Abstract
Gastroesophageal reflux disease (GERD) is a disorder due to the retrograde flow of refluxate into the esophagus. Although GERD is a common clinical diagnosis, its pathogenesis is quite complex. As a result of its multifactorial development, many patients continue to experience adverse symptoms due to GERD despite prolonged acid suppression with proton pump inhibitor therapy. The pathogenesis of GERD involves an interplay of chemical, mechanical, psychologic, and neurologic mechanisms, which contribute to symptom presentation, diagnosis, and treatment. As such, GERD should be approached as a disorder beyond acid. This review will investigate the major factors that contribute to the development of GERD, including factors related to the refluxate, esophageal defenses, and factors that promote pathologic reflux into the esophagus. In reviewing GERD pathogenesis, this paper will highlight therapeutic advances, with mention of future opportunities of study when approaching GERD.
Keywords: pepsin, bile acids, esophageal motility, ambulatory reflux monitoring, potassium competitive acid blocker
Introduction
Gastroesophageal reflux disease (GERD) is defined as the presence of troublesome reflux or erosive complications in the esophagus due to retrograde reflux of gastric contents.1 While classically considered a disease of excess acid, up to 50% of patients with GERD experience little to no relief with pharmacologic acid suppression.2,3 As more is uncovered regarding the pathophysiology and etiologies underlying GERD, it is evident that the development of reflux disease extends beyond acidity of the refluxate. Variance in symptom presentation and response to treatment can be attributed to the composition of refluxate as well as factors at the level of the esophagus, such as structural, mechanical, biochemical, and physiological aspects (Fig. 1). This paper reviews pathophysiologic mechanisms of GERD, with a focus on potential areas of future diagnostic tools and treatment options targeting varied mechanisms of GERD beyond acid suppression.
Figure 1.
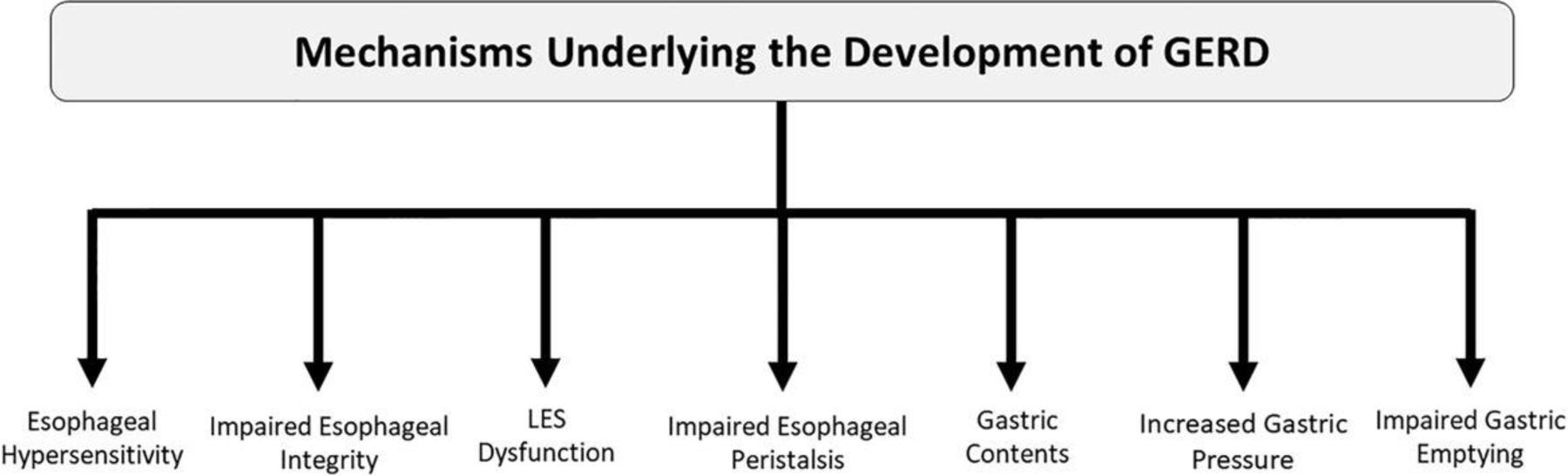
Mechanisms underlying the development of GERD.
Composition of refluxate
In GERD, the major component driving esophageal mucosal damage is the refluxate. Refluxate is able to overcome built-in defenses of the esophageal epithelium due to its potency, composition, and time of exposure to the esophageal lining.3,4 Refluxate is made up of varying levels of acid, bile, pepsin, food contents, and normal gut microbiota.4 The mechanism by which each component affects the esophageal mucosa is distinct, and thus the etiology of GERD may be specific to the predominant refluxate component and its mechanism of action (Fig. 2).
Figure 2.
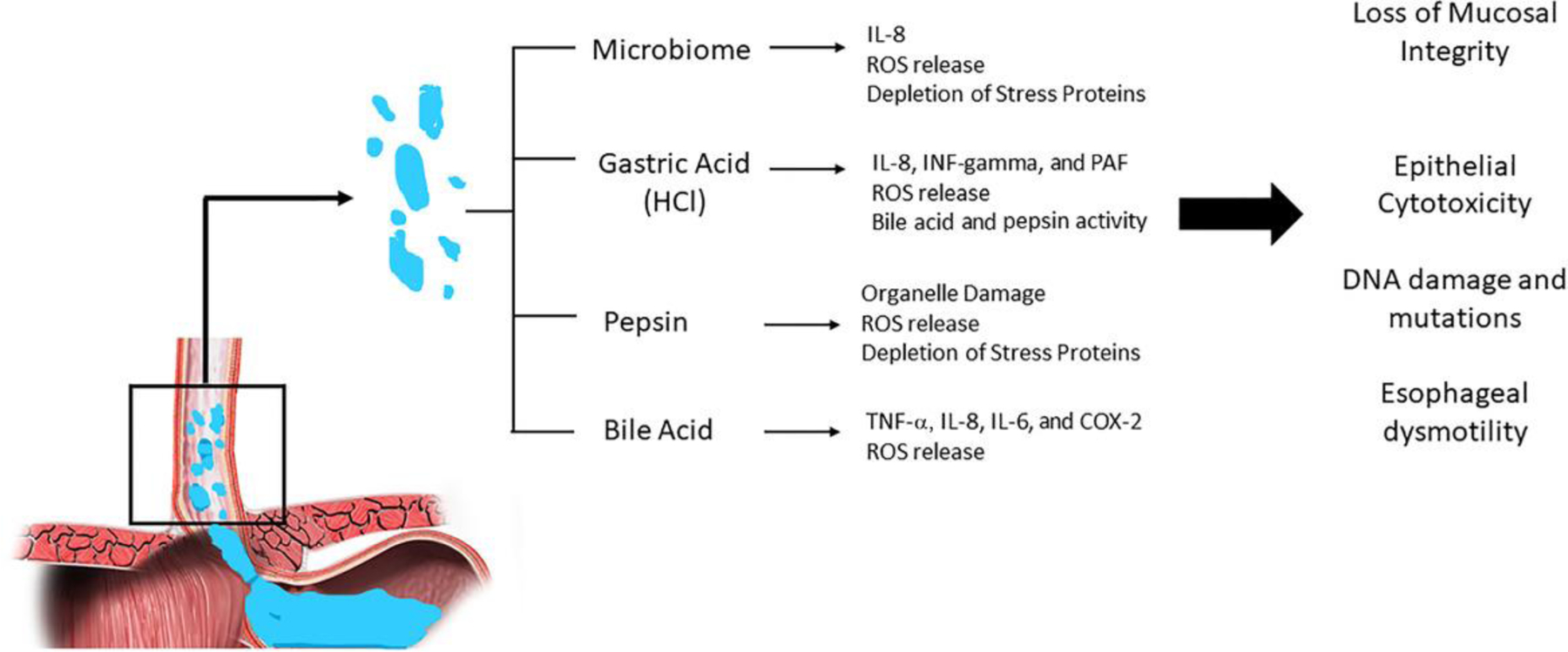
Effect of distinct refluxate components on the esophageal mucosa.
