Abstract
Cytokine-directed monocyte infiltration is involved in multiple pathological processes. Immuno-isolating matrices that can sequester cell-released chemokines in a microenvironment may prolong the viability and functionality of implanted materials. We describe a self-assembling peptide-based hydrogel that can capture the cytokine CCL2 released in the extracellular space by immune cells and stromal cells. The shear-responsive matrix can absorb and retain this signaling molecule needed for the chemotaxis of the infiltrating monocytes and their differentiation into phagocytic macrophages. Such cytokine-sequestering biomaterials may be useful as adjunctive materials with the delivery of exogenous implants or cell suspensions for tissue regeneration, without the administration of systemic immunosuppressants. Our work highlights the versatility of nanofibrous peptide hydrogels for modulating the biological response in tissue niches.
Introduction
Role of cytokines in the innate immune system
The innate immune system is an evolutionarily ancient defense1 in eukaryotes which is the first responder to injury or pathologic insult. The vascular network supplies phagocytic cells of the innate immune system (such as monocytes) into damaged tissue microenvironments where they may proliferate or differentiate (e.g., into macrophages)2 to respond to pathogens,3 to clear tissue debris,4 and promote angiogenesis.5 However, the innate immune system is less selective than the adaptive immune system, and an overly aggressive inflammatory response by phagocytic cells of the immune system, such as macrophages, can lead to or exacerbate autoimmune diseases such as rheumatoid arthritis6 and atherosclerosis.7
The signaling among the cells of the innate immune system and the other somatic cells is often mediated by cytokines.8 Interactions of various cytokines with their cognate cell-surface receptors provide the basis for design of therapeutic targets for modulation of immune responses. For example, adalimumab, a monoclonal antibody against the cytokine tumor necrosis factor, is used to treat autoimmune conditions as varied as rheumatoid arthritis9,10 and ulcerative colitis.11
CCL2—a multifaceted cytokine
Chemokine C─C motif ligand 2 (CCL2), also known as monocyte chemoattractant protein 1, is a cytokine released mostly by myeloid cells, such as monocytes and dendritic cells, to recruit other myeloid cells and antigen-recognizing T cells.12 During bacterial infection, CCL2 facilitates the release of monocytes from the bone marrow into the blood stream.13 CCL2-mediated chemotaxis of monocytes from the blood stream into damaged tissue sites is generally a normo-physiologic response by the host organism14 and can ultimately improve the viability and functionality of tissue-engineered grafts.15
However, in maladaptive contexts, CCL2 has been linked to failure of transplanted organs16 and inflammatory diseases such as autoimmune encephalomyelitis17 and atherosclerosis.18,19 Certain CCL2 polymorphisms have been shown to correlate with increased risk of myocardial infarction in humans.20 After epileptic seizures, CCL2─CCR2 signaling results in resident microglial activation and infiltration of blood-derived monocytes—leading to neuronal cell death via STAT3 phosphorylation/activation.21 Ethanol-induced microglial activation, facilitated through the CCL2─CCR2 axis, can cause neuroinflammation and neurodegeneration.22 In murine models, the interaction between CCL2 produced by tumor cells and its cognate receptor CCR2 in extravasating monocytes has been implicated in the metastasis of breast cancer23—which explains the previously observed correlation of elevated CCL2 expression with progression of breast carcinoma in humans.24 CCL2 released by Schwann cells can recruit inflammatory cells and can facilitate perineural invasion in solid tumors.25
Disruption of CCL2─CCR2 binding through small peptides
Phage-display based techniques have identified small peptides, based on the sequence and structure of CCR2, that act as antagonists of CCL2.26 Although, minimizing the CCL2─CCR2 interaction seems rational, the host still needs CCL2 in other tissue sites for protection against other injury/pathogenic insult.27,28 Thus, a more conservative localized approach would be a sequestrating scaffold that absorbs CCL2 released in a microenvironment to minimize acute inflammation locally. Lin et al. demonstrated that polyethylene glycol hydrogels linked to affinity antagonist peptides against CCL2 can capture the extracellular cytokine and thus can immuno-isolate the hydrogel against inflammatory monocytes.29 The linker chemistry and spacer geometry have to be optimized for attachment of the affinity peptides to the polymeric hydrogel while retaining its efficacy. The affinity peptides themselves may be prone to rapid diffusion, enzymatic cleavage, and biodegradation in vivo due to their small size. An efficient alternative to using poly-disperse polymeric carriers would be to append an affinity peptide to a similarly short self-assembling peptide that forms a hydrogel/depot that is retained in vivo.
