Abstract
Boiling has important effects on the texture of chicken eggs, but its effects on egg nutrients have not been systematically investigated. In this study, changes in the metabolites of egg yolks boiled under different heating intensities were quantified and compared by metabolomic analysis. A total of 797 metabolites were identified, and the abundance of 162 metabolites changed significantly after boiling. The significant reduction of L-lysine and D-fructose suggested that Maillard reactions occurred in over-boiled egg yolks. Egg yolk endogenous enzymes might induce a partial hydrolysis of proteins and phospholipids during the warm-up period of boiling, as the abundance of dipeptides, lysophospholipids, and free fatty acids was significantly increased in boiled egg yolks. Boiling increased the detectable abundance of fat-soluble vitamins, riboflavin, and biotin, possibly by altering the complex structure of protein-lipid-lipophilic compounds or denaturing vitamin-binding proteins. The results of metabolomic analyses provide important information for understanding the nutritional changes of egg yolk boiled under different heating intensities.
Keywords: Egg yolk, Metabolome, Maillard reaction, Phospholipids, Vitamins
Graphical abstract
Highlights
-
•
Metabolites of boiled egg yolks at different heating intensities were compared.
-
•
797 metabolites were quantified, 162 metabolites changed significantly after boiling.
-
•
Maillard reactions occur in over-boiled egg yolk as the reduce of L-lysine and D-fructose.
-
•
Endogenous enzymes may induce partial hydrolysis of proteins and phospholipids.
-
•
Boiling increased the detectable fat-soluble vitamins, riboflavin and biotin.
1. Introduction
Chicken egg yolk contains about 30.3% high-quality lipids, 17.3% proteins, and is rich in vitamins and minerals (USDA, FoodData Central, FDC ID:748236). Egg yolk not only improves the daily diet nutrition, but also has the health effects of anti-inflammatory activity, antioxidant activity, immune enhancement, folic acid supplement, etc (Xiao et al., 2020a; Yu et al., 2014). Almost all egg yolks consumed undergo heat treatment (either being cooked at home or processed in a factory) before being served on the table. Heat treatment remodels the texture and organoleptic properties of egg yolks by denaturing egg yolk proteins and altering their digestive properties. At the same time, heat treatment ensures the safety of the egg yolk by reducing allergenicity or killing potential microbial contamination (Claude et al., 2016; Gomaa and Boye, 2015). In recent years, the effects of heating on the nutrients, taste, and biological activity of eggs have been widely investigated. For example, a study found that the contents of lipids, vitamin A, vitamin E, and protein in fried liquid egg were the highest; steaming and baking had little effect on the amino acid composition of liquid egg; microwaving and frying increased the content of total essential and nonessential amino acids in liquid egg (Tang et al., 2021). Asensio-Grau et al. (2018) investigated the lipid and protein digestibility and bioaccessibility of lutein and zeaxanthin in eggs, showing that under conditions of exocrine pancreatic insufficiency, poaching improved the digestibility of lipids and proteins compared to boiling or omelet preparations. Meanwhile, boiling and poaching resulted in the higher bioavailabilities of lutein and zeaxanthin compared to those in omelet preparations under exocrine pancreatic insufficiency conditions. However, current studies have focused on proteins and lipids, and changes in small molecular compounds (metabolites) in egg yolk during heating have not been systematically explored.
High-throughput metabolome analysis is an efficient tool for the analysis of small molecule compounds (metabolites) in food. Metabolomic technology has been widely used in food composition analyses, authenticity and traceability studies, quality and safety control, and nutrition research (Wishart, 2008). Numerous studies have shown that metabolomics plays an important role in revealing functional foods for metabolic diseases. Metabolomics can be used to detect changes in body metabolism before and after functional food interventions, identify effective metabolites, and predict intervention effects and mechanisms (Fu et al., 2022). In addition, H-NMR-based metabolomic studies of milk showed that colostrum is richer in branched-chain amino acids, choline, and other nutrients that are beneficial to the growth and development of newborn mammals compared to regular milk (O'Callaghan et al., 2021). Wen et al. (2022) used the integrated transcriptome and metabolome to analyze the metabolic processes of Tricholoma matsutake fruiting bodies during cold storage and found that the abundance of monomers associated with protein, carbohydrate, and lipid catabolic metabolites mainly increased during cold storage. Li et al. (2021) identified a total of 249 metabolites between raw, steamed, boiled, and air-fried tilapia fillets, and 42 differential metabolites were screened from pairwise comparisons. Further analysis found that amino acids, phospholipids, and nucleotides were the main contributors to changes in flavor and nutritional properties of heated tilapia fillets. These advances suggested that metabolomic analyses can provide new insights into the mechanisms of food quality changes during storage and processing.
