Abstract
Background
Prone position has already been demonstrated to improve survival in non-COVID acute respiratory distress syndrome and has been widely performed in COVID-19 patients with respiratory failure, both in non-intubated and intubated patients. However, the beneficial effect of the prone position in COVID-19 pneumonia still remains controversial. Therefore, we aimed to evaluate the effectiveness and safety of the prone position compared with the non-prone in non-intubated and intubated COVID-19 patients, respectively.
Methods
We searched the MEDLINE, EMBASE, and Cochrane databases, as well as one Korean domestic database, on July 9, 2021, and updated the search 9 times to September 14, 2022. Studies that compared prone and non-prone positions in patients with COVID-19 were eligible for inclusion. The primary outcomes were mortality, need for intubation, and adverse events.
Results
Of the 1259 records identified, 9 randomized controlled trials (RCTs) and 23 nonrandomized studies (NRSs) were eligible. In the non-intubated patients, the prone position reduced the intubation rate compared with the non-prone position in 6 RCTs (n = 2156, RR 0.81, P = 0.0002) and in 18 NRSs (n = 3374, RR 0.65, P = 0.002). In the subgroup analysis according to the oxygen delivery method, the results were constant only in the HFNC or NIV subgroup. For mortality, RCTs reported no difference between prone and non-prone groups, but in NRSs, the prone position had a significant advantage in mortality [18 NRSs, n = 3361, relative risk (RR) 0.56, P < 0.00001] regardless of the oxygen delivery methods shown in the subgroup analysis. There was no RCT for intubated patients, and mortality did not differ between the prone and non-prone groups in NRSs. Adverse events reported in both the non-intubated and intubated groups were mild and similar between the prone and non-intubated groups.
Conclusion
For non-intubated patients with COVID-19, prone positioning reduced the risk of intubation, particularly in patients requiring a high-flow oxygen system. However, the survival benefit was unclear between the prone and non-prone groups. There was insufficient evidence to support the beneficial effects of prone positioning in intubated patients.
Trial registration This study was registered in the Prospective Register of Systematic Reviews on February 16, 2022 (Registration No.: CRD42022311150).
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-022-00953-z.
Keywords: Prone position, COVID-19, Acute respiratory distress syndrome
Background
Pulmonary involvement is common in COVID-19 patients and approximately 10–20% of hospitalized patients with COVID-19 had severe respiratory failure requiring mechanical ventilation [1]. Interventions to reduce mortality risk have been actively attempted in COVID-19 patients with respiratory failure, and the prone position is one of them.
Randomized trials and meta-analysis supported that the prone position showed favorable outcomes, including improved oxygenation, respiratory mechanics, and survival in patients with moderate-to-severe non-COVID-19 acute respiratory distress syndrome (ARDS) [2–4]. Similarly, in patients with COVID-19, several studies reported that prone positioning showed improved oxygenation [5–7] and reduced mortality [8, 9]. However, patients included in those studies varied in severity and degree of oxygen requirement, from nasal prong to mechanical ventilation. In particular, it is interesting that the awake-prone position was applied in many COVID-19 patients who were not critically ill, but had an oxygen demand and the possibility of respiratory failure. A recent meta-analysis showed that an awake-prone position reduced the risk of intubation, especially in COVID-19 patients requiring advanced respiratory support [10]. However, the result was mainly driven by one large trial, and two additional large randomized trials have been published recently. They have shown conflicting results regarding the effectiveness of prone position in patients with high-flow oxygen therapy or non-invasive ventilation [11, 12].
For intubated patients, relatively fewer studies had been performed compared to those for non-intubated patients and most studies compared oxygenation status before and after the application of prone position. Since prone positioning was considered to be performed in severe respiratory failure patients if possible, few observational studies compared outcomes of patients with and without prone position. However, the effect of prone position on mortality was inconsistent between studies [9, 13] and there has been no meta-analysis or systemic review of these comparisons.
Therefore, this study aimed to evaluate the efficacy and safety of the prone position in COVID-19 patients with respiratory failure and to analyze which prone position could be recommended among non-intubated and intubated patients, respectively.
Methods
This study followed the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [14] (Additional file 1). This study was registered in the Prospective Register of Systematic Reviews (PROSPERO) on February 16, 2022 (registration number CRD42022311150).
Eligibility criteria
The inclusion criteria were as follows: (1) population—studies targeting patients with moderate-to-severe COVID-19; (2) intervention and comparator—studies comparing prone position to non-prone position; (3) outcomes—studies reporting the clinical outcomes including mortality, need for invasive mechanical ventilation, adverse events; (4) studies published after 2020; (5) study designs—randomized clinical trials (RCTs) or nonrandomized studies (NRSs) with a comparator group; and (6) full-text articles in English or Korean language. The exclusion criteria were as follows: (1) studies that did not target patients with confirmed COVID-19; (2) studies that did not compare the prone position to the non-prone position; (3) studies that did not report our outcomes of interest; and (4) duplicated studies.
Information sources and search strategy
We searched the following electronic databases: international databases (Ovid MEDLINE, Ovid EMBASE, the Cochrane Central Register of Controlled Trials), and the Korean domestic database (KMBASE) on July 9, 2021. Since new evidence on the prone position of COVID-19 patients is continuously produced, we updated the search 9 times from September 10, 2021, to September 14, 2022. We searched Ovid-MEDLINE for updates and reference lists of previously published reviews. We used Boolean operators such as (2019-nCoV OR COVID-19 OR Wuhan) AND (prone position OR prone posture OR proning). The search strategy is presented in Additional file 2.
Selection process
Four authors (HJL, JoK, JP, and JuK) independently screened the retrieved citations by title and abstract in COVIDENCE (https://www.covidence.org/) according to the inclusion and exclusion criteria. Full texts were assessed for the final decision of inclusion or exclusion by two authors (HJL and JoK). If an agreement was not reached between the two authors, it was reached through discussion with the third author (MC).
Data items and extraction
The following data were extracted from the eligible studies using an electronic spreadsheet (Microsoft Excel) of data abstraction form: first author, published year, study design and setting, study location, sample size in each arm, oxygen therapy method, prone position protocol and duration, and outcomes of interest. Two authors (JP and JuK) extracted information from each included study, and two other authors (WIC and JJ) checked the data independently.
Study outcomes
The primary outcomes were mortality, the need for intubation (in the case of non-intubated patients), and adverse events. The secondary outcomes were the length of stay (LOS) in the hospital or intensive care unit (ICU), ICU-free days, and ventilator-free days.
Study risk-of-bias assessment
A validated tool was used according to the study design to evaluate the risk of bias in the included studies. The Cochrane risk-of-bias tool (RoB) 1.0 [15] was used for RCTs, and the Risk of Bias Assessment tool for Nonrandomized Studies (RoBANS) 2.0 [16] which was updated from RoBANS 1.0 [17] for nonrandomized studies. Two independent authors (WIC and JJ) conducted quality assessments of the studies, and disagreements were resolved by a third author (MC).