A self-assembling peptide with an anti-CCL2 epitope
We have previously demonstrated that short bioactive peptides, added to a terminus of the self-assembling domain (K─SLSLSLSLSLSL─K) via a glycine linker, can self-assemble into β-sheet based supramolecular nanofibers (see Fig. 1), which can be ionically cross-linked into thixotropic hydrogels at physiological pH and ionic strength in aqueous media. Similar peptide hydrogels have been successfully used for repair and regeneration of soft tissues.30,32-40 The presence of the bioactive domains at the termini facilitates scaffold-based signaling and other bio-modulatory activities, obviating the need for added signaling molecules. In this work, we have attached a CCL2-binding moiety (WKNFQTI) from the second extracellular loop of the murine G-protein coupled receptor CCR229 to a previously described self-assembling domain K─(SL)6─K.31,39,41-43 We design, synthesize and characterize this CCL2-sequestering self-assembling peptide [SLaM: K─(SL)6─K─G─WKNFQTI] that spontaneously forms a hydrogel in aqueous media, yielding an effective biomaterial for localized immuno-isolation, without the addition of polymeric additives. The design of the material may potentially yield a biocompatible and biodegradable matrix that can slow down chemotaxis of monocytes and macrophages.
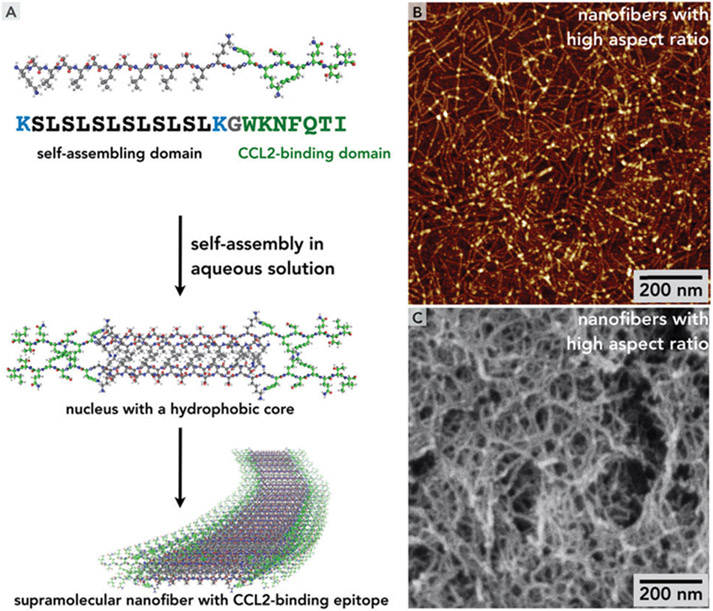
Fig. 1.
(A) Multi-step self-assembly of SLaM peptides lead to formation of a supramolecular nanofiber stabilized by a hydrophobic core formed by leucine side chains and hydrogen bonds along the principal axis, canonical for β-sheets. Similar self-assembly schemes have been used in the literature.30,31 (B) Atomic force microscopy (AFM) on diluted solutions of SLaM (2 mg mL−1) show interconnected nanofibrous structure. (C) Scanning electron micrograph of critical-point-dried and sputter-coated (with gold) SLaM hydrogel (20 mg mL−1) shows the internal fibrous mesh.
