Abstract
Eimeria magna is a common pathogen in rabbits, which results in lethargy, weight loss, diarrhea, and even death in severe cases after infection. The current method for preventing rabbit coccidiosis is to add anticoccidial drugs to the diet. However, there are many concerns about drug resistance and drug residues. In our study, the rEmMIC2 and rEmMIC3 proteins were cloned and expressed to evaluate potential as recombinant subunit vaccine candidate antigens. The protective effects of rEmMIC2 and rEmMIC3 were evaluated by the relative weight gain ratio, oocyst decrease rate, anticoccidial index, feed conversion ratio, pathological alterations, clinical symptoms, specific IgG antibody, and cytokine levels in rabbits. The molecular weights of rEmMIC2 and rEmMIC3 were 18.69 kDa and 17.47 kDa, respectively. After the coccidia challenge, the control groups showed anorexia and soft poop, whereas the experimental group showed few anorexia symptoms. Significantly different from the control group, the relative weight gain ratios of the immunized rEmMIC2 and rEmMIC3 groups were 78.37% and 75.29%, respectively, and the oocyst reduction was 77.95% and 76.09%, respectively, and the anticoccidial index was 171.12 and 169.29, respectively. IgG antibody, IFN-γ, IL-4, IL-10, and IL-17 levels were significantly increased in the experimental group. The results showed that rEmMIC2 and rEmMIC3 have potential as vaccine candidate antigens.
Keywords: Rabbit, Eimeria magna, rEmMIC2, rEmMIC3, Candidate antigens
Introduction
Rabbit coccidiosis is caused by Eimeria spp. (Li et al. 2020; Tao et al. 2017; Xie et al. 2021). It is widely distributed and infects rabbits of various breeds and ages. At 30 to 90 days, the mortality rate of infected rabbits is as high as 60% (Pilarczyk et al. 2020; Jing et al. 2012; Mäkitaipale et al. 2017; Paul and Friend 2019). Eimeria magna is a mildly pathogenic common rabbit coccidia, which results in anorexia, weight loss, diarrhea, poor feed conversion, and even death in severe cases (Pakandl 2009). Now, the method for preventing rabbit coccidiosis is to add anticoccidial drugs to the diet, but long-term drug prophylaxis leads to drug resistance and drug residue problems (Cai et al. 2015; Bachene et al. 2018). Therefore, the application of vaccine in rabbit coccidiosis may be the best replacement strategy. Currently, there is no commercial vaccine for rabbit coccidia, and vaccine development has focused on precocious line vaccines (Fang et al. 2019; Akpo et al. 2012; Li et al. 2020; Cui et al. 2018). However, precocious line vaccines are costly to produce and carry a high risk of reversal (Venkatas and Adeleke 2019), requiring the development of new, low-cost, safe vaccines (Blake et al. 2017).
The schizont stage of coccidia seriously threatens the health of the host; it destroys the intestinal epithelium continuously (Pakandl 2009; Ryley and Robinson 1976). Microneme proteins play essential roles in the invasion of coccidia into the host. They are secreted by microneme organelles that promote adhesion between parasites and the surface of host cells and perform the dynamics required for host cell invasion (Carruthers and Tomley 2008). Microneme proteins were found to effectively protect hosts from parasites in Toxoplasma gondii and chicken coccidia (Lee et al. 2018; Sathish et al. 2011; Zhao et al. 2021a, b); however, no studies have been reported on rabbit coccidia.
Till now, only two recombinant subunit vaccines against E. magna have been studied (Pu et al. 2022). In our study, we cloned and expressed E. magna microneme protein 2 (EmMIC2) and microneme protein 3 (EmMIC3) from the transcriptome data. The potential of rEmMIC2 and rEmMIC3 as vaccine candidate antigens was evaluated in rabbits by pathological alterations, clinical symptoms, relative weight gain ratio, oocyst decrease rate, feed conversion ratio, anticoccidial index, and specific IgG antibody and cytokine levels in serum.
Materials and methods
Animals and parasites
Xianyong Liu from China Agricultural University provided the Beijing strain of E. magna. They were preserved in heirloom culture at our laboratory. Sporulated oocysts of E. magna were stored in a 2.5% potassium dichromate solution at 4 °C and passed through rabbits before the coccidia challenge.
Forty-eight coccidia-free New Zealand White rabbits were born and bred in this laboratory. Rabbits were weaned after 18 days and then fed infant formula milk powder and high-temperature formula pellets (made in our laboratory), during which time drinking water was boiled and fed (Shi et al. 2016; Wei et al. 2020). Existing feeds were replaced with anticoccidial drug-free feed 5 days before the coccidia challenge.
Serum
Negative sera were isolated from rabbits free of coccidia infection. Positive sera were isolated from rabbits infected with E. magna (1 × 105 oocysts per rabbit) for 10 days. Blood was collected, and serum was isolated every 7 days from the start of immunization until sacrifice. All serum samples were stored at − 20 °C in the laboratory.
Bioinformatic analysis
BLASTP (Zhang et al. 2020a) was used to study sequence similarity. IEDB (González-Díaz et al. 2014) was used to predict B-cell antigenic epitopes and conserved domains (Marchler-Bauer et al. 2012) to predict conserved structural domains. Secondary structure prediction and amino acid sequence alignment were performed using SnapGene 4.3.6.
