrant, dysregulated KC proliferation, altered differentiation and marked angiogenesis, cutaneous cancers are rarely reported with psoriatic plaques.
Aberrant extracellular matrix (ECM)–immune cell interactions leading to dysregulated organization and deposition of ECM have been recently advocated as an important player in psoriatic disease. ECM components including laminins by affecting adhesion, migration and proliferation of immune cells via integrin signalling, cysteine-rich angiogenic protein 61 (CCN-1) by promoting pro-inflammatory cascade mediated by NFκB activation, osteopontin by enhancing Th17 and Th1 responses, have been held responsible for chronic progression of psoriasis.[166]
Unraveling the facets responsible for an imbalance between immune system remodeling and ECM dynamics is an interesting area of research which can inform a possible shift of future treatment paradigms towards development of newer therapeutics that take into account immune cell–ECM interactions.
Mechanotransduction in Psoriasis
The part played by mechanotransduction/mechanical forces – the translation of physical cues into biochemical signaling networks leading to acquisition of aberrant phenotypes by psoriatic KCs – has also been highlighted recently.[167] It is the weakened KC response to the mechanical forces, as implied by the Koebner phenomenon, that is responsible for entry of immune cells into the epidermis.[168,169] These mechano-regulated signaling pathways (Wnt pathway, yes-associated protein/[transcriptional coactivator with PDZ-binding motif (TAZ) pathway, activator protein-1 pathway) and associated molecules (transient receptor potential channels, calcium-sensing receptor, polycystin) are paving the path for further investigation towards their diagnostic and therapeutic utility in psoriasis.
RNA Interactome
Immunostimulatory RNAs (e.g. double-stranded or long noncoding RNA) generated through deregulated processing pathways along with their associated binding proteins (RNA sensors) are emerging as an important component of inflammation-related immune response pathways involving disease-associated molecular pattern sensing.[170,171,172,173,174,175] The paradigm shift in RNA metabolism-associated interactome is beginning to unravel the novel RBPs and splicing factors in context of psoriatic inflammatory pathways.
Thus, psoriatic disease is not merely a skin disease but a skin disorder with a substantial systemic inflammatory component which forms the foundations and risks for metabolic syndrome. Complex interplay of psoriasis with associated comorbidities is primed by systemic inflammation so management of psoriasis requires multispecialty care.[177,178,179] Large-scale single-cell profiling of human psoriatic skin lesions has unveiled a re-induction of prenatal cellular programs mediated by vascular endothelial cells in concert with macrophages, an interaction needed for lymphocyte recruitment and angiogenesis, illustrating an evolution of a dynamic cutaneous cellular topology during immune-mediated inflammation.[180]
Table 1 enlists all the players including inciting antigens, cellular elements, cytokines and other factors/mediators/networks involved in psoriasis. The pathogenesis of psoriasis includes the innate and adaptive arms of the immune system, whose cells interact with tissue cells leading to the production of large amounts of a myriad cytokine that, in turn, create, sustain and amplify numerous reverberating inflammatory and proliferative circuits. Understanding these components, their intricate interactions and complex interplay hold the potential to reshape psoriatic therapeutics in ways currently unimagined.
Table 1.
Main auto-antigens, cellular elements, cytokines and other factors/mediators/networks involved in psoriasis
| Auto-antigens: | |
| Cathelicidin antimicrobial peptide LL-37 | |
| Type I Keratins (K16 and K17) | |
| Melanocytic protein thrombospondin type-1 motif-like 5 (ADAMTSL5) | |
| Lipid neoantigens generated by phospholipase A2 (PLA2) group IV D (PLA2G4D) | |
| HNRNPA1 | |
| Ezrin, maspin, peroxiredoxin 2, heat shock protein 27, exhibiting homologies to Streptococcal antigens | |
|
| |
| Main cell types | |
|
| |
| Adaptive immune cells | Innate immune cells |
| 1. T cells: | Dendritic cells (DCs): |
| (A) CD4+T helper (Th) and CD8+cytotoxic T (Tc) cells | (A) Plasmacytoid dendritic cells (pDCs) |
| Th1 and Tc1 cells Th17 and Tc17 cells |
(B) Myeloid dendritic cells (mDCs) |
| Th22 and Tc22 cells | Neutrophils |
| Th9 cells | Mast cells |
| Follicular T-helper cells Regulatory T cells (Tregs) |
Natural killer cells (NK cells) and NK-T cells |
| Tissue-resident memory T cell | Innate lymphoid cells (ILC)-3: expressing NKp44 |
| (B) γδ T cells | Keratinocytes (KCs) |
| 2. B cells: Regulatory B cells (Bregs) | Macrophages |
|
| |
| Main cytokines | |
|
| |
| Interleukin (IL)-17 (IL-17A to IL-17F) | |
| IL-23/IL-12 | |
| IL-22, IL-24, IL-20 | |
| Tumour necrosis factor (TNF-α), IFN-γ | |
| IL-26 | |
| IL-29(Interferon (IFN)-λ1) | |
| IL-36 | |
| IL-19 | |
| IL-37 | |
| Angiogenic molecules | |
| Vascular endothelial growth factor (VEGF) | |
| Endothelial cell stimulating angiogenesis factor (ESAF) | |
| Platelet-derived growth factors (PDGF) | |
| von Willebrand factor (vWF) | |
| Angiopoietin-1 (ANG1) | |
| Hypoxia inducible factor (HIF)-1α | |
| Epidermal sphingolipids including ceramide and sphingosine-1-phosphate | |
|
| |
| Growth Factors | |
| Epidermal growth factor-receptor (EGFR) and its ligands (EGF family, TGF-α, amphiregulin and betacellulin) | |
| Insulin growth factor (IGF)-1 receptor | |
| Keratinocyte growth factor receptor (KGFR) and its ligand KGF | |
| NGF and its high-affinity receptors such as TrkA and p75 | |
|
| |
| Extracellular matrix components | |
|
| |
| Laminins | |
| Cysteine-rich angiogenic protein 61 (CCN-1) | |
| Osteopontin | |
|
| |
| Mechanotransduction signaling pathways and associated molecules | |
|
| |
| Mechanotransduction signalling pathways and associated molecules | |
| Wnt pathway | |
| Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) pathway | |
| Activator protein-1 pathway | |
| Transient receptor potential channels | |
| Calcium sensing receptor | |
| Polycystin | |
|
| |
| Immunostimulatory ribonucleic acids (RNAs) | |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge the technical expertise of Miss Smrity Sahu (PhD students) for making wonderful sketches and table and for student all the latest references. We also extend our special thanks to Dr. Debajyoti Chatterjee (Assistant Professor, Histopathology) for providing us with histopathology photo-micrographs.