Acid
Commonly referred to as “acid reflux,” GERD is often considered a disease of acid refluxing from the stomach into the esophagus. Acid, also known as hydrochloric acid (HCl), is in fact one of the more toxic components of gastric juice and often is a primary driver of esophageal irritation and reflux symptoms.4 The mechanism by which acid leads to mucosal injury has been well studied. At the cellular level, HCl-induced damage of the esophageal mucosa is in part due to its effects on potential difference at the esophageal mucosa. Generally, the esophageal epithelium is able to maintain an electrochemical gradient measured in vitro as potential difference. However, as shown in animal models, an increase in luminal HCl leads to a significant increase in potential difference. Consequently, regulation of cell volume becomes less efficient, which can be seen histologically as a loss of cellular integrity, with evidence of cellular edema and necrosis within the epithelial lining.5 More recent studies have also identified more indirect inflammatory effects of HCl on the esophageal epithelium. Proinflammatory mediators implicated in animal and human models include interleukins, such as interleukin (IL)-8, platelet-activating factor, and interferon-gamma. Release of these mediators leads to the recruitment of immune cells to the esophageal mucosa and to a cascade of inflammatory pathways that result in the production of reactive oxygen species (ROS) and further cell damage.6–8
Factors that enhance gastric acid–induced mucosal damage include both chemical and mechanical features. In particular, the presence of the acid pocket may predispose individuals to the development of GERD. As a highly acidic zone just distal to the gastric cardia, the acid pocket is an unbuffered region that forms in both patients with and without GERD. Its discovery was based on evidence that the frequency of reflux events tends to increase postprandially despite an increase in pH that results from the buffering effects of food.9 Although this phenomenon has been shown in those without GERD, the presence and increased size of the acid pocket are more prevalent in patients with GERD. Moreover, particular factors that disrupt esophageal clearance, including the presence of a hiatal hernia, have been associated with increased acid pocket size, such that a hiatal hernia may allow for increased pooling of acid and thus increase esophageal acid exposure.10
In regards to symptom presentation, gastric acid increases sensitivity to reflux and thus enhances the perception of reflux symptoms.11 Evidence shows that acid-induced hypersensitivity may be more significant within more proximal regions of the esophagus compared with its distal portions.12 This increased sensitivity can likely be explained by acid’s damaging effects on the esophageal mucosa, leading to impaired mucosal barriers and increasing exposure of mucosal afferent nerves to toxic refluxate. The anatomy and pathophysiology underlying reflux-related hypersensitivity will be discussed further in this review.
Bile acid
Although acid has been identified as one of the major mediators of cellular injury in GERD, not all cases of GERD are characterized by acidic pH of the refluxate. In patients with GERD undergoing pH monitoring, a percentage of patients will show a transient increase in pH above 7.0, suggestive of a predominant alkalinizing agent.13 Prior studies have demonstrated a correlation between bile acid concentrations and alkaline pH events during esophageal pH monitoring.14 As detergent molecules, bile acids are capable of solubilizing cell membranes; however, their ability to permeate through cell membranes is dependent on a neutralized and thus lipophilic state. In the presence of an acid, bile acids become protonated and thus do not hold a charge. This permits bile acids to pass across cell membranes. As such, the cytotoxic effects of bile acids may be enhanced in the presence of acid, suggesting a mechanistic role of bile acids in GERD in the setting of duodenogastroesophageal reflux.15 Furthermore, bile acids also have been shown to increase hydrogen ion absorption in the esophagus, which may explain the relationship found between bile acid concentration and the severity of reflux symptoms reported.16 Moreover, higher concentrations of and longer exposure to bile acids both show a correlation with the progressive esophageal mucosal injury, with specific bile acids having greater esophageal toxicities.17
Nevertheless, the relationship between bile and gastric acids in GERD pathophysiology may be less direct. Although studies have demonstrated a relationship between duodenogastroesophageal reflux and gastric reflux coexistence with increased severity of mucosal injury, findings suggest the effects of gastric acid exposure and those of bile acids likely occur as two distinct events. In this way, bile acid reflux has not been shown to correlate with esophageal acid exposure as determined by using pH monitoring.18 Furthermore, a poor response to proton pump inhibitor (PPI) therapy does not indicate the presence of biliary reflux.19 Still, the reverse may be true such that the presence of bile acid reflux may predict a poor response to PPIs.20 Moreover, as previously mentioned, the simultaneous presence of both bile acid and gastric acid exposure is associated with increased severity of GERD.20,21 Whether these effects are synergistic or mechanistically distinct is unclear; however, the role of bile acid detection and its treatment may lead to further symptom improvement in the settings of both severe and/or refractory GERD.
Specifically, in the setting of erosive esophagitis and Barrett’s esophagus (BE), various inflammatory factors have been associated with bile acids. In particular, the expression of IL-6, IL-8, COX-2, and TNF-α may be enhanced, with associated recruitment of inflammatory cells. This increase in proinflammatory cytokines and cells was not observed in an acid-only cohort, suggesting a direct role of bile acids in esophageal damage.22 More-over, there is evidence that bile acids, when in the presence of an acidic environment, can lead to an increase in oxidative stress by inducing the release of ROS. Release of ROS can lead to DNA damage and thus increases the risk of cellular metaplasia.23,24 Overall, it is evident that bile acids may play a major role in the pathogenesis of GERD and thus can serve as a potential diagnostic biomarker as well as a therapeutic target. A recent multicenter placebo-controlled trial of 280 patients with GERD reported that addition of a bile acid sequestrant (IW 3718) to single-dose PPI therapy led to the reduction of heartburn and regurgitation.25 The mechanisms by which bile acids can lead to esophageal damage are many and require further study in order to better understand the utility of bile acid sequestrants in refractory GERD.
