Abstract
Overnutrition-induced obesity and metabolic dysregulation are considered major risk factors contributing to breast cancer. The origin of both obesity and breast cancer can retrospect to early development in human lifespan. Genistein (GE), a natural isoflavone enriched in soybean products, has been proposed to associate with a lower risk of breast cancer and various metabolic disorders. Our study aimed to determine the effects of maternal exposure to soybean dietary GE on prevention of overnutrition-induced breast cancer later in life and explore potential mechanisms in different mouse models. Our results showed that maternal dietary GE treatment improved offspring metabolic functions by significantly attenuating high-fat diet-induced body fat accumulation, lipid panel abnormalities and glucose intolerance in mice offspring. Importantly, maternal dietary GE exposure effectively delayed high-fat diet-simulated mammary tumor development in female offspring. Mechanistically, we found that maternal dietary GE may exert its chemopreventive effects through affecting essential regulatory gene expression in control of metabolism, inflammation and tumor development via, at least in part, regulation of offspring gut microbiome, bacterial metabolites and epigenetic profiles. Altogether, our findings indicate that maternal GE consumption is an effective intervention approach leading to early-life prevention of obesity-related metabolic disorders and breast cancer later in life through dynamically influencing the interplay between early-life gut microbiota, key microbial metabolite profiles and offspring epigenome.
Keywords: Breast cancer, Obesity, Soybean genistein, Maternal prevention, Gut microbiome, Epigenetics
1. Introduction
Obesity has become a major epidemic in the United States and worldwide that threatens human health. Although obesity has genetic factors, overnutrition caused by a modern dietary pattern such as a Western diet or high fat diet (HFD) is considered a major risk factor contributing to obesity [1]. Obesity can lead to a series of metabolic syndromes and is also closely associated with a variety of human chronic diseases such as diabetes, cardiovascular diseases and cancer including breast cancer [1,2]. Studies have shown obesity or overweight is a risk factor for postmenopausal women with estrogen receptor (ER)-positive breast cancer as well as for an aggressive subtype of breast cancer, triple negative breast cancer (TNBC), which commonly affects young pre-menopausal women, African Americans/Hispanics, and/or those with a BRCA1 germline mutation [2,3]. It is suggested that obesity might promote breast tumorigenesis through negative interference with metabolism and inflammation processes [4]. However, to date, the precise mechanisms underlying how obesity regulates breast cancer development have not been fully elucidated.
The “Developmental Origins” hypothesis based on the interaction between early developmental plasticity, the environmental factors and the outcome of health and disease has been widely accepted over the last decades [5]. Both obesity and breast cancer have been well studied with respect to their developmental origins [6,7]. For example, maternal nutrition imbalance including both overnutrition and undernutrition status may cause maladaptation to the detrimental environment leading to dysregulation of metabolic systems and obesity later in the offspring’s life [6]. Epidemiolocal studies indicate that maternal obesity may also increase breast cancer risk among daughters due to a high birth weight [8]. Thus, the beneficial maternal diets and balanced nutritional status would likely impact early developmental processes and reverse adverse fetal programming leading to an altered susceptibility to later-life health outcomes such as a reduced risk of obesity or breast cancer in the offspring.
Soybean products are popular in the global food market and their beneficial effects on human health are substantial. For example, consumption of soybean foods is correlated with reducing risks of cardiovascular diseases and diabetes through control of cholesterol levels and regulation of glucose metabolism, respectively [9,10]. As the Asian Pacific population accounts for the largest consumer of soy food worldwide, Asian women benefit from their eating habits with a lower rate of breast cancer development and relapse than their Western counterparts [11]. The major bioactive compounds in soybean are isoflavones. Genistein (GE) is the most abundant and accounts for approximately 47% of total soy isoflavones found in soybean products [12]. Studies from our and many other labs have shown that soybean GE can prevent various types of breast cancer both in vitro and in vivo [13-16]. Beneficial effects of GE against obesity have been well demonstrated in both human and animal studies via various mechanisms such as reducing oxidative stress/inflammation, inducing apoptosis and differentiation, and inhibiting adipogenesis, etc [9,17,18]. Importantly, GE has shown transgenerational impacts [19]. Maternal consumption of GE can lead to beneficial health outcomes in mouse offspring such as reduced risk in developing breast cancer, obesity and cardiovascular diseases [20-22]. However, the mechanisms underlying how maternal bioactive GE exhibits its preventive properties in the offspring remain uncertain.
The gastrointestinal system is the most important habitation for indigenous intestinal microbiota. Diets and nutrition greatly affect the gut microbiota composition. Substantial human and animal studies have shown the altered composition and diversity of gut microbiota caused by high-energy diets in obese populations, indicating a direct link between functional microbial imbalance and obesity [23]. Accordingly, recent studies show that dysbiosis of gut bacteria is also linked to breast cancer [24]. The gut microbiome of breast cancer patients is significantly altered relative to that of healthy women, indicating that certain bacteria may be associated with breast cancer development and/or therapeutic responses. Importantly, studies indicate that the gut microbiome is primarily established during early life [25]. Thus, maternal microbiota could serve as an original resource for early establishment of gut microbiota in the offspring. Soy consumption can result in a favorable shift in gut microbial communities and beneficial bacterial metabolites contributing to its protective effects against human chronic diseases [26]. Our previous studies also showed that soybean GE is a potent epigenetic modulator that can exhibit its tumor suppressing effects via regulation of key tumor-related gene expression through epigenetic mechanisms [13-15,20,27]. We therefore hypothesize that dynamic interaction may exist between early-life gut microbiome, metabolome and epigenetic mechanisms by maternal dietary GE intervention, which may in turn influence the health outcome of later-life such as the risks of obesity and its related breast cancer later in offspring life.
In the current studies, we aimed to determine the effects of maternal soybean GE diet on prevention of obesity-related breast cancer through mechanistic interplay between maternal GE altered early-life gut microbiome, metabolites and epigenetic mechanisms. Our results showed that maternal soybean GE diet reduced the risk of HFD-induced metabolic disorders and delayed HFD-accelerated mammary tumor development in female offspring of several mouse models through, at least in part, affecting early-life gut microbiota, important microbial metabolites and offspring epigenome that may contribute to key regulatory gene expression profile changes in the offspring. This study provides knowledgeable mechanisms underlying maternal nutrition-mediated developmental origins of obesity and breast cancer as well as interactions between these two maladies, which will facilitate exploration of a novel in utero preventive strategy through natural bioactive products.
2. Materials and methods
2.1. Animal models, diets and treatment groups
We used a breast cancer transgenic mouse model, C3(1)-SV40 Tag (FVB-Tg[C3-1-Tag]cJeg/JegJ) (SV40), and a wild-type mouse model, C57BL/6J (C57), in this study. SV40 mice actively express Tag gene in mammary gland leading to spontaneous mammary tumor development starting around 16 weeks of age in female mice [28]. Animals were originally purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and bred in our laboratory. Mice were housed in groups in the Animal Facility at the University of Alabama at Birmingham (UAB) and were maintained in conventional housing conditions (12-h dark/12-h light cycle, 24±2°C temperatures, and 50±10% humidity).
For maternal treatment, dams (10 mice/group) were treated with either AIN93 modified control (phytoestrogen-free modified AIN-93G diet with 7% corn oil substituted for 7% soybean oil; TestDiet, St. Louis, MO, USA) or soybean GE diet (modified AIN-93G diet supplemented with 250 mg GE per kg diet; TestDiet) from the mother’s prepubertal age (4 weeks) until pregnancy and weaning as shown in Fig. 1A. Ten female mice were mated with male mice in pairs at 12 weeks of age and male mice were separated after conception. Because breast cancer prevails in women, only female offspring were used in this study. Female pups born from either control- or GE-treated mothers were weaned at 4 weeks of age, separated half equally, and treated with either control or a commercially available HFD (45% of calories from fat compared to 18% in control AIN93 diet, TestDiet). The detailed information of all diets used in this study has been provided in Supplementary Data 1. Due to different female pup size in different groups, female offspring number in each treatment group ranged from 10 to 20 mice resulting in an average of 15 experimental mice/group.