Results and discussion
Material properties of the self-assembling peptide hydrogel
The nanofibers are stabilized by the hydrophobic effect derived from packing of non-polar leucine side chains away from the aqueous medium into the core of the nanofiber. The directionality of the high-aspect-ratio nanofibers is governed by a series of canonical β-sheet-associated hydrogen bonds between the amide functionalities on adjacent chains which point to the principal axis of the nanofiber (Fig. 1A). Although the elastic modulus of individual nanofibers can be as high as > 100 MPa, they are relatively soft as the non-covalent interactions stabilizing the nanostructure are reversible.31
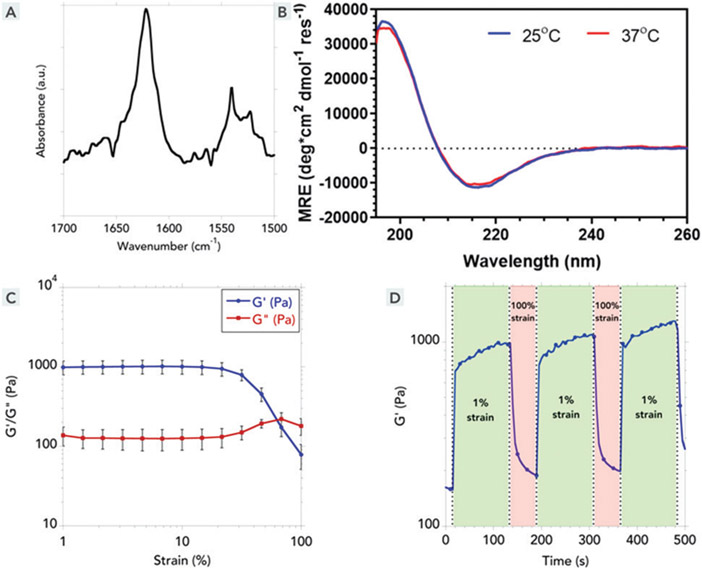
The nanofibers can form higher order entangled mesh-like networks (Fig. 1B and C) when they are ionically cross-linked by multivalent counterions, resulting in a hydrogel with an underlying β-sheet backbone, verified by FTIR and circular dichroism (Fig. 2A and B).31,44 These secondary interactions were stable over a range of temperatures suggesting off-the-shelf stability at 25 °C room temperature conditions and 37 °C in the body (Fig. 2B). These ionic cross-links are relatively weak and the bulk hydrogels generally have storage moduli between 100 and 1000 Pa. In case of SLaM, ionically crosslinked hydrogels had storage moduli around 1000 Pa (Fig. 2C), which decreases with increasing strain amounts. Consequently, at higher strains these intermolecular bonds and cross-links can break resulting in a decreased G′ (Fig. 2C). Upon relief of high shear strain, these bonds reform relatively rapidly (in less than a second) in a repeatable fashion (Fig. 2D). Thus, the supramolecularly assembled bulk-scale network structure in the hydrogel can break into constitutive multimeric blocks under high shear strain, resulting in the liquefaction of the hydrogel. Once the shear strain is lowered, the nanofibers re-self-assemble and the ionic cross-links among the nanofibers are rapidly re-established—which at bulk scale corresponds to the reconstitution of the hydrogel (Fig. 2D), which are cytocompatible with stromal cells such as fibroblasts (Fig. 3A).
Fig. 2.
Biophysical and material properties of the SLaM hydrogel. (A) Fourier transform infrared spectrum (in an attenuated total reflectance set-up) of an air-dried SLaM hydrogel shows typical β-sheet-specific amide-I peak at 1622 cm−1 (B) Circular dichroism spectroscopy, complementarily, shows the secondary structure of the nanofibers in solution. The minima at ~215 nm correspond to a β-sheet secondary structure, which did not change appreciably at two different temperatures (25 °C and 37 °C). (C) SLaM hydrogels can be aspirated with a syringe. To probe its shear-response properties, we used oscillatory rheometry. At low shear strain, G′ > G″ and at high shear strain, G″ > G′. Liquefaction of the hydrogel at high shear strain explains why it can be easily syringe-aspirated. Data shown as average of four runs (error bars correspond to standard deviation). (D) To be used as an injectable hydrogel, it’s equally crucial that the hydrogel liquefied during aspiration and injection regains its storage modulus after the shear strain is removed. We performed multiple strain cycles and observed that each time after strain removal the solution undergoes rapid gelation (shown by the jump in G′ immediately after switch from 100% strain to 1% strain).
Fig. 3.
Cytocompatibility and in vitro efficacy of the peptide hydrogel and solution. (A) SLaM does not exhibit any cytotoxicity towards 3T3 fibroblasts, (B) in a transwell chemotaxis assay, CCL2 solution was used to induce migration of THP-1 monocytes across the transwell. Migration of the monocytes was attenuated in the presence of SLaM hydrogel in the lower chamber in the transwell set-up (see the Experimental section for details). (C) Through ELISA, the ability of SLaM hydrogel to sequester CCL2 in solution was measured. CCL2 has a high affinity for SLaM hydrogels, depicted in a cartoon in (D) (pdb code 1DOK was used for the model of CCL2). For all experiments, n = 4; different Greek letters indicate statistically significant difference, p < 0.05.