Cloning of EmMIC2 and EmMIC2 genes
Based on the transcriptome data of E. magna, specific primers for EmMIC2 (GenBank: OM891512) and EmMIC3 (GenBank: OM891513) genes were designed and synthesized (EmMIC2 forward primer, 5′-CGGAATTCATGAACTGCGGACGACT-3′; reverse primer, 5′ CCAAGCTTTTACTTGGGCAGCCAA 3′; EmMIC3 forward primer, 5′ CGGGATCC-ATGATTCCCTTGAAGCGA 3′; reverse primer, 5′ CCAAGCTT-TCATACCGAGGAAGAAGGTT 3′). E. magna total RNAs were extracted and reverse transcribed into cDNA, which was stored at − 80 °C for later use. The EmMIC2 and EmMIC3 genes were ligated into the pMD-19 T (TaKaRa, Dalian, China) cloning vector for sequencing (Sangon Biotech, Shanghai, China).
Expression and purification of EmMIC2 and EmMIC3 genes
Fragments of EmMIC2 and EmMIC3 were cloned into the pET-32a ( +) vector. After correct sequencing, the recombinant plasmid was extracted and transferred into E. coli BL21 (DE3), and recombinant protein expression was induced (1 mM IPTG). The target proteins were purified using His-Trap HP columns (Cytiva, USA).
Western blotting
Recombinant proteins were transferred to nitrocellulose membranes (NC membranes, Biosharp, Hefei, China) after electrophoresis (SDS-PAGE). After incubation with 5% non-fat dried skim milk for 2 h, rabbit serum (positive and negative serum diluted 1:100 with 0.01 M PBS) was used as the primary antibody, followed by an HRP-labeled goat anti-rabbit IgG antibody. Finally, the bound antibodies were detected according to the BeyoECL Moon kit.
Immunization and challenge infection
A total of 48 healthy 35-day-old coccidia-free New Zealand rabbits (861.11 ± 96.45 g) were randomly grouped (8 rabbits per group), including the experimental group, vector protein (pET-32a) control group (VP), saponin adjuvant group (SA), challenged control (CC), and unchallenged control groups (UC) (Table 1). The SA group was immunized with 1 mg of saponin. The experimental and VP groups were immunized with 1 mg of saponins and immunized with 100 μg of rEmMIC2, rEmMIC3, and vector protein, respectively. The UC and CC groups were injected with 1 mL of PBS. Fourteen days after the second injection, rabbits in each group were orally attacked with 1 × 105 sporulated oocysts of E. magna, except for the UC group. All rabbits were sacrificed 14 days after the challenge to evaluate the effect of immunization (Fig. 1).
Table 1.
Immunization procedure
| Groups | Immunization dosages | Challenge (63 days of age) |
|---|---|---|
| Unchallenged control | 1 ml PBS | / |
| Challenged control | 1 ml PBS | 1 × 105 E. magna |
| Adjuvant control | 1 mg saponin | 1 × 105 E. magna |
| Vector protein | 100 μg pET-32a( +) + 1 mg saponin | 1 × 105 E. magna |
| Recombinant EmMIC2 | 100 μg rEmMIC2 + 1 mg saponin | 1 × 105 E. magna |
| Recombinant EmMIC3 | 100 μg rEmMIC3 + 1 mg saponin | 1 × 105 E. magna |
Immunization was administered subcutaneously in the neck, with a total volume of 1 mL
Fig. 1.
Immunization, coccidia challenge, and sampling. The yellow triangle indicates the age at which the weighing and blood collection were performed. The red asterisk indicates the age at which the rabbit was immunized with protein. The green pentagon indicates that the rabbit was infected with E. magna at that age. Blue arrows indicate all rabbits were sacrificed
Evaluation of protection effects
The clinical symptoms of each group were observed daily after the challenge. The protective effect was assessed based on clinical symptoms, pathological alterations, the relative weight gain ratio (%), the oocyst decrease rate (%), the anticoccidial index (ACI), and the feed conversion ratio (%) (McManus et al. 1968; Johnson et al. 1970). Calculated as follows: (1) the relative weight gain ratio = (average weight gain in the experimental group/average weight gain in the unchallenged control group) × 100%. (2) All groups of stools were collected in the rectum, and then the number of oocysts in the feces was counted after the challenge infection using a McMaster egg-counting chamber. Oocyst decrease rate = (challenge control OPG—experimental group OPG)/(challenge control OPG) × 100%. (3) Anticoccidial index = (relative weight gain ratio + survival rate)—(lesion value + oocyst value). (4) The feed conversion ratio = the total feed consumption after the challenge/the total weight gain after the challenge.
Detection of specific antibody IgG, and cytokines levels
Serum was collected once a week from the first immunization. All sera were tested for specific IgG antibody levels using indirect ELISA (Shen et al. 2020). After the second immunization, the serum was used to determine cytokine levels (IL-4, IL-10, IL-17, IFN-γ, and TGF-β1) (Elabscience, Wuhan, China).
Data analysis
All data were expressed as mean ± standard deviation, and the method of comparing differences between multiple groups was one-way analysis of variance (one-way ANOVA). Statistical analyses were performed using IBM SPSS Statistics version 20.0. GraphPad Prism version 8.0.2 was used for graphing.