Fig. 1.
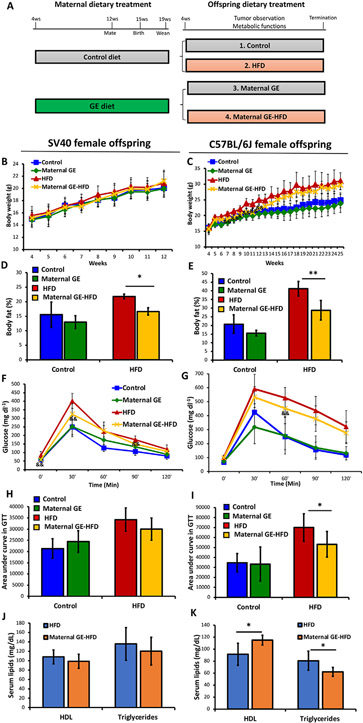
Maternal GE improved metabolic health in mice offspring. A, Maternal dietary treatment plan. Female SV40 transgenic mice or C57 mice (10 dams/group) were administered either control diet or GE diet (250 mg/kg) from 4 weeks of age until conception and weaning. Female offspring were fed either control (grey) or HFD diet (pink) throughout their lifespan resulting in 4 treatment groups in the offspring (~15 female offspring/group). B-K, Metabolic data from SV40 (left panel) and C57 female offspring mice (right panel). Body weight was monitored throughout 12 weeks of age in SV40 and 25 weeks in C57 offspring mice, respectively (n=~15 mice/group). Body fat composition, glucose tolerance test (GTT) and lipid profiles were tested at 12 weeks of age in SV40 and 21 weeks in C57 offspring mice, respectively (n=5 mice/group). B and C, Body weight. D and E, Body fat composition (fat mass/body weight). F and G, GTT. Blood glucose levels were measured at 0, 30, 60, 90 and 120 min after injection with 10% glucose intraperitoneally. H and I, GTT quantification by areas under the curve. J and K, Serum lipid profiles for HDL and triglyceride levels. Columns, mean; Bars, SE; *, P<.05, **, P<.01 significant to the indicated group. &, maternal GE-HFD significantly different from the HFD group.
As shown in Fig. 1A, four study groups were resulted: (1) Control, (2) HFD, (3) Maternal GE, and (4) Maternal GE-HFD. Obesity and mammary tumor development were monitored and recorded weekly in the offspring. All experiments and procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at UAB (Animal Project Numbers: 20653, 20671).
2.2. Obesity and metabolic parameters
Body weight was measured on a weekly basis in all female offspring mice (~15 mice/group). Quantitative magnetic resonance was used to measure fat and lean mass as well as the water content in live animals at the UAB Small Animal Phenotyping Core. Glucose tolerance test (GTT) was used to determine glucose metabolism status. Animals were fasted overnight and injected with 10% glucose intraperitoneally (2 mg glucose/g body weight). Blood glucose levels were measured using glucometer from the tail vein at 0, 30, 60, 90, and 120 min after injection. The Area Under the Curve was calculated using trapezoidal sum in calculus to quantify GTT results. Blood lipid profiles including total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides were tested using PTS Diagnostics Lipid Panel. Body composition, GTT test and blood lipid profiles were tested in 5 randomly selected live animals from each group at tumor-free stage (SV40 mice at 12 weeks of age and C57 mice at 21 weeks of age, respectively).
2.3. Mammary tumor development evaluation
We used tumor latency as the primary outcome for SV40 transgenic mouse model and tumor incidence was measured weekly. The end point for SV40 mice was 20 weeks based on our previous studies [14,20] and the instruction provided by the Jackson Laboratory. Because wild-type C57 mice are phenotypically normal, 5 randomly selected C57 female offspring mice were injected with the mixture of ~106 murine mammary tumor cells, E0771 (ATCC, Manassas, VA, USA), and same volume of Matrigel Matrix (Thermo Fisher Scientific, Wilmington, DE, USA) under mammary pads at 21 weeks to bear syngeneic breast tumor as done previously [14,15]. All C57 offspring mice including 5 engrafted and the rest tumor-free mice were sacrificed 4 weeks post injection at 25 weeks of age. We used tumor weight as the primary outcome and tumor volume was measured weekly in C57 mice. Tumor volumes were measured by a caliper weekly and calculated using the formula: tumor volume (cm3) = (length × width2) × 0.523 as described previously [14,15,26]. At the endpoint, the mammary tumors were collected, weighed and used for further analyses.
2.4. Cell culture, treatment and MTT assay
Murine mammary tumor cell line, E0771, was grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were maintained in a humidified environment of 5% CO2 and 95% air at 37°C. To determine the effects of sodium butyrate (Sigma, St. Louise, MO, USA) on cellular proliferation, aliquots of 5 × 103 E0771 cells were seeded in triplicate in 96-well plates. After 24 h seeding, attached E0771 cells were treated with 0, 1, 5, 10, and 20 mM sodium butyrate for 3 days. The medium with sodium butyrate was replaced every 24 h for the duration of the experiment. Control cells received equal amounts of PBS in the medium. MTT solution (Sigma) was added to the medium to achieve a final concentration of 1 mg/ml. The cells were incubated at 37 °C and dissolved in 100 μl DMSO after 2 h incubation. The absorbance of the cell lysates in DMSO solution was read at 570 nm by a microplate reader.
2.5. Quantitative real-time RT-PCR
Quantitative real-time PCR reactions were performed to test specific gene expression at transcriptional level. These selected target genes include tumor-related genes (tert, Myc, Cyclin D1, Pten), obesity-related genes (peroxisome proliferator activated receptor gamma [Pparg], leptin [Lep], leptin receptor [Lepr], Adiponectin), inflammation-related genes (C-reactive protein [Crp], interleukin 6 [Il6], tumor necrosis factor [Tnf], Nfkb1) and epigenetic modulators (DNA methyltransferase 1 [Dnmt1], DNA methyltransferase 3a [Dnmt3a], DNA methyltransferase 3b [Dnmt3b], histone deacetylases 1 [Hdac1]). Primer sequences and detailed RT-PCR procedures were provided in Supplementary Data 3.
2.6. Proteome profiler mouse cytokine array
The Proteome Profiler Mouse Cytokine Array Panel A (R&D Systems, Minneapolis, MN, USA) was used to profile cytokines in serum samples according to the manufacturer’s protocol. The array membranes were imaged by Amersham Imager 680 RGB (Cytiva Life Science). Pixel densitometry analysis in each spot of the array was carried out using ImageJ Software version 1.8.0_172 (https://imagej.nih.gov/ij/).
2.7. Gut microbiome analysis by 16S rRNA sequencing
Fecal samples were collected from five randomly selective C57 female offspring mice at 8 weeks of age after 4 weeks HFD treatment. Total genomic DNA was extracted and purified from fecal samples using Fecal DNA Isolation Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. The concentration and purification of all DNA samples were determined by Qubit 4 Fluorometer (Thermo Fisher). Fecal DNAs were used to perform PCR to amplify the V3-V4 regions of the bacterial 16S rRNA gene using the unique barcoded primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and the reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Amplicons were purified using the QIAquick Gel Extraction Kit (Qiagen, Germantown, MD, USA). Microbial 16S rDNA was sequenced on NextGen sequencing Illumina MiSeq platform as done previously [29]. The raw sequencing data were de-multiplexed, quality-filtered, clustered and analyzed using Quantitative Insight into Microbial Ecology data analysis package (version 1.7.0). The reads were assigned to operational taxonomic units (OTUs) of representative sequences at the 97% similarity using UPARSE version 7.1. The relative abundance of each OTU was analyzed at the phylum, class, order, family, genus and species levels. We performed the microbial community-level analyses and the single-taxon analyses. Bacterial taxonomy assignment and diversity analysis were calculated using R package phyloseq [30] to compare the bacterial species richness between different treatment groups (HFD vs. Maternal-GE HFD). Chao1, Simpson and the Shannon index were analyzed for alpha diversity and the Bray-Curtis dissimilarity index for the beta diversity analysis. Linear regression models were used to analyze alpha diversity indices for each sample. Differences in beta-diversity were tested using Bray-Curtis distance matrix and permutational multivariate analysis of variance. To identify individual taxa that are significantly associated with different treatment, single-taxon analysis was performed using the negative binomial model. We used the false-discovery rate (FDR) method to adjust the P values for multiple tests that controls the FDR, the expected proportion of false discoveries amongst the rejected hypotheses. The raw data of alpha-diversity metrices and OTU abundance was provided in Supplementary Data 2. Principal component analysis (PCA) was performed to determine the overall microbiota composition in different groups. PCA plots were visualized using EMPeror version 0.9.3-dev. Hierarchical clustering heat-map based on significant OTU abundance was prepared in RStudio. This work utilized the UAB Microbiome/Gnotobiotics shared facility, which is supported by the O’Neal Comprehensive Cancer Center (P30 CA013148).