Attenuation of CCL2-mediated monocyte chemotaxis by SLaM hydrogel
CCL2 dose-dependently induced transwell migration of THP-1 monocytes (Fig. 3B). In presence of SLaM hydrogel in contact with CCL2 solution, the monocyte migration is reduced (100 ng mL−1 CCL2 in presence of SLaM hydrogel is as effective as 10 ng mL−1 CCL2 solution without the peptide hydrogel) (Fig. 3B). See the methods section for further experimental details.
Significance of blocking CCL2─CCR2 signaling
In blood, CCR2+ (classical) monocytes differ from CCR− (patrolling) monocytes in their migratory potential and inflammatory activities.45 For example, CCR2+ monocytes can enter atherosclerotic plaques and contribute to the plaque progression,46 where they can differentiate into macrophages and proliferate.47 Thus if we can block CCL2─CCR2 binding in a local immune microenvironment (such as in a atherosclerotic plaque), we may be able to slow down the autoimmune pathologic cascade.
CCL2 sequestration by SLaM hydrogels
We measured the binding of SLaM with CCL2 via ELISA. After incubation of CCL2 solution in contact with SLaM hydrogels, majority of the CCL2 molecules in the solution were sequestered in the hydrogel (Fig. 3C and D). These results indicate that the mechanism of binding of CCL2 to SLam is dependent and similar to WKNFQTI binding only, with little dependence on the self-assembling moiety.
Limitations of the study and future directions
To our knowledge, this is the first instance where a CCL2-binding motif is part of constitutive ECM-mimic nanofibers in a peptide hydrogel. We have demonstrated that the scaffold can bind and sequester CCL2 and slow down CCL2-mediated monocyte chemotaxis. Future work aims to demonstrate the therapeutic potential of such self-assembled peptide hydrogels in an choronic inflammatory disease model having established preliminary biocompatibility, Fig. S5 (ESI†).
Experimental
Peptide synthesis and purification
Solid phase peptide synthesis was used to synthesize two peptides: a putative CCL2-binding peptide (WKNFQTI) and a self-assembling peptide containing the epitope (K─(SL)6─K─G─WKNFQTI), referred here as SLaM. Rink amide resin served as the insoluble supporting matrix. At each step piperidine was used to remove Fmoc protecting group of amino acid at N-terminus while neutral Oxyma and di-isopropyl-carbodiimide (DIC) acted as coupling agents. The N-terminus was protected with an acetyl group. After synthesis, protecting groups of side chains were removed from main chain by trifluoroacetic acid followed by an ether wash. The final peptide was purified by dialysis and lyophilization. Purity of the peptides were tested by high performance liquid chromatography (Fig. S1 in the ESI†) and verified by ESI (electrospray ionization) mass spectrometry (Fig. S2, ESI†).
Analysis of the ultrastructure
Scanning Electron Microscopy (SEM) was used to characterize the underlying microstructure of the nanofibrous hydrogel formed by the self-assembly of SLaM. 2% (w/v) hydrogel (i.e., 20 mg mL−1) was fixed with 2% glutaraldehyde and dehydrated using critical point drying (CPD) to preserve internal structure. Dehydrated samples were sputter coated with gold-platinum before observation under SEM (JEOL JSM-7900F high-performance FE-SEM machine) (Fig. 1B).
Atomic force microscopy (AFM) provides a method to detect individual nanofibers which show uniform width and consistent height. Sample solution was prepared by diluting 2% hydrogel in deionized water to reach 0.2%. Diluted solution was spin-coated on a flat mica disk and washed with DI water several times. The mica disc was fixed on magnet disc using carbon tape and dried overnight. The prepared sample was scanned in air on AFM (Bruker Dimension ICON AFM machine in ScanAsyst PeakForce mode) (Fig. 1C).
Spectroscopic characterization
Fourier transform infrared spectroscopy was carried out on a dried film of SLaM hydrogel in an attenuated total reflectance (ATR) set-up and characterized via a PerkinElmer Spectrum 100 FTIR Spectrometer (scan range: 1500–1700 cm−1) (Fig. 2A).
Circular dichroism (CD) spectroscopy is a useful way to determine the secondary structure of peptides in solution. The spectrum of 0.02% solution of SLaM was collected from 190 nm to 260 nm in a Jasco J810 CD Spectropolarimeter and molar residual ellipticity was calculated from the observed ellipticity to account for peptide length and concentration (Fig. 2B, see Fig. S3, ESI† for the CD spectrum of the mimic alone).
