Abstract
Salivary gland extract (SGE) from Ixodes ricinus ticks inhibited the killing of Borrelia afzelii spirochetes by murine macrophages. SGE also reduced the production of two major defense molecules of phagocytes, superoxide and nitric oxide. It is likely that the suppression of macrophage microbicidal mechanisms contributes to the inhibitory effect of tick saliva on the killing of B. afzelii spirochetes, thus facilitating the transmission of this important pathogen.
Hard ticks feed on their hosts for several days or even weeks, providing an opportunity for the host immune system to affect the ticks. In naive hosts, mostly nonspecific mechanisms of innate immunity connected with inflammation play a role at the tick feeding site.
Ticks have evolved strategies to modulate host immune defenses. Tick saliva contains an array of pharmacologically active molecules with antihemostatic, vasoactive, and immunomodulatory properties (16, 21). Tick saliva or salivary gland extracts (SGE) inhibited activation of the alternative pathway of complement (15) and prevented phagocytosis and other functions of neutrophils (17). The inhibitory effect of tick SGE on NK cells (8, 10) and interferon (6) has been reported. Recently, histamine-binding proteins have been identified in the saliva of ixodid tick species (14).
Tick feeding exerts a pronounced effect on the cytokine regulation of host immunity (21). Production of macrophage proinflammatory cytokines is usually suppressed, as is secretion of Th1 cytokines. Th2 cytokines are up-regulated, indicating the polarization of the immune response toward Th2 lymphocytes (3).
There is increasing evidence supporting the idea that tick-borne pathogens take advantage of the anti-inflammatory effect of tick saliva to facilitate their transmission (7). It was shown that the transmission of Borrelia burgdorferi by a tick bite is much more efficient than transmission of the pathogen by a syringe injection (5).
In this study we focused on the effect of tick saliva on macrophages, which represent the first line of host defense against the spirochete.
Specific-pathogen-free female BALB/c mice, 6 to 10 weeks old, purchased from Charles River, Sulzfeld, Germany, were used in this study. SGE was prepared from adult Ixodes ricinus ticks from the colony of the Institute of Parasitology, Academy of Sciences of the Czech Republic, České Budějovice, Czech Republic. Ticks were screened for B. burgdorferi sensu lato by PCR, with negative results. Ticks were fed in groups of 20 mating pairs within retaining cells attached to the backs of guinea pigs. After 5 days engorged female ticks were removed, and the salivary glands were dissected from the live ticks and pooled. After being washed in phosphate-buffered saline (PBS), the salivary glands were homogenized in 1 ml of PBS by sonication and were clarified by centrifugation at 10,000 × g for 10 min. The protein concentration was determined using a protein estimation kit (Bio-Rad, Richmond, Calif.). Salivary glands with a tissue weight of 0.196 g obtained from 20 ticks represented a protein concentration of 338 μg/ml. Aliquots of the SGE preparation were stored at −70°C. SGE at the concentration of 20 μg/ml, exerting the highest inhibitory effect on mechanisms of natural immunity (8), was used throughout the experiments. This concentration had no effect on macrophage viability as determined by the trypan blue exclusion test.
The CB 43 strain of Borrelia afzelii, isolated from an I. ricinus female (19), was grown in Barbour-Stoenner-Kelly-H medium (Sigma) supplemented with 6% rabbit serum. The fourth passage was used in the experiments.
