Abstract
This case report describes the evaluation and management of a 32-year-old woman who presented shortly after a fetal demise at 23 weeks of gestation with multiple symptoms, including bloody vaginal discharge. Although the initial diagnostic concern was for metastatic malignancy, the patient was ultimately determined to have disseminated tuberculosis. Genital tuberculosis is common worldwide, yet guidelines for evaluation are limited. This report highlights the relationship between pregnancy-reactivated tuberculosis, and guides clinicians on diagnostic and management considerations in the peripartum period.
Keywords: Genital tuberculosis, Peri-partum period, Oncology, Ovarian cancer, Fetal demise
Highlights
-
•
Genital tuberculosis is common worldwide, yet guidelines for evaluation are limited.
-
•
Diagnosis relies on a high degree of clinical suspicion, leaving patients undiagnosed until advanced stages, often complicating fertility outcomes.
-
•
Providers should consider tuberculosis in a reproductive-aged patients with unusual presentations, especially in the peripartum period.
1. Introduction
Worldwide, genital tuberculosis is a common form of extrapulmonary tuberculosis, accounting for up to 9% of all cases of non-pulmonary disease [1]. However, the burden of genital tuberculosis is frequently underestimated in women, particularly in high-resource settings. Genital tuberculosis is often a chronic indolent disease with low-grade symptoms that are classically non-specific and overlooked [2]. In fact, many cases are diagnosed during infertility workup [3]. Rates of genital tuberculosis contributing to infertility vary by geographic location, with incidence as high as 3–16% in higher-prevalence regions such as the Indian subcontinent [4].
Current guidelines for the evaluation of genital tuberculosis are limited. Diagnosis relies on a high degree of clinical suspicion, leaving many patients undiagnosed until advanced stages, when severe fibrosis and adhesions may complicate desired pregnancy outcomes. This case report highlights the importance of considering genital tuberculosis when assessing gynecologic pathology. It also underscores the importance of having a reasonably low index for suspicion of infection, including tuberculosis reactivation, in the peripartum period.
2. Case Presentation
A 32-year-old woman with a history of chronic vestibular migraines suffered a fetal demise at 23 weeks of gestation secondary to premature preterm rupture of membranes, with acute placental abruption noted on pathology. The pregnancy had been the result of in vitro fertilization (IVF), for which she had previously undergone hysterosalpingography, which was suggestive of blockage of the fallopian tubes. She denied a history of pelvic inflammatory disorder or sexually transmitted infections.
Seven weeks later, she presented with an abrupt worsening of her chronic headache symptoms, now accompanied by nausea and dizziness, leaving her bedbound. She did not report weakness or other focal neurological symptoms. She also endorsed bloody mucoid vaginal discharge starting one week prior to the fetal demise, which continued despite the pregnancy loss. On presentation, she endorsed several weeks of mild persistent fevers that had been evaluated by her primary medical provider with an unrevealing workup. Outpatient ultrasonography did not show retained placental products, and genital cultures for gonorrhea and chlamydia were negative.
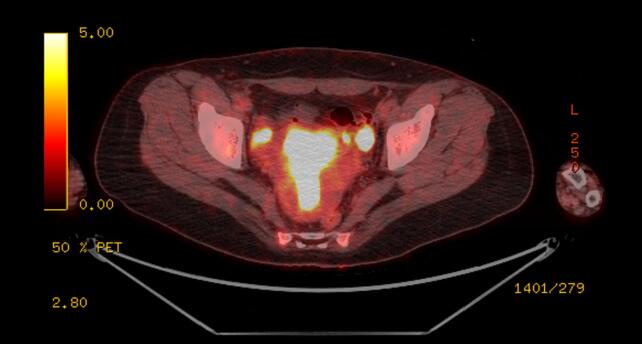
Given the severity and change in quality of the headaches, brain magnetic resonance imaging (MRI) was obtained, which showed multiple rim-enhancing lesions associated with vasogenic edema (Fig. 1). She was admitted to the neurology service. Her physical and neurologic exams were normal. Cerebrospinal fluid (CSF) studies did not reveal a lymphocytic pleocytosis, and cultures for bacteria, mycobacteria, and fungi were negative. CSF pathology was negative for malignancy. MRI of the spine demonstrated abnormal T2 signals at C4 and T10, concerning for metastatic lesions. Computed tomography (CT) of the chest, abdomen and pelvis revealed a 0.5 mm right upper lobe ground-glass pulmonary nodule, left fallopian tube dilation concerning for salpingitis, and an attenuating 1.6 cm structure in the right adnexa (Fig. 2). The patient underwent positron emission tomography (PET/CT), which showed intense heterogenous hypermetabolic activity in the endometrium, cervix, and left external iliac nodes, with thickened left fallopian tube showing intense metabolic uptake (Fig. 3). The previously observed right adnexal mass had no uptake and was felt to be non-specific. The reported T10 spinal lesion was also metabolically active.