Results
EmMIC2 and EmMIC3 were cloned and analyzed by sequencing
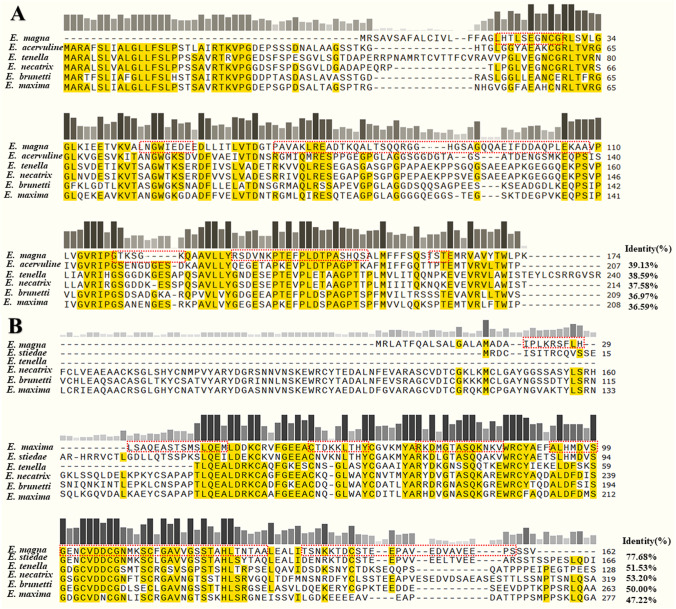
The EmMIC2 gene open reading frame (ORF) contains a total of 453 nucleotides; 151 amino acids were encoded, with a molecular weight of approximately 18.69 kDa; B-cell antigenic epitope prediction showed that antigenic epitopes occur at amino acids 19–28, 46–53, 66–108, 118–123, 131–150, and 160–162 (Fig. 2A). The amino acid sequence of the EmMIC2 gene was compared with the amino acid sequences of Eimeria acervuline (Gene Bank: XP_013253324), Eimeria tenella (Gene Bank: AAD05566), Eimeria necatrix (Gene Bank: XP_013440003), Eimeria brunetti (Gene Bank: BAM16293), and Eimeria maxima (Gene Bank: CBX60033) in multiple sequences (Fig. 2A). The EmMIC2 gene was found to have low identities (36.59–39.13%) with all five species of the Eimeria genus, and the highest identity with E. maxima was only 39.13%. The EmMIC3 gene ORF contained 432 nucleotides and 144 amino acids, with a molecular weight of approximately 17.47 kDa. B-cell antigenic epitope prediction showed that antigenic epitopes occur at amino acids 21–44, 57–65, 73–8, 93–130, 136–159 (Fig. 2B). The conserved domains predicted that positions 45–129 were microneme adhesive repeat (MAR) domains. The amino acid sequences of the EmMIC3 gene were compared with the amino acid sequences of Eimeria stiedae (Gene Bank: QIS60154), E. tenella (Gene Bank: APF30041), E. acervulina (Gene Bank: XP_013253056), E. brunetti (Gene Bank: CDJ47617), and E. maxima (Gene Bank: XP_013337599) (Fig. 2B). The EmMIC3 gene was found to have low identities (47.22–77.68%) with all five species of the genus Eimeria, and the highest identity with E. stiedae was 77.68%.
Fig. 2.
Comparison of amino acid sequences of EmMICs. A Amino acid sequence alignment of EmMIC2 with E. acervulina, E. maxima, E. bruneti, and E. tenella. B Amino acid sequence alignment of EmMIC3 with E. stiedae, E. tenella, E. acervulina, E. maxima, and E. bruneti. Aligned used Clustal Omega. The yellow background indicates amino acids that are identical to EmMICs, and the higher the bar, the darker the color indicates higher identity. The red dashed box shows the predicted B-cell antigenic epitopes
Expression and purification of recombinant proteins
After the constructed pET32a ( +)-EmMIC2 and pET32a ( +)-EmMIC3 recombinant plasmids were transferred into BL21 (DE3) cells, IPTG was used to successfully express the recombinant proteins at a concentration of 1 mmol/L for 8 h at 37 °C. Two protein bands were detected on SDS-PAGE gels with a molecular weight of approximately 35 kDa (including the vector protein). Single bands for rEmMIC2 and rEmMIC3 were obtained after purification and enrichment.
West blotting analyses
Western blotting was performed to identify rEmMIC2 and rEmMIC3 using an anti-E. magna rabbit serum as the primary antibody. Two specific rEmMIC2 and rEmMIC3 bands were identified in the anti-E. magna rabbit serum (Fig. 3, lane 1, and lane 3). No specific bands were detected in the negative sera (Fig. 3, lane 2, and lane 4).
Fig. 3.

Western blot analyses of rEmMIC2 and rEmMIC3. Lane 1: rEmMIC2 was probed with a positive serum sample against E. magna. Lane 3: rEmMIC3 was probed with a positive serum sample against E. magna. Lane 2: rEmMIC2 was probed with negative serum. Lane 4: rEmMIC3 was probed with negative serum
Protective effects of rEmMIC2 and rEmMIC3 against challenge infection with E. magna
No significant adverse effects were observed in each group after immunization. After the coccidia challenge, the CC, SA, and VP groups showed symptoms of depression, soft stools, and diarrhea. The immunized rEmMIC2 and immunized rEmMIC3 groups occasionally exhibited anorexia but no signs of soft bowel movement. Necropsy of the jejunum and ileum showed obvious bleeding spots in the CC, SA, and VP groups, whereas no obvious lesions were found in the immunized rEmMIC2 and rEmMIC3 groups (Fig. 4).
Fig. 4.