The killing assay was performed according to the guidelines of Modolell et al. (12). Peritoneal cells (PC) were recovered from BALB/c mice by lavaging the peritoneum with 4 ml of cold RPMI 1640 medium. Macrophages represented 25% of nucleated cells as determined by flow cytometry analysis using anti-F4/80 antibody (Serotec, Kidlington, United Kingdom). After being washed, the cells were seeded at 2 × 105/100 μl per well of a 96-well tissue culture plate (Nunc, Roskilde, Denmark) in RPMI 1640 medium supplemented with 10% fetal calf serum and 5 × 10−5 M 2-mercaptoethanol without antibiotics and incubated at 37°C and 3.5% CO2 for 1 h. A volume of 50 μl of SGE diluted in culture medium was added to some wells, and the cells were incubated for a further 2 h. A volume of 50 μl of the appropriate dilution of PBS was added to control wells. The spirocheticidal activity of PC was measured as the release of 14C-labeled nucleotides into the culture supernatant after interaction of prelabeled spirochetes with PC. A total of 108 spirochetes were incubated with 10 μCi of [14C]adenine (ICN Biochemicals, Irvine, Calif.) for 72 h in 10 ml of Barbour-Stoenner-Kelly medium. The spirochetes were washed three times in RPMI 1640 medium and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, and 2 × 106 spirochetes in a volume of 50 μl/well were added to wells with PC pretreated with SGE or PBS as described above. Superoxide dismutase (SOD; Sigma) at a final concentration of 500 IU/ml or N-monomethyl-arginine (NMMA; Sigma) at a final concentration of 0.1 mM was added instead of SGE to some wells. After incubation at 37°C and 3.5% CO2 for 24 h, cultures were frozen and thawed, and after centrifugation (11,000 × g, 10 min) 100 μl of the supernatant was measured in a liquid scintillation counter. The specific killing was calculated as follows: [(cpm of cultures with PC − cpm of cultures without PC)/[(cpm of spirochetes − cpm of cultures without PC)] × 100, where cpm is counts per minute. Freezing and thawing under the described conditions had no effect on the spontaneous release of 14C-labeled nucleotides or the viability of the spirochetes.
PC for the measurement of nitric oxide (NO) production were plated at 2 × 105/0.2 ml in 96-well culture plates and incubated overnight. After being rinsed, the cells were incubated with the medium alone or with the medium containing 20 μg of SGE per ml for 2 h. Then spirochetes were added, 2 × 105 or 2 × 106 per well, and the levels of nitrite in the supernatants of the cultures were assessed at 24 h by the Griess reaction (1). Cell culture supernatants (50 μl) were mixed with 50 μl of Griess reagent and incubated at room temperature for 10 min. Absorbance was measured with the Titertek Multiskan enzyme-linked immunosorbent assay reader at 540 nm. The concentration of NO2 was determined by comparing it with a standard curve generated with dilutions of NaNO2.
The nitroblue tetrazolium (NBT) assay for determination of superoxide (O2−) production was performed according to the procedure of Rook et al. (18), with modifications. PC prepared as described above for the NO assay were preincubated with SGE for 2 h and stimulated with spirochetes (2 × 105 or 2 × 106/well) for 1 h at 37°C. Together with spirochetes, NBT (Sigma) was added to each well at a final concentration of 1 mg/ml. After centrifugation (150 × g, 5 min) the supernatant was discarded, and nonreduced NBT was rinsed with 70% methanol in PBS. Reduced formazan was dissolved by the addition of 120 μl of 2 M KOH and 140 μl of dimethyl sulfoxide (Sigma) to each well. After mixing, the absorbance was measured at 690 nm.
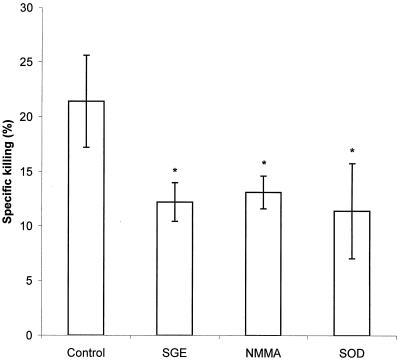
The killing assay based on the release of radioactivity from [14C]adenine-labeled spirochetes after their destruction in macrophages (Fig. 1) showed that whereas the specific killing in the control group was 21.4%, treatment with SGE reduced the destruction of spirochetes by 43% (specific killing in this group was 12.2%). Spontaneous release of radioactivity from the bacteria into the culture medium was 20.1% of the total incorporated radioactivity, while spontaneous release in the presence of SGE was 23.9%. These results are supported by our observations that SGE markedly increases the number of motile bacteria that can be enumerated after 24 h of incubation with murine macrophages (data not shown).