Fig. 1.
Magnetic resonance imaging: brain imaging showing multiple rounded enhancing lesions with concern for metastases.
Fig. 2.
Computed tomography: 1.6 cm rounded high attenuation structure in the right adnexa of uncertain etiology.
Fig. 3.
PET-CT: Thickened left fallopian tube and endometrium,with intense uptake.
With a concern for metastatic malignancy, tumor markers, including cancer antigen (CA)-125, carcinoembryonic antigen (CEA), and human chorionic gonadotropin (HCG) levels, were obtained and were all normal. An endometrial biopsy showed evidence of granulomatous inflammation but was negative for acid-fast bacilli (AFB) staining and for malignancy. Active cancer was thought to be unlikely.
During hospitalization, vaginal discharge continued, but the patient's other symptoms, including headache, dizziness, and fever, markedly improved, and she was discharged without definite diagnosis. On outpatient evaluation, she endorsed a history of untreated latent tuberculosis and serum QuantiFERON-TB Gold test was positive. She was then referred to the infectious diseases outpatient clinic. She reported she was born in India and moved to the United States eight years previously. She did not have any known tuberculosis exposures. She reported a prior positive screen for tuberculosis when she underwent IVF, and at that time, chest radiography did not show evidence of active tuberculosis. Serologic testing for other infections, including coccidioidomycosis, syphilis, and HIV, were all negative.
Given her ongoing bloody mucoid vaginal discharge and multiple radiographic findings, there was a concern for disseminated infection. The patient ultimately underwent repeat endometrial and endocervical biopsies: although AFB stains were again negative, this time both culture and PCR were ultimately positive for M. tuberculosis. The patient also had three induced sputum analyses for AFB smear, culture, and mycobacterium tuberculosis (MTB) PCR which were all negative, indicating she was not contagious.
A diagnosis of disseminated tuberculosis was made, with involvement of the endometrium, fallopian tubes, and tuberculomas of the brain and spinal column. Based on guidelines from the Infectious Disease Society of America, she was treated with a four-drug regimen consisting of rifampin, isoniazid, pyrazinamide, and ethambutol [5]. Vaginal discharge resolved after several weeks. Repeat CT and MRI four months after treatment initiation showed overall improvement, and a repeat endometrial biopsy revealed resolution of granulomatous inflammation. Given her presumed CNS involvement, treatment was narrowed to rifampin and isoniazid to complete one year of therapy.
3. Discussion
The global prevalence of genital tuberculosis is estimated at eight to ten million people [1]. However, its burden in women remains underestimated. This is in part due to the condition's challenging diagnosis. There is no single test for genital tuberculosis; instead, high clinical suspicion, physical exam, and persistent testing may yield the diagnosis [6]. While the morphology of genital organs infected with tuberculosis varies, the fallopian tubes classically show the earliest changes, affected in almost 90% of cases [2].
Well documented in the literature is the difficulty of distinguishing tuberculosis and suspected gynecologic malignancy. Presenting symptoms, including abdominal pain and weight loss, are often similar; and clinical findings run in parallel, with ascites, retroperitoneal lymphadenopathy, and elevated CA-125 levels [4]. Patients may present with adnexal masses, particularly in the case of ovarian tuberculosis. In one study from Turkey, among 612 surgeries performed for suspected ovarian malignancy between 2000 and 2011, 3.3% had pathologically confirmed tuberculosis but benign disease [7]. These tuberculosis cases exhibited similar intra-operative findings, with widespread adhesions and adnexal masses. Similar findings have been reported elsewhere [8].
In this patient, the initial concern was for metastatic gynecologic malignancy. The malignancies commonly encountered in pregnancy are those typical of the reproductive age (i.e., breast, hematologic, melanoma, cervical, lung). Given her post-partum state, gestational trophoblastic disease (e.g. choriocarcinoma) was considered in the differential diagnosis, yet urine and serum HCG were within normal limits. The patient was considered to be at low risk of primary malignancy given her young age and non-significant family history. The granulomatous inflammation on biopsy – while not unique to tuberculosis – was a clue to the diagnosis of systemic disseminated disease, which was ultimately identified on PCR testing.