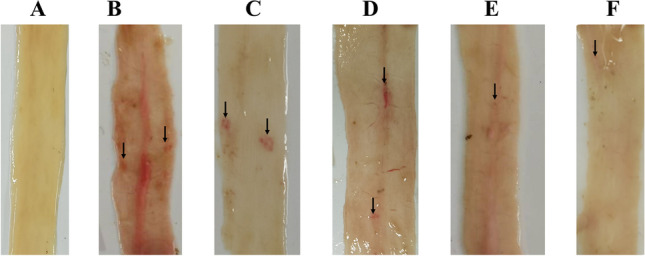
Necropsy. A UC, B CC, C VP, D SA, E rEmMIC2, and F rEmMIC3
Average weight gain was observed in the CC group (204.37 g ± 48.44 g), the immunized rEmMIC2 group (330.62±83.51 g), and the immunized rEmMIC3 group (317.75±99.13 g) (Table 2, P < 0.05). The relative weight gain ratios in the CC, SA, and VP groups were 40.59%, 48%, and 47.11%, respectively. The relative weight gain ratios were 78.37% and 75.29% in the immunized rEmMIC2 and rEmMIC3 groups, respectively, compared to that in the CC group (Table 2, P < 0.05). The feed conversion ratio was 3.31:1 in the UC group, 6.85:1 in the challenged group, and 4.23:1 and 4.40:1 in the immunized rEmMIC2 and rEmMIC3 groups, respectively. The immunized rEmMIC2 and rEmMIC3 groups were significantly lower than the CC group, with an oocyst decrease rate of 77.95% and 76.09%, respectively. In addition, ACI showed that the recombinant proteins had protective effects (Table 2).
Table 2.
Protective efficacy of recombinant protein against challenge infection with E. magna
| Groups | Average weight gain before challenge/g | Average weight gain after challenge/g | Relative weight gain ratio (%) | Oocyst output (× 104) | Oocyst reduction ratio (%) | Mean lesion scores | ACI | Feed conversion ratio |
|---|---|---|---|---|---|---|---|---|
| Unchallenged control | 631.25 ± 51.46a | 421.88 ± 38.26a | NA | NA | NA | 0 | 200 | 3.31:1 |
| Challenged control | 612.50 ± 94.11a | 204.37 ± 48.44b | 40.59 | 2.81 ± 1.19a | NA | 1.25 | 88.09 | 6.85:1 |
| Adjuvant control | 647.50 ± 123.4a | 202.50 ± 73.96b | 48.00 | 2.84 ± 0.83a | − 1.17 | 1.37 | 94.25 | 6.91:1 |
| Vector protein | 606.87 ± 94.33a | 198.75 ± 79.40b | 47.11 | 2.78 ± 0.81a | 1.06 | 1.12 | 95.86 | 7.04:1 |
| rEmMIC2 | 646.25±84.92a | 330.62±83.51c | 78.37 | 0.62 ± 0.52b | 77.95 | 0.62 | 171.12 | 4.23:1 |
| rEmMIC3 | 603.75±92.34a | 317.50±99.13c | 75.29 | 0.67 ± 0.60b | 76.09 | 0.5 | 169.29 | 4.40:1 |
Values represent the mean ± S.D. of 8 rabbits. Statistical significances between the means of different treatment groups were analyzed with SPSS software, and a significant difference was considered at P < 0.05. In the same column, a significant difference (P < 0.05) between groups was shown with different letters, and no significant difference (p > 0.05) between groups was shown with the same letter
Determination of antibody and cytokine levels
As shown in Fig. 5, the CC and SA groups maintained low IgG levels from the first immunization until the end of the trial. IgG levels were higher in the VP group but lower than those in the recombinant protein groups. The immunized rEmMIC2 and rEmMIC3 protein groups showed a rapid increase in IgG levels after the first vaccination and stable levels after the second vaccination.
Fig. 5.
Changes in antibody levels in serum of rEmMIC2 immunized group and the rEmMIC3 immunized group
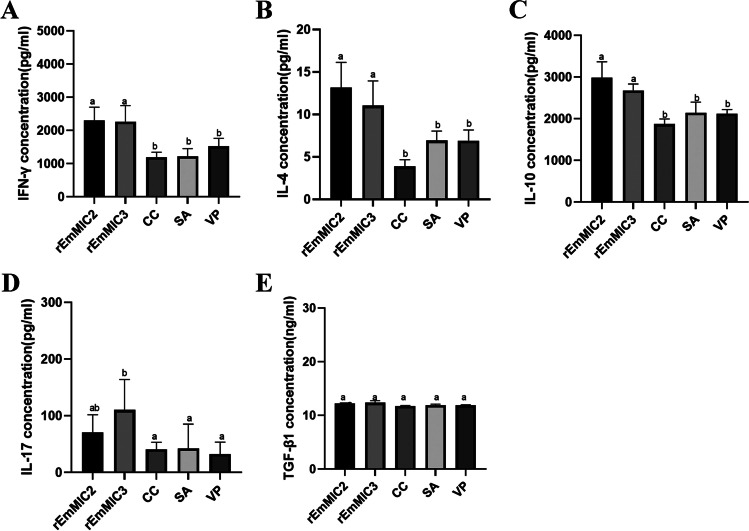
There was no significant difference between the experimental and control groups in TGF-β1 (P > 0.05) (Fig. 6E). IFN-γ, IL-4, IL-10, and IL-17 levels were significantly different from those in the control group (P < 0.05) (Fig. 6A, B, C, D).