Pepsin
The role of pepsin in mucosal injury of the esophagus is less well defined. As a peptidase enzyme, pepsin breaks down proteins into smaller peptides, allowing for protein absorption or further protein breakdown within the intestines. Depending on its protein substrate, pepsin activity is generally optimized at an environmental pH of approximately 2.0, maintains some activity at pH 2.0–6.5, and enters a “dormant phase” at a pH between 6 and 8, whereby pepsin remains inactive but is capable of reactivating upon acidification of its surrounding environment.26
Despite its broad protein substrate specificity, pepsin is responsible for less than 20% of the protein digestion that occurs within the digestive tract.27 Nevertheless, given the variety of proteins that pepsin is able to hydrolyze, release of pepsin into the esophagus and its contiguous structures can lead to damage of these organs. Unlike the stomach, the esophagus lacks a mucus layer to protect it from autodigestion by pepsin. By contrast, if pepsin leaks into the intestine, the alkaline pH of the small intestine quickly deactivates active pepsin into its dormant form. Therefore, in comparison to its more distal structures within the gastrointestinal (GI) tract, the esophagus lacks such protective mechanisms against pepsin. Upon reflux into the esophagus and extraesophageal tissues, pepsin is able to bind to and damage surface epithelial cells that line these organs.28 Activation of pepsin can occur by way of two distinct mechanisms: (1) activation upon exposure to acidic refluxate, or (2) intracellular activation upon epithelial cell uptake. Pepsin with the other components of refluxate can cause direct cell damage by destroying extracellular protein and intercellular junctions.29 However, pepsin can also cause indirect cell damage by disrupting cellular defenses. For instance, in a 2006 study by Johnston et al., depletion of squamous epithelial proteins Sep70 and Sep53 was observed upon exposure of laryngeal epithelial cells to both acid and pepsin; however, depletion of these key stress proteins was not seen when epithelial cells were exposed to acid alone.30 Moreover, there is evidence that pepsin may have inhibitory effects on intracellular defense enzymes, leading to oxidative stress, inflammation, and programmed cell death.31 Pepsin has also been found to cause direct damage to intracellular organelles, including disruptions in mitochondrial and Golgi apparatus functions.32,33 Overall, pepsin as a component of gastric refluxate can cause epithelial damage and cell death by various mechanisms. Given its ability to remain dormant and reactivate upon exposure to the appropriate environmental settings, pepsin and its effects on GERD may be refractory to acid-suppressing therapies. As such, pepsin has the potential to serve as a key diagnostic and therapeutic target in future GERD management. Currently, findings regarding the role of salivary pepsin as a diagnostic marker have been variable, suggesting that further investigation may be helpful in defining the clinical context in which pepsin detection may be of greatest utility.34–36 In regards to GERD therapy, prior studies suggest that alginates, widely used in the treatment of gastroesophageal reflux, reduce pepsin activity.37,38
Esophageal defenses against gastric refluxate
Structural integrity of the esophageal epithelium
The esophageal mucosa consists of nonkeratinized squamous epithelium divided into three functionally distinct layers, including the proliferating stratum basalis, a metabolically active stratum spinosum, and an enucleated stratum corneum. Despite its 20–30 cell layers, there are key characteristics of the esophageal mucosa that make it unable to combat noxious refluxate components. Lacking mucus-secreting cells and bicarbonate production, this esophageal lining cannot create the biochemical environment necessary to neutralize refluxed contents.39 As previously described, pepsin and bile acids are able to work synergistically with acid, such that acid provides an optimal environment for their caustic activities and absorption, respectively. Without neutralizing systems at hand, the esophagus is more prone to damage due to the aforementioned toxic effects of gastric refluxate.
Nevertheless, the absence of neutralization mechanisms within the esophagus does not explain the varying symptom presentations related to GERD. As previously mentioned, factors at the levels of the refluxate and the esophagus contribute to GERD pathogenesis and presentation. In regards to the refluxate, the actions of pepsin and gastric acid on the esophageal epithelium rely on “breaks” within the cell wall. As with any epithelium, the esophageal epithelium consists of intercellular junctional complexes that serve to maintain epithelial integrity and allow for cell–cell transport and signal transmission. Insult to cell-to-cell junctional complexes by noxious stimuli leads to increased intercellular permeability and thus further breakdown of the esophageal wall. Loss of intercellular junctions can lead to further epithelial compromise, as loose intercellular connections allow toxic contents to more easily access exposed underlying structures that compose the esophageal wall. In particular, refluxate components can cause noxious stimulation of neurochemical pathways found within the lamina propria of the esophagus. In order to gain access to the chemoreceptors of esophageal afferent neurons, pepsin and acids require intercellular pathways by way of dilated intercellular spaces (DIS). The presence of DIS on electron microscopy has been described as a sensitive marker for esophageal damage due to GERD.40,41 However, other studies have demonstrated that there may be no correlation between the DIS and GERD and GERD subtypes, such as nonerosive reflux disease (NERD).42,43 Moreover, the presence of DIS may not be as specific to GERD as previously described, since some studies have observed similar distribution of DIS in asymptomatic patients as can be seen in those with GERD.44 Furthermore, although the resolution of DIS has been observed after PPI therapy,45 other stressors, including physical, chemical, and psychological stressors, have been associated with the development of DIS.46,47 Given the conflicting findings regarding the usefulness of DIS detection in the setting of GERD diagnosis and treatment, the role of endoscopic biopsy in GERD is currently primarily limited to cases of erosive and eosinophilic esophagitis and surveillance for BE. Nevertheless, more recent studies have shown evidence that more subtle junctional changes may be associated with the development of GERD.48 Mucosal integrity (also known as mucosal impedance) technology is a novel method to assess the integrity of the esophageal mucosa during endoscopy. Initial studies suggest that mucosal integrity is able to distinguish esophageal conditions, such as GERD, eosinophilic esophagitis, and achalasia in real time.49,50 With further study and experimental evidence, detection of these molecular and ultrastructural alterations may assist in GERD diagnosis and potentially improve targeted therapies.
Neuronal afferents in the esophageal mucosa
Upon reaching neurohormonal targets via DIS, gastric acid acts on specific sensory receptors within the esophageal wall. These acid-sensitive receptors include three major families: the acid-sensing ion channel (ASIC), the P2X, and the transient receptor potential (TRP) families.51 Of the TRP receptors, transient receptor potential vanilloid 1 (TRPV1) receptor has been identified as one of the major receptors involved in the perception of acid-induced esophagitis. Upon stimulation by noxious stimuli, afferent neurons transmit the signal as action potentials along two described pathways: (1) the vagal stimulation pathway and (2) via spinal afferents. The spinal afferents carry pain sensation to the thalamus by way of the dorsal root ganglion at levels C1 to L2. By contrast, vagal afferents send signals to the central nervous system (CNS) via the nodose and jugular ganglia with synapsis at the nucleus of the solitary tract.52 These neuronal pathways can be used clinically as potential therapeutic targets when discussing GERD treatment, as described below.