Pregnancy has been associated with an increased incidence and severity of many infectious diseases, particularly viruses, including influenza and seasonal respiratory viruses and SARS-Cov-2 [6,9,10]. In addition, many other non-viral infections that typically present with limited pulmonary disease, including mycobacterial infections such as tuberculosis and endemic fungal infections such as coccidioidomycosis, may present with disseminated infection in the context of pregnancy. The relationship between pregnancy and tuberculosis reactivation is a complicated one and not fully understood [[11], [12], [13]]. The incidence of tuberculosis among pregnant women in the United States was 26.6 per 100,000 births from 2003 to 2011, as compared to 5.1–3.4 over that same period in the general population [14,15]. Perhaps better referred to as a “unique immune condition” than a true state of immune suppression, immunomodulatory changes during pregnancy have been hypothesized to contribute to this discrepancy, with pregnancy-related hormonal changes modulating the maternal immune system [[16], [17], [18], [19]].
For example, altered immune processes, such as the suppression of pro-inflammatory cytokines, may play an important role in the cell-mediated immunity responsible for the latency of tuberculosis [16]. Others comment on the cortisol-mediated vascular permeability of pregnancy, which may facilitate hematogenous dissemination of a reactivated tuberculosis infection [16,17,20]. Tuberculosis reactivation during pregnancy may result in maternal signs and symptoms of pulmonary or extrapulmonary disease, or – as in the case with this patient – may also result in spontaneous abortion, fetal malformation, or fetal demise [17,20]. Reactivated tuberculosis that does not affect the pregnancy may be more likely to present during the postpartum immune reconstitution period, when proinflammatory cytokines surge to above their pre-pregnancy levels [18].
Tuberculosis can be difficult to diagnose, especially in the peri-partum period, and no single laboratory test is sufficient for the diagnosis of genital tuberculosis. In this patient, a high clinical suspicion was required for appropriate investigation. She ultimately underwent three different endometrial biopsies for an appropriate diagnosis. Her AFB smears were always negative, consistent with the low sensitivity of this test (quoted as low as 38.8% in some of the literature) [21,22]. Conversely, MTB culture has a high sensitivity (96%), although this test takes as long as 4–6 weeks to result [6,22]. MTB PCR is a helpful diagnostic test to include with culture as well [6].
Treatment of most forms of tuberculosis in pregnancy is similar to that in non-pregnant hosts. For this patient, with the clinical suspicion of neurologic disease given tuberculomas on brain MRI, the treatment duration was extended to 12 months of combination therapy. If she had still been pregnant, guidelines typically indicate treatment with a three-drug regimen, typically for nine months, excluding pyrazinamide, for which detailed teratogen data is not available [5]. Practice guidelines for the management of patients with tuberculosis, including pregnant patients, are published and updated on a regular basis [5].
This case report discusses the overlap between genital tuberculosis, suspected metastatic malignancy, and pregnancy. Health care providers should consider the possibility of tuberculosis in a patient of reproductive age who has unusual presentation of disease, especially in the peripartum period.
Contributors
Isabel Beshar was involved in patient care, was responsible for the conception of the case report, and drafted the manuscript.
Ashley S. Moon was involved in patient care, participated in the conception of the case report, and edited the manuscript.
Ruchita Pendse contributed to data interpretation and revised the article for important intellectual content.
Andrew B. Nevins was involved in patient care, contributed to data interpretation, and revised the article critically for important intellectual content.
Babak Litkouhi was involved in patient care, contributed to conception of the case report, and revised the article critically for important intellectual content.
All authors approved the final submitted manuscript.
Funding
No funding from an external source supported the publication of this case report.
Patient consent
The patient consented to the publication of this report and accompanying images.
Provenance and peer review
This article was not commissioned and was peer reviewed.
Declaration of Competing Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Contributor Information
Isabel Beshar, Email: ibeshar@stanford.edu.
Ashley S. Moon, Email: ashmoon@stanford.edu.
Ruchita Pendse, Email: Rpendse@montefiore.org.
Andrew B. Nevins, Email: anevins@stanford.edu.
Babak Litkouhi, Email: blitkouhi@stanford.edu.