2.8. Serum short-chain fatty acids (SCFAs) by liquid chromatography-mass spectrometry (LC-MS) analysis
Concentrations of serum SCFAs in serum were determined using ultraperformance LC-MS (Bruker Impact II) in the Metabolomics Center at the University of Missouri. Detailed procedures were provided in Supplementary Data 3.
2.9. Epigenetic profiling
To determine overall epigenetic profiles, nuclear protein and genomic DNAs from mammary tumors were extracted from 5 randomly selected SV40 and C57 female offspring mice by using the nuclear extraction kit (Epigentek, Brooklyn, NY, USA) and DNeasy Blood and Tissue Kit (Qiagen), respectively. HDAC and DNMT activity assays were evaluated for the relevant epigenetic enzymatic activities, and global DNA methylation status was determined by the MethylFlash Global DNA Methylation (5-methylcytosine [5-mC]) ELISA Easy Kit (Epigentek) according to the manufacturer’s protocols. Nuclear protein was used for detection of epigenetic enzymatic activities and genomic DNAs were for global DNA Methylation status. The enzymatic activities or the percentage of methylated DNA were proportional to the optical density (OD) intensity through ELISA reaction. The colorimetric changes were detected by using a microplate reader at 450 nm. Protein expression of Hdac1 was tested by western blot and analysis method has been provided in Supplementary Data 3.
2.10. Statistical analysis
Gut microbiome analyses have been handled by biostatisticians and briefly described above. Statistical significance between the values of control and treatment groups was evaluated by one-way ANOVA followed by Tukey’s test for multiple comparisons via GraphPad Prism 9.1.2 version. Statistical significance between the numbers of subjects was evaluated by Chi-square and Fisher’s exact test. Values were presented as mean±SD (standard deviation) and P<.05 was considered statistically significant.
3. Results
3.1. Maternal GE diet attenuated HFD-induced metabolic disorders in female offspring
Because soybean products have been well studied due to their preventive effects on obesity and the relevant metabolic disorders, we therefore extended our studies to test the effectiveness of maternal exposure to soybean GE diet on improving metabolic health in mouse progenies. The concentration of maternal GE in animal diet has been proven safe in both mothers and offspring [14,15,20,21], which can be converted to human intake [31] achievable by taking GE-enriched soybean products such as tofu, miso and soy protein (Supplementary Table in Supplementary Data 3), indicating that this concentration is physiologically relevant for future human study. Two mouse models have been applied in this study: transgenic SV40 model with FVB/N genetic background [28] and obesity-related wild-type C57 mice [32]. The use of these models facilitates better understanding of the effects of maternal GE diet on HFD-induced obesity and breast cancer in different mouse strains with different genetic background.
As shown in Fig. 1A, dams were administered either control or soybean GE diet throughout their early life, pregnancy and lactation, which represents a persistent eating habit in mothers. The weaned mice were then exposed to normal or detrimental dietary stimuli so that the offspring metabolic protective effects were exclusively obtained from the mothers. As we focused on obesity and its related breast cancer, we only investigated female offspring in this study. We monitored body weight and determined the relevant metabolic parameters up to 12 weeks in SV40 mice because this model begins to spontaneously develop mammary tumor as early as 10–12 weeks and tumor growth greatly interferes with normal metabolism. Thus, the long-term metabolic alterations were observed in C57 offspring mice up to 25 weeks of age. Our results showed that maternal GE diet can reduce body weight (Fig. 1C) in C57 offspring female mice, especially in HFD-treated offspring at early age. This effect was observed but not obvious in SV40 female offspring (Fig. 1B), which may be due to limited HFD exposure. Consistently, maternal GE significantly reduced body fat composition in both SV40 (Fig. 1D) and C57 female offspring mice exposed to HFD (Fig. 1E).
As obesity constantly causes impaired glucose metabolism, we next sought to determine whether maternal GE can attenuate HFD-induced glucose metabolism abnormity in the offspring using GTT test. We found maternal GE can significantly decrease blood glucose levels at 0, 30, and 90 min after glucose surge in HFD-treated SV40 mice (Fig. 1F). However, the overall effect of maternal GE on glucose metabolism was not statistically significant in SV40 offspring mice through a quantification method, area under the curve for GTT result (Fig. 1H). As expected, maternal GE significantly reversed HFD-induced glucose intolerance in C57 offspring mice (Figs. 1G and I). Because the metabolic protective effects of maternal GE were more prominent when the offspring were exposed to overnutrition stress such as HFD, we therefore focused our research on the HFD-treated offspring in the following studies.
We next found maternal GE can significantly improve lipid profiles by increasing blood levels of “good lipid,” HDL, but decreasing “bad lipid,” triglycerides, in C57 female offspring (Fig. 1K), especially when a postnatal HFD diet was administered. This effect was not prominent in SV40 mice (Fig. 1J). Nevertheless, we did observe similar trends against overnutrition stress and obesity traits in SV40 offspring mice with FVB/N background as seen in C57 offspring mice, suggesting this preventive effect could be universal and independent of genetic background. Although the metabolic protection of maternal GE is more prominent in C57 than SV40 offspring, we believe these disparities are more likely due to a short-term exposure to HFD in SV40 offspring mice when the examinations took place. Taken together, our results suggest that maternal soybean diet exhibits its metabolic protective effects against HFD through reducing excess body fat accumulation and improving glucose metabolism and lipid profiles in the offspring.
3.2. Maternal GE diet inhibited HFD-promoted mammary tumor development
We used the preclinical transgenic SV40 mouse model and allograft mouse model carried by C57 mice to determine the risk association between obesity and breast cancer and how maternal GE diet influences the interactions between these maladies during early development. The SV40 mouse model can spontaneously develop mammary tumor driven by the trans-oncogene [28], whereas the C57 breast allograft model facilitates better understanding of the effects of maternal GE diet on HFD-induced obesity and the related breast cancer, and also helps to rule out potential impacts from transgenes. Furthermore, both mouse models generate ERα-negative mammary tumor representing a special type of aggressive breast cancer in humans, TNBC, which is closely associated with high body mass index and obesity in pre-menopausal women [3]. Thus, the use of these model systems provides the best approach to understanding maternal dietary GE intervention on early prevention of obesity-related breast cancer, especially obesity-susceptible hormone-negative breast cancer such as TNBC, through different mechanisms.