The role of neuronal afferents in the symptom presentation has become of particular interest as it relates to nonerosive GERD. GERD itself is an umbrella term that encompasses two subcategories that are based on the degree of structural damage and pathological findings that result. These subtypes of GERD include NERD and erosive reflux disease.1 While erosive reflux disease can be identified endoscopically, as evidenced by inflammation, erosive mucosal breaks, and columnarization of the squamous epithelium, patients with NERD present GERD symptoms but have no endoscopic findings suggestive of esophageal irritation. Prior studies have suggested that in the setting of NERD, symptoms may develop as a result of a hypersensitivity of esophageal sensory neurons to refluxed contents.44,51,52 To better understand the pathophysiology underlying this sensitivity, studies have demonstrated that sensory neurons within the esophageal wall may be positioned more superficially, as compared with patients with esophagitis and BE. As such, despite having intact epithelial lining, patients with NERD more often report symptoms of heartburn, as compared with those with either BE or esophagitis. Furthermore, desensitization of nociceptors may be at play in cases of esophagitis and BE. BE in particular has been characterized by hyposensitivity to noxious stimuli.53,54
Many advances have been made in recognizing reflux hypersensitivity, as it is now identified as one of the five functional esophageal disorders based on the Rome IV criteria.55 In contrast to NERD, however, patients with reflux hypersensitivity demonstrate normal, physiologic levels of reflux. Given the varying phenotypes by which reflux can present, treatment of gastroesophageal reflux can become more complex than previously thought. Therapeutic advances have been made in targeting neuronal pathways involved in the perception of GERD-related symptoms. Esophageal neuromodulators, including selective serotonin reuptake inhibitors and tricyclic antidepressants, have shown promising efficacy in the treatment of PPI-refractory heartburn.56,57 Furthermore, psychological interventions, such as esophageal-directed hypnotherapy and cognitive behavioral therapy, seem to be effective for functional esophageal disorders.58–61
Reduced esophageal clearance of refluxate
Impaired anterograde flow of esophageal and gastric contents can lead to increased exposure time of the esophageal mucosa to the refluxate. This subsequently results in increased mucosal damage due to caustic components. Factors that contribute to adequate esophageal clearance include both chemical and mechanical mechanisms, including gland secretions and esophageal motility patterns, respectively. Impairments at these levels can result in reduced esophageal clearance that contributes to GERD development.
Salivary gland secretions.
The role of saliva in promoting esophageal clearance is primarily by way of acid neutralization. The secretion of saliva is activated by a specific esophagosalivary reflex, such that the presence of acid within the esophagus activates esophageal chemoreceptors to stimulate the salivary glands.62 Saliva itself does not necessarily affect the motility patterns of esophageal clearance; however, its neutralizing effects have shown to be associated with increased clearance as compared with controls in animal models. Further supporting the role of saliva in esophageal clearance is the concept of nocturnal GERD. During sleep, salivary secretion is significantly reduced. This correlates with an increased frequency of nocturnal reflux events and an increase in esophageal acid exposure.63 Furthermore, various chronic medical disorders and medications that reduce salivary secretion have been associated with increased reflux episodes and subsequent development of esophageal reflux disease.64
Esophageal dysmotility.
The relationship between esophageal dysmotility and GERD has been well studied, with findings that suggest a bidirectional influence. Many patients with GERD often present with impaired esophageal motility, including impaired peristalsis and lower esophageal sphincter (LES) dysfunction. These dysmotility patterns can slow esophageal transit time and thus decreased clearance of esophageal contents.65,66 Factors that contribute to esophageal dysmotility include intrinsic dysmotility, such as seen in autoimmune and neuromuscular disorders, and extrinsic mechanical causes, such as the presence of a hiatal hernia. While it is evident that esophageal dysmotility can lead to decreased reflux clearance and thus increased exposure time to toxic refluxate components, existing injury to the esophageal mucosa by acid can also lead to further adverse effects on the progression of GERD and symptom presentation. Prior studies suggest a direct association between the degree of mucosal injury with increased acid exposure and severity of esophageal motor dysfunction. The resulting esophageal dysmotility can lead to impaired acid clearance and decreased LES pressure, and thus further exacerbate symptoms and progression of the disease.67 The severity of esophagitis has also been linked indirectly to LES pressure and effective peristalsis.68,69 Thus, an interplay of effects exists between esophageal dysmotility and reflux, as the two have been shown to contribute to the development and progression of each other. More specifically, GERD has been associated with particular metrics of esophageal dysmotility as measured by manometry, including worsened distal contractile integrity, peristalsis break, lower LES pressure, lower peristaltic wave amplitudes, and ineffective esophageal motility.66,69
While the relationship between esophageal motility and progression of esophagitis has been well documented, there are limited available therapeutic options that address esophageal dysmotility as it contributes to reflux disease. Data generally do not demonstrate reliable symptom improvement in GERD with prokinetic agents, such as dopamine receptor antagonists, GABAB receptor agonists, 5-hydroxytryptamine (5-HT) agonists, and acetylcholine receptor agonists.70 Furthermore, these agents can lead to clinically significant side effects. Cisapride, in particular, was previously identified as an effective therapy for GERD; however, owing to its significant cardiac side effects, cisapride was removed from the market as a medical option for GERD.71 Moreover, despite its effective prokinetic properties in patients with delayed gastric emptying, metoclopramide’s centrally acting effects on D2 receptors can lead to significant side effects, including restlessness, dystonia, and tardive dyskinesia.72
However, there is still a potential for prokinetic agents in GERD management without posing major side effect risks. Some studies have shown that mosapride, a selective 5-HT4 receptor agonist, may augment the effects of PPIs when coadministered in certain patient populations, such as those with erosive esophagitis;73,74 however, many other studies have not been able to reproduce this significant synergistic effect with mosapride.75,76 Rikkunshito, a traditional Japanese medicine that promotes gastric motility through the release of ghrelin, may have some effectiveness in treating refractory GERD as it also has synergistic effects on gastric motility when combined with PPIs and may be effective as monotherapy for GERD.77–80 Further study of prokinetic agents in the setting of GERD may give light to future advances in the management of refractory GERD.
