Abstract
Ozone is a strong oxidizing agent, capable of promoting therapeutic effects such as antimicrobial, anti inflammatory, antioxidant and healing activities, with low probability of toxicity when used in a specific dosage range. The aim of this study was to conduct a literature review to assess clinical trials available over the past 10 years regarding the effectiveness and safety of ozone therapy to treat dermatological disorders. The search for bibliographic material was carried out through PubMed, Cochrane Library and Google Scholar electronic databases. The inclusion criteria covered only controlled clinical trials published from 2011 to 2021 and written in English. The 18 selected clinical trials included 1279 patients (ranging from 12 to 400 patients per study), of which 1185 patients were adults and 94 were children. Ozone therapy was evaluated regarding the treatment of diabetic foot ulcers, digital ulcers, chronic venous leg ulcers, atopic dermatitis, skin burns, onychomycosis, tinea pedis, cutaneous leishmaniasis, balanitis xerotica obliterans and multiple common warts. Only three studies, addressing the treatment of cutaneous leishmaniasis, skin ulcers and skin burns, evidenced lack of effectiveness of ozone treatment. Mild adverse effects occurred in three clinical trials, whereas severe side effects occurred in only one clinical trial, regarding skin ulcers. Therefore, ozone therapy may be suggested as an alternative or complementary treatment in some types of dermatological conditions specially affecting refractory patients. Though, a greater number of high-quality clinical trials is needed to clearly establish the safety of ozone therapy in dermatological disorders.
KEY WORDS: Clinical trial, dermatology, ozone, review, skin diseases
Introduction
The ozone gas (O3) is a strong oxidizing agent with therapeutic properties, such as anti-inflammatory,[1] antioxidant[2] and healing[3,4] effects, besides activity against bacteria,[5] viruses,[6] fungi,[7] and protozoa.[8] Thus, ozone therapy has been suggested as an alternative treatment in dentistry[9] and in medical fields, such as dermatology. Its use has been proposed in cases of acne, eczema, atopic dermatitis, psoriasis, herpes zoster, pyoderma, mycosis, and also in skin wound healing.[10]
Although some studies evidence ozone toxicity,[11,12] it is estimated that only chronic exposure (0.7 mg/day) is capable to produce toxic effects due to the constant oxidative stress generation. In contrast, acute exposure to low or moderate levels (1–10 mg/day) generates moderate oxidative stress for a short time, which is able to stimulate the body's antioxidant system without causing injury.[10,13,14]
There are three main ways of topically applying ozone: Gaseous ozone, ozone-saturated solutions and ozonized vegetable oils. Gaseous ozone can be administered through a plastic bag containing a mixture of ozone and oxygen, which must cover the affected skin area during the treatment session. Otherwise, ozone hydrotherapy consists of washing the injured skin with ozonized solutions, such as ozonized water, while ozonized vegetable oils can be applied directly to the skin or by the use of dressings. Ozone can also be applied through intravenous, intramuscular, subcutaneous, intradermal, intraarticular and rectal routes of administration.[15,16] Another form of application is through autohemotherapy, which consists of the removal and ozonation of the patient's blood and subsequent intravenous reintroduction.[15]
In this context, the use of ozone therapy as an alternative dermatological treatment can be proposed, as it could benefit patients who are refractory or intolerant to conventional therapy.[17] Therefore, the aim of this article was to conduct a literature review to assess clinical trials available over the past 10 years regarding the effectiveness and safety of ozone therapy to treat dermatological conditions. The present investigation is the first to propose a compilation of data from current evidence in controlled clinical trials on the risk-benefit of using ozone specifically in dermatology.
Methodology
The type of review conducted was a literature systematized review. The search for bibliographic material was carried out until December 2021 through PubMed, Cochrane Library and Google Scholar databases using the terms (ozone therapy OR ozonized oils) AND (skin OR dermatology). The inclusion criteria for articles' selection covered only controlled clinical trials with an approach focused on the treatment of skin, hair or nail conditions and articles published from 2011 to 2021. Articles written in languages other than English were excluded.