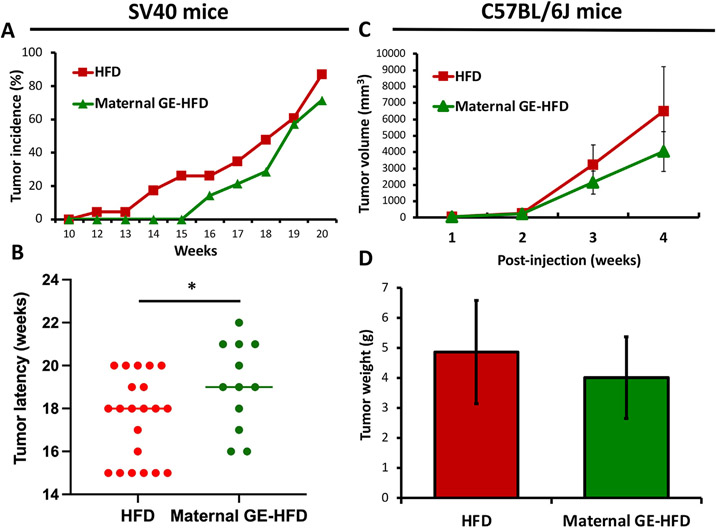
As illustrated in Fig. 2A and B, we found that maternal GE diet can significantly inhibit HFD-induced spontaneous mammary tumor development and delay tumor latency in SV40 offspring mice. Similar protective effects were observed in C57 allografts showing maternal GE can reduce engrafted breast tumor growth and tumor weight (Figs. 2C and D). Although the tumor parameters in C57 were not statistically significant, we observed an obvious inhibitory trend in tumor growth by maternal GE intervention. This may be due to the nature of the xenograft or allograft models, while the tumors are introduced in an artificial manner that could not represent normal tumorigenesis process. In summary, our results suggest that maternal GE can delay postnatal overnutrition-simulated mammary tumor promotion leading to early-life breast cancer prevention in the offspring.
Fig. 2.
Maternal dietary GE inhibited mammary tumor growth in HFD-treated female progenies. A-B, SV40 female offspring (21 mice/HFD and 12 mice/Maternal GE-HFD group). A, tumor incidence. B, tumor latency. C-D, C57 female offspring mice in HFD or maternal GE-HFD group (5 mice/group) were injected with ~106 E0771 cells under mammary pads to bear breast allografts at 21 weeks and sacrificed at 25 weeks after 4-weeks of injection. C, Tumor volume change after injection. D, Tumor weight at the endpoint. *, P<.05, significantly to HFD group.
3.3. Maternal GE diet altered key tumor-, obesity-, and inflammation-related gene expression in offspring mice
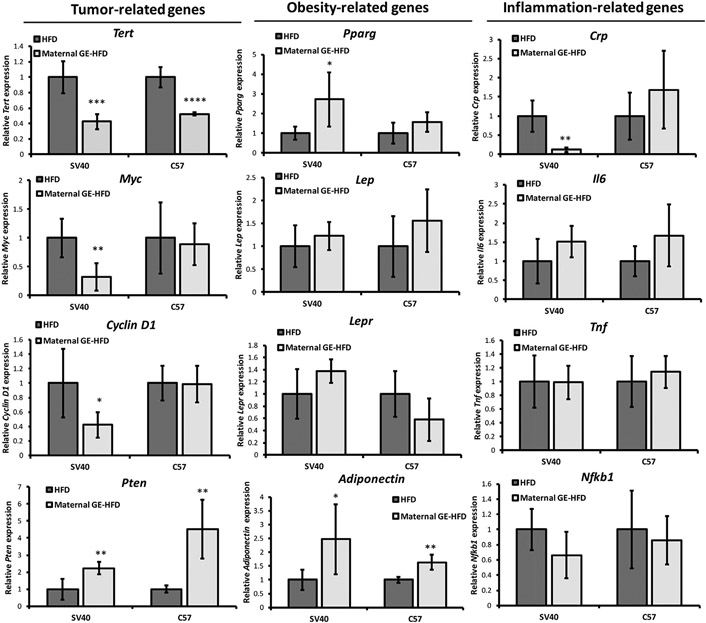
To determine the potential molecular mechanisms underpinning maternal soybean diet-induced preventive effects on HFD-stimulated breast cancer, we assessed a series of key regulatory genes that have been extensively studied in regulation of molecular processes during obesity and cancer development. These genes include tumor-related genes such as oncogenes (Tert, Cyclin D1 and Myc) and tumor suppressor gene, Pten, as well as obesity- and adipogenesis-related genes such as Pparg, Lep, Lepr and Adiponectin. As obesity is known to associate with chronic inflammation with elevated inflammatory cytokines [4], we also tested several key pro-inflammatory genes such as Crp, Il6, and Tnf and Nfkb1. We evaluated these gene expressions at transcriptional level in mammary tumors or adipose tissues of unaffected mammary glands in the offspring by either HFD or maternal soybean GE-HFD treatment.
As illustrated in Fig. 3 left panel, maternal GE can significantly decrease oncogene expression in Tert, Myc and Cyclin D1 genes but increase tumor suppressor Pten expression in both transgenic SV40 and allograft C57 mammary tumors. These gene expression patterns were more obviously affected in mammary tumors in SV40 mice than engrafted tumors in C57 mice. This could be partially due to the fact that transgenic mouse model may more closely approximate human breast pathogenesis since oncogene activation is frequently seen in malignant human breast cancer [33].
Fig. 3.
Maternal GE altered gene expression of selected key regulatory genes. Realtime RT-PCR was used to detect relative gene expressions including tumor-related genes (Tert, Myc, Cyclin D1 and Pten, left panel), obesity-related genes (Pparg, Lep, Lepr and Adiponectin, middle panel) as well as inflammation-related genes (Crp, Il6, Tnf and Nfkb1, right panel) in response to HFD or maternal GE diet-HFD treatment. Tumor-related genes were tested in mammary tumors, whereas obesity-related and inflammation-related genes were detected in unaffected mammary adipose tissues. Data were in 5 biological repeats from 3 independent experiments and were normalized to Gapdh and calibrated to the levels in control samples as 1. Columns, mean; Bars, SE; *P<.05, **P<.01, ***P<.001, ****P<.0001, significantly different from the HFD group.
Secondly, we evaluated several key obesity-related gene expression in the offspring mammary adipose tissues (Fig. 3, middle panel). Pparg is considered one of the most important regulators for adipogenesis and lipogenesis. Activation of Pparg is able to reverse obesity-induced inflammatory response contributing to improved glucose metabolism and insulin sensitivity [34]. Lep-coded leptin is a hormone produced by adipose cells that can decrease food intake and increase energy expenditure. Leptin resistance is a potential mechanistic link to obesity development [35]. Our results showed that maternal GE intervention increased Pparg, Lep and Lepr expression in the offspring adipose tissues. We also found significant elevation of Adiponectin expression in response to maternal GE treatment in both mouse models. Adiponectin is a protein hormone secreted by adipocytes that plays a crucial role in protecting against obesity-related diseases such as diabetes, insulin resistance and cardiovascular diseases [36]. These gene expression changes may contribute to maternal GE-induced preventive effects on improving metabolic functions against HFD.
Lastly, we tested gene expression of several key pro-inflammatory genes such as Crp, Il6, and Tnf and Nfkb1 in the adipose tissues of unaffected mammary glands (Fig. 3, right panel). However, we did not observe obvious expression changes in most of the tested pro-inflammatory genes in the offspring mammary fat tissues. We speculate that maternal GE-induced anti-inflammatory effect may not predominantly exhibit in the adipose tissues of normal mammary glands. In summary, our findings suggest that maternal GE can regulate these key gene expressions that may in turn contribute to its effects on prevention of HFD-stimulated obesity, metabolic disorders and breast tumorigenesis in the offspring.
3.4. Serum cytokine profiling in response to maternal GE treatment
A body of evidence indicates that consumption of soybean products has shown excellent protective effect against diet-induced obesity through regulation of oxidative and inflammatory responses [17,37]. Although there was no obvious gene expression change of the pro-inflammatory factors in the offspring mammary adipose tissues when exposed to maternal GE (Fig. 3, right panel), we believe maternal GE may induce anti-inflammatory responses ubiquitously in the circulatory system. We next sought to determine whether maternal GE could influence circulating cytokines and chemokines that play critical roles in important biological functions of inflammatory response in the offspring serum.