FIG. 1.
Effect of SGE and inhibitors of NO and O2− on the killing of B. afzelii by PC. Data are the means of three cultures ± standard deviations. An asterisk shows where the difference versus control cultures is significant at a P value of <0.05.
Since it has been shown by Modolell et al. (12) that specific inhibitors of nitric oxide and superoxide production decrease the specific killing of B. burgdorferi spirochetes in macrophages, we compared the effect of NMMA and SOD with that of SGE on the borreliacidal activity of PC (Fig. 1). Both inhibitors and SGE exerted comparable and significant effects (P < 0.05). NMMA reduced the specific killing by 39% and SOD reduced killing by 47% (spontaneous release of radioactivity from spirochetes incubated with the inhibitors was 23.0 and 25.1%, respectively).
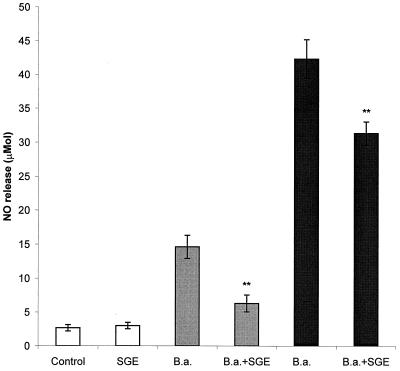
Subsequently we tested the hypothesis that SGE reduces the killing of Borrelia spirochetes by the inhibition of macrophage killing mechanisms. In the first experiment (Fig. 2), PC were pretreated with SGE for 2 h and stimulated with live spirochetes in a ratio of 1 or 10 spirochetes per cell. The production of NO was determined after 24 h of incubation. In comparison with untreated controls, SGE significantly reduced the production of NO (P < 0.01). While NO induced with the lower dose of spirochetes was reduced by 57%, the higher level of NO induced with the higher dose of bacteria was lowered by 26%.
FIG. 2.
Effect of SGE on the production of nitric oxide by PC stimulated with B. afzelii (B.a.). Spirochetes were added in two effector-to-target ratios, 1:1 (gray bars) and 1:10 (black bars). Columns represent the means of three cultures ± standard deviations. Double asterisks show where the difference versus B. afzelii-infected, SGE-untreated cultures is significant at a P value of <0.01.
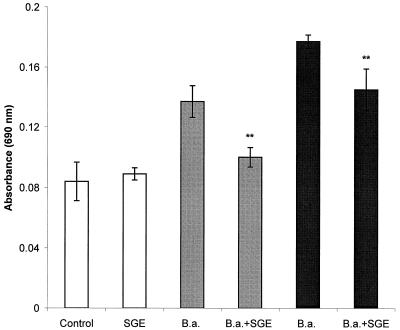
Similarly, the SGE effect on the production of superoxide and other products of the respiratory burst was estimated. PC were treated with SGE and stimulated with spirochetes in the same way as for the determination of NO, but the cells were incubated with B. afzelii for only 1 h (Fig. 3). SGE lowered the absorbance of reduced formazan (expressing the production of reactive oxygen intermediates) by 27% (spirochete/PC ratio, 1:1) or 18% (ratio, 10:1). In both cases the difference between untreated and SGE-treated groups was significant at a P value of <0.01.
FIG. 3.
Effect of SGE on the production of reactive oxygen intermediates (mainly superoxide) by PC stimulated with B. afzelii (B,a.). Spirochetes were added in two effector-to-target ratios, 1:1 (gray bars) and 1:10 (block bars). Columns represent the means of three cultures ± standard deviations. Double asterisks show where the difference versus B. afzelii-infected, SGE-untreated cultures is significant at a P value of <0.01.