Reversed gastroesophageal gradient
A fourth concept contributing to the development of GERD is the idea of reversed gastroesophageal gradient. The esophagus is primarily housed within the thoracic cavity; thus, in normal esophageal physiology, intraesophageal pressure is equal to intrathoracic pressure and lower than intra-abdominal pressure. Therefore, in order to propel contents into the stomach (into a higher-pressure compartment), the LES must maintain its tonic contraction to combat the gastroesophageal pressure gradient. However, when the LES fails to maintain tonicity, such as with an intrinsically hypotonic LES or a hiatal hernia, or under circumstances whereby intraabdominal pressure is increased, such as is the case in obesity or pregnancy, retrograde flow of refluxate from the stomach into the esophagus can occur.70,81
In focusing on the role of the LES in promoting retrograde flow of gastric contents, the concept of transient LES relaxations (TLESR) should be understood as a physiologic phenomenon that occurs whereby gastric distention induces spontaneous vagal-mediated relaxation of both the LES and crural diaphragm. These brief episodes of relaxation occur without preceding peristalsis and allow for venting of gas from the stomach.82 The data on TLESR in GERD are mixed, such that there is evidence that patients with GERD experience TLESR just as frequently as healthy individuals; however, other studies have shown a more frequent rate of TLESR occurrence in patients with GERD.83,84 Although the relationship between TLESR and GERD is not clear, the physiological mechanisms underlying TLESRs have been used to manufacture pharmacologic agents that enhance LES contractility and function. Classes of TLESR-reducing agents include GABAB receptor agonists, metabotropic glutamate receptor 5 (mGluR5), CCK receptor agonists, cannabinoid receptor agonists, and nitric oxide synthase inhibitors.70 Of these agents, GABAB receptor agonists, such as baclofen, have demonstrated the greatest efficacy in reducing reflux events related to TLESR. GABAB is an inhibitory neurotransmitter that acts centrally on vagal afferents and the nucleus of the solitary tract and peripherally by inhibiting signals at the level of vagal mechanoreceptors involved in the autonomic pathway underlying TLESRs. Baclofen has demonstrated efficacy in reducing both acid- and nonacid-related reflux symptoms likely because of its effects on maintaining adequate LES tonicity and reducing the frequency of TLESRs.85,86 Despite these promising findings, baclofen is used in select cases of GERD, and, as a centrally and peripherally acting agent, it poses risks of CNS side effects, including fatigue, nausea, emesis, and dizziness.87
As discussed throughout this review, many pharmacologic agents have been identified in the setting of GERD treatment; however, the adverse effects and inconsistent findings associated with many of these agents make it difficult to identify effective medical therapy, especially for cases of refractory GERD. Effective surgical and endoluminal and surgical antireflux interventions available for GERD can improve LES function to inhibit retrograde flow and, in some cases, lower intraabdominal pressure. The selection of antireflux intervention is based on the underlying etiology driving reflux symptoms.88,90 In cases of GERD with an associated hiatal hernia and weakened LES, one of the most effective surgeries in GERD management is the laparoscopic Nissen fundoplication (LNF) procedure.91–93 Similarly, laparoscopic Toupet fundoplication has shown promise in reducing GERD symptoms upon hiatal hernia repair and is often an alternative to LNF in cases of esophageal hypomotility.94–96 Magnetic sphincter augmentation is also a seemingly effective alternative to laparoscopic fundoplication in patients with GERD, with or without a hiatal hernia.97 Transoral incisionless fundoplication (TIF) is an endoluminal antireflux intervention option for GERD, particularly in cases without significant disruption of the antireflux barrier.98,99 Bariatric surgery is the mainstay surgical option for patients with GERD and obesity. By the nature of the procedure, bypass surgery leads to a decreased size of the stomach cavity, which limits acid production by reducing the area of acid-producing parietal cells and intra-abdominal pressure by promoting weight loss.100
Future opportunities and conclusion
As described in this review, GERD is a complex disease often based on a multifactorial pathogenesis. Understanding the mechanisms underlying GERD can allow for advances in both the diagnostic work-up and therapeutic options for GERD (Table 1). To reduce injury from refluxate, one must consider the effects of refluxate beyond acid exposure. Non-invasive methods to measure biomarkers of gastric refluxate, such as salivary pepsin, may serve an important role in the future diagnostic paradigm of GERD. Furthermore, novel bile acid sequestrants or alginates may complement acid suppression by reducing the burden of bile or pepsin. In cases where proton pump inhibition is insufficient or not tolerated, the novel potassium competitive acid blockers may provide superior acid suppression. At the same time, promising novel diagnostic and therapeutic options are emerging to augment innate esophageal defenses. Mucosal integrity may be a real-time diagnostic method to detect reduced esophageal epithelial integrity in the setting of GERD. A myriad of pharmacologic and psychological interventions are widely available to reduce nociception in states of esophageal hypersensitivity. Current treatment modalities to increase esophageal clearance are limited, and future investigation in effective and selective prokinetic or other treatment options is needed. Finally, novel surgical and endoluminal procedures, such as magnetic sphincter augmentation or TIF, show promise for restoring the antireflux barrier.
Table 1.
Current and future diagnostic tools and therapy for GERD personalized to mechanistic factor
| Factors in GERD development | Diagnostic tool | Treatment option | |
|---|---|---|---|
| Refluxate composition | Acid | Ambulatory reflux monitoring | Proton pump inhibitor H2 receptor antagonist Potassium competitive acid blockers |
| Bile | Bilitec | Bile acid sequestrant | |
| Pepsin | Salivary pepsin | Alginates | |
| Esophageal defenses | Reduced integrity of the esophageal epithelium | Dilated intracellular space on biopsy Mucosal impedance/integrity |
Alginates |
| Heightened nociception via neuronal afferents | Pharmacologic neuromodulators Esophageal-directed hypnotherapy Cognitive behavioral therapy |
||
| Reduced esophageal clearance | High-resolution manometry with impedance Multichannel intraluminal impedance and pH monitoring (MII-pH) Barium esophagram |
Prokinetic agents | |
| Reversed gastroesophageal gradient | Esophageal manometry Barium esophagram | GABA agonist Surgical fundoplication Transoral incisionless fundoplication Magnetic sphincter augmentation |
|
Note: Italics represent novel options and/or opportunities for future investigation.
In conclusion, although often regarded as a disease of acid, GERD in reality is a complex, multifactorial disease process involving an imbalance between the aggressive and defensive forces of the refluxate and esophagus, respectively. Furthermore, the manifestations of GERD can vary phenotypically as typical, atypical, and extraesophageal symptoms and can also be subcategorized diagnostically by endoscopy and biopsy.101 Thus, when discussing appropriate treatment, it is important to first elucidate the patient’s symptoms and consider adjunctive diagnostic studies to help identify contributing factors. Potentially half of patients suffering from heartburn do not experience full relief by current mainstay PPI therapy. As such, our future approach to GERD should reach beyond that of acid reflux. As more is discovered regarding GERD pathogenesis, potential targets affecting motility, hypersensitivity, and nonacid refluxate components may become novel players in how we view and manage GERD.
Footnotes
Competing interests
R.Y. receives consulting fees from Medtronic, Iron-wood Pharmaceuticals, and Diversatek, and is on the advisory board of Phatom Pharmaceuticals. P.S. declares no competing interests.