References
- 1.Raychaudhuri SP. A cutting edge overview: Psoriatic disease. Clin Rev Allergy Immunol. 2013;44:109–13. doi: 10.1007/s12016-012-8309-z. [DOI] [PubMed] [Google Scholar]
- 2.Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17:174–8. doi: 10.1046/j.1525-1470.2000.01746.x. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Farewell VT, Pellett F, Schentag C, Rahman P. HLA is a candidate region for psoriatic arthritis. Evidence for excessive HLA sharing in sibling pairs. Hum Immunol. 2003;64:887–9. doi: 10.1016/s0198-8859(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 4.Sakkas LI, Loqueman N, Bird H, Vaughan RW, Welsh KI, Panayi GS. HLA class II and T cell receptor gene polymorphisms in psoriatic arthritis and psoriasis. J Rheumatol. 1990;17:1487–90. [PubMed] [Google Scholar]
- 5.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–91. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010;34:J314–21. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–11. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 8.Tassiulas I, Duncan SR, Centola M, Theofilopoulos AN, Boumpas DT. Clonal characteristics of T cell infiltrates in skin and synovium of patients with psoriatic arthritis. Hum Immunol. 1999;60:479–91. doi: 10.1016/s0198-8859(99)00034-8. [DOI] [PubMed] [Google Scholar]
- 9.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013;44:183–93. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 10.Raychaudhuri SK, Raychaudhuri SP. SCID mouse model of psoriasis: A unique tool for drug development of autoreactive T-cell and Th-17 cell-mediated autoimmune diseases. Indian J Dermatol. 2010;55:157–60. doi: 10.4103/0019-5154.62752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin LM, Coven TR, Bhardwaj N, Steinman R, Krueger JG. Intraepidermal lymphocytes in psoriatic lesions are activated GMP-17(TIA-1)+CD8+CD3+CTLs as determined by phenotypic analysis. J Cutan Pathol. 1998;25:79–88. doi: 10.1111/j.1600-0560.1998.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 12.Diani M, Casciano F, Marongiu L, Longhi M, Altomore A, Pigatto PD. Increased frequency of activated CD8+T cell effectors in patients with psoriatic arthritis. Sci Rep. 2019;9:10870. doi: 10.1038/s41598-019-47310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchlin C, Haas-Smith SA, Hicks D, Cappuccio J, Osterland CK, Looney RJ. Patterns of cytokine production in psoriatic synovium. J Rheumatol. 1998;25:1544–52. [PubMed] [Google Scholar]
- 14.Pap T, Sunderkötter C. Psoriasis versus psoriasisarthritis: Gemeinsamkeiten und unterschiede in der pathophysiologie [Psoriasis vs. psoriatic arthritis: Similarities and differences in the pathophysiology] Z Rheumatol. 2017;76:477–83. doi: 10.1007/s00393-017-0342-0. [DOI] [PubMed] [Google Scholar]
- 15.Winchester R, FitzGerald O. The many faces of psoriatic arthritis: Their genetic determinism. Rheumatology (Oxford) 2020;59:i4–9. doi: 10.1093/rheumatology/kez325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian S, Krueger JG, Li K, Jabbari A, Brodmerkel C, Lowes MA, et al. Meta-analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS One. 2012;7:e44274. doi: 10.1371/journal.pone.0044274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:2552–64. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrick MT, Stuart PE, Raja K, Gudjonsson JE, Tejasvi T, Yang J, et al. Genetic signature to provide robust risk assessment of psoriatic arthritis development in psoriasis patients. Nat Commun. 2018;9:4178. doi: 10.1038/s41467-018-06672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsoi LC, Stuart PE, Tian C, Gudjonsson JE, Das S, Zawistowski M, et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat Commun. 2018;8:15382. doi: 10.1038/ncomms15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan CT, Cao L, Roberson ED, Duan S, Helms CA, Nair RP, et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am J Hum Genet. 2012;90:796–808. doi: 10.1016/j.ajhg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784–95. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, et al. Molecular dissection of psoriasis: Integrating genetics and biology. J Invest Dermatol. 2010;130:1213–26. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 23.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 24.Nickoloff BJ. Keratinocytes regain momentum as instigators of cutaneous inflammation. Trends Mol Med. 2006;12:102–6. doi: 10.1016/j.molmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 26.Hertle MD, Kubler MD, Leigh IM, Watt FM. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992;89:1892–901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iizuka H, Honda H, Ishida-Yamamoto A. Epidermal remodeling in psoriasis (II): A quantitative analysis of the epidermal architecture. J Invest Dermatol. 1997;109:806–10. doi: 10.1111/1523-1747.ep12341002. [DOI] [PubMed] [Google Scholar]
- 28.Costello P, Bresnihan B, O'Farrelly C, FitzGerald O. Predominance of CD8+ T lymphocytes in psoriatic arthritis. J Rheumatol. 1999;26:1117–24. [PubMed] [Google Scholar]
