Abstract
The coronavirus disease-2019 pandemic disrupted traditional research practices with the cessation of face-to-face contact with study participants. Researchers needed to respond with alternative methods to continue nurse-led clinical research. A rapid pivot to remote processes for recruitment, enrollment, data collection, and participant incentives can enable research to continue despite restrictions on in-person activities. Technology offers innovative methods in meeting current research needs but is not without challenges and continued need for ethics evaluation.
Keywords: COVID-19, Virtual research, Virtual focus groups, Adolescent sleep research, Mindfulness research, Code of ethics for nurses, Qualitative research
Key points
-
•
During the pandemic, established programs of research were able to maintain productivity by being capable of responding swiftly to new guidelines.
-
•
The ability to adapt to new methods and new technology led to research success.
-
•
Virtual platforms created flexible options for study participant enrollment.
-
•
In some studies, virtual formats enabled increased participation and decreased participant attrition.
Introduction
The coronavirus disease-2019 (COVID-19) pandemic, with its unprecedented infection and fatality rates, disrupted daily life on a magnitude not seen in modern times. Research practices were also disrupted as in-person procedures for nonessential, non-COVID-19 research studies were temporarily suspended by institutions to maximize safety, minimize the spread of infection, and protect individuals’ health.1 , 2 These disruptions in research practices have drawn attention to the importance of contingency plans to support the continuity of nurse-led clinical research. Events such as the pandemic, which restrict in-person engagement with human subjects require modifications to methods of conducting research.1, 2, 3
The pandemic also created opportunities to address new research questions.4 Determining the gaps in our collective knowledge and responding to these needs required speed in the research process that is seldom seen. Some researchers could build on their earlier work by continuing with the same concepts of interest but adjusting to add new sample populations, adding new ways of delivering an intervention and new methods of data collection. Others responded with new study ideas where timing and relevance became crucial as the pandemic and the accompanying social forces were rapidly changing.
This paper describes several of the challenges that researchers encountered during the COVID-19 pandemic and how the authors have responded. Three research studies are highlighted, which show the following:
-
1.
Using technology to adapt and modify a research proposal
-
2.
Designing a mixed methods study to be totally virtual
-
3.
Conducting virtual focus groups
-
4.
Moving swiftly to retain relevance
In addition, helpful solutions are presented that include various aspects of the research process including subject recruitment, study design, and data collection.
Using technology to adapt and modify a research study
Readily adapting to changing public health situations requires staying abreast of current local public health guidance and relevant research regulations, as well as competence with available technology. During the pandemic, physical barriers were set that limited person-to-person contacts, administrative barriers to ambulatory care site access, temporary clinic closures, and limitations on numbers of individuals permitted in the clinic.
An original research study, initiated in December 2019 and abruptly halted in March 2020, highlights innovative methods to use technology to move research forward during the COVID-19 pandemic.
Research Case Study 1. Mobile Phones in the Bedroom: Impact on Adolescent Sleep
This descriptive, correlational study sought to explore the attitudes, behaviors, and sleep patterns of adolescents (n = 34; ages 13 to 17) who use mobile phones in the bedroom after lights out. Data were collected via an initial battery of questionnaires on attitudes and beliefs regarding sleep and mobile phone use, followed by a 7-day collection of sleep data via actigraphy (a wristwatch-type device to measure activity as a proxy for sleep), and daily sleep and nighttime mobile phone use diaries. Most of the sample (62%) completed participation before the pause of in-person research, while the remainder were engaged remotely following Institutional Review Board (IRB) approved protocol modifications that allowed for remote engagement.
The study protocol was modified to extend from solely in-person recruitment to also allow remote procedures. This enabled the research to proceed while maintaining compliance with regulatory guidelines. The safe engagement with potential research participants was important to maintain in this study. The study involved two recruitment modalities: in-person recruitment in three ambulatory care sites or via Studyfinder, a web-based recruitment database.5 A detailed description of this web-based study recruitment tool is presented in Box 1 .
Box 1. Web-Based Recruitment.
Web-Based Recruitment.
