Abstract
Background
Mpox (formerly monkeypox) is a viral disease caused by the mpox virus (MPXV), endemic in Central and West Africa and currently causing a global outbreak of international concern. Much remains unknown about sample types most suited for mpox laboratory diagnosis. While it is established that high viral loads can be found in active skin lesions (currently the recommended mpox laboratory confirmation specimen type), WHO mpox testing guidelines encourage the use of oropharyngeal swabs as an additional sample type for mpox diagnosis and suggest investigating the value of other specimens like blood samples.
Objective
In this study, we verified the value of select alternative specimen types for mpox laboratory confirmation.
Methods
We included 25 patients with MPXV-confirmed skin lesions to compare diagnostic sensitivity of MPXV PCR testing on EDTA plasma and two upper respiratory specimens: oropharyngeal swabs and saliva.
Results
In our patient cohort with MPXV-confirmed skin lesions, diagnostic sensitivity of MPXV PCR was 80% in EDTA plasma, 64% in oropharyngeal swabs, and 88% in saliva. MPXV viral loads were significantly higher in saliva compared to oropharyngeal swabs and EDTA plasma.
Discussion
The WHO recommendation to collect oropharyngeal swabs as an additional specimen for mpox diagnosis might need to be revised to include saliva wherever feasible. We suggest investigating saliva as a diagnostic specimen in the absence of active skin lesions or during the phase preceding skin manifestations. Moreover, the relatively high MPXV DNA content of saliva warrants elucidating its potential role in disease transmission.
Keywords: Monkeypox, Diagnosis, PCR, Laboratory, Specimens, Sensitivity, Saliva, Throat swab, Blood, plasma
1. Background
In response to the current worldwide mpox outbreak, laboratory capacity for MPXV detection had to be scaled up rapidly worldwide. Nevertheless, most of the knowledge on diagnostic procedures for MPXV originated in central Africa, where the disease is caused by a different MPXV clade and the clinical presentation, affected population, and mode of transmission may differ substantially from the ongoing outbreak ([1], [2], [3], [4], [5]). While diagnosis of mpox is traditionally done on skin swabs, patients can also present with detectable viral DNA in saliva, oropharyngeal, and nasopharyngeal swabs, blood, seminal fluid, urine, faeces, and anal swabs ([6], [7], [8], [9], [10], [11]).
The World Health Organization (WHO) mpox testing guidelines recommend skin lesion material as primary specimen type for mpox laboratory confirmation and encourage oropharyngeal swabs as a potentially useful additional specimen (12). However, it is not clear whether MPXV preferentially binds and replicates in the pharyngeal mucosa or in the oral cavity and salivary glands and which sampling site thus has higher diagnostic yield. The WHO also mention that “EDTA blood may support detection of MPXV but may not contain the high level of virus found in lesion samples”. To shed light on the usefulness of suggested additional specimens for mpox diagnosis, we assessed the diagnostic sensitivity of EDTA plasma, oropharyngeal swabs, and saliva collected from patients of whom the mpox diagnosis was confirmed on skin lesion swabs.
2. Material and methods
2.1. Sample collection
We used diagnostic samples from patients with active (early to late) skin lesions (including in the anogenital region) presenting for MPXV testing at our sexual health clinic at the Institute of Tropical Medicine (ITM), Antwerp, Belgium. We obtained swabs (Copan ESwab®, containing 1 mL of Liquid Amies medium; Murrieta, CA, USA) from (multiple) lesions (by sampling the lesion fluid and base) and the oropharynx. At least 1 ml of saliva was self-collected under supervision in an OMNIgene·ORAL® (DNA Genotek; Ontario, Canada) collection tube (that contains a proprietary viral DNA stabilizing solution), mostly after collection of the oropharyngeal swab. Blood was collected in a 9 ml EDTA K3E S-Monovette ® tube (Sarstedt, Nümbrecht, Germany) and centrifuged at 2500 g for 10 min to separate the EDTA plasma fraction. All samples were taken as patients presented for their initial diagnostic workup (thus, during the same visit and without instructions on food consumption and time of visit).