- 29.Laloux L, Voisin MC, Allain J, Martin N, Kerboull L, Chevalier X, et al. Immunohistological study of entheses in spondyloarthropathies: Comparison in rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 2001;60:316–21. doi: 10.1136/ard.60.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragaz A, Ackerman AB. Evolution, maturation, and regression of lesions of psoriasis. New observations and correlation of clinical and histologic findings. Am J Dermatopathol. 1979;1:199–214. doi: 10.1097/00000372-197900130-00002. [DOI] [PubMed] [Google Scholar]
- 31.Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524–8. doi: 10.1016/j.clindermatol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Altman EM, Kamino H. Diagnosis: Psoriasis or not? What are the clues. Semin Cutan Med Surg. 1999;18:25–35. doi: 10.1016/s1085-5629(99)80005-4. [DOI] [PubMed] [Google Scholar]
- 33.Nickoloff BJ, Bonish BK, Marble DJ, Schriedel KA, Di Pietro LA, Gordon KB, et al. Lessons learned from psoriatic plaques concerning mechanisms of tissue repair, remodeling, and inflammation. J Investig Dermatol Symp Proc. 2006;11:16–29. doi: 10.1038/sj.jidsymp.5650010. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Yin M, Zhang LJ. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells. 2019;8:807. doi: 10.3390/cells8080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elango T, Sun J, Zhu C, Zhou F, Zhang Y, Sun L, et al. Mutational analysis of epidermal and hyperproliferative type I keratins in mild and moderate psoriasis vulgaris patients: A possible role in the pathogenesis of psoriasis along with disease severity. Hum Genomics. 2018;12:27. doi: 10.1186/s40246-018-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand S, Gupta P, Bhardwaj R, Narang T, Dogra S, Minz RW, et al. Is psoriasis an autoimmune disease? Interpretations from an immunofluorescence-based study. J Cutan Pathol. 2017;44:346–51. doi: 10.1111/cup.12891. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb AB, Lebwohl M, Shirin S, Sherr A, Gilleaudeau P, Singer G, et al. Anti-CD4 monoclonal antibody treatment of moderate to severe psoriasis vulgaris: Results of a pilot, multicenter, multiple-dose, placebo-controlled study. J Am Acad Dermatol. 2000;43:595–604. doi: 10.1067/mjd.2000.107945. [DOI] [PubMed] [Google Scholar]
- 38.Raychaudhuri SP, Kundu-Raychaudhuri S, Tamura K, Masunaga T, Kubo K, Hanaoka K, et al. FR255734, a humanized, Fc-Silent, Anti-CD28 antibody, improves psoriasis in the SCID mouse-psoriasis xenograft model. J Invest Dermatol. 2008;128:1969–76. doi: 10.1038/jid.2008.38. [DOI] [PubMed] [Google Scholar]
- 39.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–9. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 40.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti–interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–9. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 41.McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: A 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349–56. doi: 10.1136/annrheumdis-2012-202646. [DOI] [PubMed] [Google Scholar]
- 42.Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–87. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19:179. doi: 10.3390/ijms19010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston A, Gudjonsson JE, Sigmundsdottir H, Love TJ, Valdimarsson H. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8(+) T cells. Clin Exp Immunol. 2004;138:83–93. doi: 10.1111/j.1365-2249.2004.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diluvio L, Vollmer S, Besgen P, Ellwart JW, Chimenti S, Prinz JC. Identical TCR beta-chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgaris. J Immunol. 2006;176:7104–11. doi: 10.4049/jimmunol.176.11.7104. [DOI] [PubMed] [Google Scholar]
- 46.Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Gudjonsson JE, Johnston A. Psoriasis--as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494–501. doi: 10.1016/j.it.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5:5621. doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- 48.Arakawa A, Siewert K, Stöhr J, Besgen P, Kim SM, Rühl G, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203–12. doi: 10.1084/jem.20151093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung KL, Jarrett R, Subramaniam S, Salimi M, Gutowska-Owsiak D, Chen YL, et al. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399–412. doi: 10.1084/jem.20160258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben Abdallah H, Johansen C, Iversen L. Key signaling pathways in psoriasis: Recent insights from antipsoriatic therapeutics. Psoriasis (Auckl) 2021;11:83–97. doi: 10.2147/PTT.S294173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi T, Yamasaki K. Psoriasis and antimicrobial peptides. Int J Mol Sci. 2020;21:6791. doi: 10.3390/ijms21186791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–85. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson-Huang LM, Lowes MA, Krueger JG. Putting together the psoriasis puzzle: An update on developing targeted therapies. Dis Model Mech. 2012;5:423–33. doi: 10.1242/dmm.009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haider AS, Lowes MA, Suárez-Fariñas M, Zaba LC, Cardinale I, Khatcherian A, et al. Identification of cellular pathways of “type 1,” Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J Immunol. 2008;180:1913–20. doi: 10.4049/jimmunol.180.3.1913. [DOI] [PubMed] [Google Scholar]