The use of a web-based recruitment database, such as Studyfinder, enables researchers to advertise IRB-approved research studies in a format available for potential participants to: browse available studies, seek additional information from the study team, determine eligibility, and engage in a relevant study.3 , 5, 6, 7
Social Media [for example, Facebook, Twitter, and Instagram] can be effective for recruiting: hard to reach populations, large numbers of participants, and people with specific health conditions. In addition, social media may shorten recruitment periods and be cost-effective. The use of social media as a recruitment strategy requires IRB approval with safeguards for participant privacy. Using social media may exclude those without internet or broadband access resulting in bias and limiting generalizability of findings.3 , 6
Recruitment was initially slow, with only 10% of the planned recruitment achieved after the first 4 weeks. An unanticipated boost in recruitment occurred after the Studyfinder link was posted on personal and local parent group social media pages by a parent.
-
•
Potential study participants or their parents noticed the social media posts and made inquiries about the study through the Studyfinder link.
-
•
This occurrence resulted in snowball recruitment as parents or their adolescents shared the information among peer networks, providing greater exposure within the local community with enhanced interest in study participation.
-
•
Snowball recruitment may result in selection bias limiting generalizability of results. More than half of the study participants were recruited before the cessation of in-person clinical research activities.
-
•
Study recruitment continued via Studyfinder only, which enabled participant recruitment to be completed within the original timeline. Initially, the study protocol involved in-person enrollment with signed parental consent and minor assent at the initial study visit.
-
•
After suspension of in-person research activities, remote enrollment via secure Zoom videoconference was implemented following consultation with an Office of Research Protection analyst, subsequent study modification, and IRB approval.
Remote enrollment included evaluation of screening questions, explanation of the study with participant expectations, and verbal consent and minor assent, which was followed by return of a signed consent via email (Box 2 ). This successful strategy also facilitated flexible enrollment on evenings and weekends when participants and parents were less likely to be occupied.
Box 2. Study Screening and Enrollment.
Web-based screening and enrollment tools.
Web-based screening tools, such as Survey Monkey or Qualtrix, as well as electronic data systems such as Research Electronic Data Capture (REDCap) are resources which may be used to screen potential participants safely.2 , 3 Parental consent and minor assent may occur electronically or verbally (eg, telephone; videoconference).
Secure videoconferences via BlueJeans for Health care, Go To Meeting, Microsoft Teams, or Zoom for Health care facilitate remote approaches for consent/assent (Stiles-Shields et al., 2020).2 Electronic resources (eg, DocuSign; REDCap; Qualtrix) enable electronic signatures indicating consent and minor assent.2 , 3
Electronic data capture (EDC) with Research Electronic Data Capture (REDCap) was planned and conducted from the outset of the study and continued during the suspension of in-person research. Remote options for data collection are described in Box 3 .
-
•
REDCap enabled direct data entry by participants with twice daily e-mail prompts and eliminated handling of paper forms and manual entry of data.
-
•
The wrist actigraphy watch device was issued at study enrollment and participants began the 7-day data collection immediately. However, following the transition to remote enrollment, this device was mailed to participants via the postal service and data collection began upon receipt of the device.
-
•
Distribution of research study devices that are needed to collect data is traditionally done in person; however, alternate methods of device distribution are required during a pandemic. Alternatives include the use of the postal service, shipping companies, or private couriers to transport data collection devices.
Box 3. Data Collection: Remote Options.
Remote Options for Data Collection.
Remote options for data collection include Electronic Data Capture (EDC) (eg, REDCap, ConnEDCt, Qualtrix, Survey Monkey, Excel), phone, telehealth, home-based monitoring, or videoconference modalities.1 , 2 , 8 , 9
The use of an EDC platform requires orientation, training, and testing before IRB approval. The REDCap system enables researchers to collect, store, and export data to statistical software packages for analysis.8 The ConnEDCt data base system was developed to collect and manage public health data,8 whereas the Comprehensive Adolescent Research and Engagement Studies (CARES) EDC was developed specifically for management of HIV data.10
Traditional participant incentives frequently take the forms of physical gift cards, checks, or cash payments. An adaptation is electronic gift cards or reloadable debit cards. Each can be easily purchased and sent directly to participants via email.2 , 3
Designing a study to be totally virtual
Research Case Study 2. Nurses evaluate whether a mindfulness intervention decreases stress (NEW MINDS)
Planning for the NEW MINDS (Nurses Evaluate Whether a Mindfulness Intervention Nets Decreased Stress) study began in 2019 with brainstorming sessions including members of the Research and Evidence-based Practice Council. The study team, including three clinical nurses, an advanced practice nurse, a nursing faculty member, and a statistician, collaborated with a clinical psychologist, who is a certified mindfulness-based stress reduction (MBSR) instructor. 11 The team decided to focus on two major sets of stress-related outcomes to measure the impact of mindfulness training for bedside nurses––physiologic measurements and self-reported measurements (surveys and focus groups).