Fig. 6.
Cytokines were measured in serum prior to coccidia challenge. A IFN-γ, B IL-4, C IL-10, D IL-17, and E TGF-β1
Discussion
Protozoa are characterized by aggregates of protein filopodia and specific secretory organelles (apical complexes). The microneme is the smallest secretory organelle in the root tip complex and secretes and releases microneme proteins involved in parasite adhesion and host cell invasion (Dowse and Soldati 2004; Carruthers and Tomley 2008). Both MIC2 and MIC3 were first identified in E. tenella. EtMIC2 contains have five TSP-1 structural domains with conserved transmembrane and C-terminal regions (Tomley et al. 1991). EtMIC3 contains have four conserved intramembrane repeat regions and three mutated outer repeat regions (Labbé, 2005). BLASTP analysis showed that EmMIC3 contains have MAR structural domain. This is consistent with the findings of Huang et al. (2018a) for E. acervulina and E. mitis. The MAR is a domain of proteins in MICs, and all MICs with the MAR structural domain are called MAR proteins (MCPs) (Friedrich et al. 2010). MAR structural domains are classified as I and II. The difference between them is that MARI contains an abducted α-helix/loop region, whereas MARII contains a β-finger region. Currently, only type I MAR structural domains are found in Eimeria spp. (Cowper et al. 2012; Marugan-Hernandez et al. 2017). The MAR structural domain can bind to sialic acid to help parasites attach to their host cells (Lai et al. 2011; Blumenschein et al. 2007).
When coccidia invade and migrate, the intestinal structures are destroyed, leading to impaired digestive function and reduced feed conversion (Cornelissen et al. 2009; Belli et al. 2004). In our experiment, the recombinant protein groups showed significant enhance in the relative weight gain and feed conversion ratio compared to the control groups. The results show that rEmMIC2 and rEmMIC3 can effectively reduce E. magna damage to the intestinal structure of rabbits, improve the feed conversion ratio, and reduce the feeding costs of rabbits infected with E. magna. The oocyst output of the recombinant protein groups decreased significantly, suggesting that rEmMIC2 and rEmMIC3 reduce the reproductive capacity of coccidia, thereby reducing their damage to the host intestine. The protective effects of MIC2 on E. brunetti and MIC3 on E. acervulina have been evaluated, and the results showed that the recombinant proteins MIC2 and MIC3 effectively reduced the excretion of coccidia oocysts and increased the weight gain ratio of chickens infected with coccidian (Hoan et al. 2016; Zhang et al. 2016).
Compared with the chicken coccidia vaccine, more research of the rabbit coccidia vaccine has been conducted on precocious line vaccines. In a study by Bachene et al. (2018), the oocyst reduction ratio of the precocious line vaccine was more than 90%. In another study by Fang et al. (2019), the weight gain of the immunized group after the rabbit coccidia challenge was similar to that of the unchallenged group, while the relative weight gain ratio and oocyst reduction in our study were slightly inferior compared to the precocious line vaccines. In the future, multivalent recombinant subunit vaccines (Zhang et al. 2012), live oral vector vaccines (Huang et al. 2020b), and DNA vaccines (Yan et al. 2018) will have potentially improve protective effects.
It has shown that humoral immunity is crucial for combating coccidial infections (Constantinoiu et al. 2008; Yuan et al. 2022; Ding et al. 2004; Zhao et al. 2021a, b). Serum IgG levels in the immunized rEmMIC2 and rEmMIC3 protein groups were significantly higher than those in the control group, confirming that rEmMIC2 and rEmMIC3 proteins induced humoral immunity. IFN-γ is an important cytokine in Th1-type immunity and is thought to play an essential role in coccidial immune response and infection resistance (Chapman 2014). IL-4 and IL-10 are secreted by Th2-type cells and regulate humoral immunity, effectively assisting B-cell activation (Inagaki-Ohara et al. 2006; Rothwell et al. 2004). IL-17 is secreted by Th17-type cells and plays a role in combating coccidial infections, and it could help to enhance the protective effects of vaccines (Xu and Li 2011). It has also been found that IL-17 reduced oocyst excretion and decreased intestinal damage if chicken treated with IL-17 neutralizing antibodies after coccidia infection (Zhang et al. 2013).
In our experiment, the cytokine levels in the experimental group were significantly different compared with the control group, and rEmMIC2 and rEmMIC3 could induce cellular immunity and improve the host against E. magna infection.
Conclusions
Immunization with rEmMIC2 and rEmMIC3 effectively reduced oocyst output, weight loss, and intestinal damage. The results suggest that rEmMIC2 and rEmMIC3 can serve as recombinant subunit vaccine candidates for E. magna.
Acknowledgements
The authors thank Ge Hao, Changming Xiong, and Wei He for their contributions to the sample collection.
Author contribution
HC participated in the design of the study, fed the experimental animals, and performed the experiments, statistical analysis, and manuscript writing. JYP fed the experimental animals and performed the experiments. XJ, RYZ, and BJ contributed to the sample collection. GYY participated in the design of the study. XBG, YX, RH, JX, XRP, YJR, and GYY assisted in the study design. All authors read and approved the final manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2017YFD0501200). The funder had no role in the design of the study, data collection, analysis, interpretation, or writing of the manuscript.
Data availability
The datasets supporting the conclusions of this study are included in this article.