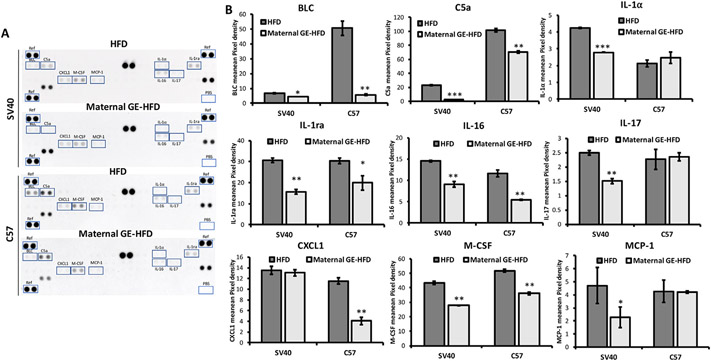
As shown in Figs. 4A and B, cytokine array data indicated that maternal GE significantly inhibited a number of pro-inflammatory factors such as C5a (Complement Component 5a), interleukins (IL-1α, IL-1ra, IL-16, IL-17), MCP-1 (Monocyte Chemoattractant Protein-1) and CXCL1 (C-X-C Motif Chemokine Ligand 1), which confirm our hypothesis that maternal GE can potentiate anti-inflammatory effects in the offspring system. We also found down-regulation of several key chemokines including B lymphocyte chemoattractant (BLC) and macrophage colony-stimulating factor (M-CSF) that regulate the immune system and tumor response. For example, maternal GE can dramatically reduce serum BLC level in mice offspring, especially in C57 mice. Highly elevated BLC level has been found in multiple malignancies including breast cancer [38]. HFD-induced chronic inflammation status has been well demonstrated to increase initiation and growth of breast cancer [2,4]. Our data support a protective role of maternal soybean intervention on obesity-related breast cancer through potentiation of anti-inflammatory effects and modulation of the immune landscape in the female offspring.
Fig. 4.
Maternal GE altered serum pro-inflammatory cytokine profiles in the offspring. A, Serum cytokine levels in SV40 and C57 offspring mice from HFD or maternal GE-HFD groups were analyzed using Proteome Profiler Mouse Cytokine Array according to the manufacturer’s specifications. Each cytokine has duplicate spots. Differentially expressed cytokines were boxed and labeled. Representative blots from an experiment were repeated three times. B, The cytokine spots on the blot were quantified by measuring pixel density of each spot using ImageJ. Columns, mean; Bars, SE; *P<.05, **P<.01, ***P<.001, significantly different from the HFD group.
3.5. Maternal soybean GE diet altered the offspring gut microbiome
We believe that maternal exposure to beneficial diets can shift the offspring gut microbiome profiles during early life, which may provide protection against postnatal nutrition stress and in turn influence the outcome of later-life health or disease status in the offspring. To validate our hypothesis, we determined the impact of maternal soybean diet on early-life gut microbiome in the offspring mice by using high-throughput bacterial 16S rRNA sequencing.
The average number of sequencing reads per sample obtained was 21,056. After quality control and data processing, a total of 354 OTUs were identified (Supplementary Data 2). Further analysis of α-diversity showed that there was no significant difference of Shannon diversity between the HFD and maternal-GE HFD offspring mice, indicated that maternal nutrients may have less impact on microbial richness and diversity in the offspring gut. Importantly, we discovered that maternal soybean GE consumption significantly affected the β-diversity and composition of gut microbiota in HFD-treated offspring mice using Bray-Curtis distance matrix (P=.021). PCA demonstrated that maternal GE significantly altered the community structure of the gut microbiota between the two offspring groups under HFD (Fig. 5A). The distribution of offspring gut bacteria was clearly separated depended on their mothers’ nutritional exposure to either control or GE diet. These results suggest that the composition of offspring gut microbiota can be changed massively in response to the maternal dietary status.
Fig. 5.
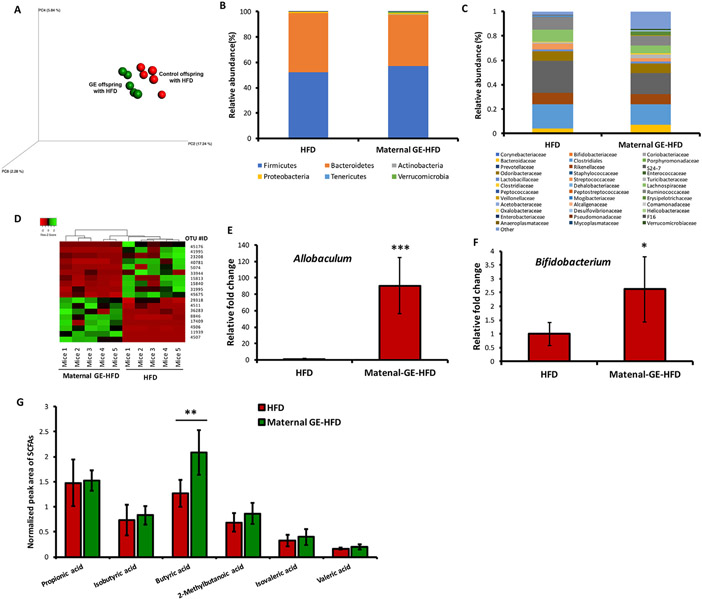
Maternal GE altered gut microbiome and key metabolites in the HFD-treated offspring. Gut microbiome analyses by 16S rRNA sequencing (n=5/group) and serum SCFA analysis by LC-MS analysis (n=6/group) in HFD-treated C57 female offspring exposure to either control or maternal GE treatment. A, 3D PCoA plot demonstrating beta diversity. B, Phylum level changes. C, Family level changes in microbial abundance in HFD and maternal GE-HFD mice. D, Heatmap results show the identified OTU with significant microbiota changes in response to maternal GE treatment. E, Relative fold change in the genus Allobaculum. F, Relative fold change in the genus Bifidobacterium. G, Normalized serum SCFAs. Columns, mean; Bars, SE; *P<.05, **P<.01, ***P<.001, significantly different from the HFD group.
At the phylum level, the offspring taxa in both groups were similar and dominated by Bacteroidetes and Firmicutes (Fig. 5B). The changes in the community structure of the gut microbiota at the family level showed more diversity in response to maternal GE (Fig. 5C). The dominant bacteria in both groups included S24-7, Clostridiales, Lachnospiraceae and Ruminococcaceae. In comparison with the HFD group, maternal supplementation with GE significantly decreased the abundance of the most dominant bacteria, S24-7, but increased Erysipelotrichaceae and Bacteroidetes.
In order to screen the key OTUs that respond to maternal GE diet out of all identified OTUs, further negative binomial model detected top 18 taxa with FDR adjusted P values lower than .05 (Supplementary Data 2). The relative abundance of these significantly altered bacterial taxonomic units were depicted in a heatmap at order level (Fig. 5D). Specifically, we discovered a significant preponderance of the genus Allobaculum in maternal GE-HFD offspring as compared to HFD offspring, which is most likely a reflection of maternal dietary differences (Fig. 5E). We also found that maternal soybean diet significantly induced enrichment of important probiotics in the offspring gut such as the genus Bifidobacterium (Fig. 5F), that has been shown to relate to gut homeostasis and healthy status [39]. We therefore speculate that the offspring may “inherit” beneficial microbial species from the mother in utero and/or during lactation to maintain a healthy and balanced gut bacterial ecosystem from childhood that benefit their future health.
3.6. Maternal soybean GE diet altered key microbial metabolites in the offspring
The gut microbiota is considered as a great bioreactor that assists metabolism and processing of food ingredients. Among those bacterially produced metabolites, short-chain fatty acids (SCFAs) are one of most important products of bacterial fermentation that are known to play key roles on maintaining a healthy status in the human colon [40,41]. Coincidentally, we found that maternal GE diet significantly increased abundance of SCFAs-producing bacteria such as Allobaculum, Bifidobacterium and Bacteroides in HFD-treated offspring. We next evaluated how these microbial changes may influence specific key metabolite production such as SCFAs in the offspring serum by LC-MS analysis. Our results suggest that maternal GE resulted in an increase in most SCFAs, especially butyric acid (Fig. 5G). Butyrate in particular is important in SCFAs and has been shown to protect against several human diseases including cancer, diabetes and obesity through mechanisms, at least in part, epigenetic regulations [42,43]. Our study indicates that maternal soybean diet can alter the taxonomic composition and abundance of certain important gut microbiota leading to subsequent bacterially produced key metabolite profile changes, contributing to its beneficial effects on improving health outcomes.