The study analyses were performed on the first 25 patients with positive MPXV PCR results on skin lesion swabs visiting the ITM sexual health clinic of whom all three alternative testing specimens (EDTA plasma, throat swab, and saliva) were available. In addition, four patients presenting at the clinic with negative MPXV PCR results on skin lesion swabs were included to verify specificity on the alternative sample types.
2.2. Laboratory diagnosis
For MPXV real-time PCR testing, DNA was either manually extracted (QIAamp DNA Mini kit, Qiagen, Hilden, Germany; using 200ul sample input and 100ul elution volume) or automatically (Maxwell®, Promega, Madison, WI, USA; using 300ul sample input and 75ul elution volume). The extraction method was kept identical within compared pairs. PCR was performed using PerfeCTa FastMix II PCR Reagents (Quantabio, Beverly, MA, USA) and MPXV generic primers/probe as described previously (13). Amplification was carried out on a QuantStudio 5 instrument (ThermoFisher Scientific, Waltham, MA, USA).
2.3. Statistical testing
Diagnostic sensitivity of alternative specimen types (EDTA plasma, oropharyngeal swab and saliva) was calculated as the number of positive results for a given specimen type divided by the total number of mpox confirmed study participants. The confidence interval of these proportions was computed with the hybrid Wilson/Brown method (Graphpad Prism 9). Sensitivities of alternative specimen types were compared using the McNemar test (Graphpad Prism online calculator). Comparison of positive MPXV PCR Ct values generated on different sample types was performed using paired t-testing or two-way ANOVA with Tukey's multiple comparisons.
3. Results
All four included patients with MPXV negative skin lesions had negative MPXV PCR testing results on the alternative specimen types. In the 25 patients with MPXV positive skin lesions, diagnostic sensitivity of plasma, oropharyngeal swab and saliva were 80% (95% CI: 0.61 to 0.91), 64% (95% CI: 0.45 to 0.80), and 88% (95% CI: 0.70 to 0.96) respectively (Table 1 ). Sensitivities between specimen types were not statistically significantly different.
Table 1.
MPXV PCR Ct value results generated on different indicated specimen types per study participant (every row represents a single patient, n = 25) with indication of oral lesion presence. (nd stands for not detected)
| Patient # | Plasma | Oropharyngeal swab | Saliva | Oral lesions |
|---|---|---|---|---|
| 1 | 35.54 | 26.77 | 30.94 | No |
| 2 | 37.72 | nd | 41.08 | No |
| 3 | 34.84 | 39.07 | 36.71 | Yes (lip) |
| 4 | 38.87 | 37.94 | 33.85 | No |
| 5 | 33.44 | nd | 37.05 | No |
| 6 | 37.51 | 25.54 | 25.71 | Yes (lip) |
| 7 | 39.90 | 32.66 | 33.61 | Yes (lip) |
| 8 | 38.41 | 35.98 | 23.85 | No |
| 9 | 37.54 | 33.86 | 25.15 | No |
| 10 | nd | nd | nd | No |
| 11 | 32.74 | 34.98 | 29.13 | No |
| 12 | nd | nd | nd | No |
| 13 | 39.16 | nd | 22.76 | No |
| 14 | 40.58 | 36.39 | 30.23 | No |
| 15 | 37.27 | 37.02 | 28.23 | Yes (sublingual) |
| 16 | nd | nd | 37.51 | No |
| 17 | 36.60 | 36.12 | 35.17 | No |
| 18 | 36.11 | nd | 37.32 | No |
| 19 | 39.77 | 31.09 | 25.43 | No |
| 20 | 35.41 | nd | 29.90 | No |
| 21 | 34.77 | 37.61 | nd | No |
| 22 | 36.59 | 31.76 | 23.02 | No |
| 23 | 35.83 | 35.16 | 25.64 | Yes (lip) |
| 24 | nd | 36.14 | 31.03 | yes (lip) |
| 25 | nd | nd | 31.11 | No |
| # positives (out of n = 25): | 20 |
16 | 22 | |
| Sensitivity: | 0.80 (0.61 to 0.91) |
0.64 (0.45 to 0.80) |
0.88 (0.70 to 0.96) |
|
| McNemar P value | vs plasma: 0.2207 | vs plasma: 0.6171 vs oropharyngeal swab: 0,0771 |
Next, PCR Cycle threshold (Ct) values were compared between specimen types on a subset of 14 (out of the total 25) included mpox patients that had positive MPXV PCR results on all tested specimens. This analysis indicates that saliva contains the highest MPXV viral loads of the alternative specimens tested (Fig. 1 ). The mean MPXV PCR Ct value in the samples from the 14 patients testing positive on all tested sample types was 37.29 (95% CI: 36.04 to 38.53) in plasma, 33.88 (95% CI: 31.58 to 36.18) in oropharyngeal swabs, and 29.05 (95% CI: 26.47 to 31.63) in saliva. In comparison, the mean MPXV PCR Ct value on skin lesion swabs of these 14 mpox patients was 21.81 (95% CI: 19.04 to 24.59), which differed significantly from the matching saliva Ct value (Paired t-test P value = 0.0005).