References
- 1.Vakil N, van Zanten SV, Kahrilas K, et al. 2006. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am. J. Gastroenterol 101: 1900–1920. [DOI] [PubMed] [Google Scholar]
- 2.Ang D, How CH & Ang TL 2016. Persistent gastrooesophageal reflux symptoms despite proton pump inhibitor therapy. Singapore Med. J 57: 546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castell DO, Murray JA, Tutuian OR, et al. 2004. Review article: the pathophysiology of gastro-oesophageal reflux disease–oesophageal manifestations. Aliment. Pharmacol. Ther 20(Suppl. 9): 14–15. [DOI] [PubMed] [Google Scholar]
- 4.Tack J & Pandolfino JE 2018. Pathophysiology of gastroesophageal reflux disease. Gastroenterology 54: 277–288. [DOI] [PubMed] [Google Scholar]
- 5.Orlando RC, Powell DW & Carney CN 1981. Pathophysiology of acute acid injury in rabbit esophageal epithelium. J. Clin. Invest 68: 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliot M, et al. 2002. Diversity in oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 50: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J, Altomare A, de la Monte S, et al. 2010. HCl-induced inflammatory mediators in esophageal mucosa increase migration and production of H2O2 by peripheral blood leukocytes. Am. J. Physiol. Gastrointest. Liver Physiol 299: G791–G798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isomoto H, Wang A, Mizuta Y, et al. 2003. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am. J. Gastroenterol 98: 551–556. [DOI] [PubMed] [Google Scholar]
- 9.Kahrilas PJ, McColl K, Fox M, et al. 2013. The acid pocket: a target for treatment in reflux disease? Am. J. Gastroenterol 108: 1058–1064. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell DR, Derakhshan MH, Robertson EV, et al. 2016. The role of the acid pocket in gastroesophageal reflux disease. J. Clin. Gastroenterol 50: 111–119. [DOI] [PubMed] [Google Scholar]
- 11.Emerenziani S, Ribolsi M, Guarino MPL, et al. 2014. Acid reflux episodes sensitize the esophagus to perception of weakly acidic and mixed reflux in non-erosive reflux disease patients. Neurogastroent. Motil 26: 108–114. [DOI] [PubMed] [Google Scholar]
- 12.Zerbib F, Duriez A, Roman S, et al. 2008. Determinants of gastro-esophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut 57: 156–160. [DOI] [PubMed] [Google Scholar]
- 13.Lin KM, Ueda RK, Hinder RA, et al. 1991. Etiology and importance of alkaline esophageal reflux. Am. J. Surg 162: 553–557. [DOI] [PubMed] [Google Scholar]
- 14.Stein HJ, Feussner H, Kauer W, et al. 1994. Alkaline gastroesophageal reflux: assessment by ambulatory esophageal aspiration and pH monitoring. Am. J. Surg 167: 163–168. [DOI] [PubMed] [Google Scholar]
- 15.Kauer WK, Peters JH, DeMeester TR, et al. 1997. Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery 122: 874–881. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui A, Rodriguez-Stanley R, Zubaidi S, et al. 2005. Esophageal visceral sensitivity to bile salts in patients with functional heartburn and in health control subjects. Dig. Dis. Sci 50: 81–85. [DOI] [PubMed] [Google Scholar]
- 17.Nehra D, Howell P, Williams CP, et al. 1999. Toxic bile acids in gastroesophageal reflux disease: influence of gastric acidity. Gut 44: 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pace F, Sangaletti O, Pallotta S, et al. 2007. Biliary reflux and non-acid reflux are two distinct phenomena: a comparison between 24-hour multichannel intraesophageal impedance and bilirubin monitoring. Scand. J. Gastroenterol 42: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 19.Gasiorowska A, Navarro-Rodriguez T, Wendel C, et al. 2009. Comparison of the degree of duodenogastroesophageal reflux and acid reflux between patients who failed to respond and those who were successfully treated with a proton pump inhitibor once daily. Am. J. Gastroenterol 104: 2005–2013. [DOI] [PubMed] [Google Scholar]
- 20.de Bortoli N, Gyawali CP, Frazzoni M, et al. 2020. Bile reflux in patients with nerd is associated with more severe heartburn and lower values of mean nocturnal base-line impedance and chemical clearance. Neurogastroenterol. Motil e13919. 10.1111/nmo.13919 [DOI] [PubMed]
- 21.Vaezi MF & Richter JE 1996. Role of acid and duo-denogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology 111: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 22.Sun D, Wang X, Gai Z, et al. 2015. Bile acids but not acidic acids induce Barrett’s esophageal. Int. J. Clin. Exp. Pathol 8: 1384–1392. [PMC free article] [PubMed] [Google Scholar]
- 23.McQuaid KR, Laine L, Fennerty MB, et al. 2011. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment. Pharmacol. Ther 34: 146–165. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak K, Payne CM, Chavarria M, et al. 2007. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut 56: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaezi MF, Fass R, Vakil N, et al. 2020. IW-3718 reduces heartburn severity in patients with refractory gastroesophageal reflux disease in a randomized trial. Gastroenterology 158: 2093–2103. [DOI] [PubMed] [Google Scholar]
- 26.Tang J 2013. Pepsin A. In Handbook of Proteolytic Enzymes Rawlings ND & Salvesen G, Eds.: 27–35. Academic Press. [Google Scholar]
- 27.Smith ME & Morton DG 2010. The stomach: basic functions. In The Digestive System 2nd ed. 39–50. Churchill Livingstone. [Google Scholar]
- 28.Johnston N, Knight J, Dettmar PW, et al. 2004. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope 114: 2129–2134. [DOI] [PubMed] [Google Scholar]
- 29.Tobey NA, Hosseini SS, Caymaz-Bor C, et al. 2001. The role of pepsin in acid injury to esophageal epithelium. Am. J. Gastroenterol 96: 3062–3070. [DOI] [PubMed] [Google Scholar]
- 30.Johnston N, Dettmar PW, Lively MO, et al. 2006. Effect of pepsin on laryngeal stress protein (Sep70, Sep53, and Hsp70) response: role in laryngopharyngeal reflux disease. Ann. Otol. Rhinol. Laryngol 115: 47–58. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez P, Piazuelo E, Sanchez T, et al. 2005. Free radicals and antioxidant systems in reflux esophagitis and Barrett’s esophagus. World J. Gastroenterol 11: 2697–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuels T, Wells C, Pearson A, et al. 2013. Curcumin and anthocyanin inhibit pepsin-mediated cell damage and carcinogenic changes in airway epithelial cells. Ann. Otol. Rhinol. Laryngol 122: 632–641. [PubMed] [Google Scholar]
- 33.Johnston N, Wells CW, Samuels TL, et al. 2009. Pepsin in nonacidic refluxate can damage hypopharyngeal epithelial cells. Ann. Otol. Rhinol. Laryngol 118: 677–685. [DOI] [PubMed] [Google Scholar]
- 34.Kim TH, Lee KJ, Yeo M, et al. 2008. Pepsin detection in the sputum/saliva for the diagnosis of gastroesophageal reflux disease in patients with clinically suspected atypical gastroesophageal reflux disease symptoms. Digestion 77: 201–206. [DOI] [PubMed] [Google Scholar]