Material properties
Oscillatory rheometry was used to evaluate thixotropic properties of hydrogel using strain sweep and shear recovery tests. 40 μL of previously prepared 2 wt% hydrogel was loaded onto the bottom plate. 4 mm geometry was used and gap length was set to 250 μm. The hydrogel was pre-strained for 5 minutes at a 1% strain at 1 rad s−1 followed by strain weep (0.1% to 100% strain at 1 Hz) for 5 minutes (four runs, Fig. 2C). For shear recovery test, the geometry oscillated between 1% and 100% shear strain (Fig. 2D).
Cytocompatibility
General cytocompatibility of SLaM was evaluated with using 3T3 fibroblasts. The cells were seeded at a density of 10 000 cells per well in a 96 well plate and cultured in DMEM (with 10% fetal bovine serum, 1% Pen-Strep) for 24 hours. Conditioned serum free media with various concentrations of the hydrogel (0.2, 0.02 and 0.002% of SLaM solution) were introduced after aspirating the complete media for 8 hours. Cells cultured in complete 3T3 fibroblast media were used as controls. Cell Counting Kit 8 (CCK8) was used to evaluate cell viability (absorbance at 450 nm and reference 650 nm in a plate reader) (Fig. 3A).
Chemotaxis of monocytes
THP-1 cells (a human leukemia monocytic cell line) were used to study cell migration in the presence of different concentrations of CCL2 with and without SLaM hydrogel (2%). 200 μL of the hydrogel was coated on ten 24 well plates and incubated at 37 °C for 30 min for hydrogel stabilization. 400 μL of condition media with 100, 10, and 1 ng mL−1 of CCL2 were added into separate wells. In the positive control wells, 200 μL of 298 mM sucrose solution was added to replace peptide hydrogel and 400 μL of 10 ng mL−1 of CCL2 conditioned media was added. THP-1 cells at a density of 200 000 cells per well were seeded in ThinCerts 8 μm inserts and the inserts were hung on the each well (at transwell set-up). The plate was incubated at 37 °C for 16 hours. After 16 hours, 200 μL of the sample from basolateral chamber was aliquoted and 20 μL of CCK8 was added to assay cell migration. The sample was incubated for an hour at 37 °C before absorbance at 450 nm was measured (reference 650 nm) (Fig. 3B).
CCL2 sequestration
50 μL of 2% hydrogel was coated on 96 well plate and 150 μL of various concentration of CCL2 diluted in HBSS (10, 5, 1, 0.5, and 0.1 ng mL−1) was added each well (n = 3). Samples were incubated at 37 °C. At 1, 4, 8, and 24 hour timepoint, 10 μL of sample was aliquoted from each well and kept in −80 °C up to the time of ELISA. Human MCP-1/CCL2 ELISA Kit, (catalog no. RAB0054-1KT, Sigma-Aldrich) was used and manufacturer’s protocol was followed (Fig. 3C and Fig. S4, ESI†).
Biocompatibility (subcutaneous implantation)
In vivo physiological biocompatibility responses toward SLaM were studied on female Wistar rats (200–225 g). Implantation was performed on dorsal subcutaneous regions. Each rat received a total of four implants (n = 4). 200 μL of 2% hydrogel was injected and explanted at 7 and 14 days after implantation for histological staining and imaging (Masson’s trichrome and H&E) (Fig. S5, ESI†).
Conclusions
Here we show that a self-assembled peptide hydrogel can sequester the monocyte-chemoattracting cytokine CCL2 and can be formulated as a shear-responsive hydrogel. Such injectable biomaterials may be useful for local immunomodulation in tissue microenvironments.
Supplementary Material
Acknowledgements
We would like to thank Candice Casillas and Dr William (Stevens Institute of Technology) for characterization of the secondary structure of the peptide by CD spectroscopy.
Funding sources
This work was supported by NIH R15 EY029504, the NJIT Undergraduate Research and Innovation (URI) Program, and NJIT Startup funds (to V. A. K.). B. S. and V. A. K. have equity interests in start-up companies attempting to translate peptides from this technological platform.