Two intervention groups were designed for comparison: a full MBSR course and an abbreviated mindfulness training program. All study activities including obtaining informed consent, study orientation, MBSR training sessions, and data collection sessions (surveys, focus groups, and physiologic measurements) were designed to be conducted in-person during defined meeting times.
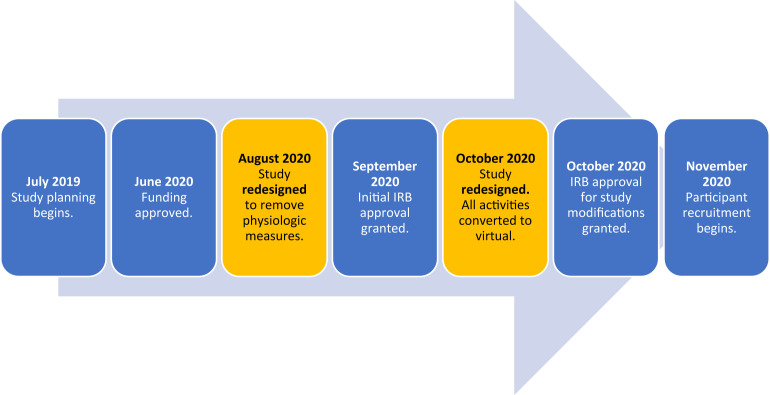
By June 2020, the team had received sufficient funding to support the cost of the mindfulness training program materials and instructor, and developed an agreement to collect all physiologic measurements using one of the study team member’s labs at no additional cost to the study team. All study protocol and supporting documents were written in preparation for submission to the IRB.
Shortly thereafter, conditions began to change regarding community transmission of COVID-19 and resulting hospitalizations. In response to local and federal restrictions, the University guidance, and other factors, research laboratory occupancy was reduced to 25% of normal operating occupancy, and funding was severely reduced. As a result, the study team received notification in late July 2020 that all outcomes based on physiologic measurements would have to be removed from the study protocol. The laboratory resources were simply not available to carry out that aspect of the study. Despite these challenges, the team decided that the importance of the mindfulness interventions (especially in the context of a pandemic) and the potential knowledge gained from self-reported measurements made it possible to simply exclude the physiologic measurements and move forward with the remainder of the study.
The team quickly adjusted the protocol and supporting documents, and in September 2020, the study was approved by the IRB. Dates and locations for the mindfulness training courses were determined and planning for recruitment began. As the study was preparing to launch, though, the notification came from the Office of Human Protections that all in-person study activities were being suspended. This meant that all in-person meetings for the study (consent, orientation, data collection, MBSR course) would have to be delayed until after the restrictions were lifted or that we would have to convert to a totally virtual study––unfamiliar territory for the majority of the study team. Considering the unknown timeline of COVID-19 and the safety of our study team and participants, conversion to a virtual format was chosen. By mid-October, a modified version of the study was resubmitted and approved by the IRB. The study timeline with redesign steps is shown in Fig. 1 .
Fig. 1.
New MINDS study timeline.
The revisions included a redesign for three major aspects of the study:
-
1.The process to obtain and document informed consent from each participant was now conducted entirely virtually, rather than in-person.
-
•A study team member set up a meeting with each interested nurse after an email exchange to check eligibility and determine the participant’s intervention group of choice (interventions were not randomly allocated due to the significant differences in scheduling commitment).
-
•Using an online survey form developed in close collaboration with the IRB, the study team member reviewed the whole consent form with the participant and answered questions and then both parties signed and dated the form.
-
•
-
2.Orientation and MBSR course sessions were now held virtually.
-
•Mindfulness training courses are traditionally held in-person to enhance the shared experience of connectedness and support among participants.
-
•The team originally intended for one group to enroll in a traditional MBSR program (in-person class held 2 to 3 h weekly for 8 weeks plus a day-long silent retreat), whereas the other group received an abbreviated, pre-recorded version of the course material meant to only take a few minutes each week after an initial in-person training session.
-
•With the study redesign, both groups’ interventions were now fully virtual.
-
•
-
3.All surveys were now sent electronically.
-
•Participants complete the surveys at their own convenience (within an allotted time frame) rather than asking all participants to attend in-person data collection sessions with printed survey forms.
-
•Focus groups were also conducted virtually.