- 35.Guo Z, Wu H, Jiang J, et al. 2018. Pepsin in saliva as a diagnostic marker for gastroesophageal reflux disease: a meta-analysis. Med. Sci. Monit 24: 9509–9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Zhao Y, Ren J, et al. 2018. Pepsin in saliva as a diagnostic marker for laryngopharyngeal reflux: a meta-analysis. Eur. Arch. Otorhinolaryngol 275: 671–678. [DOI] [PubMed] [Google Scholar]
- 37.Chater PI, Wilcox MD, Brownless IA, et al. 2015. Alginate as a protease inhibitor in vitro and in a model gut system; selective inhibition of pepsin but not trypsin. Carbohydr. Polym 131: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strugala V, Avis J, Jolliffe IG, et al. 2009. The role of an alginate suspension on pepsin and bile acids – key aggressors in the gastric refluxate. Does this have implications for the treatment of gastro-oesophageal reflux disease? J. Pharm. Pharmacol 61: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 39.Kahrilas PJ & Hogan WJ 1993. Gastroesophageal reflux disease. In Gastrointestinal Disease Pathophysiology/Diagnosis/Management Sleisenger MH & Fordtran JS, Eds.: 378–401. Philadelphia, PA: W.B. Saunders Company. [Google Scholar]
- 40.Calabrese C, Fabbri A, Bortolotti M, et al. 2003. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment. Pharmacol. Ther 18: 525–532. [DOI] [PubMed] [Google Scholar]
- 41.Tobey NA, Carson JL, Alkiek RA, et al. 1996. Dilated intercellular spaces: a morphological feature of acid reflux—damaged human esophageal epithelium. Gastroenterology 111: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 42.Vaezi MF, Slaughter JC, Smith BS, et al. 2010. Dilated intercellular space in chronic laryngitis and gastro-oesophageal reflux disease: at baseline and post-lansoprazole therapy. Aliment. Pharmacol. Ther 32: 916–924. [DOI] [PubMed] [Google Scholar]
- 43.Tadiparthi RA, Bansal A, Wani S, et al. 2011. Dilated intercellular space and lymphocytes on biopsy relate to symptoms in erosive GERD but not NERD. Aliment. Pharmacol. Ther 33: 1202–1208. [DOI] [PubMed] [Google Scholar]
- 44.Azumi T, Adachi K, Furuta K, et al. 2008. Esophageal epithelial surface in patients with gastroesophageal reflux disease: an electron microscopic study. World J. Gastroenterol 14: 5712–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue Y, Zhou LY & Lin SR 2008. Dilated intercellular spaces in gastroesophageal reflux disease patients and the changes of intercellular spaces after omeprazole treatment. Chin. Med. J 121: 1297–1301. [PubMed] [Google Scholar]
- 46.Farre R, De Vos R, Geboes K, et al. 2007. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut 56: 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang DH, Zhou LY, Dong XY, et al. 2010. Factors influencing intercellular spaces in the rat esophageal epithelium. World J. Gastroenterol 16: 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CC, Lee JW, Liu TT, et al. 2013. Relevance of ultra-structural alterations of intercellular junction morphology of inflamed human esophagus. J. Neurogastroenterol. Motil 19: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel DA, Higginbotham T, Slaughter JC, et al. 2019. Development and validation of a mucosal impedance contour analysis system to distinguish esophageal disorders. Gastroenterology 156: 1617–1626.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choksi Y, Lal P, Slaughter JC, et al. 2018. Esophageal mucosal impedance patterns discriminate patients with eosinophilic esophagitis from patients with GERD. Clin. Gastroenterol. Hepatol 16: 664–671.e1. [DOI] [PubMed] [Google Scholar]
- 51.Miwa H, Kondo T, Oshima T, et al. 2010. Esophageal sensation and esophageal hypersensitivity—overview from bench to bedside. J. Neurogastroenterol. Motil 16: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lottrup C, Olesen SS & Drewes AM 2019. The pain system in oesophageal disorders: mechanisms, clinical characteristics and treatment. Gastroenterol. Res. Pract 2011: 910420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fletcher J, Gillen D, Wirz A, et al. 2003. Barrett’s esophagus evokes a quantitatively and qualitatively altered response to both acid and hypertonic solutions. Am. J. Gastroenterol 98: 1480–1486. [DOI] [PubMed] [Google Scholar]
- 54.Weijenborg PW, Smout AJPM, Krishnadath KK, et al. 2017. Esophageal sensitivity to acid in patients with Barrett’s esophagus is not related to preserved esophageal mucosal integrity. Neurogastroenterol. Motil 29: e13066. [DOI] [PubMed] [Google Scholar]
- 55.Schmulson M 2018. How to use Rome IV criteria in the evaluation of esophageal disorders. Curr. Opin. Gastroenterol 34: 258–265. [DOI] [PubMed] [Google Scholar]
- 56.Broekaert D, Fischler B, Sifrim D, et al. 2006. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: a double-blind, placebo-controlled study. Aliment. Pharmacol. Ther 23: 365–370. [DOI] [PubMed] [Google Scholar]
- 57.Ostovaneh MR, Saeidi B, Hajifathalian K, et al. 2014. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: a double-blind placebo-controlled trial. Neurogastroenterol. Motil 26: 670–678. [DOI] [PubMed] [Google Scholar]
- 58.Riehl ME & Keefer L 2015. Hypnotherapy for esophageal disorders. Am. J. Clin. Hypn 58: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riehl ME, Kinsinger S, Kahrilas PJ, et al. 2015. Role of a health psychologist in the management of functional esophageal complaints. Dis. Esophagus 28: 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riehl ME & Chen JW 2018. The proton pump inhibitor nonresponder: a behavioral approach to improvement and wellness. Curr. Gastroenterol. Rep 20: 34. [DOI] [PubMed] [Google Scholar]
- 61.Riehl ME, Pandolfino JE, Palsson OS, et al. 2016. Feasibility and acceptability of esophageal-directed hypnotherapy for functional heartburn. Dis. Esophagus 29: 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richter JE & Vaezi MF 2021. Gastroesophageal reflux disease. In Gastrointestinal Disease Pathophysiology/Diagnosis/Management 11th ed. Sleisenger MH & Fordtran JS, Eds.: 670–690. Philadelphia, PA: Elsevier, Inc. [Google Scholar]
- 63.Helm JF 1989. Role of saliva in esophageal function and disease. Dysphagia 4: 76–84. [DOI] [PubMed] [Google Scholar]
- 64.Burgess J 2018. Salivary stimulation—could it play a role in GERD management? J. Otolaryngol. ENT Res 10: 127–130. [Google Scholar]
- 65.Diener U, Patti MG, Molena D, et al. 2001. Esophageal dysmotility and gastroesophageal reflux disease. J. Gastrointest. Surg 5: 260–265. [DOI] [PubMed] [Google Scholar]
- 66.Chrysos E, Prokopakis G, Athanasakis E, et al. 2003. Factors affecting esophageal motility in gastroesophageal reflux disease. Arch. Surg 138: 241–246. [DOI] [PubMed] [Google Scholar]
- 67.Meneghetti AT, Tedesco P, Damani T, et al. 2005. Esophageal mucosal damage may promote dysmotility and worsen esophageal acid exposure. J. Gastrointest. Surg 9: 1313–1317. [DOI] [PubMed] [Google Scholar]
- 68.Stein HJ, Eypasch EP, DeMeester TR, et al. 1990. Circadian esophageal motor function in patients with gastroesophageal reflux disease. Surgery 108: 769–778. [PubMed] [Google Scholar]