- 55.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–16. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, et al. Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 58.Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ILC3 in psoriasis. J Invest Dermatol. 2014;134:984–91. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grän F, Kerstan A, Serfling E, Goebeler M, Muhammad K. Current developments in the immunology of psoriasis. Yale J Biol Med. 2020;93:97–110. [PMC free article] [PubMed] [Google Scholar]
- 60.Petit RG, Cano A, Ortiz A, Espina M, Prat J, Muñoz M, et al. Psoriasis: From pathogenesis to pharmacological and nano-technological-based therapeutics. Int J Mol Sci. 2021;22:4983. doi: 10.3390/ijms22094983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–83. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chhabra S, Narang T, Joshi N, Goel S, Sawatkar G, Saikia B, et al. Circulating T-helper 17 cells and associated cytokines in psoriasis. Clin Exp Dermatol. 2016;41:806–10. doi: 10.1111/ced.12845. [DOI] [PubMed] [Google Scholar]
- 63.Yilmaz SB, Cicek N, Coskun M, Yegin O, Alpsoy E. Serum and tissue levels of IL-17 in different clinical subtypes of psoriasis. Arch Dermatol Res. 2012;304:465–9. doi: 10.1007/s00403-012-1229-1. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Chen X, Liu Z, Yue Q, Liu H. Expression of Th17 cytokines in skin lesions of patients with psoriasis. J Huazhong Univ Sci Technolog Med Sci. 2007;27:330–2. doi: 10.1007/s11596-007-0329-1. [DOI] [PubMed] [Google Scholar]
- 65.Chiricozzi A, Cannizzaro MV, Salandri GA, Marinari B, Pitocco R, Dattola A, et al. Increased levels of IL-17 in tear fluid of moderate-to-severe psoriatic patients is reduced by adalimumab therapy. J Eur Acad Dermatol Venereol. 2016;30:e128–9. doi: 10.1111/jdv.13403. [DOI] [PubMed] [Google Scholar]
- 66.Chiricozzi A, Suárez-Fariñas M, Fuentes-Duculan J, Cueto I, Li K, Tian S, et al. Increased expression of interleukin-17 pathway genes in nonlesional skin of moderate-to-severe psoriasis vulgaris. Br J Dermatol. 2016;174:136–45. doi: 10.1111/bjd.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem. 2012;359:419–29. doi: 10.1007/s11010-011-1036-6. [DOI] [PubMed] [Google Scholar]
- 68.Michalak-Stoma A, Bartosińska J, Kowal M, Raczkiewicz D, Krasowska D, Chodorowska G. IL-17A in the psoriatic patients' serum and plaque scales as potential marker of the diseases severity and obesity. Mediators Inflamm. 2020;2020:7420823. doi: 10.1155/2020/7420823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Girolomoni G, Mrowietz U, Paul C. Psoriasis: Rationale for targeting interleukin-17. Br J Dermatol. 2012;167:717–24. doi: 10.1111/j.1365-2133.2012.11099.x. [DOI] [PubMed] [Google Scholar]
- 71.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutowska-Owsiak D, Schaupp AL, Salimi M, Selvakumar TA, McPherson T, Taylor S, et al. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp Dermatol. 2012;21:104–10. doi: 10.1111/j.1600-0625.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 73.Koshy PJ, Henderson N, Logan C, Life PF, Cawston TE, Rowan AD. Interleukin 17 induces cartilage collagen breakdown: Novel synergistic effects in combination with proinflammatory cytokines. Ann Rheum Dis. 2002;61:704–13. doi: 10.1136/ard.61.8.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chabaud M, Lubberts E, Joosten L, van Den Berg W, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001;3:168–77. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koenders MI, Kolls JK, Oppers-Walgreen B, van den Bersselaar L, Joosten LA, Schurr JR, et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 2005;52:3239–47. doi: 10.1002/art.21342. [DOI] [PubMed] [Google Scholar]
- 76.Menter A, Krueger GG, Paek SY, Kivelevitch D, Adamopoulos IE, Langley RG. Interleukin-17 and interleukin-23: A aarrative review of mechanisms of action in psoriasis and associated comorbidities. Dermatol Ther (Heidelb) 2021;11:385–400. doi: 10.1007/s13555-021-00483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vidal S, Puig L, Carrascosa-Carrillo JM, González-Cantero Á, Ruiz-Carrascosa JC, Velasco-Pastor AM. From messengers to receptors in psoriasis: The role of IL-17RA in disease and treatment. Int J Mol Sci. 2021;22:6740. doi: 10.3390/ijms22136740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu M, Lu H, Lee YH, Wu Y, Liu K, Shi Y, et al. An interleukin-25-mediated autoregulatory circuit in keratinocytes plays a pivotal role in psoriatic skin inflammation. Immunity. 2018;48:787–98.e4. doi: 10.1016/j.immuni.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 79.Gaber T, Strehl C, Buttgereit F. Metabolic regulation of inflammation. Nat Rev Rheumatol. 2017;13:267–79. doi: 10.1038/nrrheum.2017.37. [DOI] [PubMed] [Google Scholar]
- 80.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–65. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathis D. Organismal immunometabolism: Advances in both directions. Nat Rev Immunol. 2019;19:83–4. doi: 10.1038/s41577-018-0118-z. [DOI] [PubMed] [Google Scholar]