Declarations
Ethics approval and consent to participate
The animal study protocol was reviewed and approved by the Animal Care and Use Committee of Sichuan Agricultural University (SYKY 2019–187). All animal procedures used in this study were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Bethesda, MD, USA) and the recommendations of the ARRIVE guidelines. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
The authors declare that they are aware of the publication of this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Chen and Jiayan Pu contributed equally to this work.
Contributor Information
Hao Chen, Email: chenhao2197@outlook.com.
Jiayan Pu, Email: pujaiyan@aliyun.com.
Jie Xiao, Email: xiaojjinu@163.com.
Xin Bai, Email: baixin1002@hotmail.com.
Ruoyu Zheng, Email: zhengry0831@aliyun.com.
Xiaobin Gu, Email: guxiaobin198225@126.com.
Yue Xie, Email: xyue1985@gmail.com.
Ran He, Email: ranhe1991@hotmail.com.
Jing Xu, Email: xujing90@hotmail.com.
Bo Jing, Email: jingbooo@163.com.
Xuerong Peng, Email: pxuerong@126.com.
Yongjun Ren, Email: renyj17513@126.com.
Guangyou Yang, Email: guangyou1963@126.com.
References
- Akpo Y, Kpodékon MT, Djago Y, Licois D, Youssao IAK. Vaccination of rabbits against coccidiosis using precocious lines of Eimeria magna and Eimeria media in Benin. Vet Parasitol. 2012;184:73–76. doi: 10.1016/j.vetpar.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Bachene MS, Temim S, Ainbaziz H, Bachene A, Suo X. A vaccination trial with a precocious line of Eimeria magna in Algerian local rabbits Oryctolagus cuniculus. Vet Parasitol. 2018;261:73–76. doi: 10.1016/j.vetpar.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Belli SI, Mai K, Skene CD, Gleeson MT, Witcombe DM, Katrib M, Finger A, Wallach MG, Smith NC. Characterisation of the antigenic and immunogenic properties of bacterially expressed, sexual stage antigens of the coccidian parasite, Eimeria maxima. Vaccine. 2004;22:4316–4325. doi: 10.1016/j.vaccine.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Blake DP, Pastor-Fernández I, Nolan MJ, Tomley FM. Recombinant anticoccidial vaccines-a cup half full. Infect Genet Evol. 2017;55:358–365. doi: 10.1016/j.meegid.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Blumenschein TM, Friedrich N, Childs RA, Saouros S, Carpenter EP, Campanero-Rhodes MA, Simpson P, Chai W, Koutroukides T, Blackman MJ, Feizi T. Atomic resolution insight into host cell recognition by Toxoplasma gondii. EMBO J. 2007;26:2808–2820. doi: 10.1038/sj.emboj.7601704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Zhu S, Zhao Q, Han H, Huang B, Dong H, Yu Z, Bao W, Jiang W. Drug resistance of Eimeria species isolated from five chicken farms in Shaoxing City. Acta Agriculturae Zhejiangensis. 2015;27:532–536. [Google Scholar]
- Carruthers VB, Tomley FM. Microneme proteins in apicomplexans. Molecular Mechanisms of Parasite Invasion. New York: Springer; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Constantinoiu CC, Molloy JB, Jorgensen WK, Coleman GT. Antibody response against endogenous stages of an attenuated strain of Eimeria tenella. Vet Parasitol. 2008;154:193–204. doi: 10.1016/j.vetpar.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Cornelissen JBWJ, Swinkels WJC, Boersma WA, Rebel JMJ. Host response to simultaneous infections with Eimeria acervulina, maxima and tenella: a cumulation of single responses. Vet Parasitol. 2009;162:58–66. doi: 10.1016/j.vetpar.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Cowper B, Matthews S, Tomley F. The molecular basis for the distinct host and tissue tropisms of coccidian parasites. Mol Biochem Parasitol. 2012;186:1–10. doi: 10.1016/j.molbiopara.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Cui P, Fang SF, Gu XL, Yu XJ, Liang SZ, Zhang LY, Li HF, Suo X. Selection and phylogenetic analysis of 18S rDNA from precocious line of Eimeria magna. Chin J Veterinary Sci. 2018;38:1327–1331. [Google Scholar]
- Ding X, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP. Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Infect Immun. 2004;72:6939–6944. doi: 10.1128/IAI.72.12.6939-6944.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowse T, Soldati D. Host cell invasion by the apicomplexans: the significance of microneme protein proteolysis. Curr Opin Microbiol. 2004;7:388–396. doi: 10.1016/j.mib.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Fang S, Gu X, El-Ashram S, Li X, Yu X, Guo B, Li H, Liu N, Liu X, Cui P, Suo X. Immune protection provided by a precocious line trivalent vaccine against rabbit Eimeria. Vet Parasitol. 2019;275:108927 . doi: 10.1016/j.vetpar.2019.108927. [DOI] [PubMed] [Google Scholar]