The methodological quality of the studies was assessed according to the following criteria: Blinded, randomized and controlled clinical trials, treatment period longer than 30 days (in order to evaluate effectiveness and acute toxic side effects) and sample size equal to or greater than the average number of participants involved in all studies (n = 71). Studies that fit these criteria were considered by the review authors to be at lower risk of bias.
Results
After conducting the literature search and analyzing the articles titles and abstracts, 30 clinical trials approaching the use of ozone therapy to treat dermatological diseases were identified. Among these, 12 studies were excluded for not meeting the inclusion criteria: One study was published in 2002, six studies were written in different languages than English and five studies were conducted without control groups. A total of 18 trials met the inclusion criteria and were selected to compose the present review [Figure 1]. Figure 2 presents an overview of the included clinical trials and Table 1 summarizes the most relevant data and main results from each study.[18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]
Figure 1.
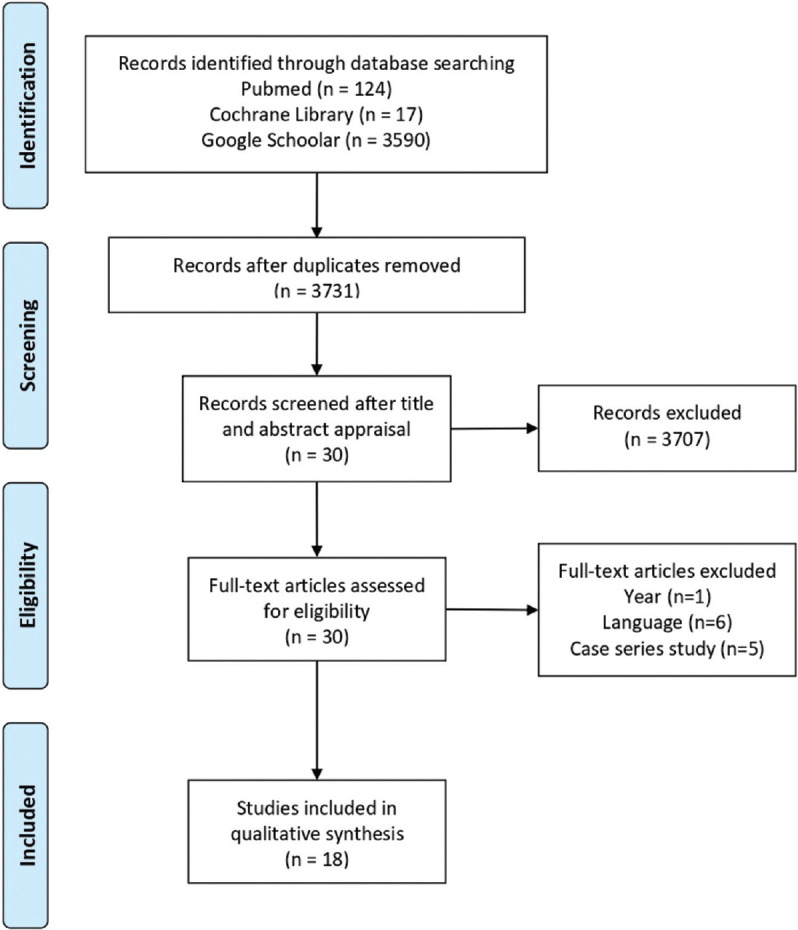
Methodology used for searching and selecting articles
Figure 2.
An overview of the clinical trials included in the present review. *( ): Contains the number of clinical trials found for each dermatological condition
Table 1.