The present paper demonstrates that I. ricinus SGE decreases the killing of spirochetes of B. afzelii by mouse PC, mainly macrophages. The results of a killing assay depend on both phagocytosis and killing of bacteria by macrophages. According to our preliminary results (data not shown), the phagocytosis of Borrelia spirochetes is partially inhibited by I. ricinus SGE. Thus, it is likely that both internalization and killing mechanisms of phagocytes are affected by tick saliva, resulting in higher survival rates of the pathogen at the tick feeding site.
To our knowledge, there is only one paper demonstrating the inhibitory effect of Ixodes dammini (current name, Ixodes scapularis) saliva on the phagocytosis of B. burgdorferi spirochetes by rat neutrophils (17). No papers have reported the effect of tick saliva on the killing of Borrelia organisms in phagocytic cells.
The bactericidal activity of professional phagocytes is realized by several mechanisms, including reactive oxygen and nitrogen intermediates. Hence we assayed the effect of SGE on two major defense molecules of phagocytic cells, superoxide and nitric oxide. SGE significantly decreased the production of NO by PC stimulated by B. afzelii spirochetes. Similar results have been obtained for the effect of I. dammini saliva on the production of NO by mouse macrophages stimulated with lipopolysaccharide (20) and for the impact of Rhipicephalus sanguineus saliva on NO produced by mouse macrophages stimulated by gamma interferon (2). In this work we took advantage of the high capacity of B. burgdorferi to stimulate the inducible NO synthase (iNOS) (11). Nitric oxide and superoxide have been shown to be efficient against B. burgdorferi spirochetes (12). In our experiments, addition of the specific inhibitor of iNOS NMMA reduced the killing of spirochetes by PC to an extent similar to that of SGE. This implies that the inhibition of the NO-dependent killing mechanism by SGE can explain in part the reduced spirocheticidal capacity of PC.
SGE reduced the production of further defense molecules connected with the respiratory burst, especially the superoxide anion. The specific inhibitor of superoxide, SOD, reduced the bactericidal activity of PC. This indicates the involvement of this mechanism in the SGE-mediated inhibitory effect on the killing of B. afzelii spirochetes. The suppressive effect of I. dammini saliva on the production of superoxide by rat neutrophils stimulated with zymosan was described by Ribeiro et al. (17). While the SGE-mediated inhibition of iNOS can be connected with the anti-inflammatory action of some Th2 cytokines (interleukin-4, interleukin-10, and transforming growth factor β), which are up-regulated by tick saliva (3, 9), the inhibitory effect of saliva on the production of superoxide is so fast that only a direct effect on phagocytic cells can come into consideration.
The events immediately following the inoculation of pathogens by ticks are poorly understood but are critical for further progress of the infection process. Phagocytic cells play an important role in elimination of B. burgdorferi spirochetes (4). However, they can also serve as shuttles carrying bacteria from the tick feeding site to the draining lymph nodes. In spite of the majority of phagocytosed spirochetes being degraded, occasionally some of them persist in phagocytic cells and can be recultured (13). Tick saliva can increase the number of surviving bacteria by the inhibition of phagocyte killing mechanisms. The inhibitory effect of tick saliva on macrophage microbicidal activity has been demonstrated using murine macrophages infected with Trypanosoma cruzi (2).
The inhibitory effect of I. ricinus SGE on the spirocheticidal activity of mouse macrophages demonstrated in the present study extends the number of mechanisms which can be suppressed by tick saliva. This suppression facilitates survival or even reproduction of a tick-transmitted pathogen at the tick feeding site and its dissemination into the body.
Acknowledgments
This work was supported by grants 524/99/1334 and 524/99/D035 from the Grant Agency of the Czech Republic and by grant 4700-3 from the Grant Agency of the Ministry of Health of the Czech Republic.