- 83.Troha K, Ayres JS. Metabolic adaptations to infections at the organismal level. Trends Immunol. 2020;411:113–25. doi: 10.1016/j.it.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lou F, Sun Y, Xu Z, Niu L, Wang Z, Deng S, et al. Excessive polyamine generation in keratinocytes promotes self-RNA sensing by dendritic cells in psoriasis. Immunity. 2020;53:204–16. doi: 10.1016/j.immuni.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Bandyopadhyay M, Larregina AT. Keratinocyte-polyamines and dendritic cells: A bad duet for psoriasis. Immunity. 2020;53:16–8. doi: 10.1016/j.immuni.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 86.Varshney P, Narasimhan A, Mittal S, Malik G, Sardana K, Saini N. Transcriptome profiling unveils the role of cholesterol in IL-17A signaling in psoriasis. Sci Rep. 2016;6:19295. doi: 10.1038/srep19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qu N, Xu M, Mizoguchi I, Furusawa J, Kaneko K, Watanabe K, et al. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Cli Dev Immunol. 2013;2013:968549. doi: 10.1155/2013/968549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol. 2009;129:302–8. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: Enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–15. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 90.Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–30. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fotiadou C, Lazaridou E, Sotiriou E, Gerou S, Kyrgidis A, Vakirlis E, et al. IL-17A, IL-22, and IL-23 as markers of psoriasis activity: A cross-sectional, hospital-based study. J Cutan Med Surg. 2015;19:555–60. doi: 10.1177/1203475415584503. [DOI] [PubMed] [Google Scholar]
- 92.Yawalkar N, Karlen S, Hunger R, Brand CU, Braathen LR. Expression of interleukin-12 is increased in psoriatic skin. J Invest Dermatol. 1998;111:1053–7. doi: 10.1046/j.1523-1747.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 93.Cheng J, Tu Y, Li J, Huang C, Liu Z, Liu D. A study on the expression of interleukin (IL)-10 and IL-12 p35, p40 mRNA in the psoriatic lesions. J Tongji Med Univ. 2001;21:86–8. doi: 10.1007/BF02888047. [DOI] [PubMed] [Google Scholar]
- 94.Lebwohl M. Interleukin-23 blockade: Another breakthrough in the treatment of psoriasis. Lancet. 2019;394:544–46. doi: 10.1016/S0140-6736(19)31513-2. [DOI] [PubMed] [Google Scholar]
- 95.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–40. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 96.Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: A novel immunological cascade with potential relevance in psoriasis. Eur J Immunol. 2009;39:3570–81. doi: 10.1002/eji.200939687. [DOI] [PubMed] [Google Scholar]
- 97.Chiricozzi A, Romanelli M, Panduri S, Donetti E, Prignano F. Relevance of in vitro 3-D skin models in dissecting cytokine contribution to psoriasis pathogenesis. Histol Histopathol. 2017;32:893–8. doi: 10.14670/HH-11-877. [DOI] [PubMed] [Google Scholar]
- 98.Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, et al. Skin inflammation induced by the synergistic sction of IL-17A, IL-22, oncostatin M, IL-1{alpha}, and TNF-{alpha} recapitulates some features of psoriasis. J Immunol. 2010;184:5263–70. doi: 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- 99.Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 100.Donetti E, Cornaghi L, Arnaboldi F, Landoni F, Romagnoli P, Mastroianni N, et al. Interleukin 22 early affects keratinocyte differentiation, but not proliferation, in a three-dimensional model of normal human skin. Exp Cell Res. 2016;345:247–54. doi: 10.1016/j.yexcr.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 101.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;74:3695–702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 102.Jiang Q, Yang G, Xiao F, Xie J, Wang S, Lu L, Cui D. Role of Th22 cells in the pathogenesis of autoimmune diseases. Front Immunol. 2021;12:688066. doi: 10.3389/fimmu.2021.688066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol (Berl) 2009;87:523–36. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 104.Tohyama M, Hanakawa Y, Shirakata Y, Dai X, Yang L, Hirakawa S, et al. IL-17 and IL-22 mediate IL-20 subfamily cytokine production in cultured keratinocytes via increased IL-22 receptor expression. Eur J Immunol. 2009;39:2779–88. doi: 10.1002/eji.200939473. [DOI] [PubMed] [Google Scholar]
- 105.Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RD. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994;96:146–51. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;5:273–9. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caldarola G, De Simone C, Carbone A, Tulli A, Amerio P, Feliciani C. TNF-alpha and its receptors in psoriatic skin, before and after treatment with etanercept. Int J Immunopathol Pharmacol. 2009;22:961–6. doi: 10.1177/039463200902200411. [DOI] [PubMed] [Google Scholar]
- 108.Wakefield PE, James WD, Samlaska CP, Meltzer MS. Tumor necrosis factor. J Am Acad deramtol. 1991;24:675–85. doi: 10.1016/0190-9622(91)70102-8. [DOI] [PubMed] [Google Scholar]
- 109.Rajzer L, Wojas-Pelc A. Rola cytokin uwalnianych przez keratynocyty w patogenezie łuszczycy [The role of cytokines released by keratinocytes in psoriasis pathogenesis] Przegl Lek. 2009;66:150–4. [PubMed] [Google Scholar]