References
- 1.Bose Mridula. Female genital tract tuberculosis: how long will it elude diagnosis? Indian J. Med. Res. 2011;134:13–14. [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma J.B. Current diagnosis and management of female genital tuberculosis. J. Obstet. Gynecol. India. 2015 Dec;65(6):362–371. doi: 10.1007/s13224-015-0780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varghese A., Fader A., Wilbur M., Salimian K., Azadi J.R., Johnson P.T., et al. Peritoneal tuberculosis: the great mimicker. Int. J. Gynecol. Cancer. 2020 Apr;30(4):546–550. doi: 10.1136/ijgc-2020-001291. [DOI] [PubMed] [Google Scholar]
- 4.Ozgu E. Tuberculosis in gynecologic oncology: still a diagnostic dilemma. Obstet. Gynecol. Int. J. [Internet]. 2014;1(2) Nov 18 [cited 2022 Jan 31]. Available from: https://medcraveonline.com/OGIJ/tuberculosis-in-gynecologic-oncology-still-a-diagnostic-dilemma.html. [Google Scholar]
- 5.Nahid P., Dorman S.E., Alipanah N., Barry P.M., Brozek J.L., Cattamanchi A., et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 2016 Oct 1;63(7):e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H.S., Park K.U., Park J.O., Chang H.E., Song J., Choe G. Rapid, sensitive, and specific detection of mycobacterium tuberculosis complex by real-time PCR on paraffin-embedded human tissues. J. Mol. Diagn. 2011 Jul;13(4):390–394. doi: 10.1016/j.jmoldx.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oge T., Ozalp S.S., Yalcin O.T., Kabukcuoglu S., Kebapci M., Arik D., et al. Peritoneal tuberculosis mimicking ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012 May;162(1):105–108. doi: 10.1016/j.ejogrb.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Devi L., Tandon R., Goel P., Huria A., Saha P.K. Pelvic tuberculosis mimicking advanced ovarian malignancy. Trop. Dr. 2012 Jul;42(3):144–146. doi: 10.1258/td.2012.120076. [DOI] [PubMed] [Google Scholar]
- 9.Uyeki T.M., Bernstein H.H., Bradley J.S., Englund J.A., File T.M., Fry A.M., et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clin. Infect. Dis. 2019 Mar 5;68(6):e1–47. doi: 10.1093/cid/ciy866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson A., Thung S.F., Norwitz E.R. 2022. H1N1 Influenza in Pregnancy: What All Obstetric Care Providers Ought to Know. :7. [PMC free article] [PubMed] [Google Scholar]
- 11.Bercovitch R.S., Catanzaro A., Schwartz B.S., Pappagianis D., Watts D.H., Ampel N.M. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin. Infect. Dis. 2011 Aug 15;53(4):363–368. doi: 10.1093/cid/cir410. [DOI] [PubMed] [Google Scholar]
- 12.Mathad J.S., Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin. Infect. Dis. 2012 Dec 1;55(11):1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsson J., Kühlmann-Berenzon S., Berggren I., Bruchfeld J. Increased risk of active tuberculosis during pregnancy and postpartum: a register-based cohort study in Sweden. Eur. Respir. J. 2020 Mar;55(3):1901886. doi: 10.1183/13993003.01886-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Messidi A., Czuzoj-Shulman N., Spence A.R., Abenhaim H.A. Medical and obstetric outcomes among pregnant women with tuberculosis: a population-based study of 7.8 million births. Am. J. Obstet. Gynecol. 2016 Dec;215(6):797.e1–797.e6. doi: 10.1016/j.ajog.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Center for Disease Control and Prevention . 2022. TB Incidence in the United States. Available from: https://www.cdc.gov/tb/statistics/tbcases.htm. [Google Scholar]
- 16.Liu K., Zhang X., Ma X., Jia X., Zhao X., Yang X., et al. A case report of cervical pregnancy after in vitro fertilization complicated by tuberculosis and a literature review. Open Med. 2020 Nov 9;15(1):1083–1086. doi: 10.1515/med-2020-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gai X., Chi H., Cao W., Zeng L., Chen L., Zhang W., et al. Acute miliary tuberculosis in pregnancy after in vitro fertilization and embryo transfer: a report of seven cases. BMC Infect. Dis. 2021 Dec;21(1):913. doi: 10.1186/s12879-021-06564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasleem A., Mahmood A., Bharat A. Vol. 5. 2020. An Unfortunate Case of Reactivation of Tuberculosis in a Postpartum Female. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., He C., Chen L. Pregnancy in a patient with endometrial tuberculosis by in vitro fertilization: a case report. J. Int. Med. Res. 2020 Nov;48(11) doi: 10.1177/0300060520967824. 030006052096782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Raya B., Michalski C., Sadarangani M., Lavoie P.M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 2020 Oct 7;(11) doi: 10.3389/fimmu.2020.575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasool G., Khan A.M., Mohy-Ud-Din R., Riaz M. Detection of mycobacterium tuberculosis in AFB smear-negative sputum specimens through MTB culture and GeneXpert ® MTB/RIF assay. Int. J. Immunopathol. Pharmacol. 2019 Jan;33 doi: 10.1177/2058738419827174. 205873841982717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew P., Kuo Y.H., Vazirani B., RHK Eng, Weinstein M.P. 2002. Are Three Sputum Acid-Fast Bacillus Smears Necessary for Discontinuing Tuberculosis Isolation? :3. [DOI] [PMC free article] [PubMed] [Google Scholar]