Effect measures and synthesis methods
Based on the data extraction results, the meta-analysis was performed as follows. Relative risks (RR) with 95% confidence intervals (CI) for discrete outcome data and mean differences (MD) with 95% CI for continuous outcome data were calculated using the random-effects model because of heterogeneity across studies. Statistical significance was set at P < 0.05. To assess between-study heterogeneity, we displayed forest plots and calculated I2 statistics with a value of > 75%, considered high heterogeneity [18]. A subgroup analysis was performed based on oxygen delivery methods [conventional oxygen therapy (COT), high-flow nasal cannula (HFNC), non-invasive ventilation (NIV), or invasive mechanical ventilation (IMV)]. When more than one oxygen delivery method was used, the studies were classified based on the method by which the majority of patients received oxygen. To assess publication bias, we generated funnel plots for the primary outcomes reported in at least ten studies and performed Egger's linear regression test. We used Review Manager (RevMan) 5.4 [19] to synthesize the data and R version 4.2.1 [20] for Egger's linear regression test.
Certainty of evidence assessment
We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) [21] to assess the certainty of the evidence of the primary outcomes. Two authors (WIC and JJ) assessed the certainty of the evidence as high, moderate, low, or very low, and discrepancies were resolved by a third author (MC).
Results
Study selection
The study selection process is illustrated in Fig. 1. A total of 1426 records were identified using the search strategy on July 9, 2021, and 347 duplicate records were removed before the screening. One hundred and eighty records were updated until September 14, 2022. Of the 1259 records, 1116 were excluded after screening using titles and abstracts. Subsequently, the full texts of the 143 reports were retrieved. After reviewing the eligibility of the original texts, 9 RCTs with 2431 patients (sample size range, 27–1121) [11, 12, 22–28] and 23 nonrandomized studies (NRSs) including 2 nonrandomized controlled trains with 744 patients (sample size range, 243–501) [29, 30], 7 prospective cohort studies with 761 patients (sample size range, 32–335) [5, 7, 31–35], and 14 retrospective cohort studies with 3119 patients (sample size range, 20–827) [6, 8, 9, 36–46] were included in our review. The list of excluded studies and reasons for exclusion are presented (Additional file 3).
Fig. 1.
PRISMA flowchart
The characteristics of the included studies are summarized in Table 1. Eleven studies originated in Europe [6, 8, 26, 27, 30, 32, 33, 37, 44–46], eight from Asia [5, 25, 34–36, 38, 40, 41], five from North America [9, 23, 28, 29, 39], four from the South America [11, 31, 42, 43], two from Africa [7, 24], and two from multiple countries [12, 22]. Seventeen studies [6, 8, 11, 12, 22, 23, 25, 27, 29, 31, 32, 34, 41, 43–46] were conducted at multiple centers and others at single centers. In most of the studies, including the all RCTs, patients were provided through the COT, HFNC, or NIV, and in three NRSs studies [9, 38, 45], through mechanical ventilation. The proning protocols varied in terms of time and frequency of sessions, such as at least 2–18 h per day or no restrictions in time and frequency. The reported proning durations varied. The average proning time per day (3–15 h per day) [11, 22, 26, 27, 29–31, 34, 40, 45], the total number of proning session (2–4 sessions) [11, 30, 40], or days in proning (2.5–13 days) [11, 12, 27, 30, 31, 40, 44, 45] were reported.
Table 1.
The basic characteristics of studies included in this review
| First author, published year | Study design | Study setting | Study location | Enrollment period | Total sample size (prone/non-prone) | Age (year, IQR or SD) (prone/non-prone) | Male (%) (prone/non-prone) | Oxygen therapy methods | Proning protocol | Location of proning | Proning duration in prone group | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daily | Total | |||||||||||
| Ehrmann [22] | RCT | Multicenter | Canada, France, Ireland, Mexico, USA, Spain | 2020.4.2.–2021.1.26 | 1121 (564/557) | 61.5 (13.3)/60.7 (14.0) | 67/66 | HFNC | As long and as frequently as possible each day | ICU, Ward, ER | 5 (IQR 1.6–8.8) h | NR |
| Jayakumar [25] | RCT | Multicenter | India | NR | 60 (30/30) | 54.8 (11.1)/57.3 (12.1) | 83.3/83.3 | Nasal prongs, face mask, non-rebreather mask, HFNC, or NIV | ≥ 6 h/day (cumulative) | ICU | ≥ 6 h in 43% of intervention group | NR |
| Kharat [26] | RCT | Single center | Switzerland | 2020.4.6.–2020.4.25 | 27 (10/17) | 54 (14)/60 (11) | 60/65 | Nasal cannula | ≤ 12 h/day | Ward | 295 (SD 216) m | NR |
| Rosén [27] | RCT | Multicenter | Sweden | 2020.10.7.–2021.2.7 | 75 (35/39) | 66 (53–74)/65 (55–70) | 64/82 | HFNC, NIV | ≥ 16 h/day | Ward, ICU | 9.0 (IQR 4.4–10.6) h | 4.2 (1.7–5.7) d |
| Taylor [28] | RCT | Single center | US | 2020.6.1.–2020.8.31 | 40 (27/13) | 56 (45–66)/60 (54–63) | 63/77 | Nasal cannula, MFNC | As long as possible and allowed to return to the supine position as necessary | Ward | NR | NR |
| Fralick [23] | RCT | Multicenter | Canada, US | 2021.3.-2021.5 | 248 (126/122) | 59.5 (45–68)/54 (44–62) | 65/63 | Nasal cannula, mask, HFNC | Four sessions/day (up to 2 h/sessions), encouraged to sleep in pone position overnight | Ward | NR | From randomization to first 72 h: 6 (1.5–12.8) h, from 72 h to 7 d: 0 (0–12) h |
| Gad [24] | RCT | Single center | Egypt | 2020.6.-2020.9 | 30 (15/15) | 49.0 (38–62)/46.0 (33–51) | 60.0/53.3 | High flow with non-rebreathing facemask | Each session for 1–2 h according to patient to tolerability with 3 h apart during waking hours | Critical care isolation | NR | NR |
| Ibarra-Estrada [11] | RCT | Multicenter | Mexico | 2020.5.5.–2021.1.26 | 430 (216/214) | 58.6 (15.8)/58.2 (15.8) | 61.1/58.9 | HFNC | As long as possible | Intermediate or intensive care unit | 4 sessions/d (3–5) 3.4 (3–3.6) h/session | 9.4 (5.6–12.9) h for 6 (3.7–9) ds |
| Alhazzani [12] | RCT | Multicenter | Multicountry | 2020.5.19–2021.5.13 | 400 (205/195) | 56.8 (12.5)/58.3 (13.2) | 73/69 | HFNC, LFNC, NPPV | 8 h/d–10 h/d with 2 to 3 breaks (1–2 h each), if needed | ICU or a monitored acute care unit | NR | 3 (1–5) d |
| Musso [30] | NRCT | Single center | Italy | Int 2020.12.16.–2021.5.30. Cont 2020.4.1.–2020.12.15 | 243 (81/162) | 68 (60–75)/69 (61–78) | 76/72 | NIV | As long as possible, at least 1 session/day lasting ≥ 8 h scheduled overnight | Subintensive care unit | 12.2 (10.1–13.8) h, 2 session/d | 6 (5–8) d |