Clinical trials using ozone therapy for the treatment of dermatological disorders
| Author (year) | Treated Disorder | Study Design; Sample Size (n) | Intervention | Treatment Period | Improvements in Clinical Conditions Compared to CG |
|---|---|---|---|---|---|
| Hassan et al. (2011)[18] | Diabetic foot ulcers | Randomized controlled clinical trial (n=60) | OG: Bagging gaseous ozone combined or not with rectal insufflation + subcutaneous injections CG: Infrared laser or ST only |
3 months | Significant increase in the healing percentage in patients treated with ozone or infrared laser, both compared to ST only Improvement of vascular parameters |
| Wainstein et al. (2011)[19] | Diabetic foot ulcers | Double-blind, randomized, placebo-controlled, multileft clinical trial (n=61) | OG: Bagging gaseous ozone +ST CG: Sham treatment with room air only + ST |
12 weeks/ Reassessment at week 24 | Significant higher proportion of patients with total closure of wounds (81% vs. 44%, P=0.03) |
| Zhang et al. (2014)[20] | Diabetic foot ulcers | Randomized controlled clinical trial (n=50) | OG: Bagging gaseous ozone + ST CG: ST only |
20 days | Significant wound size reduction (P<0.001) Higher levels of collagen content and growth factors expression in wound tissue (P<0.05) Overall effective rate: 92% (P=0.037) |
| Solovąstru et al. (2015)[21] | Venous leg ulcers | Randomized controlled clinical trial (n=29) | OG: Spray formulation containing 15% of ozonated sunflower oil+1% of α-bisabolol CG: Standard cream containing vitamin A, vitamin E, talc, and zinc oxide |
30 days | Significant reduction of the ulcer surface (P<0.05) Complete ulcer healing was observed in 25% of patients treated with ozone (P<0.05) |
| Teuvov et al. (2017)[22] | Diabetic foot ulcers | Controlled clinical trial (n=37) | OG: Intravenous ozone-saturated solution + ozonized saline in drains+dressings of wounds in post-operative period + ST CG: ST only |
According to the general condition of each patient | Decreased number of microbial bodies in smears Faster decrease of endotoxins levels Higher speed in regenerative processes of purulent wounds Reduction of bed-days |
| Hassanien et al. (2018)[23] | Digital ulcers | Randomized controlled clinical trial (n=50) | CG: Bagging gaseous ozone + calcium channel blockers CG: Calcium channel blockers |
20 days | Increase in VEGF and decrease of anti-ETAR protein in local wounds (P<0.001) Effective wound healing rate of 96% (P=0.007) |
| Izadi et al. (2019)[24] | Diabetic foot ulcers | Single-blind, randomized, controlled clinical trial (n=200) | OG: Bagging gaseous ozone+ozonized gel + subcutaneous injection of oxygen-ozone around the wound + rectal or intravenous insufflation+ST CG: ST only |
180 days or until complete wound closure | Complete wound closure in 100% of patients treated with ozone Lower mean healing time (P=0.012) Lower percentage of patients who needed amputation (P<0.05) |
| Kadir et al. (2020)[25] | Diabetic foot ulcers | Nonrandomized controlled study (n=27) | OG: Bagging gaseous ozone + standard wound care CG: Standard wound care (antimicrobial dressings) |
21 days | Decreased number of bacterial colonies (P=0.037) The wound healing assessment showed no significant difference in the results between the two groups (P=0.00) |
| Kaymaz et al. (2021)[26] | Digital ulcers | Prospective, randomized controlled study (n=25) | OG: Bagging gaseous ozone +ST CG: ST only |
4 weeks | Effectiveness rate for ulcer healing of 92% (P=0.010) Reduction of ulcer size (P=0.027), ulcer pain (P<0.01), number and duration of Reynoud’s attacks (P<0.01) and improvement of hand mobility (P=0.02) |
| Menéndez et al. (2011)[27] | Onychomycosis | Single-blind, randomized, controlled, phase III clinical trial (n=400) | OG: Ozonized sunflower oil CG: Ketoconazole cream 2% |
3 months/ Reassessment after 1 year | 90.5% of the patients were cured and 9.5% have improved their condition (P<0.0001) Decreased number of relapses after 1 year in completely recovered patients (2.8% vs 37.0%) |
| Lu et al. (2018)[28] | Foot mycosis (tinea pedis) | Single-blind, randomized, controlled clinical trial (n=60) | OG: Washing of affected feet with ozonized water + immersion in ozonized water + ozonized oil CG: naftinfine hydrochloride and ketoconazole cream |
