Abstract
Purpose
We describe a modified technique for managing a peripheral, non-infected, corneal perforation using a “Sandwich” technique that combines posterior lamellar keratoplasty, an amniotic membrane patch and a Gundersen conjunctival flap.
Observations
A 75-year-old female patient presented with Sjogren's syndrome-related corneal perforation. A mini-Descemet stripping automated endothelial keratoplasty (DSAEK) graft (5 mm) was introduced into the anterior chamber and was mobilized to plug the perforation. Then, two amniotic membrane patches were stacked over the perforation and glued. Finally, the whole area was covered with a Gundersen conjunctival flap, mobilized from the inferior conjunctiva and secured in place using interrupted 10-0 nylon sutures. A step-by-step guide is also described. At three months, the final visual acuity was 6/9. A literature review revealed ten cases in which a posterior lamellar graft was effectively employed to treat corneal perforations.
Conclusions and Importance
We described a modified approach for treating peripheral corneal perforation surgically. This “sandwich” approach is simple to replicate and can give quick healing with few visual repercussions. Our detailed guide may be utilized to obtain similar results and may be added to the array of treatment options for peripheral corneal perforation.
Keywords: Descemet stripping automated endothelial keratoplasty, Graft, Perforation, Management
1. Introduction
Corneal perforations are considered a potential sight-threatening emergency. It might arise due to local infection, inflammation, or systemic autoimmune disorder. Management choices are influenced by the location and size of the perforation and the underlying etiology and visual potential.1
Depending on the perforation's size, cyanoacrylate glue with a plastic drape plug can be used as an initial temporizing measure.2 Long-term management can include an anterior lamellar or penetrating keratoplasty.3 Recently, a novel method that includes only posterior lamellar keratoplasty graft was described using an amniotic membrane in a staged approach for impending corneal perforation. This resulted in structural support, reepithelization and ocular surface healing.4 To the best of our knowledge, only 11 cases were reported, where a tectonic posterior lamellar keratoplasty graft was used in the management of impending or actual perforation, Table 1.,4, 5, 6, 7, 8 Therefore this paper aims to add to the existing research by presenting our experience with posterior lamellar keratoplasty in the treatment of peripheral perforation cases. We have modified the original method by Graue-Hernandez et al. for impending perforation4 and termed it the “top-up sandwich technique.” The corneal surface is reinforced with a partial Gundersen flap externally in addition to a an external layer of amniotic membrane sandwiched between the perforation and a mini-posterior lamellar graft in the anterior chamber.
Table 1.
Previous cases of a tectonic posterior lamellar keratoplasty graft in managing impending or actual perforation.
| Author | Year | Region | Number of cases | Description | Type of procedure | Follow up | Outcomes | Final VA |
|---|---|---|---|---|---|---|---|---|
| Graue-Hernandez4 | 2012 | Mexico | 1 | Corneal thinning secondary to dry eye. | A tectonic DSAEK and amniotic membrane graft. | 8 months | Ocular surface healed quickly, and re-epithelization occurred over 2 weeks and lasted for follow-up. | NA |
| Nahum5 | 2016 | Israel and Italy | 3 | Case 1,2: Endothelial decompensation and sterile corneal perforation. Case 3- an impending corneal perforation. |
DSAEK | Case 1: 3 months Case 2: 1 year Case 3: 1 year |
All grafts remained clear throughout the follow-up. Case 1 underwent DALK on DSAEK with improved visual acuity. | Case 1–20/30 Case 2–20/60 Case 3–20/400 |
| Okada8 | 2016 | Japan | 1 | A corneal perforation of undetermined etiology. | Partial-thickness corneal button | 5 months | Seven weeks postoperatively, the corneal defect was closed and lasted for follow-up. | 6/6 |
| Jesse6 | 2017 | Switzerland | 1 | A perforated corneal ulcer due to neurotrophic keratopathy caused by previous recurrent herpetic keratitis. | A DSAEK graft. | 6 months | Re-bubbling was performed the day after and four 10–0 Nylon sutures were placed for 2 weeks those = The long-term follow-up showed stable and transparent cornea. | CF due to severe AMD. |
| Tourkmani7 | 2020 | United Kingdom | 4 | Case 1- perforation due to exposure keratopathy Case 2,3- perforation due to herpetic keratitis Case 4- corneal hydrops secondary to PMD. |
Cases 1–3: DSEK Case 4: hemi-DSEK | Case 1: 2 months Case 2:NA Case 3:NA Case 4:NA |
Case 1–4: Tectonic goal satisfactorily achieved. Case 4- patient underwent 2 re-floating attempts before anchoring suture placement due to repeated graft detachment. |
Case 1-NA, without visual potential. Case 2-NA Case 3-NA Case 4-NA |
DSEK; Descemet stripping endothelial keratoplasty, DSAEK; Descemet stripping automated endothelial keratoplasty, BCVA; best-corrected visual acuity, VA; visual acuity, CF; counting fingers, PMD; pellucid marginal degeneration, NA; not available, DALK; deep anterior lamellar keratoplasty.