| Qian [29] | NRCT | Multicenter | US | 2020.5.13.–2020.12.11 | 501 (243/258) | 61.6 (15.4)/60.3 (15.2) | 56.6/56.8 | HFNC, LFNC, NIV | Encouraged as often and consistently as possible | NR | 4.2 (1.8–6.7) h | NR |
| Ferrando [32] | Prospective cohort study | Multicenter | Spain, Andorra | 2020.3.12.–2020.6.9 | 199 (55/144) | 60.0 (54.0–70.0)/63.0 (55.0–71.0) | 75.9/72.7 | HFNC | > 16 h regardless of the number of sessions | ICU | NR | NR |
| Ni [5] | Prospective cohort study | Single center | China | 2020.1.31.–2020.2.15 | 52 (17/35) | 60 (12)/64 (12) | 64.7/60 | NR | ≥ 4 h/day for 10 days | Provisional ICU | NR | NR |
| Zang [35] | Prospective cohort study | Single center | China | 2020.2.1.–2020.4.30 | 60 (23/37) | 63 (59–71)/66 (60–72) | 56.5/70.3 | O2 storage mask | 1–2 h/session, 3–4 sessions/day for more than 5 consecutive days | NR | NR | 13.4 (SD 8.0) h |
| Bahloul [7] | Prospective cohort study | Single center | Tunisia | 2020.9.1.–2020.12.4 | 38 (21/17) | 61 (10)/60 (12) | 76.2/NR | Facial mask, HFNC | 2–4 h followed by 2 h of supine positioning during the day, and to sleep in a proning at night, when possible | ICU | NR | NR |
| Esperatti [31] | Prospective cohort study | Multicenter | Argentina | 2020.6.–2021.1 | 335 (187/148) | 57 (47–66)/66.5 (56.5–75) | 76/72 | HFNC | ≥ 6 h/day, no time limits for prone position | ICU | 12 (IQR 9–16) h | 5 (IQR 3–8) d |
| Sryma [34] | Prospective cohort study | NR | India | NR | 45 (30/15) | 50.9 (10.1)/57.5 (12.2) | 96.7/60 | NIV, HFNC, COT | ≥ 2 h/session, target duration of 8 h/day | NR | 7.5 (range 4–12) h on the first day | NR |
| Pierucci [33] | Prospective cohort study | Single center | Italy | 2020.3.11.–2020.4.30 | 32 (16/16) | 59 (11)/70 (15) | 81/62 | Int: spontaneously breathing Cont: HFNC, CPAP, NIV | As long as possible with intervals for meals and other personal care | |||
| Jagan [39] | Retrospective cohort study | Single center | US | 2020.3.24.–2020.5.5 | 105 (40/65) | 56.0 (14.4)/65.8 (16.3) | 50/56.9 | Non-intubation | ≥ 1 h/day for at least 5 sessions, ≥ 1 h/overnight | NR | NR | NR |
| Padrão [42] | Retrospective cohort study | Single center | Brazil | 2020.3.1.–2020.4.30 | 166 (57/109) | 51.8 (13)/61.4 (13.6) | 70/66 | Nasal cannula, venturi mask, non-rebreather mask | ≥ 4 h in the first session, stimulated twice daily to maintain proning | NR | First session < 1 h 6%, 1–2 h 14%, 2–3 h 12%, 3–4 h 10%, > 4 h 58% | NR |
| Barker [37] | Retrospective cohort study | Single center | UK | 2020.3.26.–2020.6.26 | 20 (10/10) | 59 (55–63)/65 (55–71) | 60/60 | NIV (Int 90%, Con 70%) | 30 m–2 h, repeated as many times as comfortable | ICU | NR | 120 (IQR 76–161) m, 4 (IQR 1–7) sessions/patient |
| Jouffroy [6] | Retrospective cohort study | Multicenter | France | 2020.2.20.–2020.4.24 | 379 (40/339) | 59.5 (56–64)/62 (53–69) | 90/75.2 | HFNC | 3–6 h/session, twice a day | ICU | NR | 2.5 (IQR 1.6–3.4) d, 3 (IQR 2–5) sessions |
| Loureiro-Amigo [8] | Retrospective cohort study (SEMI-COVID-19) | Multicenter | Spain | 2020.3.1.–2020.8.31 | 163 (60/103) | 66.57 [59.2–72.4]/70.81 [60.6–74.2] | 71.7/68.9 | Venturi masks, rebreathing masks | NR | Ward | NR | NR |
| Prud’homme [44] | Retrospective cohort study | Multicenter | France | 2020.3.20–2020.4.20 | 96 (48/48) | 62 (11)/61 (18) | 77.1/64.6 | COT, HFNC | ≥ 3 h/day during 3 consecutive days, 1 to 12 h/session | Non-ICU | 3–8 h in 67%, > 5 h in 38% of intervention group | 6.9 (SD 5.2) d |
| Shelhamer [9] | Retrospective cohort study | Single center | US | 2020.3.25.–2020.5.2 | 261 (62/199) | 60.0 (54.3–66.5)/66.0 (55.0–74.5) | 67.7/60.3 | IMV | ≥ 16 h in the afternoon, supine position the following morning | Traditional ICU, Converted floor ICU | NR | NR |
| Stilma [45] | Retrospective cohort study | Multicenter | Netherlands | 2020.3.1.–2020.6.1 | 734 (438/296) | Without indication for proning 65.0 (10.3)/64.2 (11.4) With indication for proning 62.6 (11.2)/66.6 (9.1) | 72.6/73.3 | IMV | NR | ICU | 15.0 (IQR 10.5–21.0) h | 3 (2–3) d |
| Tonelli [46] | Retrospective cohort study | Multicenter | Italy | 2020.3.1.–2020.6.1 | 114 (38/76) | 61 (32 − 75)/70 (33 − 80) | 66/73 | HFNC, CPAP, NIV | ≥ 3 h before back to supine | ICU | NR | NR |
| Perez-Nieto [43] | Retrospective cohort study (APRONOX study) | Multicenter | Mexico, Ecuador | 2020.5.1.–2020.6.12 | 827 (505/322) | 53.4 (13.9)/55.8 (14.5) | 73.3/71.4 | LFNC, HFNC or a non-rebreather mask | ≥ 2 h continuously | ICU 13% non-ICU 87% | NR | 12 (IQR 8–24) h |
| Koike [40] | Retrospective study | Single center | Japan | Int: 2020.10.1.–2020.12.1. Cont: 2020.12.1.–2020.3.31 | 58 (27/31) | 71 (55–77)/63 (49–70) | 90/87 | Simple O2, HFNC, NPPV | Discontinued if the patient developed intolerable respiratory distress, tachypnea > 35 bpm, or new unacceptable back pain during proning | ICU | 2 (2–3) sessions/d 180 (120–240) m | 13 (7–16) d |
| Altinay [36] | Retrospective study | Single center | Turkey | 2020.3.15.–2020.6.15 | 48 (25/23) | 62.4 (10.9)/72.6 (10.1) | 44/39.1 | Nonrebreather mask oxygen | 18 h intermittently in a day | ICU | NR | NR |
| Numata [41] | Retrospective study | Multicenter | Japan | 2020.7–2021.2 | 108 (54/54) | 68 (58–76)/70 (59–79) | 68.5/42.6 | HFNC | As long as possible, at least 3 times a day and for at least 6 h per day | Severe COVID-19 patient unit | NR | NR |
| Chen [38] | Retrospective study | Single center | China | 2020.1.9.–2020.4.10 | 40 (17/23) | 69 (56–87)/72 (54–89) | 64.7/78.3 | IMV | NR | ICU | NR | NR |
RCT, randomized controlled trial; NRCT, nonrandomized controlled trial; HFNC, high-flow nasal cannula; MFNC, medium-flow nasal cannula; ICU, intensive care unit; ER, emergency room; IQR, interquartile range; SD, standard definition; NR, not reported; NIV, non-invasive ventilation; COT, conventional oxygen therapy; ECMO, extracorporeal membrane oxygenation; IMV, invasive mechanical ventilation; CPAP, continuous positive airway pressure; LFNC, low-flow nasal cannula; NPPV, non-invasive positive pressure ventilation
Risk of bias in studies
The majority of RCTs were assessed as having a low risk of bias in all the dimensions. In more than half of the NRSs, the domains of the possibility of target group comparison and selection were rated as having a high risk of bias (Additional file 4: Fig. S1). However, serious problems did not occur because the domains of exposure measurement, blinding of assessors, outcome assessment, and selective outcome reporting were assessed as having a low risk of bias in most NRSs.