Fig. 1.
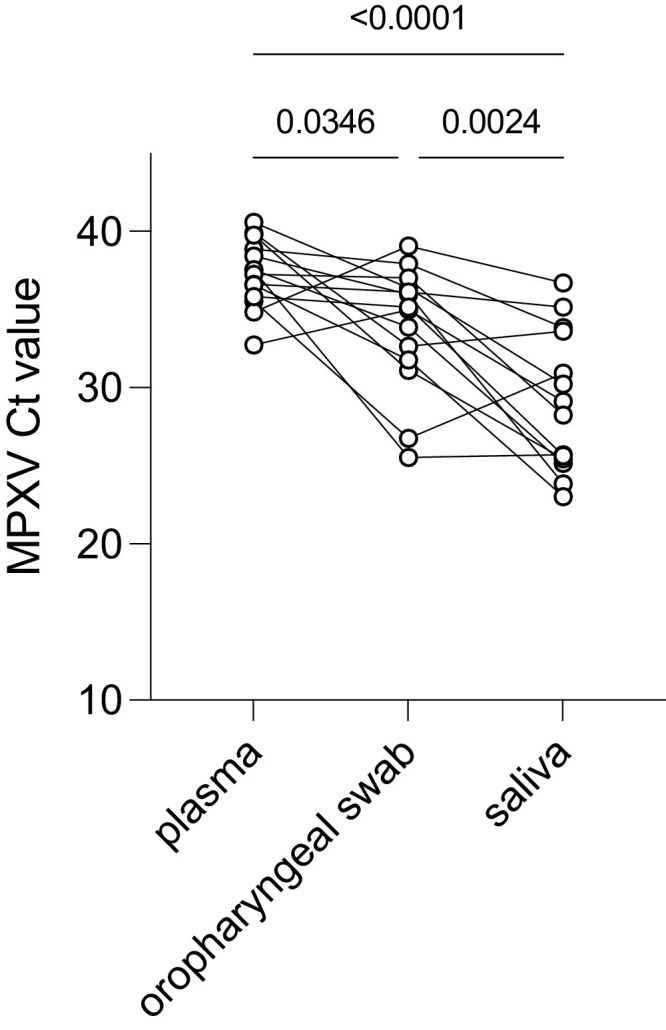
Comparison of MPXV PCR Ct values generated on different indicated sample types from mpox patients testing positive on all three sample types (n = 14 out of 25 included mpox patients. All P-values denote two-way ANOVA with Tukey's multiple comparisons (the overall ANOVA P value is < 0.0001).
Of note, we did not observe any difference in MPXV PCR testing between EDTA plasma and serum (both had an 80% sensitivity (95% CI: 0.61 to 0.91), and MPXV PCR Ct values were not different; supplementary figure 1).
4. Discussion
Our study confirms recent evidence that, during acute clinical presentation of mpox with active skin lesions, plasma samples do not contain high levels of detectable virus (9).