This study also includes step-by-step guidance to facilitate understanding of our methods, hoping other surgeons would adopt it when faced with similar situations.
2. Methods
A 75-year-old female with a history of hypertension and chronic obstructive pulmonary disease was referred to our tertiary center for a tectonic keratoplasty. She had no relevant ocular history other than bilateral cataract surgery more than a year earlier with no complications and a quiescent follow-up. The history of the present illness started four weeks prior with spontaneous ulceration. There was no evidence of corneal infiltrates. Microbial culture, sensitivity, and viral swabs were all done and returned negative. Blood work was done to rule out underlying autoimmune etiology, and the results were within normal range. The etiology was presumed to be related to underlying Sjogren disease. The patient received pulse steroid treatment over three days with subsequent oral steroids, which were weaned over six weeks.
Given the positive response and lack of active inflammation, ongoing immunosuppressive medications were not deemed necessary. Upon presentation, the patient had a 3 × 2 mm right side peripheral inferior corneal perforation. An active leak was partially sealed through the perforation wound by iris tissue, cyanoacrylic glue, and a plastic drape plug. With repeated gluing, a complete seal was created. Topical treatment included prophylactic antibiotics, prednisolone 0.5%, and lubrication. All topical treatment was preservative free. Oral Doxycycline was added. Due to the perforation's size, the irregular depth of the anterior chamber, and the eye's visual potential, surgical management was considered the definitive solution. Even more than a year after the procedure, the patient is still being followed up on by our department.
2.1. Surgical technique
The donor cornea was mounted an artificial anterior chamber (Single-use artificial chamber, Moria) and the epithelium was removed. The approximate thickness of the DSAEK was between 150 and 160 μm, measured with a hand-held pachymetry. The amniotic membrane transplant diameter was 3 mm punched using a standard dermatology punch.
A single-pass lamellar dissection was performed using a 2.75 mm metal keratome. The residual stromal bed was then punched using a 5 mm dermatology punch, and the tissue was marked and dyed with vision blue (DORC, Zuidland, The Netherlands). An anterior chamber (AC) maintainer was introduced following sterilization and draping. The anterior synechiae were broken by gentle sweeps using an iris repositor. With a complete seal of the perforation still maintained by the glue, the graft was introduced manually through a 3 mm incision. Once in the eye, the main port was sutured with 3x 10/0 Nylon sutures, the AC maintainer was removed, and the DSAEK (Descemet Stripping Endothelial Keratoplasty) corneal graft was floated by air injection at the iris plan. A 30G needle ab interno achieved graft mobilization to avoid destabilization of the glue. Once in place, the glue was removed, and more air was injected to ascertain a complete seal. The epithelium was next debrided around the perforation and two amniotic membrane discs were placed using tissue glue (Tisseel, Baxter Advanced Surgery, Deerfield, IL, US). For added stability, a Gundersen conjunctival partial flap was then mobilized from the adjacent inferior conjunctiva and pulled to cover the whole ulcerated area and secured in place by combining tissue glue and 10/0 Nylon sutures. The Gunderson flap has not receded. A bandage contact lens (BCL) was placed (Movie 1-Supplemental digital content).
The following is the supplementary data related to this article:
1
3. Result
During follow-up, the BCL was removed and the anterior chamber was formed with no leak. Anterior segment photos and anterior segment OCT are presented in Fig. 1A, Fig. 1B, Fig. 2A, Fig. 2B. Visual acuity was 6/60 at week one following surgery and final visual acuity at four months was 6/9. The patient denoted comfort as soon as week one.
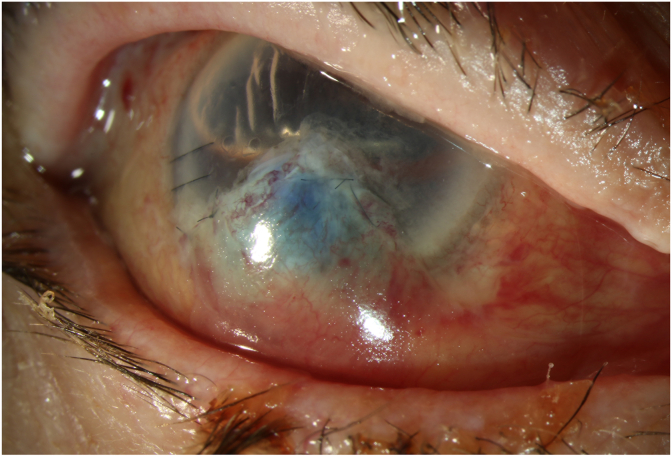
Fig. 1A.