Non-intubated group
Mortality
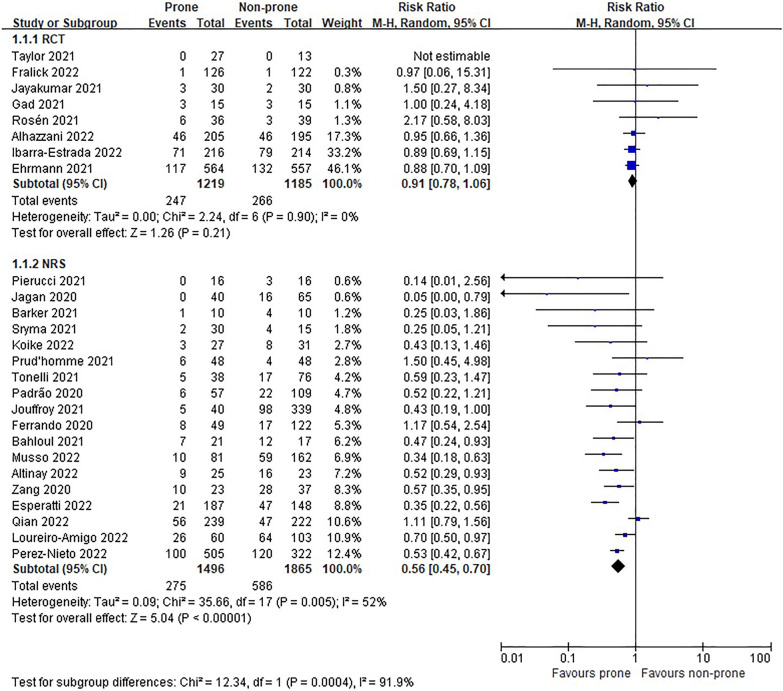
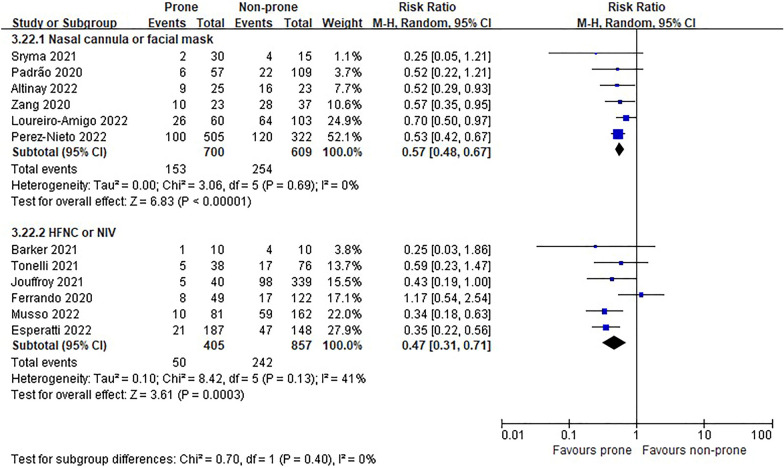
In the eight RCTs [11, 12, 22–25, 27, 28], there was no difference in morality between prone and non-prone groups (high certainty of evidence), but in the NRSs [6–8, 29–37, 39, 40, 42–44, 46], the prone position had a significant advantage of survival in the non-intubated patient group (18 NRSs, n = 3361, RR 0.56, 95% CI 0.45 to 0.70, P < 0.00001, I2 = 52%, very low certainty of evidence; Fig. 2). The subgroup analysis for the oxygen delivery method showed constant results (Figs. 3, 4). In NRSs, prone reduced mortality compared to non-prone in the nasal cannula or facial mask group (6 NRSs, n = 1309, RR 0.57, 95% CI 0.48–0.67, P < 0.00001, I2 = 0%) and the HFNC or NIV group (6 NRSs, n = 1262, RR 0.47, 95% CI 0.31–0.71, P = 0.0003, I2 = 41%). Although the funnel plot for mortality in NRSs was asymmetric, we observed no evidence of publication bias in Egger's linear regression test (P = 0.2192, Additional file 4: Fig. S2).
Fig. 2.
Mortality of non-intubated patients
Fig. 3.
Subgroup analysis of mortality by oxygen delivery methods in non-intubated patients of randomized studies
Fig. 4.
Subgroup analysis of mortality by oxygen delivery methods in non-intubated patients of non-randomized studies
Need for intubation
The intubation rate of the prone group was significantly lower than that of the non-prone group in 7 RCTs (n = 2156, RR 0.81, 95% CI 0.72 to 0.90, P = 0.0002, I2 = 0%, high certainty of evidence) [11, 12, 22, 24, 25, 27, 28] and 18 NRSs (n = 3374, RR 0.65, 95% CI 0.50 to 0.85, P = 0.002, I2 = 74%, very low certainty of evidence) [6, 29, 30, 36, 37, 39–44, 46] (Fig. 5). In the subgroup analysis according to the oxygen delivery method, proning showed advantage only in the HFNC or NIV subgroup (Figs. 6, 7). Although the funnel plot for the intubation rate of nonrandomized studies was asymmetric, we observed no evidence of publication bias in Egger's linear regression test (P = 0.8453, Additional file 4: Fig. S3).
Fig. 5.
Need for intubation of non-intubated patients
Fig. 6.
Subgroup analysis of intubation rate by oxygen delivery methods in non-intubated patients of randomized controlled trials
Fig. 7.
Subgroup analysis of intubation rate by oxygen delivery methods in non-intubated patients of non-randomized studies
Adverse events
Seven RCTs reported adverse events [11, 12, 22, 25–28]. The incidence of cardiac arrest (at any time) was similar between the prone and non-prone positions (prone vs. non-prone 3/564 vs. 1/557, p value not reported) [22] and skin breakdown and vomiting were also similar between the two groups (moderate certainty of evidence, Additional file 4: Fig. S4). Six NRSs reported adverse events in the prone group [5, 30, 34, 42, 44, 46], which were mainly mild (very low certainty of evidence, Additional file 4: Table S1).
Length of stay in hospital or ICU
Length of stays in hospital or ICU were not different between prone and non-prone groups in both RCTs and NRSs (Additional file 4: Figs. S5, S6).
ICU-free days and ventilator-free days
ICU-free days were not different in RCTs, and ventilator-free days were not different in RCTs and NRSs between prone and non-prone groups (Additional file 4: Figs. S7, S8).