4 weeks | No significant differences were observed between the two groups regarding the number of patients cured, the rate of positive results on mycological examination and the clinical signs and symptoms (P>0.05) |
| Illek et al. (2013)[29] | Atopic dermatitis | Controlled clinical trial (n=64) | OG: Ozonized olive oil+rectal insufflations of ozone-oxygen mixture + ST CG: ST only |
3 months | Significant increase in the absolute number of CD3 lymphocytes cells, total IgE and TNF-α (P<0.001) Complete remission of symptoms in both groups after the end of therapy Reappearance of symptoms after 10 months (vs. 3 months in control group) |
| Zeng et al. (2020)[30] | Atopic dermatitis | Randomized, controlled clinical trial (n=12) | OG: Ozonized water shower+topical ozonized camellia oil+cotton swabs dipped with ozonized water rotated in nasal cavities CG: Tap water shower and basal oil |
3 days | Significant mitigation of lesions (P<0.001) Significantly restoring of bacterial diversity of the skin (P=0.023) |
| Campanati et al. (2013)[31] | Skin Burns | A prospective, comparative, single-blind, non-randomized, controlled clinical trial (n=30) | OG: Ozonized oil CG: Low-molecular-weight hyaluronic acid topical gel 0.2% |
12 weeks | No significant differences were observed between the treatments (P=0.33-0.80) Patients in ozone group presented less intense hyperpigmentation of healing skin after therapy (P=0.03) |
| Martusevich et al. (2018)[32] | Skin burns | Prospective, open, controlled clinical trial (n=40) | OG: Intravenous ozonized sodium chloride solution + autohaemotherapy+rectal ozone insufflations + antioxidants with succinic acid +ST CG: ST only |
15 days | Reduction in levels of endotoxicosis markers, higher blood antioxidant activity and elevation of general microcirculation parameters for both groups, with no significant differences observed between the interventions |
| Currò et al. (2018)[33] | Balanitis xerotica obliterans | Controlled clinical trial (n=30) | OG: Cream containing ozonized olive oil with vitamin E acetate CG: No treatment |
7 days | Significant reduction of pro-inflammatory cytokines and enzymes in foreskin tissues (P<0.001) Significant increase in growth factors and other components related to epithelial integrity and tissue regeneration (P<0.001) |
| Aghaei et al. (2019)[34] | Cutaneous leishmaniasis | Randomized, controlled clinical trial (n=30) | OG: Ozone olive oil+glucantime (meglumine antimoniate) CG: Glucantime |
2 months | Reduction in lesion size in both groups, with no significant differences between them Superficial scarring or slight post-inflammatory hyperpigmentation was left after healing in ozone group |
| Ibrahim et al. (2020)[35] | Multiple common warts | Single-blind, randomized, controlled clinical trial (n=74) | OG: Subcutaneous injection of gaseous ozone in each wart CG: Subcutaneous injection of saline in each wart |
Until complete clearance or for a maximum of ten sessions/ 6 months follow up | Treatment response rate of 90.9% (P<0.001) Preservation of normal skin texture and pigmentation after recovery Lower recurrence rate after 6 months of the end of therapy (5.0% vs. 15%) |
OG=ozone group, CG=control group, ST=standard treatment, VEGF=vascular endothelial growth factor, Anti-ETAR=endothelin-1 type A receptor autoantibodies, TNF-α = alpha tumor necrosis factor
Adverse effects in patients treated with ozone therapy have been reported in 4 of the 18 evaluated clinical trials. Mild adverse effects occurred in three clinical trials,[28,34,35] whereas severe side effects occurred in only one trial.[19] In the study conducted by Wainstein et al. (2011),[19] symptoms such as osteomyelitis, fever, wound infection and pulmonary congestion were reported in five patients (8.2%). In the study developed by Lu et al. (2018),[28] only one patient (1.6%) presented skin peeling. Ibrahim et al. (2020)[35] observed symptoms such as pain at the application site, numbness (7.5%) and fatigue (5.0%) in patients who received subcutaneous injections of ozone gas. Aghaei et al. (2019)[34] noticed that some patients treated with ozone had a transient burning sensation at the application site. Moreover, this was the only study that reported adverse effects in the control group (glucantime 20 mg/kg twice daily), including arthralgia, headache and fever.