Our data also show that the upper respiratory samples cannot replace skin lesion swabs in case of symptomatic presentation with skin lesions. For such individuals with active skin lesions, diagnostic sensitivity was 64% on oropharyngeal swabs, but reached up to 88% in saliva. Despite the low number of included mpox suspects in our study, we were able to demonstrate that saliva contained higher MPXV viral loads (using MPXV PCR Ct values as a semi-quantitative proxy) compared to oropharyngeal swabs and EDTA plasma at the exact time of patient presentation for diagnostic workup (the mean time since symptom onset was 8,5 days (95% CI: 5642 to 11,32)). Of note, the presence of oral lesions (in the mouth and at the lips; n = 6/25) was not associated with the saliva MPXV Ct value (simple logistic regression p value = 0,7825).
Our observations align with published MPXV PCR data allowing additional within-patient comparison of saliva with nasopharyngeal swabs (instead of oropharyngeal swabs tested in our report, as is recommended by WHO) (6, 7). While most of the samples in those reports were not collected at the very day of diagnosis (sampling was performed only after mpox diagnosis was confirmed (7)) these data support the presence of higher MPXV DNA content in saliva compared to a swab taken from the upper respiratory mucosa (nasopharyngeal swabs in this case) shortly after clinical presentation for skin lesions and subsequent diagnosis (supplementary figure 2).
While our data provide insights in the diagnostic value of sampling sites other than the preferred skin lesion swab specimens, they cannot be extrapolated to testing of patients presenting without skin lesions (but with other primary presentations like proctitis and lymphadenopathy). In addition, prospective studies performing longitudinal follow-up of yet asymptomatic high-risk contacts of confirmed mpox cases are needed to decipher whether and which alternative samples would have a place in testing pre-symptomatic or prodromal mpox cases. We recommend testing the value of an anorectal swab in that context based on reports on asymptomatic MPXV detection in this specimen(5, 14). An advantage of saliva in such context would be its ease of (serial) self-collection which could be helpful in some settings e.g., to lower the barrier for testing and the workload and exposure of healthcare workers.
MPXV is considered a Category A, UN2814 pathogen and is hence subjected to transportation using strict biosafety procedures (such as triple packaging) by a certified shipper. It is relevant to note that the saliva in our study was collected in a viral DNA stabilizing lysing buffer. While not specifically validated by the company for MPXV inactivation, the ease of lysis of enveloped viruses in general virus-inactivating media might also enhance biosafety during sample transportation and manipulation in the laboratory. Of note, we observed equivalence of MPXV PCR diagnostic sensitivity in an additional shorter series comparing skin swabs collected in a guanidine-based transport medium to a viral transport medium without guanidine (supplementary Figure 3), and we have now urged external requesting laboratories involved in the clinical workup of mpox suspect cases to use inactivating transport media for centralized MPXV PCR testing at our institute.
5. Conclusions
In the presence of skin lesions, lesion swabs should remain the standard specimen type for mpox laboratory confirmation by PCR. In the case where an additional testing sample is considered, our data suggest that saliva provides higher sensitivity than oropharyngeal swabs and EDTA plasma. The WHO recommendation to collect oropharyngeal swabs as an additional testing specimen to skin lesion swabs might therefore need to be revised to include saliva wherever feasible. The relatively high viral load in saliva warrants further investigation into i) the potential use of saliva as a diagnostic sample (besides oropharyngeal swabs and blood) in the absence of skin lesions, and during the pre-symptomatic phase after high-risk contact, and ii) the potential role of saliva in transmission (e.g., by droplets, kissing, using saliva as lubrification during sex).
Ethical statement
Patients were informed of the use of their samples for secondary research and asked to sign an informed consent form. Ethics approval for this research was obtained through the ITM institutional review board (file number: 1596/22).
Funding statement
This work was funded by the Department of Economy, Science, and Innovation of the Flemish government.