Intubated group
Only NRSs included intubated patients [9, 38, 45]. Mortality did not differ between the prone and non-prone groups in 2 NRSs [9, 45] (Fig. 8), but 1 NRS [38] reported better survival in prone group than in non-prone group (adjusted hazard ratio 0.282, 95% CI 0.126 to 0.63) (very low certainty of evidence). Incident occurrence of peripheral line removal in two patients during positioning was reported in 1 NRS [9] (very low certainty of evidence, Additional file 4: Table S1). Hospital LOS and ICU LOS were longer in prone group than non-prone group (hospital LOS, one study [9], n = 261, MD 10.1 days, 95% CI 7.39 to 12.81 days, P < 0.00001; ICU LOS, one study [45], n = 734, MD 2.71 days, 95% CI 0.77 to 4.65 days, P = 0.006). Ventilator-free days did not differ between the prone and non-prone groups in 2 NRSs [9, 45] (Additional file 4: Fig. S9), and no studies reported ICU-free days between prone and non-prone groups.
Fig. 8.
Mortality of intubated patients in non-randomized studies
The GRADE summary of findings table of primary outcomes is reported in Table 2.
Table 2.
GRADE summary of findings table of primary outcomes
| Outcomes | Study design | Anticipated absolute effectsc (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|---|
| Risk with non-prone | Risk with prone | |||||
| Non-intubated patients | ||||||
| Mortality | RCT | 224 per 1000 | 204 per 1000 (175–238) | RR 0.91 (0.78–1.06) | 2404 (8) | ⨁⨁⨁⨁ High |
| NRS | 314 per 1000 | 176 per 1000 (141–220) | RR 0.56 (0.45–0.70) | 3361 (18) | ⨁◯◯◯ Very lowa | |
| Need for intubation | RCT | 389 per 1000 | 315 per 1000 (280–351) | RR 0.81 (0.72–0.90) | 2156 (7) | ⨁⨁⨁⨁ High |
| NRS | 412 per 1000 | 267 per 1000 (206–350) | RR 0.65 (0.50–0.85) | 3374 (18) | ⨁◯◯◯ Very lowa | |
| Adverse events | RCT | 24 per 1000 | 23 per 1000 (16–34) | RR 0.97 (0.66–1.43) | 7011 (6) | ⨁⨁⨁◯ Moderateb |
| NRS | Cases in the prone group were reported as follows: desaturation or hemodynamic worsening 0/30 [34], back pain 2/30 [34] and 3/57 [42], bloating sensation 2/30 [34], gastric distension and vomit 0/81 [30], peripheral line removal 2/57 [42] and 2/81 [30], nasal skin ulceration 2/81 [30], major adverse events 0/48 [44], overall adverse events 0/17 [5] and 0/38 [46] | 716 (6) | ⨁◯◯◯ Very lowa,b | |||
| Intubated patients | ||||||
| Mortality | NRS | 525 per 1000 | 504 per 1000 (441–573) | RR 0.96 (0.84–1.09) | 995 (2) | ⨁◯◯◯ Very lowa |
| Chen et al. [38] reported better survival in prone group than in non-prone group (adjusted hazard ratio 0.282, 95% CI 0.126 to 0.63) | 40 (1) | |||||
| Adverse events | NRS | Cases in the prone group were reported as follows: endotracheal tube dislocation 0/62 [9], peripheral line removal 2/62 [9] | 261 (1) | ⨁◯◯◯ Very lowa,b | ||
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
CI, confidence interval; RR, risk ratio; RCT, randomized controlled trial; NRS, nonrandomized studies
aDowngrade for risk-of-bias concern in the domains of possibility of target group comparisons, target group selection, and confounder
bNo more than 300 events
cThe risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
Discussion
In this analysis, we divided patients with moderate-to-severe COVID-19 into intubated and non-intubated groups and investigated the benefit of the prone position. In summary, we found that prone position reduced the risk of intubation in non-intubated patients, particularly those supplied with high-flow oxygen systems. However, prone position did not reduce the risk of mortality in both the intubated or non-intubated groups. In non-intubated patients, survival benefit was only observed in observational studies, not in randomized trials. Moreover, there are no randomized controlled trials comparing prone to supine positions in intubated patients. Only a few observational cohort studies were included and did not show statistically better survival. In addition, ventilator-free days were significantly shorter in the prone position group. As a result, there is still insufficient evidence to support the beneficial effect of prone position in intubated patients.
However, it would be considered unethical to assign patients to the non-prone group since large randomized controlled trials and meta-analyses have already shown the beneficial effect of the prone position in patients with moderate and severe non-COVID ARDS [3, 47, 48]. In addition, although two different phenotypes of COVID-19 ARDS have been proposed, several studies have suggested similar clinical features between COVID-19 and non-COVID ARDS [49]. Compliance was higher in the COVID-19 initially, but decreased 3–7 days after onset with no difference from non-COVID-19 ARDS [50]. In addition, pathological characteristics and distribution of compliance were similar among studies of COVID-19 and non-COVID-19 ARDS [51, 52]. They also suggested that treatment previously considered for non-COVID-19 ARDS may apply to COVID-19 patients with respiratory failure [51]. In addition, observational cohort studies demonstrated that improved oxygenation and increased Pao2/Fio2 ratio after prone positioning even remained significantly higher after returning to the supine position [9, 53, 54]. Static lung compliance was also increased after prone positioning with reduced driving pressure [54]. In the analysis of lung computed tomography in COVID-19 ARDS, regional hyperinflation decreased, and inflation distribution was more homogenous in the prone position, which was also similar to other ARDS [55]. Based on previous experiences from non-COVID-19 ARDS and improvement of oxygenation in observational studies, guidelines recommended implementing the prone position in intubated patients with COVID-19. Further research is needed to evaluate the effect of prone position in intubated patients.
Meanwhile, studies on awake-prone positioning in non-intubated patients were conducted more actively, including randomized controlled trials. We included the most recently updated trials in this meta-analysis. Among non-intubated patients, the prone position group had a reduced risk of intubation. In the subgroup analysis according to oxygen delivery methods, prone positioning reduced intubation rates only in more severely ill patients receiving a high-flow oxygen system or non-invasive ventilation, and the risk of intubation was similar between the prone and non-prone groups in patients with low-flow oxygen. However, since there were only three randomized trials and a small number of patients included in the low-flow oxygen subgroup, it is insufficient to evaluate the effect of prone position. Therefore, more randomized trials will be needed. There was no difference in the mortality between prone and non-prone groups among randomized trials regardless of oxygen supply methods, which was consistent with previous meta-analysis [10]. There was also no significant difference in the length of ICU and hospital stays between the prone and non-prone groups.
Before COVID-19, there were only a few case series and retrospective observational studies about awake-prone positioning on acute respiratory failure [56–59]. Those studies showed that a prone position improved oxygenation, but a detailed investigation has not been done. Among non-intubated COVID-19 patients, improvement in oxygenation was also observed with prone positioning [5–7]. A physiologic study showed that dead space and shunt were reduced. As a result, V/Q mismatch was improved in the prone position, similar to the mechanism in the intubated patients [60]. Reduced intubation risk might be due to improvement of oxygenation and respiratory mechanics. Nevertheless, mortality rates were similar between the two groups in randomized trials. One suggestion why the mortality benefit was not achieved in the prone position group was low adherence to prone position in the awake-prone groups [37]. The duration of the awake-prone position depended on the patient’s effort, unlike when performed in intubated patients, who were usually sedated for prone position. In this analysis, durations were not stated in the studies and, if noted, varied with a median of 5 to 9 h. However, recent studies showed that a longer duration of prone position was associated with better outcomes [11, 22, 31], and Esperatti et al. suggested performing prone position for at least 8 h per day to reduce the risk of mortality [31]. Therefore, there is a possibility that the duration of prone positioning was not sufficient to achieve survival gain. However, other factors affecting the duration of prone position, such as poor medical conditions, also influence survival, so the effect of duration on mortality should be assessed more carefully.