Discussion
A general analysis of clinical trials results suggests a positive response to the use of ozone therapy in the investigated dermatological conditions. The patients received ozone therapy by gaseous ozone[18,19,20,23,24,25,26] or incorporated in different vehicles such as water,[22,28,30] gel[24] and oil.[21,27,28,29,30,31,33,34] Only in three clinical trials ozone was not used topically, but were used through subcutaneous,[35] intralesional,[22] intravenous[22,32] and rectal[32] routes. Only one study used the autohemotherapy procedure with ozone.[32] Otherwise, three interventions associated different routes of administration of injectable ozone with its topical application.[18,24,29]
Effectiveness
Considering the outcomes of effectiveness presented in Table 1, ozone therapy has achieved significantly superior results in statistical analyzes when compared to control groups in the treatment of the following dermatological disorders: Diabetic foot ulcers,[19,20,24] chronic venous leg ulcers,[21] digital ulcers,[23,26] onychomycosis,[27] atopic dermatitis,[29,30] balanitis xerotica obliterans[33] and multiple common warts.[35] Besides, the studies conducted by Menéndez et al. (2011),[27] Izadi et al. (2019)[24] and Ibrahim et al. (2020)[35] were considered to be at a lower risk of bias in their results analysis compared to the other studies. They met all the following criteria: Blinded, randomized and controlled clinical trials, longer treatment period (30 days or more) and larger sample size (74–400 patients).
Another study approaching the treatment of diabetic foot ulcers reported superior results for ozone therapy (n = 20) in relation to the control group (n = 17), which used the standard treatment (removal of necrotic and purulence-impregnated tissues).[22] However, it is pertinent to report that the authors do not exhibit statistical analyzes that indicate whether this superiority is significant.
Only six studies did not show superiority of ozone treatment when compared to the control groups.[18,25,28,31,32,34], however the ozone was considered an interesting alternative treatment in three of them.[18,28,31] Hassan et al. (2011)[18] observed that both infrared laser and ozone were effective in promoting the healing of diabetic foot wounds, with similar results. Therefore, ozone therapy could be considered as an alternative to laser treatment. The use of ozone therapy in the treatment of tinea pedis showed similar effectiveness to the treatment with naftinfine associated with ketoconazole, suggesting again the possibility of using ozone as an alternative therapy.[28] Likewise, the trial developed by Campanati et al. (2013)[31] reported a significant decrease in burning sensation, erythema, tension and itching in burned patients treated with ozone and also in the group treated with hyaluronic acid topical gel, without significant differences between the treatments. Therefore, ozone therapy was not inferior to the standard treatment and could be used alternatively. In addition, patients who received ozone therapy presented a significantly less intense hyperpigmentation of healing skin compared to control group (P = 0.03).
Only three studies evidenced lack of effectiveness of ozone therapy.[25,32,34] In the trial conducted by Kadir et al. (2020)[25] about skin ulcers, there was no significant difference between treatments in the wound healing assessment, despite a significant reduction in the number of wound bacterial colonies. In the evaluation of ozone therapy to treat skin burns, Martusevich et al. (2018)[32] observed a reduction in levels of endotoxicosis markers and proteinases, an increase in general microcirculation parameters and higher blood antioxidant activity in patients treated with ozone plus standard therapy. However, there were no significant differences reported in relation to the use of standard treatment alone, evidencing that ozone therapy was not effective for the patients observed in this study. In the treatment of cutaneous leishmaniasis, the use of ozone associated with glucantime showed similar results to the use of glucantime alone, suggesting the ineffectiveness of ozone therapy under the conditions of this clinical trial.[34]
Adverse effects
The alternative use of ozone therapy remains controversial due to its potential for toxicity,[11,12] which is described to occur when criteria such as therapeutic window, exposure time, administration routes and appropriate application methods are not taken into account.[10,36] The forms of ozone application for which adverse effects were observed include bagging gaseous ozone,[19] ozonized water,[28] ozonized oil[28,34] and gas subcutaneous injections.[35] Severe symptoms in patients treated with ozone were reported in only one study, conducted by Wainstein et al. (2011).[19] However, according to the authors, there was no causal association between the symptoms and ozone intervention. Skin peeling, reported by Lu et al. (2018),[28] is a mild and possibly expected symptom, since the intervention occurred through topical administration and may be related to the disease course itself. Moreover, this adverse effect disappeared a few days later, probably due to integumentary tissue adaptation to ozone treatment. The pain and numbness at the application site, that was observed by Ibrahim et al. (2020),[35] occurred possibly due to the method of application being performed through subcutaneous injections, a relatively invasive route of administration. In spite of a few clinical trials report the occurrence of adverse effects, in 7 of the 18 studies analyzed, the authors appear not to evaluate the occurrence of unwanted effects during ozone treatment.[18,20,22,23,25,26,32] Thereby, there is a lack of complete data regarding the safety of ozone therapy in these seven investigated clinical trials.