Abbreviations
- SEM
Scanning electron microscopy
- AFM
Atomic force microscopy
- HPLC
High-performance liquid chromatography
- ESI
Electrospray ionization
- TNF
Tumor necrosis factor
- CCL2
Chemokine C─C motif ligand 2
- ELISA
Enzyme-linked immunosorbent assay
Footnotes
Conflicts of interest
B. S. and V. A. K. have equity interests in start-up companies attempting to translate peptides from this technological platform.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tb02250c
References
- 1.Buchmann K, Front. Immunol, 2014, 5, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kratofil RM, Kubes P and Deniset JF, Arterioscler., Thromb., Vasc. Biol, 2017, 37, 35–42. [DOI] [PubMed] [Google Scholar]
- 3.Serbina NV, Jia T, Hohl TM and Pamer EG, Annu. Rev. Immunol, 2008, 26, 421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn TA and Vannella KM, Immunity, 2016, 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton HJ, Armaiz-Pena GN, Gonzalez-Villasana V, Lopez-Berestein G, Bar-Eli M and Sood AK, Cancer Res., 2014, 74, 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y and Pope RM, Curr. Pharm. Des, 2005, 11, 569–580. [DOI] [PubMed] [Google Scholar]
- 7.Jaipersad AS, Lip GY, Silverman S and Shantsila E, J. Am. Coll. Cardiol, 2014, 63, 1–11. [DOI] [PubMed] [Google Scholar]
- 8.Borish LC and Steinke JW, J. Allergy Clin. Immunol, 2003, 111, S460–S475. [DOI] [PubMed] [Google Scholar]
- 9.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, Sharp J, Perez JL and Spencer-Green GT, Arthritis Rheum., 2006, 54, 26–37. [DOI] [PubMed] [Google Scholar]
- 10.Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, Nemcova D, Mouy R, Sandborg C, Bohnsack J, Elewaut D, Foeldvari I, Gerloni V, Rovensky J, Minden K, Vehe RK, Weiner LW, Horneff G, Huppertz HI, Olson NY, Medich JR, Carcereri-De-Prati R, McIlraith MJ, Giannini DH, Martini A and Giannini E, Pediatric Rheumatology Collaborative Study and O. Pediatric Rheumatology International Trials, N. Engl. J. Med, 2008, 359, 810–820. [DOI] [PubMed] [Google Scholar]
- 11.Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B, Kampman W, Lazar A and Thakkar R, Gut, 2011, 60, 780–787. [DOI] [PubMed] [Google Scholar]
- 12.Deshmane SL, Kremlev S, Amini S and Sawaya BE, J. Interferon Cytokine Res, 2009, 29, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serbina NV and Pamer EG, Nat. Immunol, 2006, 7, 311–317. [DOI] [PubMed] [Google Scholar]
- 14.Shi C and Pamer EG, Nat. Rev. Immunol, 2011, 11, 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS and Breuer CK, Proc. Natl. Acad. Sci. U. S. A, 2010, 107, 4669–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogliari AC, Caldara R, Socci C, Sordi V, Cagni N, Moretti MP, Dell’acqua A, Mercalli A, Scavini M, Secchi A, Bonifacio E, Bosi E and Piemonti L, Am. J. Transplant, 2008, 8, 1303–1311. [DOI] [PubMed] [Google Scholar]
- 17.Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J and Prinz M, Brain, 2009, 132, 2487–2500. [DOI] [PubMed] [Google Scholar]
- 18.Boring L, Gosling J, Cleary M and Charo IF, Nature, 1998, 394, 894–897. [DOI] [PubMed] [Google Scholar]
- 19.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A and Mallat Z, Circulation, 2008, 117, 1649–1657. [DOI] [PubMed] [Google Scholar]
- 20.McDermott DH, Yang Q, Kathiresan S, Cupples LA, Massaro JM, Keaney JF Jr., Larson MG, Vasan RS, Hirschhorn JN, O’Donnell CJ, Murphy PM and Benjamin EJ, Circulation, 2005, 112, 1113–1120. [DOI] [PubMed] [Google Scholar]
- 21.Tian DS, Peng J, Murugan M, Feng LJ, Liu JL, Eyo UB, Zhou LJ, Mogilevsky R, Wang W and Wu LJ, J. Neurosci, 2017, 37, 7878–7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, Wang H, Xu M, Frank JA and Luo J, J. Neuroinflammation, 2018, 15, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA and Pollard JW, Nature, 2011, 475, 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K and Toi M, Cancer, 2001, 92, 1085–1091. [DOI] [PubMed] [Google Scholar]