Complications that may occur in the prone position include dislocation of the endotracheal tube and vascular lines, transient hypotension, vomiting, and pressure sores [61]. However, there were no serious adverse events such as unstable hemodynamics and removal of the endotracheal tube both in the intubated and non-intubated patients in our study. The incidence of minor complications was also similar between the two groups. The prone position can be a safe and effective intervention for patients with respiratory failure.
This study had several limitations. First, RCTs were limited to the non-intubated group. Therefore, there is a lack of evidence to evaluate the effect of the prone position in intubated patients. Second, the severity of the included patients varied within and among studies. The oxygen supply method in non-intubated patients was particularly heterogeneous, from the nasal cannula to the high-flow oxygen system and non-invasive ventilation. So we performed subgroup analysis by dividing the studies into two groups: low-flow and high-flow oxygen systems, and found that the reduced risk of intubation was observed only in more severe patients with high-flow oxygen systems or non-invasive ventilation. Third, most studies did not present the cycle and duration of prone positioning or were inconsistent, particularly for non-intubated patients. According to the previous guidelines, there were only recommendations for intubated patients to maintain a prone position for at least 16 h. Further studies to evaluate the effective duration of the prone position in non-intubated patients should be considered, although heterogeneous disease severities and oxygen requirements may make the investigation difficult.
Conclusions
For non-intubated patients with COVID-19, prone positioning reduced the risk of intubation, particularly in patients requiring a high-flow oxygen system. However, the survival benefit was unclear between the prone and non-prone groups. There was insufficient evidence to support the beneficial effects of prone positioning in intubated patients, because only a few observational studies compared prone position and non-prone position. Further well-designed randomized controlled trials will be needed.
Supplementary Information
Additional file 3. List of excluded studies after full-text screening.
Additional file 4. Forest plots, table. Risk of bias of included randomized controlled trials (a, b) and non-randomized studies (c, d). (a) Risk of bias graph of randomized controlled trials: review authors' judgements about each risk of bias item presented as percentages across all included studies. (b) Risk of bias graph of randomized controlled trials: review authors' judgements about each risk of bias item for each included study. (c) Risk of bias graph of non-randomized studies: review authors' judgements about each risk of bias item presented as percentages across all included studies. (d) Risk of bias graph of non-randomized studies: review authors' judgements about each risk of bias item for each included study. Figure S2. Contour-enhanced funnel plot for mortality of non-intubation patients in non-randomized studies. Figure S3. Contour-enhanced funnel plot for intubation rate of non-intubation patients in non-randomized studies. Figure S4. Adverse events in randomized controlled trials. Table S1. Adverse events in non-randomized studies. Figure S5. Hospital length of stay of non-intubated patients. Figure S6. ICU length of stay of non-intubated patients. Figure S7. ICU-free days of non-intubated patients in randomized controlled trials. Figure S8. Ventilator-free days of non-intubated patients. Figure S9. Ventilator-free days of intubated patients in non-randomized studies.
Acknowledgements
The authors would like to thank the Korean Academy of Tuberculosis and Respiratory Diseases and the Korean Society of Critical Care Medicine for their contributions to this paper: Suk-Kyung Hong, HakJae Lee, In Seok Jeong, and Jae-Seung Jung.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- COT
Conventional oxygen therapy
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- HFNC
High-flow nasal cannula
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- LOS
Length of stay
- MD
Mean differences
- NIV
Non-invasive ventilation
- NRS
Nonrandomized study
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized clinical trial
- RoB
Cochrane risk-of-bias tool
- RoBANS
Risk of Bias Assessment tool for Nonrandomized studies
- RR
Relative risk
Author contributions
HJL and JoK contributed equally to this work. Study concept and design: HJL, JoK, and JuK. Literature search and resolving discrepancies: MC. Data extraction and quality assessment of the included studies: WIC and JJ. Data analysis and interpretation: HJL, JoK, WIC, and JJ. Drafting of the manuscript: HJK and JoK. Revising the manuscript: HJL, JoK, and JuK. All authors have read and approved the final manuscript.
Others
This manuscript followed the reporting protocol, especially the PICO or major processes for conducting systematic reviews.
Funding
This research was supported by the National Evidence-based Collaborating Agency (Grant Nos. NP21-004 and NA22-009). The funding sources had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyeon-Jeong Lee and Junghyun Kim have contributed equally to this work
References
- 1.Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761. doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 3.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez R, Trenchs X, Klamburg J, Castedo J, Serrano JM, Besso G, Tirapu JP, Santos A, Mas A, Parraga M, et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med. 2008;34(8):1487–1491. doi: 10.1007/s00134-008-1119-3. [DOI] [PubMed] [Google Scholar]
- 5.Ni Z, Wang K, Wang T, Ni Y, Huang W, Zhu P, Fan T, Wang Y, Wang B, Deng J, et al. Efficacy of early prone or lateral positioning in patients with severe COVID-19: a single-center prospective cohort. Precis Clin Medi. 2020;3(4):260–271. doi: 10.1093/pcmedi/pbaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouffroy R, Darmon M, Isnard F, Geri G, Beurton A, Fartoukh M, Tudesq JJ, Nemlaghi S, Demoule A, Azoulay E, et al. Impact of prone position in non-intubated spontaneously breathing patients admitted to the ICU for severe acute respiratory failure due to COVID-19. J Crit Care. 2021;64:199–204. doi: 10.1016/j.jcrc.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahloul M, Kharrat S, Hafdhi M, Maalla A, Turki O, Chtara K, Ammar R, Suissi B, Hamida CB, Chelly H, et al. Impact of prone position on outcomes of COVID-19 patients with spontaneous breathing. Acute Crit Care. 2021;36(3):208–214. doi: 10.4266/acc.2021.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loureiro-Amigo J, Suarez-Carantona C, Oriol I, Sanchez-Diaz C, Coloma-Conde A, Manzano-Espinosa L, Rubio-Rivas M, Otero-Perpina B, Ferreiro-Mazon Jenaro MM, Coduras-Erdozain A et al. Prone position in COVID-19 patients with severe acute respiratory distress syndrome receiving conventional oxygen therapy: a retrospective study. Arch Bronconeumol. 2021;58(3):277–80. [DOI] [PMC free article] [PubMed]
- 9.Shelhamer MC, Wesson PD, Solari IL, Jensen DL, Steele WA, Dimitrov VG, Kelly JD, Aziz S, Gutierrez VP, Vittinghoff E, et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: a cohort study and analysis of physiology. J Intensive Care Med. 2021;36(2):241–252. doi: 10.1177/0885066620980399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Luo J, Pavlov I, Perez Y, Tan W, Roca O, Tavernier E, Kharat A, McNicholas B, Ibarra-Estrada M, et al. Awake prone positioning for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Lancet Respir Med. 2022;10(6):573–583. doi: 10.1016/S2213-2600(22)00043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarra-Estrada M, Li J, Pavlov I, Perez Y, Roca O, Tavernier E, McNicholas B, Vines D, Marín-Rosales M, Vargas-Obieta A, et al. Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. Crit Care. 2022;26(1):84. doi: 10.1186/s13054-022-03950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhazzani W, Parhar KKS, Weatherald J, Al Duhailib Z, Alshahrani M, Al-Fares A, Buabbas S, Cherian SV, Munshi L, Fan E, et al. Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure: a randomized clinical trial. JAMA. 2022;327(21):2104–2113. doi: 10.1001/jama.2022.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stilma W, van Meenen DMP, Valk CMA, de Bruin H, Paulus F, Serpa Neto A, Schultz MJ. On Behalf Of The P-CCG: incidence and practice of early prone positioning in invasively ventilated COVID-19 patients-insights from the PRoVENT-COVID observational study. J Clin Med. 2021;10(20):4783. doi: 10.3390/jcm10204783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:14651858. [DOI] [PMC free article] [PubMed]
- 16.Kim SY, Seo HJ, Lee YJ, Park JE. Study Design Algorithm for Medical literature of Intervention (DAMI) and Risk of Bias for Nonrandomized Studies (RoBANS) ver 2.0 by HIRA. Seoul: Health Insurance Review & Assessment Service; 2013. pp. 65–66. [Google Scholar]
- 17.Kim S, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, Jang BH, Son HJ. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collaboration TC: Review Manager (RevMan), 5.4 edn. The Cochrane Collaboration; 2020.