- 110.Johnson-Huang LM, McNutt NS, Krueger JG, Lowes MA. Cytokine-producing dendritic cells in the pathogenesis of inflammatory skin diseases. J Clin Immunol. 2009;29:247–56. doi: 10.1007/s10875-009-9278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci U S A. 2005;102:19057–62. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–94. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004;279:32633–42. doi: 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]
- 114.Silvagni E, Missiroli S, Perrone M, Patergnani S, Boncompagni C, Bortoluzzi A, et al. From bed to bench and back: TNF-α, IL-23/IL-17A, and JAK-dependent inflammation in the pathogenesis of psoriatic synovitis. Front Pharmacol. 2021;12:672515. doi: 10.3389/fphar.2021.672515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhuang L, Ma W, Cai D, Zhong H, Sun Q. Associations between tumor necrosis factor-α polymorphisms and risk of psoriasis: A meta-analysis. PLoS One. 2013;8:e68827. doi: 10.1371/journal.pone.0068827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sarkar S, Panda S, Kim B, Raychaudhuri SK, Ghosh A, Raychaudhuri SP. Risk of tuberculosis with anti-tumor necrosis factor-alpha therapy in patients with psoriasis and psoriatic arthritis in Indian population. Indian J Dermatol Venereol Leprol. 2020;86:1–7. doi: 10.4103/ijdvl.IJDVL_791_18. [DOI] [PubMed] [Google Scholar]
- 117.Rajabi Dehnavi P, Eftekhari SM, Kadkhodaei A, Kefayat A. CD137: A member of the TNFR family- in psoriasis skin lesions in comparison with normal skin specimens. Iran J Pathol. 2021;16:103–8. doi: 10.30699/IJP.2020.118767.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Albanesi C, Scarponi C, Cavani A, Federici M, Nasorri F, Girolomoni G. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma- and interleukin-4-induced activation of human keratinocytes. J Invest Dermatol. 2000;115:81–7. doi: 10.1046/j.1523-1747.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 119.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: Mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–41. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnson-Huang LM, Suárez-Fariñas M, Pierson KC, Fuentes-Duculan J, Cueto I, Lentini T, et al. A single intradermal injection of IFN-γ induces an inflammatory state in both non-lesional psoriatic and healthy skin. J Invest Dermatol. 2012;132:1177–87. doi: 10.1038/jid.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fu LX, Chen T, Guo ZP, Cao N, Zhang LW, Zhou PM. Enhanced serum interferon-lambda 1 interleukin-29 levels in patients with psoriasis vulgaris. An bras Dermatol. 2021;96:416–21. doi: 10.1016/j.abd.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397:754–66. doi: 10.1016/S0140-6736(21)00184-7. [DOI] [PubMed] [Google Scholar]
- 123.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397:1301–15. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- 124.Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: Role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961–71. doi: 10.2353/ajpath.2008.070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ben-Bassat H. Biological activity of tyrosine kinase inhibitors: Novel agents for psoriasis therapy. Curr Opin Investig Drugs. 2001;2:1539–45. [PubMed] [Google Scholar]
- 126.Forsberg S, Ostman A, Rollman O. Regeneration of human epidermis on acellular dermis is impeded by small-molecule inhibitors of EGF receptor tyrosine kinase. Arch Dermatol Res. 2008;300:505–16. doi: 10.1007/s00403-008-0853-2. [DOI] [PubMed] [Google Scholar]
- 127.Krueger JG, Krane JF, Carter DM, Gottlieb AB. Role of growth factors, cytokines, and their receptors in the pathogenesis of psoriasis. J Invest Dermatol. 1990;94:135S–40S. doi: 10.1111/1523-1747.ep12876121. [DOI] [PubMed] [Google Scholar]
- 128.Poumay Y, Mitev V. Members of the EGF receptor family in normal and pathological epidermis. Folia Med (Plovdiv) 2009;51:5–17. [PubMed] [Google Scholar]
- 129.Krane JF, Gottlieb AB, Carter DM, Krueger JG. The insulin-like growth factor I receptor is overexpressed in psoriatic epidermis, but is differentially regulated from the epidermal growth factor receptor. J Exp Med. 1992;175:1081–90. doi: 10.1084/jem.175.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Finch PW, Murphy F, Cardinale I, Krueger JG. Altered expression of keratinocyte growth factor and its receptor in psoriasis. Am J Pathol. 1997;151:1619–28. [PMC free article] [PubMed] [Google Scholar]
- 131.Yoshida A, Kanno H, Watabe D, Akasaka T, Sawai T. The role of heparin-binding EGF-like growth factor and amphiregulin in the epidermal proliferation of psoriasis in cooperation with TNFalpha. Arch Dermatol Res. 2008;300:37–45. doi: 10.1007/s00403-007-0809-y. [DOI] [PubMed] [Google Scholar]
- 132.McKay IA, Leigh IM. Altered keratinocyte growth and differentiation in psoriasis. Clin Dermatol. 1995;13:105–14. doi: 10.1016/0738-081x(95)93817-8. [DOI] [PubMed] [Google Scholar]
- 133.Powell TJ, Ben-Bassat H, Klein BY, Chen H, Shenoy N, McCollough J, et al. Growth inhibition of psoriatic keratinocytes by quinazoline tyrosine kinase inhibitors. Br J Dermatol. 1999;141:802–10. doi: 10.1046/j.1365-2133.1999.03152.x. [DOI] [PubMed] [Google Scholar]