3.7. Sodium butyrate treatment inhibited mammary tumor proliferation in vitro
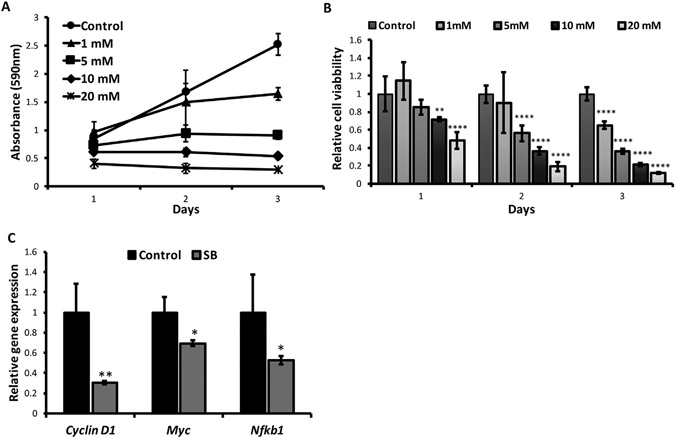
To determine the causality mechanisms linking maternal soybean GE to early-life microbiota and microbially produced key metabolites in improving health outcomes later in life, we tested the impact of sodium butyrate (SB), a key microbial metabolite that has shown significant increment after maternal GE treatment (Fig. 5G), on in vitro proliferation of breast cancer cells. The MTT assay was performed in murine mammary tumor cells, E0771, treated with various concentrations of SB as shown in Figs. 6A and B. We observed that SB induced a time- (Fig. 6A) and dose-dependent (Fig. 6B) manner on cell growth inhibition. As illustrated in Fig. 6B, the growth of breast tumor cell was significantly inhibited at 10 mM and 20 mM of SB from the first day of the treatment. 5 mM of SB treatment led to a significant suppression of cell proliferation from the second day of the treatment, whereas 1 mM of SB treatment exhibited significant inhibitory effects after 3-day treatment. In addition, we found 5 mM of SB treatment caused significant down-regulation of important tumor promoting genes such as Cyclin D1 and Myc as well as key pro-inflammatory gene, Nfkb1, in E0771 cells (Fig. 6C). These results provide potential causal mechanisms unpinning maternal GE diet-induced preventive outcomes through reshaping early-life microbiota and the subsequent beneficial microbial metabolites such as SB in the offspring system, leading to improved metabolic health and breast cancer outcome later in life.
Fig. 6.
Sodium butyrate (SB) inhibited tumor proliferation in murine mammary tumor E0771 cells. A and B, E0771 cells were treated with 0, 1, 5, 10, and 20 mM SB for 3 d and cellular proliferation was determined by MTT assay. A, MTT absorbance at 590 nm at different time and doses of SB treatment. B, Relative cell viability. C, Realtime RT-PCR was used to detect relative gene expressions of Cyclin D1, Myc and Nfkb1 at 5 mM of 3-day SB treatment. All experiments were performed in triplicate and data were from 3 independent experiments. Columns, mean; Bars, SE; *P<.05, **P<.01, ***P<.001, ****P<.0001, significantly different from the control group.
3.8. Maternal GE intervention influenced offspring epigenetic profiles
Although soybean GE exhibits its protective effects against obesity and cancer via various mechanisms, studies from our and other labs showed that soybean GE is a potent epigenetic effector that affects key gene expression through regulation of important epigenetic modulators such as DNMT or HDAC [13-15,20,44]. We next investigated whether maternal soybean diet could affect offspring epigenetic profiling attributing to its inhibitory effects on HFD-stimulated mammary tumor in the female offspring.
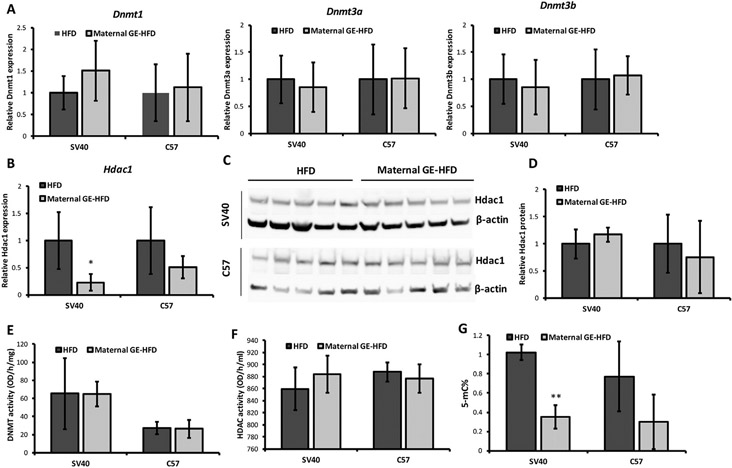
Our results showed that maternal GE did not influence gene expression of three conserved Dnmts that are responsible for both maintenance (Dnmt1) and de novo DNA methylation (Dnmt3a and Dnmt3b) in the offspring mammary tumors of both mouse models (Fig. 7A). Although maternal GE can induce significant down-regulation of Hdac1 mRNA expression in SV40 offspring (Fig. 7B), this inhibitory effect was not able to detect at the protein level (Figs. 7C and D). Consistently, neither DNMT nor HDAC activities were affected by maternal GE treatment (Figs. 7E and F). Interestingly, maternal GE resulted in a global demethylation status in the offspring tumors of both mouse models, especially in SV40 mice (Fig. 7G). Although a majority of the tested epigenetic changes were negative, we believe the different tumor proliferative stages in the individual animals may affect dynamitic epigenetic states. The precise epigenomic status could be determined by whole-genome bisulfite sequencing or chromatin immunoprecipitation-sequencing.
Fig. 7.
Maternal GE influenced epigenetic profiles in the offspring mammary tumors. A and B, Realtime RT-PCR was used to detect relative gene expression in Dnmt1, Dnmt3a, Dnmt3b and Hdac1. C, Nuclear protein was extracted from mammary tumors in female offspring. Hdac1 protein level was evaluated by western-blot from 5 randomly selective animals administered by HFD or maternal GE-HFD. D, Quantification of Hdac1 protein. All protein levels were normalized to β-actin as an internal control and calibrated to HFD group. Representative photograph from an experiment was repeated three times. E, DNMT activity. F, HDAC activity. G, Genome DNA was extracted and global DNA methylation status was determined by evaluation of 5-mC percentage. Data were obtained from 3 independent experiments in 5 biological repeats. Columns, mean; Bars, SE; *P<.05, **P<.01, significantly different from the HFD group.
Collectively, these results indicate that maternal soybean diet can induce a loss of global DNA methylation in the offspring epigenome that are independent of regulation of DNMT expression or enzymatic activities but rather likely through an inherited epigenetic regulation during early life.
4. Discussion
In modern society, overnutrition-caused obesity featured by an increased body mass index and metabolic disorders has become a major risk factor for development of many human chronic diseases including breast cancer [2]. Evidence supporting developmental origins of both obesity and breast cancer provides promising prevention approaches that center on maternal dietary intervention. In the current study, we focused on determination of the effects of maternal bioactive soybean GE intervention on prevention of HFD-induced obesity and its related breast cancer, and elucidation of the potential mechanisms through regulation of key regulatory genes/pathways, gut microbiome and epigenetics in the offspring. Our studies provide strong evidence indicating that maternal soybean GE diet is highly effective in reducing HFD-induced metabolic dysregulations and delaying HFD-accelerated mammary tumor development in the female offspring of several mouse models. Mechanistically, maternal GE consumption can significantly affect the composition of early-life gut microbiota, the signature of microbially produced metabolites, the global DNA methylation status and subsequent key regulatory gene profiles in the offspring, contributing to maternal GE-induced beneficial health outcome later in the offspring’s life.