-
•
By transforming to a fully virtual study, we found a variety of benefits and challenges which will help to inform future studies.
Benefits
-
•
Virtual training made accessibility much easier––no one had to worry about driving to attend the course at a physical location, and participants could join from practically anywhere as long as connection was available. For example, one participant in the full MBSR group drove to the beach to get the most relaxing experience for the full-day silent retreat.
-
•
The study team and the participant had immediate access to the signed consent document by using the virtual consent process, and there was no need for secure storage protocols for paper-based consent forms.
-
•
Data collection via electronic surveys allowed participants to enter and submit their responses privately without any involvement of the study team (other than sending survey invitations and reminders). This removed the possibility of data entry mistakes. Also, no need for storing paper forms again.
-
•
Conducting virtual focus groups may have allowed participants to speak more freely and honestly than they would have in-person.
Challenges
-
•
Participants were not able to meet in-person with each other or with the study team for the whole duration of the study. This resulted in some frustration related to the lack of personal connection.
-
•
Materials such as yoga mats and meditation cushions are generally provided during in-person mindfulness training programs. Because there was no physical contact with any participant, these materials could not be distributed; therefore, participants may or may not have had the proper equipment at home to fully participate in the training exercises.
-
•
All participants were required to have audio and video capabilities for all meetings––this may have excluded a subgroup of the population without Internet or technology access at home.
-
•
Although it has its advantages, allowing participants to join virtually from anywhere for all study activities made eliminating distractions a challenge. Each participant was in the setting of their choice rather than a controlled, consistent environment. It is possible that participants in a chaotic environment (at work, with children and/or pets in the background, etc.) may have struggled to focus and learn from the mindfulness interventions to the same degree as participants in a secluded/quiet learning environment.
-
•
In-person MBSR courses are the “gold standard” intervention when it comes to mindfulness training.11 Because all study activities were converted to virtual meetings, neither of our intervention groups met the traditional course standards.
With the study changes made and approved, recruitment eventually launched in November 2020 and the interventions began in January 2021.
In addition to the challenges surrounding the conversion to a virtual study, the difficult personal and work-related situations caused by the COVID-19 pandemic led to some study recruitment and retention challenges as well. Out of the 45 participants that we intended to enroll in the “brief” mindfulness training course, 39 nurses (in two waves of 29 then 10) joined the study and 25 of them completed the intervention and participated in pre- and post-intervention data collection. For the full MBSR course group, 14 nurses were enrolled out of the 15 available spots, and 10 of them completed the intervention and data collection. Increased time commitment, changing schedules, and staffing challenges at work during surges of COVID-19 hospitalization as well as personal/family conflicts explained the majority of study non-completion.
Conducting virtual focus groups
The qualitative data for the NEW MINDS mixed methods study was collected in semi-structured focus group interviews lasting approximately 1 h, conducted 3 months post-intervention, and repeated at 9 months post-intervention. Initial focus groups and follow-up focus groups were facilitated by two consistent nurse researchers. Focus groups were conducted via a virtual platform as necessitated by the COVID-19 pandemic. This format presented potential challenges to the facilitators, as well as unexpected benefits.
Benefits
Virtual focus groups offered a significant advantage to participants, and consequently, the overall quality of the study. Based on feedback from participants:
-
1.
The virtual format allowed for easier participation in the focus groups, as participants only needed to accommodate a designated time, and not a designated location. Some participants lived an hour or more away from the hospital that served as the study location, and reported that they would not have been inclined to travel back to the hospital for the focus groups.
-
2.
Conducting focus groups virtually eliminated the need for facilitators to reserve appropriate meeting space for the focus group in advance, and avoid potential space scheduling conflicts.
-
3.
Participant feedback supported the conclusion that rather than functioning as a barrier, the virtual format instead enabled increased participation in the focus groups, and decreased participant attrition.
Similarly, the increased flexibility and convenience afforded by the virtual platform to participants also enabled the focus group facilitators to offer a greater variety of time/day options for focus groups without considerations for travel time, child care, or other constraints. The range of times and days increased access for participants by accommodating their individual work shifts and personal schedules. Participants who were isolating or on quarantine were still able to participate and attend virtually as well.
Finally, a benefit to the virtual format of the study was the integrated recording feature of the virtual platform. The integrated recording capability streamlined the focus group recording process, necessary for transcription and qualitative analysis while ensuring that each participant’s active consent was recorded.