- 25.Bakst RL, Xiong H, Chen CH, Deborde S, Lyubchik A, Zhou Y, He S, McNamara W, Lee SY, Olson OC, Leiner IM, Marcadis AR, Keith JW, Al-Ahmadie HA, Katabi N, Gil Z, Vakiani E, Joyce JA, Pamer E and Wong RJ, Cancer Res., 2017, 77, 6400–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MY, Byeon CW, Hong KH, Han KH and Jeong S, FEBS Lett., 2005, 579, 1597–1601. [DOI] [PubMed] [Google Scholar]
- 27.Depaolo RW, Lathan R, Rollins BJ and Karpus WJ, Infect. Immun, 2005, 73, 6514–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winter C, Taut K, Srivastava M, Langer F, Mack M, Briles DE, Paton JC, Maus R, Welte T, Gunn MD and Maus UA, J. Immunol, 2007, 178, 5828–5838. [DOI] [PubMed] [Google Scholar]
- 29.Lin CC, Boyer PD, Aimetti AA and Anseth KS, J. Controlled Release, 2010, 142, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar VA, Taylor NL, Shi S, Wang BK, Jalan AA, Kang MK, Wickremasinghe NC and Hartgerink JD, ACS Nano, 2015, 9, 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar B, Siddiqui Z, Nguyen PK, Dube N, Fu W, Park S, Jaisinghani S, Paul R, Kozuch SD, Deng D, Iglesias-Montoro P, Li M, Sabatino D, Perlin DS, Zhang W, Mondal J and Kumar VA, ACS Biomater. Sci. Eng, 2019, 5, 4657–4670. [DOI] [PubMed] [Google Scholar]
- 32.Hartgerink JD, Beniash E and Stupp SI, Science, 2001, 294, 1684–1688. [DOI] [PubMed] [Google Scholar]
- 33.Shah RN, Shah NA, Del Rosario Lim MM, Hsieh C, Nuber G and Stupp SI, Proc. Natl. Acad. Sci. U. S. A, 2010, 107, 3293–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webber MJ, Tongers J, Newcomb CJ, Marquardt KT, Bauersachs J, Losordo DW and Stupp SI, Proc. Natl. Acad. Sci. U. S. A, 2011, 108, 13438–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berns EJ, Sur S, Pan L, Goldberger JE, Suresh S, Zhang S, Kessler JA and Stupp SI, Biomaterials, 2014, 35, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar VA, Liu Q, Wickremasinghe NC, Shi S, Cornwright TT, Deng Y, Azares A, Moore AN, Acevedo-Jake AM, Agudo NR, Pan S, Woodside DG, Vanderslice P, Willerson JT, Dixon RA and Hartgerink JD, Biomaterials, 2016, 98, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendricks MP, Sato K, Palmer LC and Stupp SI, Acc. Chem. Res, 2017, 50, 2440–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore AN and Hartgerink JD, Acc. Chem. Res, 2017, 50, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen PK, Gao W, Patel SD, Siddiqui Z, Weiner S, Shimizu E, Sarkar B and Kumar VA, ACS Omega, 2018, 3, 5980–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkar B, Nguyen PK, Gao W, Dondapati A, Siddiqui Z and Kumar VA, Biomacromolecules, 2018, 19, 3597–3611. [DOI] [PubMed] [Google Scholar]
- 41.Aulisa L, Dong H and Hartgerink JD, Biomacromolecules, 2009, 10, 2694–2698. [DOI] [PubMed] [Google Scholar]
- 42.Moore AN, Lopez Silva TL, Carrejo NC, Origel Marmolejo CA, Li IC and Hartgerink JD, Biomaterials, 2018, 161, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen PK, Sarkar B, Siddiqui Z, McGowan M, Iglesias-Montoro P, Rachapudi S, Kim S, Gao W, Lee E and Kumar VA, ACS Appl. Bio Mater, 2018, 1, 865–870. [DOI] [PubMed] [Google Scholar]
- 44.Dong H, Paramonov SE, Aulisa L, Bakota EL and Hartgerink JD, J. Am. Chem. Soc, 2007, 129, 12468–12472. [DOI] [PubMed] [Google Scholar]
- 45.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M and Ley K, Science, 2010, 327, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ and Randolph GJ, J. Clin. Invest, 2007, 117, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R and Swirski FK, Nat. Med, 2013, 19, 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.