REFERENCES
- 1.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 2.Ferreira B R, Silva J S. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-γ-induced macrophage microbicidal activity. Vet Immunol Immunopathol. 1998;64:279–293. doi: 10.1016/s0165-2427(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira B R, Silva J S. Successive tick infestations selectively promote a T-helper 2 cytokine profile in mice. Immunology. 1999;96:434–439. doi: 10.1046/j.1365-2567.1999.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgilis K, Steere A C, Klempner M S. Infectivity of Borrelia burgdorferi correlates with resistance to elimination by phagocytic cells. J Infect Dis. 1991;163:150–155. doi: 10.1093/infdis/163.1.150. [DOI] [PubMed] [Google Scholar]
- 5.Gern L, Schaible U E, Simon M M. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J Infect Dis. 1993;167:971–975. doi: 10.1093/infdis/167.4.971. [DOI] [PubMed] [Google Scholar]
- 6.Hajnicka V, Kocakova P, Slovak M, Labuda M, Fuchsberger N, Nuttall P A. Inhibition of the antiviral action of interferon by tick salivary gland extract. Parasite Immunol. 2000;22:201–206. doi: 10.1046/j.1365-3024.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones L D, Hodgson E, Nuttall P A. Enhancement of virus transmission by tick salivary glands. J Gen Virol. 1989;70:1895–1898. doi: 10.1099/0022-1317-70-7-1895. [DOI] [PubMed] [Google Scholar]
- 8.Kopecký J, Kuthejlová M. Suppressive effect of Ixodes ricinus salivary gland extract on mechanisms of natural immunity in vitro. Parasite Immunol. 1998;20:169–174. [PubMed] [Google Scholar]
- 9.Kopecký J, Kuthejlová M, Pechová J. Salivary gland extract from Ixodes ricinus ticks inhibits production of interferon-γ by the upregulation of interleukin-10. Parasite Immunol. 1999;21:351–356. doi: 10.1046/j.1365-3024.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- 10.Kubeš M, Fuchsberger N, Labuda M, Žuffová E, Nuttall P A. Salivary gland extracts of partially fed Dermacentor reticulatus ticks decrease natural killer cell activity in vitro. Immunology. 1994;82:113–116. [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Seiler K P, Tai K-F, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modolell M, Schaible U E, Ritting M, Simon M M. Killing of Borrelia burgdorferi by macrophages is dependent on oxygen radicals and nitric oxide and can be enhanced by antibodies to outer surface proteins of the spirochete. Immunol Lett. 1994;40:139–146. doi: 10.1016/0165-2478(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery R R, Nathanson M H, Malawista S E. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. J Immunol. 1993;150:909–915. [PubMed] [Google Scholar]
- 14.Paesen G C, Adams P L, Harlos K, Nuttall P A, Stuart D I. Tick histamine-binding proteins: isolation, cloning and three dimensional structure. Mol Cell. 1999;3:661–671. doi: 10.1016/s1097-2765(00)80359-7. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro J M C. Ixodes dammini: salivary anti-complement activity. Exp Parasitol. 1987;64:347–353. doi: 10.1016/0014-4894(87)90046-4. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro J M C. How ticks make a living. Parasitol Today. 1995;11:91–93. doi: 10.1016/0169-4758(95)80162-6. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro J M C, Weiss J J, Telford S R., III Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp Parasitol. 1990;70:382–388. doi: 10.1016/0014-4894(90)90121-r. [DOI] [PubMed] [Google Scholar]
- 18.Rook G A W, Steele J, Umar S, Dockrell H M. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by γ-interferon. J Immunol Methods. 1985;82:161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- 19.Štěpánová-Tresová G, Kopecký J, Kuthejlová M. Identification of Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii in Ixodes ricinus ticks from Southern Bohemia using monoclonal antibodies. Zentbl Bakteriol. 1999;289:797–806. doi: 10.1016/s0934-8840(00)80005-5. [DOI] [PubMed] [Google Scholar]
- 20.Urioste S, Hall L E, Telford III S R, Titus R. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J Exp Med. 1994;180:1077–1085. doi: 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikel S K, Bergman D. Tick-host immunology: significant advances and challenging opportunities. Parasitol Today. 1997;13:383–389. doi: 10.1016/s0169-4758(97)01126-5. [DOI] [PubMed] [Google Scholar]