Anterior segment photography at day 1 post-operatively.
Anterior segment photography presents a sealed perforation by the “Sandwich” technique which combines posterior lamellar keratoplasty, an amniotic membrane patch, and a Gundersen conjunctival flap.
Fig. 1B.
Anterior segment photography at 6 weeks post-operatively.
Anterior segment photography presents a sealed wound with a satisfactory result.
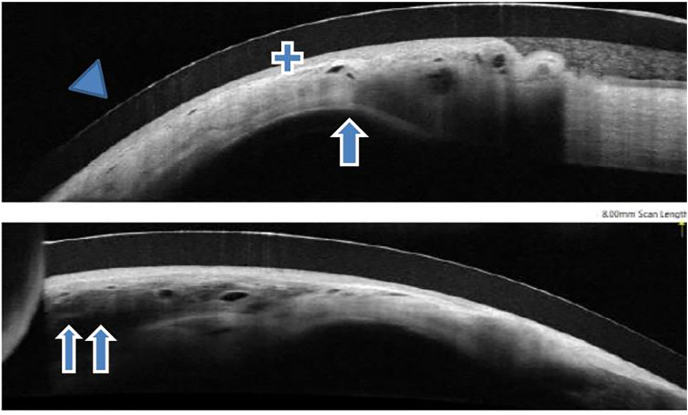
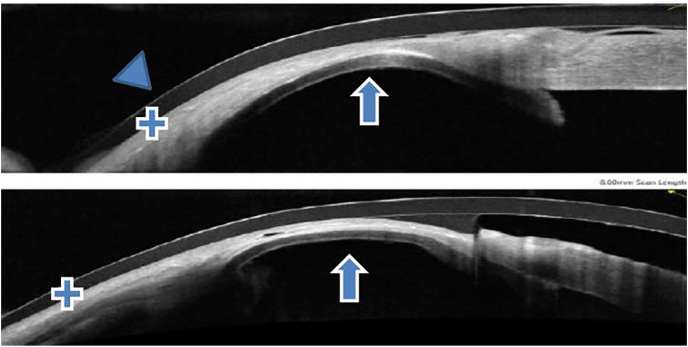
Fig. 2A.
Anterior Segment OCT (Optical Coherence Tomography) photos 1 week post-operatively.
Fig. 2B.
Anterior Segment OCT (Optical Coherence Tomography) photos at 6 weeks post-operatively. Arrow head: contact lens Single arrow: DSAEK button Double arrow: amniotic membrane transplantation Plus sign: conjunctiva.
4. Discussion
In this study, we demonstrate the effectiveness of a posterior lamellar graft in the treatment of corneal perforation. According to the literature, most grafts were larger than 8 mm, however similar to our case, Tourkmani et al. also employed a smaller graft (Hemi-DSAEK) to seal off the inferior perforation in a young patient with pellucid marginal degeneration.7 We propose that the DSAEK graft be added to the arsenal of treatments for corneal perforation. We chose a tectonic DSAEK due to considerations of perforation's location, visual potential, age, co-morbidities, and anesthetic risk. The surgeon could consider suture pull-through to pass needles to intended site of placement and drag the lenticule directly to that location. Similar to Hernandez et al., who strengthen the superior cornea with the amniotic membrane,4 we stabilize the perforation with double amniotic membranes and an additional Gundersen partial flap. This benefits from adding an extra layer of protection, promoting cornea healing underneath, and improving the patient's comfort.
In contrast to full tectonic (penetrating keratoplasty) or partial (deep anterior lamellar keratoplasty), tectonic DSAEK procedure will not induce significant corneal astigmatism. Furthermore, it is characterized by a rapid healing time, reduces the need for general anesthetic, has no graft sutures-related complications, and is associated with less immunological rejection.9 In our case, we introduced the tissue through a 3 mm incision by pulling it with endoglide forceps. Other authors suggested that the graft might be inserted through the perforation wound, removing the necessity for the main corneal incision utilized in traditional DSAEK.5 In our experience, this is the sole option if the perforation is more significant than 4 mm in diameter. The size chosen for the corneal graft was sufficient to completely seal the perforation without extending to affect the visual axis or excessively cover normally functioning endothelial cells while leaving as much recipient endothelium as possible untouched. We recommend choosing a graft size at least 1.5 mm wider than the perforation's largest diameter. In addition, maintaining a temporary perforation seal throughout surgery is paramount during the graft introduction and mobilization. Using cyanoacrylate gluing can facilitate easier manipulation while the glue can be removed after graft introduction, mobilization and floating with air injection into the AC. Alternatively, with more extensive perforations, the graft tissue could be placed over the perforation and pushed into the anterior chamber directly.