- 20.Team RC . R: a language and environment for statistical computing. 4.2.1. Vienna: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 21.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Ehrmann S, Li J, Ibarra-Estrada M, Perez Y, Pavlov I, McNicholas B, Roca O, Mirza S, Vines D, Garcia-Salcido R, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fralick M, Colacci M, Munshi L, Venus K, Fidler L, Hussein H, Britto K, Fowler R, da Costa BR, Dhalla I, et al. Prone positioning of patients with moderate hypoxaemia due to covid-19: multicentre pragmatic randomised trial (COVID-PRONE) BMJ. 2022;376:e068585. doi: 10.1136/bmj-2021-068585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gad GS. Awake prone positioning versus non invasive ventilation for COVID-19 patients with acute hypoxemic respiratory failure. Egypt J Anaesth. 2021;37(1):85–90. doi: 10.1080/11101849.2021.1889944. [DOI] [Google Scholar]
- 25.Jayakumar D, Ramachandran P, Rabindrarajan E, Vijayaraghavan BKT, Ramakrishnan N, Venkataraman R. Standard Care versus awake prone position in adult nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 infection-a multicenter feasibility randomized controlled trial. J Intensive Care Med. 2021;36(8):918–924. doi: 10.1177/08850666211014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharat A, Dupuis-Lozeron E, Cantero C, Marti C, Grosgurin O, Lolachi S, Lador F, Plojoux J, Janssens JP, Soccal PM, et al. Self-proning in COVID-19 patients on low-flow oxygen therapy: a cluster randomised controlled trial. ERJ Open Res. 2021;7(1):00692–2020. [DOI] [PMC free article] [PubMed]
- 27.Rosén J, von Oelreich E, Fors D, Jonsson Fagerlund M, Taxbro K, Skorup P, Eby L, Campoccia Jalde F, Johansson N, Bergström G, et al. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. Crit Care. 2021;25(1):209. doi: 10.1186/s13054-021-03602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor SP, Bundy H, Smith WM, Skavroneck S, Taylor B, Kowalkowski MA. Awake prone positioning strategy for nonintubated hypoxic patients with COVID-19: a pilot trial with embedded implementation evaluation. Ann Am Thorac Soc. 2021;18(8):1360–1368. doi: 10.1513/AnnalsATS.202009-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian ET, Gatto CL, Amusina O, Dear ML, Hiser W, Buie R, Kripalani S, Harrell FE, Jr, Freundlich RE, Gao Y, et al. Assessment of awake prone positioning in hospitalized adults with COVID-19: a nonrandomized controlled trial. JAMA Intern Med. 2022;182(6):612–621. doi: 10.1001/jamainternmed.2022.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musso G, Taliano C, Molinaro F, Fonti C, Veliaj D, Torti D, Paschetta E, Castagna E, Carbone G, Laudari L, et al. Early prolonged prone position in noninvasively ventilated patients with SARS-CoV-2-related moderate-to-severe hypoxemic respiratory failure: clinical outcomes and mechanisms for treatment response in the PRO-NIV study. Crit Care. 2022;26(1):118. doi: 10.1186/s13054-022-03937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esperatti M, Busico M, Fuentes NA, Gallardo A, Osatnik J, Vitali A, Wasinger EG, Olmos M, Quintana J, Saavedra SN, et al. Impact of exposure time in awake prone positioning on clinical outcomes of patients with COVID-19-related acute respiratory failure treated with high-flow nasal oxygen: a multicenter cohort study. Crit Care (Lond, Engl) 2022;26(1):16. doi: 10.1186/s13054-021-03881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrando C, Mellado-Artigas R, Gea A, Arruti E, Aldecoa C, Adalia R, Ramasco F, Monedero P, Maseda E, Tamayo G, et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020;24(1):597. doi: 10.1186/s13054-020-03314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierucci P, Ambrosino N, Di Lecce V, Dimitri M, Battaglia S, Boniello E, Portacci A, Resta O, Carpagnano GE. Prolonged active prone positioning in spontaneously breathing non-intubated patients With COVID-19-associated hypoxemic acute respiratory failure with PaO(2)/FiO(2) >150. Front Med. 2021;8:626321. doi: 10.3389/fmed.2021.626321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sryma P, Mittal S, Mohan A, Madan K, Tiwari P, Bhatnagar S, Trikha A, Dosi R, Bhopale S, Viswanath R, et al. Effect of proning in patients with COVID-19 acute hypoxemic respiratory failure receiving noninvasive oxygen therapy. Lung India. 2021;38(7 Supplement 1):S6–S10. doi: 10.4103/lungindia.lungindia_794_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zang X, Wang Q, Zhou H, Liu S, Xue X. Efficacy of early prone position for COVID-19 patients with severe hypoxia: a single-center prospective cohort study. Intensive Care Med. 2020;46(10):1927–1929. doi: 10.1007/s00134-020-06182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altinay M, Sayan I, Turk HS, Cinar AS, Sayın P, Yucel T, Islamoglu S, Ozkan MT, Cetiner I. Effect of early awake prone positioning application on prognosis in patients with acute respiratory failure due to COVID-19 pneumonia: a retrospective observational study. Braz J Anesthesiol. 2022;72(2):194–199. doi: 10.1016/j.bjane.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker J, Pan D, Koeckerling D, Baldwin AJ, West R. Effect of serial awake prone positioning on oxygenation in patients admitted to intensive care with COVID-19. Postgrad Med J. 2021;98(1159):360–364. doi: 10.1136/postgradmedj-2020-139631. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Zhang J, Feng H, Wan F, Zhang Y, Tan L. Prone positioning in intubated and mechanically ventilated patients with SARS-CoV-2. J Clin Anesth. 2021;71:110258. doi: 10.1016/j.jclinane.2021.110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagan N, Morrow LE, Walters RW, Klein LP, Wallen TJ, Chung J, Plambeck RW. The positioned study: prone positioning in nonventilated coronavirus disease 2019 patients—a retrospective analysis. Crit Care Explor. 2020;2(10):e0229. doi: 10.1097/CCE.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koike T, Hamazaki N, Kuroiwa M, Kamiya K, Otsuka T, Sugimura K, Nishizawa Y, Sakai M, Miida K, Matsunaga A, et al. Detailed changes in oxygenation following awake prone positioning for non-intubated patients with COVID-19 and hypoxemic respiratory failure—a historical cohort study. Healthcare (Basel, Switzerland) 2022;10(6):1006. doi: 10.3390/healthcare10061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Numata K, Kobayashi D, Hosoda T, Saito Y, Minoura A, Yamazaki S, Fujitani S. Efficacy of awake prone positioning for severe illness coronavirus disease 2019 patients: a propensity score-adjusted cohort study. Acute Med Surg. 2022;9(1):e734. doi: 10.1002/ams2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padrão EMH, Valente FS, Besen B, Rahhal H, Mesquita PS, de Alencar JCG, da Costa MGP, Wanderley APB, Emerenciano DL, Bortoleto FM, et al. Awake prone positioning in COVID-19 hypoxemic respiratory failure: exploratory findings in a single-center retrospective cohort study. Acad Emerg Med. 2020;27(12):1249–1259. doi: 10.1111/acem.14160. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Nieto OR, Escarraman-Martinez D, Guerrero-Gutierrez MA, Zamarron-Lopez EI, Mancilla-Galindo J, Kammar-Garcia A, Martinez-Camacho MA, Deloya-Tomas E, Sanchez-Diaz JS, Macias-Garcia LA, et al. Awake prone positioning and oxygen therapy in patients with COVID-19: The APRONOX study. Eur Respir J. 2021;15:15. doi: 10.1183/13993003.00265-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prud'homme E, Trigui Y, Elharrar X, Gaune M, Loundou A, Lehingue S, Boyer A, Lefebvre L, Dols AM, Chanez P, et al. Effect of prone positioning on the respiratory support of nonintubated patients with COVID-19 and acute hypoxemic respiratory failure: a retrospective matching cohort study. Chest. 2021;160(1):85–88. doi: 10.1016/j.chest.2021.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stilma W, van Meenen DMP, Valk CMA, de Bruin H, Paulus F, Serpa Neto A, Schultz MJ. On behalf of the P-CCG: incidence and practice of early prone positioning in invasively ventilated COVID-19 patients-insights from the PRoVENT-COVID observational study. J Clin Med. 2021;10(20):19. doi: 10.3390/jcm10204783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonelli R, Pisani L, Tabbì L, Comellini V, Prediletto I, Fantini R, Marchioni A, Andrisani D, Gozzi F, Bruzzi G, et al. Early awake proning in critical and severe COVID-19 patients undergoing noninvasive respiratory support: a retrospective multicenter cohort study. Pulmonology. 2021. [DOI] [PMC free article] [PubMed]
- 47.Mancebo J, Fernández R, Blanch L, Rialp G, Gordo F, Ferrer M, Rodríguez F, Garro P, Ricart P, Vallverdú I, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173(11):1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 48.Beitler JR, Shaefi S, Montesi SB, Devlin A, Loring SH, Talmor D, Malhotra A. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 2014;40(3):332–341. doi: 10.1007/s00134-013-3194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, Aldecoa C, Martínez-Pallí G, Martínez-González MA, Slutsky AS, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beloncle F, Studer A, Seegers V, Richard JC, Desprez C, Fage N, Merdji H, Pavlovsky B, Helms J, Cunat S, et al. Longitudinal changes in compliance, oxygenation and ventilatory ratio in COVID-19 versus non-COVID-19 pulmonary acute respiratory distress syndrome. Crit Care. 2021;25(1):248. doi: 10.1186/s13054-021-03665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goligher EC, Ranieri VM, Slutsky AS. Is severe COVID-19 pneumonia a typical or atypical form of ARDS? And does it matter? Intensive Care Med. 2021;47(1):83–85. doi: 10.1007/s00134-020-06320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749–776. doi: 10.1016/j.ccc.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss TT, Cerda F, Scott JB, Kaur R, Sungurlu S, Mirza SH, Alolaiwat AA, Kaur R, Augustynovich AE, Li J. Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: a retrospective observational cohort study. Br J Anaesth. 2021;126(1):48–55. doi: 10.1016/j.bja.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy A, Behera S, Pande A, Bhattacharjee A, Bhattacharyya A, Baidya DK, Anand RK, Ray BR, Subramaniam R, Maitra S. Physiological effect of prone positioning in mechanically ventilated SARS-CoV-2- infected patients with severe ARDS: an observational study. J Anaesthesiol Clin Pharmacol. 2022;38(Suppl 1):S120–s124. doi: 10.4103/joacp.joacp_282_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Protti A, Santini A, Pennati F, Chiurazzi C, Ferrari M, Iapichino GE, Carenzo L, Dalla Corte F, Lanza E, Martinetti N, et al. Lung response to prone positioning in mechanically-ventilated patients with COVID-19. Crit Care. 2022;26(1):127. doi: 10.1186/s13054-022-03996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valter C, Christensen AM, Tollund C, Schønemann NK. Response to the prone position in spontaneously breathing patients with hypoxemic respiratory failure. Acta Anaesthesiol Scand. 2003;47(4):416–418. doi: 10.1034/j.1399-6576.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 57.Feltracco P, Serra E, Barbieri S, Persona P, Rea F, Loy M, Ori C. Non-invasive ventilation in prone position for refractory hypoxemia after bilateral lung transplantation. Clin Transplant. 2009;23(5):748–750. doi: 10.1111/j.1399-0012.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 58.Feltracco P, Serra E, Barbieri S, Milevoj M, Michieletto E, Carollo C, Rea F, Zanus G, Boetto R, Ori C. Noninvasive high-frequency percussive ventilation in the prone position after lung transplantation. Transplant Proc. 2012;44(7):2016–2021. doi: 10.1016/j.transproceed.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 59.Scaravilli V, Grasselli G, Castagna L, Zanella A, Isgrò S, Lucchini A, Patroniti N, Bellani G, Pesenti A. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Liu L, Xie J, Wang C, Zhao Z, Chong Y, Yuan X, Qiu H, Zhao M, Yang Y, Slutsky AS. Prone position improves lung ventilation-perfusion matching in non-intubated COVID-19 patients: a prospective physiologic study. Crit Care. 2022;26(1):193. doi: 10.1186/s13054-022-04069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188(11):1286–1293. doi: 10.1164/rccm.201308-1532CI. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 3. List of excluded studies after full-text screening.
Additional file 4. Forest plots, table. Risk of bias of included randomized controlled trials (a, b) and non-randomized studies (c, d). (a) Risk of bias graph of randomized controlled trials: review authors' judgements about each risk of bias item presented as percentages across all included studies. (b) Risk of bias graph of randomized controlled trials: review authors' judgements about each risk of bias item for each included study. (c) Risk of bias graph of non-randomized studies: review authors' judgements about each risk of bias item presented as percentages across all included studies. (d) Risk of bias graph of non-randomized studies: review authors' judgements about each risk of bias item for each included study. Figure S2. Contour-enhanced funnel plot for mortality of non-intubation patients in non-randomized studies. Figure S3. Contour-enhanced funnel plot for intubation rate of non-intubation patients in non-randomized studies. Figure S4. Adverse events in randomized controlled trials. Table S1. Adverse events in non-randomized studies. Figure S5. Hospital length of stay of non-intubated patients. Figure S6. ICU length of stay of non-intubated patients. Figure S7. ICU-free days of non-intubated patients in randomized controlled trials. Figure S8. Ventilator-free days of non-intubated patients. Figure S9. Ventilator-free days of intubated patients in non-randomized studies.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.