Barriers
Despite the overwhelmingly positive experience with the virtual platform for the focus groups, this format posed several potential barriers in executing focus groups. By anticipating these challenges and adapting accordingly, the facilitators were able to overcome them.
-
1.
One such concern was the ability to ensure the anonymity of focus group participants in the virtual format. This was addressed by the facilitators disabling the Zoom platform’s display of participants’ names in their respective video windows.
-
2.
The virtual format presented a possible barrier to study participants with decreased comfort/familiarity with technology, and opened up the potential for technological difficulties. In preparation for this possibility, one of the focus group facilitators was designated to assist with technological difficulties if needed, whereas the other facilitator conducted the interview. Fortunately, these problems did not materialize, as all facilitators and participants had prior experience using the virtual platform.
-
3.
Facilitators were tasked with creating a comfortable, welcoming focus group environment over a virtual platform while establishing rapport with and among participants.
Benefits and challenges to using virtual methods for conducting qualitative research have been reported by other researchers who have made similar observations.12 , 13 Ultimately, in this study, focus group facilitators determined that they were able to create an appropriate atmosphere for active participation and honest feedback from participants in the focus group despite the virtual format.
Unexpected Findings
The greatest unexpected finding to arise from the pandemic-imposed process of conducting focus groups virtually was that facilitators were still able to cultivate meaningful connections with and among participants and foster a safe and open space for discussion through virtual means. The significance of this finding, while not conclusive, cannot be overlooked, especially regarding its potential bearing upon other studies that may require qualitative data collection and analysis to be conducted virtually.
Moving swiftly to retain relevance
Adaptability during the pandemic was essential for research productivity. This was true for bench researchers who had the vision to adapt their labs and equipment to contribute in new ways to address the public health crisis; as well as for researchers in the social sciences and nursing. Researchers moved swiftly to meet immediate lifesaving needs, as well as to study the human experience in real time.
The COVID-19 pandemic has challenged nurses and all health care professionals to examine how we balance the obligation to provide safe care to those in need while also meeting obligations to self-care. In addition, long-standing infection control practices were altered as we struggled to find and maintain adequate supplies of personal protective equipment (PPE). As the months progressed, supplies became available but another valuable resource challenge emerged. In the fall of 2021, the shortage of registered nurses to provide care to the sick and to the well in all practice settings was a problem without a solution in site.
The ANA Code of Ethics for Nurses has a long tradition of providing moral guidance for all nurses in all practice domains.14 “The Code is one standard that is universally applicable to all nurses.”15 For these reasons, it provided an excellent framework to study how nurses were practicing, coping, and the resources they needed to continue.
Research Case Study 3. Ethics in an era of the pandemic.
Nurse researchers at our institution set out to understand the experience of nurses during the first stage of COVID-19 by studying the relevance of the ANA Code of Ethics to their practice during the pandemic.16
A descriptive study using qualitative methods was conducted with nurses participating in one of six virtual focus groups. Twenty-three registered nurses participated. They represented acute care and ambulatory care settings; cared for adults, children and families; cared for patients with COVID-19 infections, many of who were critically ill. The ANA Code of Ethics was shared and participants were asked to identify which provisions, if any, seemed most relevant to their practice during COVID-19. Findings indicated that most of the provisions had relevance. There was a strong appreciation for collaborative efforts and a strong sense of obligation and commitment. Nurses served as advocates for patients, families, and communities. They witnessed increased suffering and death and identified the need for palliative care education and struggled to maintain a work–life balance.16
In studying the experience of a pandemic as it is occurring, research processes and timelines needed to move faster than usual for findings to maintain their relevance. In this study, nurse researchers were able to move swiftly by using their knowledge and expertise in bioethics and qualitative research methods to design and implement the study within 3 months. In addition, moving quickly on research dissemination through publications and presentations became equally important. Writing of the initial manuscript began while data analysis was underway and attention to abstract submission deadlines to national conferences was paramount.
Summary
The COVID-19 pandemic altered traditional methods of conducting clinical research and required nurse researchers to adapt research protocols to the new guidelines. In-person research was largely replaced by remote processes with integration of electronic resources into all aspects of research approaches. Researcher preparedness, flexibility, and perseverance were strengths that promoted research success. In addition, prompt assessment of barriers and facilitators to continued research amid an uncertain environment was critically important.