- Friedrich N, Santos JM, Liu Y, Palma AS, Leon E, Saouros S, Kiso M, Blackman MJ, Matthews S, Feizi T, Soldati-Favre D. Members of a novel protein family containing microneme adhesive repeat domains act as sialic acid-binding lectins during host cell invasion by apicomplexan parasites. J Biol Chem. 2010;285:2064–2076. doi: 10.1074/jbc.M109.060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Díaz H, Pérez-Montoto LG, Ubeira FM. Model for vaccine design by prediction of B-epitopes of IEDB given perturbations in peptide sequence, in vivo process, experimental techniques, and source or host organisms. J Immunol Res. 2014;2014:768515 . doi: 10.1155/2014/768515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoan TD, Zhang Z, Huang J, Yan R, Song X, Xu L, Li X. Identification and immunogenicity of microneme protein 2 (EbMIC2) of Eimeria brunetti. Exp Parasitol. 2016;162:7–17. doi: 10.1016/j.exppara.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Huang X, Liu J, Tian D, Li W, Zhou Z, Huang J, Song X, Xu L, Yan R, Li X. The molecular characterization and protective efficacy of microneme 3 of Eimeria mitis in chickens. Vet Parasitol. 2018;258:114–123. doi: 10.1016/j.vetpar.2018.06.020. [DOI] [PubMed] [Google Scholar]
- Huang H, Jiang Y, Zhou F, Shi C, Yang W, Wang J, Kang Y, Cao X, Wang C, Yang G. A potential vaccine candidate towards chicken coccidiosis mediated by recombinant Lactobacillus plantarum with surface displayed EtMIC2 protein. Exp Parasitol. 2020;215:107901 . doi: 10.1016/j.exppara.2020.107901. [DOI] [PubMed] [Google Scholar]
- Inagaki-Ohara K, Dewi FN, Hisaeda H, Smith AL, Jimi F, Miyahira M, Abdel-Aleem ASF, Horii Y, Nawa Y. Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun. 2006;74:5292–5301. doi: 10.1128/IAI.02024-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing F, Yin G, Liu X, Suo X, Qin Y. Large-scale survey of the prevalence of Eimeria infections in domestic rabbits in China. Parasitol Res. 2012;110:1495–1500. doi: 10.1007/s00436-011-2653-4. [DOI] [PubMed] [Google Scholar]
- Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Labbé M, De Venevelles P, Girard-Misguich F, Bourdieu C, Guillaume A, Pery P. Eimeria tenella microneme protein EtMIC3: identification, localisation and role in host cell infection. Mol Biochem Parasitol. 2005;140:43–53. doi: 10.1016/j.molbiopara.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lai L, Bumstead J, Liu Y, Garnett J, Campanero-Rhodes MA, Blake DP, Palma AS, Chai W, Ferguson DJ, Simpson P, Feizi T. The role of sialyl glycan recognition in host tissue tropism of the avian parasite Eimeria tenella. PLoS Pathog. 2011;7:e1002296 . doi: 10.1371/journal.ppat.1002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kang HJ, Lee DH, Kang SM, Quan FS. Virus-like particle vaccines expressing Toxoplasma gondii rhoptry protein 18 and microneme protein 8 provide enhanced protection. Vaccine. 2018;36:5692–5700. doi: 10.1016/j.vaccine.2018.08.016. [DOI] [PubMed] [Google Scholar]
- Li TS, Zou Y, Ma YT, Ma YY, Chen H, Liang XX, Cong W, Sun XL, Zhu XQ. Molecular characterization of Eimeria spp. and Blastocystis in rabbits in Shandong Province. China Parasitol Res. 2020;119:1547–1551. doi: 10.1007/s00436-020-06652-0. [DOI] [PubMed] [Google Scholar]
- Mäkitaipale J, Karvinen I, Virtala AMK, Näreaho A. Prevalence of intestinal parasites and risk factor analysis for Eimeria infections in Finnish pet rabbits. Vet Parasitol: Reg Stud Rep. 2017;9:34–40. doi: 10.1016/j.vprsr.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F. CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res. 2012;41:D348–D352. doi: 10.1093/nar/gks1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugan-Hernandez V, Fiddy R, Nurse-Francis J, Smith O, Pritchard L, Tomley FM. Characterization of novel microneme adhesive repeats (MAR) in Eimeria tenella. Parasites Vectors. 2017;10:1–9. doi: 10.1186/s13071-017-2454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EC, Campbell WC, Cuckler AC. Development of resistance to quinoline coccidiostats under field and laboratory conditions. J Parasitol. 1968;54:1190–1193. doi: 10.2307/3276989. [DOI] [PubMed] [Google Scholar]
- Pakandl M. Coccidia of rabbit: a review. Folia Parasitol. 2013;56:153–166 . doi: 10.14411/fp.2009.019. [DOI] [PubMed] [Google Scholar]
- Paul GC, Friend DG. Clostridial enterotoxemia and coccidiosis in weanling cottontail rabbits (Sylvilagus audubonii, Sylvilagus floridanus, Sylvilagus nuttallii) from Colorado, USA. J Wildl Dis. 2019;55:189–195. doi: 10.7589/2018-02-026. [DOI] [PubMed] [Google Scholar]
- Pilarczyk B, Tomza-Marciniak A, Pilarczyk R, Januś E, Stanek P, Seremak B, Sablik P. The effect of the sex, age, and breed of farmed rabbits and the choice of management system on the extensity and intensity of Eimeria infection. Vet World. 2020;13:1654 . doi: 10.14202/vetworld.2020.1654-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J, Xiao J, Bai X, Chen H, Zheng R, Gu X, Xie Y, He R, Xu J, Jing B, Peng X. Prokaryotic expression of Eimeria magna SAG10 and SAG11 genes and the preliminary evaluation of the effect of the recombinant protein on immune protection in rabbits. Int J Mol Sci. 2022;18:10942. doi: 10.3390/ijms231810942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol. 2004;173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- Ryley JF, Robinson TR. Life cycle studies with Eimeria magna Pérard, 1925. Z Parasitenkd. 1976;50:257–275. doi: 10.1007/BF02462971. [DOI] [PubMed] [Google Scholar]