- 69.Liu L, Li S, Zhu K, et al. 2019. Relationship between esophageal motility and severity of gastroesophageal reflux disease according to the Los Angeles classification. Medicine 98: e15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang YK, Hsu WH, Wang SSW, et al. 2013. Current pharmacological management of gastroesophageal reflux disease. Gastroenterol. Res. Pract 2013: 983653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Achem SR & Robinson M 1998. A prokinetic approach to treatment of gastroesophageal reflux disease. Dig. Dis 16: 38–46. [DOI] [PubMed] [Google Scholar]
- 72.Ramirez B & Richter JE 1993. Promotility drugs in the treatment of gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther 7: 5–20. [DOI] [PubMed] [Google Scholar]
- 73.Madan K, Ahuja V, Kashyap PC, et al. 2004. Comparison of efficacy of pantoprazole alone versus pantoprazole plus mosapride in therapy of gastroesophageal reflux disease: a randomized trial. Dis. Esophagus 17: 274–278. [DOI] [PubMed] [Google Scholar]
- 74.Futagami S, Iwakiri K, Shindo T, et al. 2010. The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying. J. Gastroenterol 45: 413–421. [DOI] [PubMed] [Google Scholar]
- 75.Yamaji Y, Isomura Y, Yoshida S, et al. 2014. Randomized controlled trial comparing the efficacy of mosapride plus omeprazole combination therapy to omeprazole monotherapy in gastroesophageal reflux disease. J. Dig. Dis 15: 469–476. [DOI] [PubMed] [Google Scholar]
- 76.Sirinawasatien A & Kantathavorn N 2019. Efficacy of four weeks treatment of omeprazole plus mosapride combination therapy compared with that of omeprazole monotherapy in patients with proton pump inhibitor-refractory gastroesophageal reflux disease: a randomized controlled trial. Clin. Exp. Gastroenterol 12: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tominaga K, Iwakiri R & Fujimoto K 2012. Rikkunshito improves symptoms in PPI-refractory GERD patients: a prospective, randomized, multicenter trial in Japan. J. Gastroenterol 47: 284–292. [DOI] [PubMed] [Google Scholar]
- 78.Tatsuta M & Iishi H 1993. Effect of treatment with liu-jun-zi-tang (TJ-43) on gastric emptying and gastrointestinal symptoms in dyspeptic patients. Aliment. Pharmacol. Ther 7: 459–462. [DOI] [PubMed] [Google Scholar]
- 79.Arai M, Matsumura T & Tsuchiya N 2012. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology 59: 62–66. [DOI] [PubMed] [Google Scholar]
- 80.Hayakawa T, Arakawa T, Kase Y, et al. 1999. Liu-Jun-Zi-Tang, a Kampo medicine, promotes adaptive relaxation in isolated guinea pig stomachs. Drugs Exp. Clin. Res 25: 211–218. [PubMed] [Google Scholar]
- 81.Costanzo LS 2010. Gastrointestinal physiology. In Physiology Vol. 1. Philadelphia, PA: Saunders, Inc. [Google Scholar]
- 82.Hershcovici T, Mashimo H & Fass R 2011. The lower esophageal sphincter. Neurogastroenterol. Motil 23: 819–830. [DOI] [PubMed] [Google Scholar]
- 83.Schneider JH, Kuper MA, Konigsrainer A, et al. 2010. Transient lower esophageal sphincter relaxation and esophageal motor response. J. Surg. Res 159: 714–719. [DOI] [PubMed] [Google Scholar]
- 84.Ribolsi M, Holloway RH, Emerenziani S, et al. 2014. Impedance-high resolution manometry analysis of patients with nonerosive reflux disease. Clin. Gastroenterol. Hepatol 12: 52–57. [DOI] [PubMed] [Google Scholar]
- 85.Grossi L, Spezzaferro M, Sacco LF, et al. 2008. Effect of baclofen on oesophageal motility and transient lower oesophageal sphincter relaxations in GORD patients: a 48-h manometric study. Neurogastroenterol. Motil 20: 760–766. [DOI] [PubMed] [Google Scholar]
- 86.Li S, Shi S, Chen F, et al. 2014. The effects of baclofen for the treatment of gastroesophageal reflux disease: a meta-analysis of randomized controlled trials. Gastroenterol. Res. Pract 2014: 307805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Falk GW 2010. Inhibition of transient lower esophageal sphincter relaxation in GERD: will lesogaberan advance the field? Gastroenterology 139: 377–379. [DOI] [PubMed] [Google Scholar]
- 88.Yadlapati R, Hungness ES & Pandolfino JE 2018. Complications of antireflux surgery. Am. J. Gastroenterol 113: 1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yadlapati R, Vaezi MF, Vela MF, et al. 2018. Management options for patients with GERD and persistent symptoms on proton pump inhibitors: recommendations from an expert panel. Am. J. Gastroenterol 113: 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gawron AJ, Bell R, Abu Dayyeh BK, et al. 2020. Surgical and endoscopic management options for patients with GERD based on proton pump inhibitor symptom response: recommendations from an expert U.S. panel. Gastrointest. Endosc 92: 78–87.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johansson KE & Tibbling L 1988. Esophageal body motor disturbances in gastroesophageal reflux and the effects of fundoplication. Scand. J. Gastroenterol. Suppl 155: 82–88. [DOI] [PubMed] [Google Scholar]
- 92.Kornmo TS & Ruud TE 2008. Long-term results of laparoscopic Nissen fundoplication due to gastroesophageal reflux disease. A ten year follow-up in a low volume center. Scand. J. Surg 97: 227–230. [DOI] [PubMed] [Google Scholar]
- 93.Broeders JA, Rijnhart-de Jong HG, Draaisma WA, et al. 2009. Ten-year outcome of laparoscopic and conventional Nissen fundoplication: randomized clinical trial. Ann. Surg 250: 698–706. [DOI] [PubMed] [Google Scholar]
- 94.Bona D, Aiolfi A, Asti E, et al. 2020. Laparoscopic Toupet fundoplication for gastroesophageal reflux disease and hiatus hernia: proposal for standardization using the “critical view” concept. Updates Surg 72: 555–558. [DOI] [PubMed] [Google Scholar]
- 95.Erenoglu C, Miller A & Schirmer B 2003. Laparoscopic Toupet versus Nissen fundoplication for the treatment of gastroesophageal reflux disease. Int. Surg 88: 219–225. [PubMed] [Google Scholar]
- 96.Qin M, Ding G & Yang H 2013. A clinical comparison of laparoscopic Nissen and Toupet fundoplication for gastroesphageal reflux disease. J. Laparoendosc. Adv. Surg. Tech. A 23: 601–604. [DOI] [PubMed] [Google Scholar]
- 97.Ayazi S, Chowdhury N, Zaidi AH, et al. 2020. Magnetic sphincter augmentation (MSA) in patients with hiatal hernia: clinical outcome and patterns of recurrence. Surg. Endosc 34: 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cadiére GB, Buset M, Muls V, et al. 2008. Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J. Surg 32: 1676–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Testoni PA, Mazzoleni G & Testoni SGG 2016. Transoral incisionless fundoplication for gastro-esophageal reflux disease: techniques and outcomes. World J. Gastrointest. Pharmacol. Ther 7: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan A, Kim A, Sanossian C, et al. 2016. Impact of obesity treatment on gastroesophageal reflux disease. World J. Gastroenterol 22: 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yadlapati R & Pandolfino JE 2020. Personalized approach in the work-up and management of gastroesophageal reflux disease. Gastrointest. Endosc. Clin. N. Am 30: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]