Our literature review of the other report 11 cases is summarized in Table 1. Three of these cases had perforation owing to herpetic keratitis, three had undetermined etiology, two had endothelial decompensation, one had exposure keratitis, one had dry eye disease and one had corneal hydrops attributable to Pellucid marginal degeneration. The surgeries included a DSAEK tectonic graft, an amniotic membrane graft, and a hemi-DSAEK.7 Overall, using a DSAEK as a tectonic graft was successful in achieving a stable cornea surface with rapid healing (mean follow-up of 6.8 ± 3.71 months; mean final visual acuity of 6/15, Only two cases (2/11, 18%) needed a secondary procedure for rebuilding and sutures anchoring of the grafts. Moreover, It is important to use smear and tissue culture to rule out infection in suspected cases and, if necessary, antibiotic management. The use of antivirals in herpetic keratitis, the management of neurotrophic keratopathy in case it is the cause, and immunosuppressive treatment in autoimmune diseases. Furthermore, it is important to emphasize the importance of a correct diagnosis and management of the underlying disease for the long-term survival and outcome of the tectonic graft.
In conclusion, we present a modified technique for managing a peripheral, non-infected, corneal perforation using a “Sandwich” technique that combines posterior lamellar keratoplasty, an amniotic membrane patch and a Gundersen conjunctival partial flap. This “sandwich” approach is Straightforward to replicate and can give quick healing with few visual repercussions. Our detailed guide may be utilized to obtain similar results and may be added to the array of treatment options for peripheral corneal perforation.
Patient consent
Consent to publish this case report has been obtained from the patient in writing. This report does not contain any personal identifying information.
Disclosures authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Funding
No financial support was received for this submission.
Declaration of competing interest
The authors have no conflicts of interest.
Acknowledgements
None.
References
- 1.Deshmukh R., Stevenson L.J., Vajpayee R. Management of corneal perforations: an update. Indian J Ophthalmol. 2020;68(1):7–14. doi: 10.4103/ijo.IJO_1151_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okabe M., Kitagawa K., Yoshida T., et al. Application of 2-octyl-cyanoacrylate for corneal perforation and glaucoma filtering bleb leak. Clin Ophthalmol. 2013;7:649–653. doi: 10.2147/OPTH.S43106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabison E.E., Doan S., Catanese M., Chastang P., Ben M'hamed M., Cochereau I. Modified deep anterior lamellar keratoplasty in the management of small and large epithelialized descemetoceles. Cornea. 2011 Oct;30(10):1179–1182. doi: 10.1097/ICO.0b013e3182031c81. [DOI] [PubMed] [Google Scholar]
- 4.Graue-Hernandez E.O., Zuñiga-Gonzalez I., Hernandez-Camarena J.C., Jaimes M., Chirinos-Saldaña P., Navas A., et al. Tectonic DSAEK for the management of impending corneal perforation. Case Rep Ophthalmol Med. 2012;2012 doi: 10.1155/2012/916528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahum Y., Bahar I., Busin M. Tectonic Descemet stripping automated endothelial keratoplasty for the management of sterile corneal perforations in decompensated corneas. Cornea. 2016 Dec;35(12):1516–1519. doi: 10.1097/ICO.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 6.Jesse M., Lange A.P. Treatment of a perforated corneal ulcer with tectonic DSAEK: a case report. Klin Monbl Augenheilkd. 2017;234(4):448–450. doi: 10.1055/s-0043-100637. [DOI] [PubMed] [Google Scholar]
- 7.Tourkmani A.K., Ansari A.S., Hossain P.N., Konstantopoulos A., Anderson D.F. Tectonic Descemet stripping endothelial keratoplasty for the management of corneal perforation: a case series. Cornea. 2020 Dec;39(12):1571–1575. doi: 10.1097/ICO.0000000000002424. [DOI] [PubMed] [Google Scholar]
- 8.Okada A., Sano I., Ikeda Y., Fujihara E., Tanito M. Patch grafting using a cryopreserved Descemet stripping automated endothelial keratoplasty flap for treating corneal perforation. Case Rep Ophthalmol. 2016 Apr;7(1):202–207. doi: 10.1159/000445796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dapena I., Ham L., Melles G.R.J. Endothelial keratoplasty: DSEK/DSAEK or DMEK--the thinner the better? Curr Opin Ophthalmol. 2009 Jul;20(4):299–307. doi: 10.1097/ICU.0b013e32832b8d18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1