Limitations
Although evidence suggesting ozone therapy effectiveness has been found for most of the clinical trials, several studies presented one or more limitations, such as very small sample size,[30] absence of blinding[18,21,22,23,25,26,29,30,32,33] and randomization,[22,25,29,31,32,33] failure to verify homogeneity between the control and treatment groups,[18,19] inability to reduce variances due to individual characteristics,[30] high rate of loss to follow-up,[19] no long-term follow-up,[20,23,25,26,30,32,33] treatment by different operators at different centers and impossibility of promoting the same standard treatment conditions for all patients due to different economic situations,[24] as well as inability to control environmental factors.[25]
Conclusion
This is the first review article to compile data from clinical trials on the use of ozone to treat dermatological conditions, which provides a source of evidence that will help in clinical decision-making situations. Since 15 from the 18 selected trials showed the effectiveness of ozone treatment, it may be suggested that ozone therapy could be used as an alternative or complementary treatment in some types of dermatological conditions, such as diabetic foot ulcers, chronic venous leg ulcers, digital ulcers, mycoses, atopic dermatitis, skin burns, balanitis xerotica obliterans and multiple common warts, specially affecting refractory patients. However, based on the available evidence, the use of ozone as a first-choice treatment for dermatological disturbances cannot yet be recommended. There is a dearth of conclusive results on its safety, especially in long-term treatments. Health professionals must evaluate the risk-benefit ratio of this therapy for the patients, considering clinical history, comorbidities and response to conventional therapy. A greater number of high quality, double-blind, randomized and controlled clinical trials is needed to reinforce evidence of effectiveness and clearly establish the safety of ozone therapy in dermatological disorders.
Financial support and sponsorship
The authors thank “Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq”(project number 423066/2018-8) and “Universidade Federal do Rio Grande do Sul”.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tartari AP, Moreira FF, Pereira MC, Carraro E, Cidral-Filho FJ, Salgado AI, et al. Anti-inflammatory effect of ozone therapy in an experimental model of rheumatoid arthritis. Inflammation. 2020;43:985–93. doi: 10.1007/s10753-020-01184-2. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez ZB, Álvarez RG, Guanche D, Merino N, Rosales FH, Cepero SM, et al. Antioxidant mechanism is involved in the gastroprotective effects of ozonized sunflower oil in ethanol-induced ulcers in rats. Mediators Inflamm. 2007;2007:65873. doi: 10.1155/2007/65873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valacchi G, Zanardi I, Lim Y, Belmonte G, Miracco C, Sticozzi C, et al. Ozonated oils as functional dermatological matrices: Effects on the wound healing process using SKH1 mice. Int J Pharm. 2013;458:65–73. doi: 10.1016/j.ijpharm.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 4.Oğuzkan SB, Kaya ST, Cesur A, Karagül B, Uğraş S, Uğraş HI. Determination of effect on cutaneous wound healing of ozonated hazelnut oil. Proceedings. 2018;2:1537. [Google Scholar]
- 5.Giuliani G, Ricevuti G, Galoforo A, Franzini M. Microbiological aspects of ozone: Bactericidal activity and antibiotic/antimicrobial resistance in bacterial strains treated with ozone. Ozone Ther. 2018;3:48–51. [Google Scholar]
- 6.Petry G, Rossato LG, Nespolo J, Kreutz LC, Bertol CD. In vitro inactivation of Herpes virus by ozone. Ozone Sci Eng. 2014;36:249–52. [Google Scholar]