Researchers conducting clinical research during a pandemic can benefit from: acknowledging the challenges faced; staying virtually connected with one another, participants, study sites, and advisory board members; and regular brainstorming to benefit from the team’s combined ingenuity, resources, skills, and networks. Taken together, these approaches can maintain study integrity, while promoting recruitment, enrollment, and engagement with research participants, so that data may be collected and analyzed and findings may be disseminated within (or close to) the study timeline.
The question looms as to the long-term impact of remote research practices and if modifications adopted during the COVID-19 pandemic will become standard operating procedures. This recent public health crisis should serve as a call to action for research teams to evaluate current processes and procedures, team members’ skill sets and knowledge of current and emerging technological advancements. Although technology offers innovative methods in meeting current research needs, it is not without challenges and continued need for ethics evaluation.
Clinics care points
-
•
Embracing the use of technology in clinical research can potentially help with all stages of a study: subject recruitment and enrollment, providing interventions, and delivering incentives.
-
•
Virtual platforms may provide focus group facilitators the ability to make connections with and among participants and foster a safe and open space for discussion.
-
•
In studying the experience of a pandemic as it is occurring, research processes and timelines need to move faster than usual for findings to maintain their relevance.
Footnotes
Disclosure Statement: The authors (C. Snyder, S. Acri, R. Allen, and M.L. Kanaskie) have no disclosures or funding to report and no commercial or financial conflicts of interest.
References
- 1.Omary M.B., Eswaraka J., Kimball S.D., et al. The COVID-19 pandemic and research shutdown: Staying safe and productive. JCI. 2020;130(6):1–8. doi: 10.1172/JCI138646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiles-Shields B., Plevinsky J.M., Psihogios A.M., et al. Considerations and future directions for conducting clinical research with pediatric populations during the COVID-19 pandemic. JPP. 2020;45:720–724. doi: 10.1093/jpepsy/jsaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saberi P. Research in the time or coronavirus: Continuing ongoing studies in the midst of the COVID-19 pandemic. AIDS Behav. 2020;24(8):2232–2235. doi: 10.1007/s10461-020-02868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speroni K.G., Hess R. Pandemic challenges: Keeping nursing research alive. JONA. 2021;51(7/8):364–365. doi: 10.1097/NNA.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 5.Studyfinder. 2022 https://studyfinder.psu.edu/researchers Available at. Accessed date June 21, 2022. [Google Scholar]
- 6.Topolovec-Vranic J., Natarajan K. The use of social media in recruitment for medical research studies: A scoping review. J Med Internet Res. 2016;18(11):e286. doi: 10.2196/jmir.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelinas L., Pierce R., Winkler S., et al. Using social media as a research recruitment tool: Ethical issues and recommendations. Am J Bioeth. 2017;17:3–14. doi: 10.1080/15265161.2016.1276644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris P.A., Taylor R., Thielke R., et al. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruth C.J., Huey S.L., Krisher J.T., et al. An electronic data capture framework (ConnEDCt) for global and public health research: Design and implementation. J Med Internet Res. 2020;22(8):e18580. doi: 10.2196/18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comulada W.S., Tang W., Swendeman D., et al. Development of an electronic data collection system to support a large-scale HIV behavioral intervention trial: protocol for an electronic data collection system. JMIR Res Protoc. 2018;7(12):e10777. doi: 10.2196/10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leader Mindful. What is Mindfulness-Based Stress Reduction (MBSR)? https://www.mindfulleader.org/ Available at: Accessed June 23, 2022.
- 12.Schlegel E.C., Tate J.A. Pickler RH, Smith, LH. Practical strategies for qualitative inquiry in a virtual world. J Adv Nurs. 2021;77(10):4035–4044. doi: 10.1111/jan.15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pocock T., Smith M., Wiles J. Recommendations for Virtual Qualitative Health Research During a Pandemic. Qual Health Res. 2021;31(13):2403–2413. doi: 10.1177/10497323211036891. [DOI] [PubMed] [Google Scholar]
- 14.American Nurses Association . American Nurses Association; Silver Spring (MD): 2015. Code of ethics for nurses with Interpretative Statements. [Google Scholar]
- 15.Fowler M.D. Faith and Ethics, Covenant and Code. J Christ Nurs. 2017;34(4):216–224. doi: 10.1097/CNJ.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 16.Dellasega C. Kanaskie ML Nursing ethics in an era of pandemic. Appl Nurs Res. 2021;62:151508. doi: 10.1016/j.apnr.2021.151508. [DOI] [PMC free article] [PubMed] [Google Scholar]