- Sathish K, Sriraman R, Subramanian BM, Rao NH, Balaji K, Narasu ML, Srinivasan VA. Plant expressed EtMIC2 is an effective immunogen in conferring protection against chicken coccidiosis. Vaccine. 2011;29:9201–9208. doi: 10.1016/j.vaccine.2011.09.117. [DOI] [PubMed] [Google Scholar]
- Shen N, Wei W, Chen Y, Ren Y, Xiong L, Tao Y, Gu X, Xie Y, Peng X, Yang G. An antibody persistent and protective two rSsCLP-based subunit cocktail vaccine against Sarcoptes scabiei in a rabbit model. Vaccines. 2020;8:129. doi: 10.3390/vaccines8010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Tao G, Bao G, Suo J, Hao L, Fu Y, Suo X. Stable transfection of Eimeria intestinalis and investigation of its life cycle, reproduction and immunogenicity. Front Microbiol. 2016;7:807. doi: 10.3389/fmicb.2016.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao G, Wang Y, Li C, Gu X, Cui P, Fang S, Suo X, Liu X. High pathogenicity and strong immunogenicity of a Chinese isolate of Eimeria magna Pérard, 1925. Parasitol Int. 2017;66:207–209. doi: 10.1016/j.parint.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Tomley FM, Clarke LE, Kawazoe U, Dijkema R, Kok JJ. Sequence of the gene encoding an immunodominant microneme protein of Eimeria tenella. Mol Biochem Parasitol. 1991;49:277–288. doi: 10.1016/0166-6851(91)90071-D. [DOI] [PubMed] [Google Scholar]
- Venkatas J, Adeleke MA. A review of Eimeria antigen identification for the development of novel anticoccidial vaccines. Parasitol Res. 2019;118:1701–1710. doi: 10.1007/s00436-019-06338-2. [DOI] [PubMed] [Google Scholar]
- Wei W, Shen N, Xiao J, Tao Y, Luo Y, Angel C, Gu X, Xie Y, He R, Jing B, Peng X. Expression analysis and serodiagnostic potential of microneme proteins 1 and 3 in Eimeria stiedai. Genes. 2020;11:725. doi: 10.3390/genes11070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Xiao J, Zhou X, Gu X, He R, Xu J, Jing B, Peng X, Yang G. Global transcriptome landscape of the rabbit protozoan parasite Eimeria stiedae. Parasites Vectors. 2021;14:1–17. doi: 10.1186/s13071-021-04811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Li X. Vaccination of chickens with DNA vaccine expressing Eimeria tenella MZ5-7 against coccidiosis. Vet Parasitol. 2011;177:6–12. doi: 10.1016/j.vetpar.2010.11.041. [DOI] [PubMed] [Google Scholar]
- Yan M, Cui X, Zhao Q, Zhu S, Huang B, Wang L, Zhao H, Liu G, Li Z, Han H, Dong H. Molecular characterization and protective efficacy of the microneme 2 protein from Eimeria tenella. Parasite. 2018;25:60 . doi: 10.1051/parasite/2018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Liu J, Hu X, Yang S, Zhong S, Yang T, Zhou Y, Zhao G, Jiang Y, Li Y. Alterations in the jejunal microbiota and fecal metabolite profiles of rabbits infected with Eimeria intestinalis. Parasites Vectors. 2022;15:1–16. doi: 10.1186/s13071-022-05340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ma L, Liu R, Zhang Y, Zhang S, Hu C, Song M, Cai J, Wang M. Eimeria tenella heat shock protein 70 enhances protection of recombinant microneme protein MIC2 subunit antigen vaccination against E. tenella challenge. Vet Parasitol. 2012;188:239–246. doi: 10.1016/j.vetpar.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu R, Song M, Hu Y, Pan B, Cai J, Wang M. Eimeria tenella: interleukin 17 contributes to host immunopathology in the gut during experimental infection. Exp Parasitol. 2013;133:121–130. doi: 10.1016/j.exppara.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Yang X, Liu L, Wang S, Lu M, Ehsan M, Gadahi JA, Song X, Xu L, Yan R. The molecular characterization and immunity identification of microneme 3 of Eimeria acervulina. J Eukaryot Microbiol. 2016;63:709–721. doi: 10.1111/jeu.12318. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhou Z, Huang J, Sun X, Haseeb M, Ahmed S, Shah MAA, Yan R, Song X, Xu L, Li X. Molecular characterization of a potential receptor of Eimeria acervulina microneme protein 3 from chicken duodenal epithelial cells. Parasite. 2020;27:18. doi: 10.1051/parasite/2020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhu S, Zhao Q, Huang B, Liu G, Li Z, Wang L, Dong H, Han H. Molecular characterization and protective efficacy of a new conserved hypothetical protein of Eimeria tenella. Parasite. 2021;28:40. doi: 10.1051/parasite/2021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Ming S, Sun L, Wang B, Li H, Zhang X, Zhao X. Identification and characterization of Eimeria tenella microneme protein (EtMIC8) Microbiol Spectr. 2021;9:e00228–e321. doi: 10.1128/Spectrum.00228-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this study are included in this article.