A wealth of evidence from epidemiological and animal studies indicates an important role of maternal diets and nutrition on influencing later-life health outcomes [6,19,45]. In light of these factors, nutrient supply during maternal life including prenatal and postnatal early-life stages could be especially important to reverse adverse fetal programming and alter susceptibility to certain diseases later in the offspring’s life. Soy is one of the most pervasive agricultural products worldwide. It serves as a key source of vegetable protein and oil, and can be made into food products like tofu, soy sauce, meat substitutes, etc. The major bioactive compounds in soy are isoflavones and GE is the predominant isoflavone in soy-derived foods and dietary supplements [12]. In addition to its profound preventive effects against various types of human diseases such as obesity and cancer [9-11], studies in experimental animals demonstrate that maternal GE has potential transgenerational effects in reducing risks of certain diseases including obesity or breast cancer in the offspring [19-22]. These results are consistent with the epidemiological evidence in Asian population with a high amount of soy consumption but low risk of breast cancer incidence throughout multi-generations [46]. However, to date, there is no study investigating how maternal soybean GE diet influences the incidence of obesity-related breast cancer in the offspring as well as the interactions between these maladies during early development, and exploring potential mechanisms underpinning these phenomena. For the first time, we addressed these challenges in this study.
We tested our hypothesis in determining the preventive effects of maternal soybean GE on later-life obesity and obesity-induced breast cancer in a transgenic SV40 mouse model and obesity-related wild-type C57 mice. Importantly, our results indicate, for the first time, that maternal GE may beneficially attenuate postnatal overnutrition-induced metabolic disorders and its stimulated mammary tumor progression in both mouse models, suggesting that this preventive effect is profound and independent of genetic background and tumor etiology. The effective maternal GE dose against offspring breast tumor from this study is equivalent to ~200 mg GE/60 kg human daily [31]. This amount can be achieved by consuming GE-enriched soybeans and the derived products such as tofu, miso, natto et al., indicating that the GE dose used in this study is physiologically available and can be translated to future human studies. A dietary recommendation is provided to instruct human intake of the selective common soybean products that corresponds to effective GE amount in our study (Supplementary Table in Supplementary Data 3).
It has been suggested that obesity contributes to breast tumorigenesis by increasing local and circulating proinflammatory cytokines and mediators leading to a chronic inflammatory environment that promotes cancer invasion and metastasis [47]. Our results showed that maternal GE significantly affected essential regulatory gene expression patterns in specific tissue compartments of the offspring such as tumor-related genes in mammary tumor (tert, Myc, Cyclin D1 and Pten), obesity related-genes (Pparg and Adiponectin) in mammary adipose tissue and key pro-inflammatory cytokines/chemokines in the circulation system. Maternal soybean-induced chemoprotective effects are most likely elicited through its universal impacts on ameliorating chronic inflammatory response in obese state, which may in turn improve glucose/lipid metabolism and potentiate anti-tumor effects leading to prevention of HFD-induced obesity and breast cancer later in life.
As maternal GE does not directly interfere with postnatal nutrition stimuli such as HFD in the offspring, the protective phenotypes that are persistently transmitted to the next generation are likely mediated by specific modulators directly exposed to GE during maternal life and postnatal early life. Various studies have shown that the initial colonization of gut microbiota occurs during early life through, for example, the microbial transmission from the mothers to the offspring in utero, the postnatal “infection” throughout lactation and early childhood stages [25,48]. Substantial human and animal studies have shown the altered composition and diversity of gut microbiota in the offspring from obese mothers could play a pivotal role in programming the offspring to develop metabolic disorders from their early life [49,50]. We therefore hypothesize that maternal beneficial nutritional exposure may influence early-life microbial colonization and development, and thereby alter long-term health outcomes later in life. We evaluated how maternal GE diet predominated the early-life gut microbiota establishment. As expected, maternal GE diet resulted in significant changes of early-life intestinal microbiota compositions in the offspring mice. For example, Bifidobacterium, an important probiotic that has been used to reduce blood glucose in diabetes patients, was significantly enriched in maternal GE-treated offspring. The significant abundance such as Allobaculum and Bacteroides at the genus level was also observed in maternal GE-treated offspring mice with HFD. These enriched bacterial species are considered the producers of SCFAs [51], a group of multifaceted mediators that maintain homeostasis in healthy colon through, at least in part, epigenetic mechanisms [40-43]. Further studies confirmed an elevated level of most SCFAs, especially SB in maternal GE-treated offspring serum. Importantly, our in vitro studies provide potential causal linkage between the beneficial microbial metabolite, SB, and its inhibitory effects on breast cancer proliferation and inflammatory response that may explain the preventive outcomes of maternal GE. These results indicate maternal GE may reverse HFD-induced dysbiosis of gut microbiome and produce beneficial bacterially produced metabolites in the offspring who are predisposed to obesity and obesity-related breast cancer. Our results are coherent with the previous reports from Zhou et al. showing improved metabolic functions and altered microbiome by maternal GE treatment in both male and female C57 offspring, especially in male offspring [52,53]. This sex-dependent metabolic response by maternal GE treatment might be due to differential microbial changes between the male and female offspring [54]. Coincidently, our parallel study also observed the similar but rather more prominent metabolic protective effects of maternal GE in male offspring (manuscript in preparation). Further comparative microbiome analysis will unveil more insights regarding the gender disparity that may also apply in humans. Thus, our study provides key evidence pointing toward the early-life microbiome and microbial metabolome as key mechanistic mediators in regulation of the developmental origins of health and disease.
It has become increasingly apparent that dysregulation of epigenetic mechanisms frequently occurs during disease initiation including breast tumorigenesis and abnormal body-fat accumulation such as obesity [55]. Since epigenetic mechanisms are frequently regulated by environmental factors, maternal diets and early-life nutrition may play important roles in influencing early-life epigenetic reprogramming processes leading to different phenotypic changes such as a reduced disease risk of certain diseases in the offspring. Although soybean GE is believed to act as an epigenetic modulator to influence DNA methylation and histone modification processes [13-15,27,44], maternal GE does not directly alter gene expression of major epigenetic enzymes nor their activities in the weaned offspring. However, we did observe a global demethylation state in the offspring tumor, suggesting this hypomethylation status may originate from epigenetic reprogramming of DNA methylation patterns during the embryonic developmental stage leading to an altered DNA methylation state in the offspring. Dolinoy et al. found that in utero GE exposure alters offspring susceptibility to obesity by permanently altering the fetal epigenome during early embryonic development [21]. Our recent study also showed that maternal GE can induce epigenetic inheritance in key tumor suppressor genes, contributing to its breast cancer prevention potential later in offspring life [20]. Thus, we believe that maternal soybean diet can alter global DNA methylation status during the offspring’s early life. This altered epigenetic state and conserved phenotypes could sustain throughout generations through an inherited epigenetic regulation machinery. Because maternal GE can also increase the level of bacterially produced beneficial metabolites such as butyrate that is considered as an epigenetic modulator in regulation of gene expression [43], we speculate there is a dynamic crosstalk existing between the initial colonizing microbiota, metabolome and the epigenome of the host at critical early developmental stages [56]. Their interaction may eventually influence key regulatory gene expressions or signal pathways that affect later-life health and disease outcomes. Exploration of the crosstalk linkage between these factors requires further in-depth studies, which will shed light on studying developmental plasticity of human diseases and facilitate development of early-life disease prevention strategies through maternal dietary intervention.
Breast cancer and obesity-related diseases affect hundreds of thousands of people world-wide and elucidation of its prevention through natural bioactive products will have global impact. Our studies elucidated the potential mechanisms linking maternal soybean dietary GE intervention to its preventive potential on obesity and the related breast cancer later in life through an interactive regulation between the early-life gut microbiome, microbial metabolites and the offspring epigenome. This study may lead to translational disease chemoprevention potential that benefits human health by appropriate administration of maternal diets and nutrition. Future study will focus on exploration of the crosstalk mechanisms between maternal diet, the early-life microbiota, the offspring metabolome and epigenome through, for example, function analysis of specific metabolites or candidate bacteria on epigenetic profiles in gnotobiotic animals. Furthermore, a pilot clinical trial will be necessary to test the feasibility of this novel dietary regimen in obese pregnant women. Finally, this study has high significance for potentially developing a novel maternal supplementary regimen that may benefit human health from early life.