- 7.Geweely NS. Antifungal activity of ozonized olive oil (Oleozone) Int J Agric Biol. 2006;8:670–5. [Google Scholar]
- 8.Hernández F, Hernández D, Zamora Z, Díaz M, Ancheta O, Rodriguez S, et al. Giardia duodenalis: Effects of an ozonized sunflower oil product (Oleozon) on in vitro trophozoites. Exp Parasitol. 2009;121:208–12. doi: 10.1016/j.exppara.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Suh Y, Patel S, Kaitlyn R, Gandhi J, Joshi G, Smith NL, et al. Clinical utility of ozone therapy in dental and oral medicine. Med Gas Res. 2019;9:163–7. doi: 10.4103/2045-9912.266997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz A, Kontorschikova CN, Malesnikov OV, Sánchez GM, Re L, Gribkova IA, et al. Madrid: Asociación Española de Profesionales Médicos en Ozonoterapia, AEPROMO; 2011. Guia Para El Uso Médico Del Ozonio: Fundamentos Terapéuticos e Indicaciones. [Google Scholar]
- 11.Magzamen S, Moore BF, Yost MG, Fenske RA, Karr CJ. Ozone-related respiratory morbidity in a low-pollution region. J Occup Environ Med. 2017;59:624–30. doi: 10.1097/JOM.0000000000001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raza A, Dahlquist M, Lind T, Ljungman PLS. Susceptibility to short-term ozone exposure and cardiovascular and respiratory mortality by previous hospitalizations. Environ Heal. 2018;17:37. doi: 10.1186/s12940-018-0384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travagli V, Zanardi I, Silvietti A, Bocci V. A physicochemical investigation on the effects of ozone on blood. Int J Biol Macromol. 2007;41:504–11. doi: 10.1016/j.ijbiomac.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Re L, Martínez-Sánchez G, Bordicchia M, Malcangi G, Pocognoli A, Morales-Segura MA, et al. Is ozone pre-conditioning effect linked to Nrf2/EpRE activation pathway in vivo? A preliminary result. Eur J Pharmacol. 2014;742:158–62. doi: 10.1016/j.ejphar.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Wang X. Emerging roles of ozone in skin diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:114–23. doi: 10.11817/j.issn.1672-7347.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Travagli V, Zanardi I, Bocci V. Topical applications of ozone and ozonated oils as anti-infective agents: An insight into the patent claims. Recent Pat Antiinfect Drug Discov. 2009;4:130–42. doi: 10.2174/157489109788490271. [DOI] [PubMed] [Google Scholar]
- 17.Clavo B, Santana-Rodriguez N, Gutierrez D, Lopez JC, Suarez G, Lopez L, et al. Long-term improvement in refractory headache following ozone therapy. J Altern Complement Med. 2013;19:453–8. doi: 10.1089/acm.2012.0273. [DOI] [PubMed] [Google Scholar]
- 18.HHassan AS, Zakarya O, Al Sheimy WM, Eldin NE. Therapeutic effecacy of ozone and low level laser in treatment of diabetic foot ulcers. Zagazig Med J. 2011;17:109–22. [Google Scholar]
- 19.Wainstein J, Feldbrin Z, Boaz M, Harman-Boehm I. Efficacy of ozone-oxygen therapy for the treatment of diabetic foot ulcers. Diabetes Technol Ther. 2011;13:1255–60. doi: 10.1089/dia.2011.0018. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Guan M, Xie C, Luo X, Zhang Q, Xue Y. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 2014;2014:273475. doi: 10.1155/2014/273475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solovąstru LG, Stîncanu A, De Ascentii A, Capparé G, Mattana P, Vâţă D. Randomized, controlled study of innovative spray formulation containing ozonated oil and α-bisabolol in the topical treatment of chronic venous leg ulcers. Adv Ski Wound Care. 2015;28:406–9. doi: 10.1097/01.ASW.0000470155.29821.ed. [DOI] [PubMed] [Google Scholar]