- 134.Raychaudhuri SK, Raychaudhuri SP, Weltman H, Farber EM. Effect of nerve growth factor on endothelial cell biology: Proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch Dermatol Res. 2001;293:291–5. doi: 10.1007/s004030100224. [DOI] [PubMed] [Google Scholar]
- 135.Raychaudhuri SP, Sanyal M, Weltman H, Kundu-Raychaudhuri S. K252a, a high-affinity nerve growth factor receptor blocker, improves psoriasis: An in vivo study using the severe combined immunodeficient mouse-human skin model. J Invest Dermatol. 2004;122:812–9. doi: 10.1111/j.0022-202X.2003.12602.x. [DOI] [PubMed] [Google Scholar]
- 136.Raychaudhuri SP, Raychaudhuri SK. The regulatory role of nerve growth factor and its receptor system in fibroblast-like synovial cells. Scand J Rheumatol. 2009;38:207–15. doi: 10.1080/03009740802448866. [DOI] [PubMed] [Google Scholar]
- 137.Rosengren S, Corr M, Boyle DL. Platelet-derived growth factor and transforming growth factor beta synergistically potentiate inflammatory mediator synthesis by fibroblast-like synoviocytes. Arthritis Res Ther. 2010;12:R65. doi: 10.1186/ar2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: A new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470–7. doi: 10.1111/j.1749-6632.2009.04652.x. [DOI] [PubMed] [Google Scholar]
- 139.Jiang M, Fang H, Shao S, Dang E, Zhang J, Qiao P, et al. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019;33:13241–53. doi: 10.1096/fj.201900642R. [DOI] [PubMed] [Google Scholar]
- 140.Shi Z, Qiu X, Zheng L, Liu X, Hong D, He M, et al. ULK1 inhibition as a targeted therapeutic strategy for psoriasis by regulating keratinocytes and their crosstalk with neutrophils. Front Immunol. 2021;12:3096. doi: 10.3389/fimmu.2021.714274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rawat A, Rinchai D, Toufiq M, Marr AK, Kino T, Garand M, et al. A neutrophil-driven inflammatory signature characterizes the blood transcriptome fingerprint of psoriasis. Front Immunol. 2020;11:587946. doi: 10.3389/fimmu.2020.587946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chiang CC, Cheng WJ, Korinek M, Lin CY, Hwang TL. Neutrophils in psoriasis. Front Immunol. 2019;10:2376. doi: 10.3389/fimmu.2019.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schön MP, Broekaert SM, Erpenbeck L. Sexy again: The renaissance of neutrophils in psoriasis. Exp Dermatol. 2017;26:305–11. doi: 10.1111/exd.13067. [DOI] [PubMed] [Google Scholar]
- 144.Herster F, Bittner Z, Archer NK, Dickhöfer S, Eisel D, Eigenbrod T, et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat Commun. 2020;11:105. doi: 10.1038/s41467-019-13756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ottaviani C, Nasorri F, Bedini C, de Pità O, Girolomoni G, Cavani A. CD56bright CD16(-) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–28. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 146.Polese B, Zhang H, Thurairajah B, King IL. Innate Lymphocytes in Psoriasis. Front Immunol. 2020;11:242. doi: 10.3389/fimmu.2020.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sato Y, Ogawa E, Okuyama R. Role of innate immune cells in psoriasis? Int J Mol Sci. 2020;21:6604. doi: 10.3390/ijms21186604. doi: 10.3390/ijms21186604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nussbaum L, Chen YL, Ogg GS. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol. 2021;184:14–24. doi: 10.1111/bjd.19380. [DOI] [PubMed] [Google Scholar]
- 150.Weiss E, Mamelak AJ, La Morgia S, Wang B, Feliciani C, Tulli A, et al. The role of interleukin 10 in the pathogenesis and potential treatment of skin diseases. J Am Acad Dermatol. 2004;50:657–78. doi: 10.1016/j.jaad.2003.11.075. [DOI] [PubMed] [Google Scholar]
- 151.Debes GF, Mc Gettigan SE. Skin-associated B cells in health and inflammation. J Immunol. 2019;202:1659–66. doi: 10.4049/jimmunol.1801211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Micali G, Verzì AE, Broggi G, Caltabiano R, Musumeci ML, Lacarrubba F. Evaluation of capillary density in psoriasis: An intrapatient study and literature review. PloS One. 2021;16:e0247835. doi: 10.1371/journal.pone.0247835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lacarrubba F, Pellacani G, Gurgone S, Verzì AE, Micali G. Advances in non-invasive techniques as aids to the diagnosis and monitoring of therapeutic response in plaque psoriasis: A review. Int J Dermatol. 2015;54:626–34. doi: 10.1111/ijd.12870. [DOI] [PubMed] [Google Scholar]
- 154.Liew SC, Das-Gupta E, Chakravarthi S, Wong SF, Lee N, Safdar N, et al. Differential expression of the angiogenesis growth factors in psoriasis vulgaris. BMC Res Notes. 2012;5:201. doi: 10.1186/1756-0500-5-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Batani A, Brănişteanu DE, Ilie MA, Boda D, Ianosi S, Ianosi G, et al. Assessment of dermal papillary and microvascular parameters in psoriasis vulgaris using in vivo reflectance confocal microscopy. Exp Ther Med. 2017;15:1241–6. doi: 10.3892/etm.2017.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu Y, Yang Y, Zhang J, Zhang H, Ma C, Tang X, et al. Anti-angiogenic efficacy of PSORI-CM02 and the associated mechanism in psoriasis in vitro and in vivo. Front Immunol. 2021;12:649591. doi: 10.3389/fimmu.2021.649591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Akhtar T, Wani WY, Kamal MA, Kaur R. Role of angiogenic growth factors in psoriasis: A review. Curr Drug Metab. 2018;19:910–6. doi: 10.2174/1389200219666180416162942. [DOI] [PubMed] [Google Scholar]