Authors’ contributions
Conceptualisation: EF, MVE, KV, IB, LL, ITM MPX study group; Methodology and patient care: JC, FV, IB, LL, SVH, TV, SB, NB, IDB, CK, PS, EF; Formal analysis: KV, BKMJ; Writing: KV; Writing, review and editing: KV, MVE, DVDB, BKMJ, KKA, JVG, IDB, NB, SB, TV, SVH, LL, IB
Declaration of Competing Interest
None
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105372.
Contributor Information
ITM MPX study group:
Christophe Van Dijck, Kadrie Ramadan, Karin Van Looveren, Jolien Baeyens, Cindy Van Hoyweghen, Marianne Mangelschots, Sandra Coppens, Leo Heyndrickx, Johan Michiels, Tessa De Block, Marie Laga, Jef Vanhamel, Bea Vuylsteke, Emmanuel Bottieau, Leen Vandenhove, Philippe Selhorst, Antonio Mauro Rezende, Hilde Smet, Hanne Rasson, Jacob Verschueren, Tom Platteau, Anne Hauner, and Betty Willems
Appendix. Supplementary materials
References
- 1.Perez Duque M., Ribeiro S., Martins J.V., Casaca P., Leite P.P., Tavares M., et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inigo Martinez J., Gil Montalban E., Jimenez Bueno S., Martin Martinez F., Nieto Julia A., Sanchez Diaz J., et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill. 2022;27(27) doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girometti N., Byrne R., Bracchi M., Heskin J., McOwan A., Tittle V., et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect. Dis. 2022 doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan D., Sze S., Nazareth J., Martin C.A., Al-Oraibi A., Baggaley R.F., et al. Monkeypox in the UK: arguments for a broader case definition. Lancet. 2022;399(10344):2345–2346. doi: 10.1016/S0140-6736(22)01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Baetselier I., Van Dijck C., Kenyon C., Coppens J., Michiels J., de Block T., et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat. Med. 2022 doi: 10.1038/s41591-022-02004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antinori A., Mazzotta V., Vita S., Carletti F., Tacconi D., Lapini L.E., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiro-Mestres A., Fuertes I., Camprubi-Ferrer D., Marcos M.A., Vilella A., Navarro M., et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27(28) doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarin-Vicente E.J., Alemany A., Agud-Dios M., Ubals M., Suner C., Anton A., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022 doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veintimilla C., Catalan P., Alonso R., de Viedma D.G., Perez-Lago L., Palomo M., et al. The relevance of multiple clinical specimens in the diagnosis of monkeypox virus, Spain, June 2022. Euro Surveill. 2022;27(33) doi: 10.2807/1560-7917.ES.2022.27.33.2200598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominik Nörz T.T.B., Tang Hui Ting, Grewe Ilka, Hermanussen Lennart, Matthews Hanna, Pestel Julia, Degen Olaf, Günther Thomas, Grundhoff Adam, Fischer Nicole, Addo Marylyn M., Jordan Sabine, Hertling Sandra, Unger Stephan, Schäfer Guido, Schewe Knud, Hoffmann Christian, Aepfelbacher Martin, Pfefferle Susanne, Wiesch Julian Schulze zur, Schmiedel Stefan, Lütgehetmann Marc. Clinical characteristics and comparison of longitudinal qPCR results from different specimen types in a cohort of ambulatory and hospitalized patients infected with monkeypox virus. J. Clin. Virol. 2022 doi: 10.1016/j.jcv.2022.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., et al. Monkeypox virus infection in humans across 16 Countries - April-June 2022. N. Engl. J. Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 12.WHO interim guidance for laboratory testing for the monkeypox virus. 23 May 2022.
- 13.Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time pcr assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods. 2010;169(1):223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferre V.M., Bachelard A., Zaidi M., Armand-Lefevre L., Descamps D., Charpentier C., et al. Detection of Monkeypox virus in anorectal swabs from asymptomatic men who have sex with men in a sexually transmitted infection screening program in Paris, France. Ann. Intern. Med. 2022;175(10):1491–1492. doi: 10.7326/M22-2183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