- 22.Teuvov AA, Baziev AM, Lovpache ZN, Teunikova IS, Chudopal SM. Ozone therapy in the comprehensive treatment of diabetic foot syndrome? Biomed Pharmacol J. 2017;10:1871–8. doi: 10.13005/bpj/1306. [Google Scholar]
- 23.Hassanien M, Rashad S, Mohamed N, Elawamy A, Ghaly MS. Non-invasive oxygen-ozone therapy in treating digital ulcers of patients with systemic sclerosis. Acta Reumatol Port. 2018;43:210–6. [PubMed] [Google Scholar]
- 24.Izadi M, Kheirjou R, Mohammadpour R, Aliyoldashi MH, Moghadam SJ, Khorvash F, et al. Efficacy of comprehensive ozone therapy in diabetic foot ulcer healing. Diabetes Metab Syndr. 2019;13:822–5. doi: 10.1016/j.dsx.2018.11.060. [DOI] [PubMed] [Google Scholar]
- 25.Kadir K, Syam Y, Yusuf S, Zainuddin M. Ozone therapy on reduction of bacterial colonies and acceleration of diabetic foot ulcer healing. Home Healthc Now. 2020;38:215–20. doi: 10.1097/NHH.0000000000000889. [DOI] [PubMed] [Google Scholar]
- 26.Kaymaz S, Karasu U, Alkan H, Ulutaş F, Yaşar CA, Ök ZD, et al. Efficacy of local oxygen-ozone therapy for the treatment of digital ulcers refractory to medical therapy in systemic sclerosis: A randomized controlled study. Mod Rheumatol. 2021:roab117. doi: 10.1093/mr/roab117. doi: 10.1093/mr/roab117. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON® in patients suffering from onychomycosis. Mycoses. 2011;54:272–7. doi: 10.1111/j.1439-0507.2010.01898.x. [DOI] [PubMed] [Google Scholar]
- 28.Lu J, Guo M, Ligui H, Wu K, Xiang Y, Huang J, et al. Efficacy of combination of ozonated water with oil for treatment of tinea pedis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:147–51. doi: 10.11817/j.issn.1672-7347.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Illek YY, Zaytseva GA, Galanina AV, Vasilieva YA, Rybakova TN, Suslova EY. Immunomodulating and anti-relapse effects of ozone therapy in atopic dermatitis in preschool and primary school children. Sovrem Tehnol v Med. 2013;5:89–91. [Google Scholar]
- 30.Zeng J, Dou J, Gao L, et al. Topical ozone therapy restores microbiome diversity in atopic dermatitis. Int Immunopharmacol. 2020;80:106191. doi: 10.1016/j.intimp.2020.106191. [DOI] [PubMed] [Google Scholar]
- 31.Campanati A, De Blasio S, Giuliano A, Xiang Y, Huang J, Ding S, et al. Topical ozonated oil versus hyaluronic gel for the treatment of partial- to full-thickness second-degree burns: A prospective, comparative, single-blind, non-randomised, controlled clinical trial. Burns. 2013;39:1178–83. doi: 10.1016/j.burns.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Martusevich AK, Peretyagin SP, Ruchin MV, Struchkov AA. Ozone therapy in patients with burn disease. J Biomed Sci Eng. 2018;11:27–35. [Google Scholar]
- 33.Currò M, Russo T, Ferlazzo N, Caccamo D, Antonuccio P, Arena S, et al. Anti-inflammatory and tissue regenerative effects of topical treatment with ozonated olive oil/Vitamin E acetate in balanitis xerotica obliterans. Molecules. 2018;23:645. doi: 10.3390/molecules23030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aghaei M, Aghaei S, Sokhanvari F, Ansari N, Hosseini SM, Mohaghegh M-A, et al. The therapeutic effect of ozonated olive oil plus glucantime on human cutaneous leishmaniasis. Iran J Basic Med Sci. 2019;22:25–30. doi: 10.22038/ijbms.2018.29232.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim AM, Elkot RA, Khashaba SA. Successful treatment of multiple common warts with intralesional ozone. Dermatologic Surg. 2020;46:928–33. doi: 10.1097/DSS.0000000000002174. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz A, Sánchez GM, Sabah F. 2nd ed. Madrid: International Scientific Committee of Ozone Therapy (ISCO3); 2015. Madrid Declaration On Ozone Therapy. [Google Scholar]