Supplementary Material
Funding
This work was supported by grants from the National Institutes of Health (NCI R01 CA178441 and NCI R01 CA204346 to TOT, NCCIH K01 AT009373 to YL), United States Department of Agriculture, National Institute of Food and Agriculture (USDA NIFA, 2021-67017-34007 to YL) and the startup funding from the Department of Pharmacology and Toxicology at the University of Alabama at Birmingham, and the Department of Obstetrics, Gynecology & Women’s Heath, the Department of Surgery at the University of Missouri.
Footnotes
Ethics approval and consent to participate
Animal procedures in this study were approved by UAB IACUC (Animal Project Numbers: 20653, 20671).
Declaration of Competing Interests
The authors declare no potential conflicts of interest.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jnutbio.2022.109119.
References
- [1].Apovian CM . The clinical and economic consequences of obesity. Am J Manag Care 2013;19:s219–28. [PubMed] [Google Scholar]
- [2].Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast 2004;13:85–92. [DOI] [PubMed] [Google Scholar]
- [3].Sun H, Zhou J, Chen L, Zu X, Wen G, Zhong J. Triple-negative breast cancer and its association with obesity. Mol Clin Oncol 2017;7:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book 2013;33:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barker DJ. The origins of the developmental origins theory. J Intern Med 2007;261:412–17. [DOI] [PubMed] [Google Scholar]
- [6].Parlee SD, MacDougald OA. Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity. Biochim Biophys Acta 2014;1842:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab 2006;17:340–8. [DOI] [PubMed] [Google Scholar]
- [8].Silva Idos S, De Stavola B, McCormack V. Collaborative group on pre-natal risk factors and subsequent risk of breast cancer, birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med 2008;5:e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cederroth CR, Vinciguerra M, Gjinovci A, Kühne F, Klein M, Cederroth M, et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008;57:1176–85. [DOI] [PubMed] [Google Scholar]
- [10].Ramdath DD, Padhi EM, Sarfaraz S, Renwick S, Duncan AM. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients 2017;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kucuk O. Soy foods, isoflavones, and breast cancer. Cancer 2017;123:1901–3. [DOI] [PubMed] [Google Scholar]
- [12].Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer 2007;57:1–10. [DOI] [PubMed] [Google Scholar]
- [13].Li Y, Liu L, Andrews LG, Tollefsbol TO. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int J Cancer 2009;125:286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li Y, Meeran SM, Patel SN, Chen H, Hardy TM, Tollefsbol TO. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol Cancer 2013;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li Y, Chen H, Hardy TM , Tollefsbol TO. Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PLoS One 2013;8:e54369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res 1998;58:3833–8. [PubMed] [Google Scholar]
- [17].Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol 2013;698:31–8. [DOI] [PubMed] [Google Scholar]
- [18].Zhang M, Ikeda K, Xu J, Yamori Y, Gao X, Zhang B. Genistein suppresses adipogenesis of 3T3-L1 cells via multiple signal pathways. Phytother Res 2009;23:713–18. [DOI] [PubMed] [Google Scholar]
- [19].Li Y, Saldanha SN, Tollefsbol TO. Impact of epigenetic dietary compounds on transgenerational prevention of human diseases. AAPS J 2014;16:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen M, Li S, Srinivasasainagendra V, Sharma M, Li Z, Tiwari H. et al. Maternal soybean genistein on prevention of later-life breast cancer through inherited epigenetic regulations. Carcinogenesis 2022;43:bgac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect 2006;114:567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bonacasa B, Siow RC, Mann GE. Impact of dietary soy isoflavones in pregnancy on fetal programming of endothelial function in offspring. Microcirculation 2011;18:270–85. [DOI] [PubMed] [Google Scholar]
- [23].Kvit KB, Kharchenko NV. Gut microbiota changes as a risk factor for obesity. Wiad Lek 2017;70:231–5. [PubMed] [Google Scholar]
- [24].Chen J, Douglass J, Prasath V, Neace M, Atrchian S, Manjili MH. et al. The microbiome and breast cancer: a review. Breast Cancer Res Treat 2019;178:493–6. [DOI] [PubMed] [Google Scholar]
- [25].Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med 2016;22:713–22. [DOI] [PubMed] [Google Scholar]
- [26].Paul B, Royston KJ, Li Y, Stoll ML, Skibola CF, Wilson LS. et al. Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition. PLoS One 2017;12:e0189756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li Y, Tollefsbol TO. Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Curr Med Chem 2010;17:2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Green JE, Shibata MA, Yoshidome K, Liu ML, Jorcyk C, Anver MR, et al. The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene 2000;19:1020–7. [DOI] [PubMed] [Google Scholar]
- [29].Sharma M, Arora I, Stoll ML, Li Y, Morrow CD, Barnes S, et al. Nutritional combinatorial impact on the gut microbiota and plasma short-chain fatty acids levels in the prevention of mammary cancer in Her2/neu estrogen receptor-negative transgenic mice. PLoS One 2020;15:e0234893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013;8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008;22:659–61. [DOI] [PubMed] [Google Scholar]
- [32].Wong SK, Chin KY, Suhaimi FH, Fairus A, Ima-Nirwana S. Animal models of metabolic syndrome: a review. Nutr Metab (Lond) 2016;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Osborne C, Wilson P, Tripathy D. Oncogenes and tumor suppressor genes in breast cancer: potential diagnostic and therapeutic applications. Oncologist 2004;9:361–77. [DOI] [PubMed] [Google Scholar]
- [34].Stienstra R, Duval C, Müller M, Kersten S. PPARs, obesity, and inflammation. PPAR Res 2007;2007:95974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Myers MG, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010;21:643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci 2017;18:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Incir S, Bolayirli IM, Inan O, Aydın MS, Bilgin IA, Sayan I, et al. The effects of genistein supplementation on fructose induced insulin resistance, oxidative stress and inflammation. Life Sci 2016;158:57–62. [DOI] [PubMed] [Google Scholar]
- [38].Kazanietz MG, Durando M, Cooke M. CXCL13 and its receptor CXCR5 in cancer: inflammation, immune response, and beyond. Front Endocrinol (Lausanne) 2019;10:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther 2005;22:495–512. [DOI] [PubMed] [Google Scholar]
- [40].Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- [41].Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 2019:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer R. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 2008;27:104–19. [DOI] [PubMed] [Google Scholar]
- [43].Berni Canani R, Di Costanzo M, Leone L. The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clin Epigenetics 2012;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res 2005;11:7033–41. [DOI] [PubMed] [Google Scholar]
- [45].Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol 2001;30:15–23. [DOI] [PubMed] [Google Scholar]
- [46].Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst 2003;95:906–13. [DOI] [PubMed] [Google Scholar]
- [47].Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med 2013;64:45–57. [DOI] [PubMed] [Google Scholar]
- [48].Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the "sterile womb" and "in utero colonization" hypotheses: implications for research on the pioneer infant microbiome. Microbiome 2017;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr 2018;172:368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mann PE, Huynh K, Widmer G. Maternal high fat diet and its consequence on the gut microbiome: a rat model. Gut Microbes 2018;9:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mischke M, Plösch T. The gut microbiota and their metabolites: potential implications for the host epigenome. Adv Exp Med Biol 2016;902:33–44. [DOI] [PubMed] [Google Scholar]
- [52].Zhou L, Xiao X, Zhang Q, Zheng J, Li M, Yu M, et al. Improved glucose and lipid metabolism in the early life of female offspring by maternal dietary genistein is associated with alterations in the gut microbiota. Front Endocrinol (Lausanne) 2018;9:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhou L, Xiao X, Zhang Q, Zheng J, Deng M. Maternal genistein intake mitigates the deleterious effects of high-fat diet on glucose and lipid metabolism and modulates gut microbiota in adult life of male mice. Front Physiol 2019;10:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhou L, Xiao X, Zhang Q, Zheng J, Li M, Wang X, et al. Gut microbiota might be a crucial factor in deciphering the metabolic benefits of perinatal genistein consumption in dams and adult female offspring. Food Funct 2019;10:4505–21. [DOI] [PubMed] [Google Scholar]
- [55].Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004;429:457–63. [DOI] [PubMed] [Google Scholar]
- [56].Li Y. Epigenetic mechanisms link maternal diets and gut microbiome to obesity in the offspring. Front Genet 2018;9:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.