- 158.Nickoloff BJ, Mitra RS, Varani J, Dixit VM, Polverini PJ. Aberrant production of interleukin-8 and thrombospondin-1 by psoriatic keratinocytes mediates angiogenesis. Am J Pathol. 1994;144:820–8. [PMC free article] [PubMed] [Google Scholar]
- 159.Gong J, Yang H, Qi D, Tang X. The association of serum vascular endothelial growth factor levels and psoriasis vulgari. Medicine. 2020;99:e21565. doi: 10.1097/MD.0000000000021565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Elias PM, Arbiser J, Brown BE, Rossiter H, Man MQ, Cerimele F, et al. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: Implications for the pathogenesis of psoriasis. Am J Pathol. 2008;173:689–99. doi: 10.2353/ajpath.2008.080088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jiang M, Li B, Zhang J, Hu L, Dang E, Wang G. Vascular endothelial growth factor driving aberrant keratin expression pattern contributes to the pathogenesis of psoriasis. Exp Cell Res. 2017;360:310–9. doi: 10.1016/j.yexcr.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 162.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 163.Rioux G, Ridha Z, Simard M, Turgeon F, Guérin SL, Pouliot R. Transcriptome profiling analyses in psoriasis: A dynamic contribution of keratinocytes to the pathogenesis. Genes (Basel) 2020;11:1155. doi: 10.3390/genes11101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bocheńska K, Gabig-Cimińska M. Unbalanced sphingolipid metabolism and Its implications for the pathogenesis of psoriasis. Molecules. 2020;25:1130. doi: 10.3390/molecules25051130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Georgescu SR, Tampa M, Caruntu C, Sarbu MI, Mitran CI, Mitran MI, et al. Advances in understanding the immunological pathways in psoriasis. Int J Mol Sci. 2019;20:739. doi: 10.3390/ijms20030739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bhattacharjee O, Ayyangar U, Kurbet AS, Ashok D, Raghavan S. Unraveling the ECM-immune cell crosstalk in skin diseases. Front Cell Dev Biol. 2019;7:68. doi: 10.3389/fcell.2019.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Malakou LS, Gargalionis AN, Piperi C, Papadavid E, Papavassiliou AG, Basdra EK. Molecular mechanisms of mechanotransduction in psoriasis. Ann Transl Med. 2018;6:245. doi: 10.21037/atm.2018.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Seetharaman S, Etienne-Manneville S. Integrin diversity brings specificity in mechanotransduction. Biol Cell. 2018;110:49–64. doi: 10.1111/boc.201700060. [DOI] [PubMed] [Google Scholar]
- 169.Aubin F. What's new in dermatological research? Ann Dermatol Vééréol. 2011;138:S233–40. doi: 10.1016/S0151-9638(11)70096-5. [DOI] [PubMed] [Google Scholar]
- 170.Liu G, Gack MU. Distinct and orchestrated functions of RNA sensors in innate immunity. Immunity. 2020;53:26–42. doi: 10.1016/j.immuni.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mc Whirter SM, Jefferies CA. Nucleic acid sensors as therapeutic targets for human disease. Immunity. 2020;53:78–97. doi: 10.1016/j.immuni.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 172.Song JK, Yin SY, Li W, Li XD, Luo Y, Luo Y, et al. An update on the role of long non-coding RNAs in psoriasis. Chin Med J (Engl) 2020;134:379–89. doi: 10.1097/CM9.0000000000001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yan J, Song J, Qiao M, Zhao X, Li R, Jiao J, et al. Long noncoding RNA expression profile and functional analysis in psoriasis. Mol Med Rep. 2019;19:3421–30. doi: 10.3892/mmr.2019.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Elamir AM, Shaker OG, El-Komy MH, Mahmoud Sharabi M, Aboraia NM. The role of LncRNA MALAT-1 and MiRNA-9 in psoriasis. Biochem Biophys Rep. 2021;26:101030. doi: 10.1016/j.bbrep.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ghafouri-Fard S, Eghtedarian R, Taheri M, Rakhshan A. The eminent roles of ncRNAs in the pathogenesis of psoriasis. Noncoding RNA Res. 2020;5:99–108. doi: 10.1016/j.ncrna.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tang L, Liang Y, Xie H, Yang X, Zheng G. Long non-coding RNAs in cutaneous biology and proliferative skin diseases: Advances and perspectives. Cell Prolif. 2020;53:e12698. doi: 10.1111/cpr.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Farber EM, Raychaudhuri SP. Concept of total care: A third dimension in the treatment of psoriasis. Cutis. 1997;59:35–9. [PubMed] [Google Scholar]
- 178.Raychaudhuri SK, Chatterjee S, Nguyen C, Kaur M, Jialal I, Raychaudhuri SP. Increased prevalence of the metabolic syndrome in patients with psoriatic arthritis. Metab Syndr Relat Disord. 2010;8:331–4. doi: 10.1089/met.2009.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Siegel D, Devaraj S, Mitra A, Raychaudhuri SP, Raychaudhuri SK, Jialal I. Inflammation, atherosclerosis, and psoriasis. Clin Rev Allergy Immunol. 2013;44:194–204. doi: 10.1007/s12016-012-8308-0. [DOI] [PubMed] [Google Scholar]
- 180.Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, Goh I, et al. Developmental cell programs are co-opted in inflammatory skin disease. Science. 2021;371:eaba6500. doi: 10.1126/science.aba6500. [DOI] [PMC free article] [PubMed] [Google Scholar]