Metabolome analyses have been implemented for the study of egg storage and processing. The effects of breeds of laying hens (Rhode Island Red and Black Australian chickens) and feeds (mixed and fermented feeds) on egg composition have been studied through metabolomic analyses. Quantitative data from 138 egg yolk and 132 egg white metabolites showed that both breeds and feeds had significant effects on egg yolk and white qualities. Further analyses screened out three metabolites (erythritol, threonol, and urea) that were significantly altered in the yolk by the feed, and the yolk was enriched in sugar alcohols and fatty acids upon feeding fermented feeds (Goto et al., 2019). A non-targeted metabolomic analysis identified 43 and 16 different metabolites in the white and yolk of duck eggs with different preservation periods, respectively. The major metabolites in the yolk were amino acids, carbohydrates, and lipids, and the representative white metabolites were amino acids, benzene, and indoles. Further comparative analyses revealed that the metabolite composition changed, with various nutrients such as amino acids, fatty acids, nucleotides, sugars, and vitamins being degraded, while ammonia, biogenic amines, and flavor substances were produced with longer preservation times (Liu et al., 2022a). Metabolome analyses revealed that during the high-density ultrasound-induced depolymerization of yolk granules, the abundance of 14 metabolites changed significantly in the supernatant of yolk granules, suggesting that the process of yolk granule depolymerization was accompanied by substance dissolution (Geng et al., 2021). Additionally, the water-soluble metabolites in raw and boiled eggs were compared, and amino acid and fructose concentrations in boiled egg whites were significantly higher than those in raw egg whites, while metabolites in boiled egg yolks showed little changes (Ogura et al., 2020).
Boiled eggs represent the most common form of home cooking, and the nutritional changes of various boiled eggs at different heating intensities are important concerns for consumers when choosing cooking methods. Therefore, in this work, quantitative metabolomic analysis was performed to reveal changes in metabolite levels in boiled egg yolk under different heating intensities. Through the analysis of high-throughput quantitative metabolite data, this study is expected to provide insights into properties such as the texture, color, and nutrition of boiled egg yolks.
2. Materials and methods
2.1. Materials and reagents
Chicken eggs were provided by Fengji Food Group Co., Ltd. (Mianyang, Sichuan, China). Formic acid, ammonium formate, and ammonia (LCMS grade) were all purchased from Aladdin (Shanghai, China). Methanol and acetonitrile (LCMS grade) were supplied by Merck (USA). All other reagents (analytical reagents) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Preparation of egg yolk samples
Fresh eggs were selected and heated using different modes in an egg steamer (ZDQ-B06R2, Foshan Xiaoxiong Electric Co., Ltd., Foshan, Guangdong, China). Room temperature (25 °C) was the starting point of heating, and the heating temperature curves (measured) are shown in Fig. S1. After boiling, the egg yolks were collected, marked as hot spring egg yolk (HEY), soft-boiled egg yolk (SEY), normal-boiled egg yolk (NEY), and over-boiled egg yolk (OEY). Eggs marked as fresh egg yolk (FEY) were compared as a reference. The egg-cooking procedure was repeated 3 times, and 5 yolks were collected and mixed for each repetition; in total, 15 yolks were collected and mixed randomly into 3 replicates for each treatment group.
2.3. Sample extraction for metabolomic analysis
The egg yolk sample (20 mg) was added to a 400 μL solution (methanol: water = 7:3, V/V; containing the internal standard, Table S1). The egg yolk sample solution was vortexed (3 min) and sonicated (10 min) in an ice bath and then vortexed again for 1 min. The fully mixed sample solution was placed in a freezer (−20 °C) for 30 min, and the supernatant (extracts) were collected by centrifugation (12000×g, 10 min, 4 °C). Finally, 200 μL of extracts were used for subsequent LC-MS/MS analyses. Three replicate samples from each group were processed and extracted individually.
2.4. LC-MS/MS analysis of metabolites
The metabolomic analysis of egg yolk extracts was carried out using an ultra-performance liquid chromatography (UPLC) device (ExionLC AD, SCIEX, Darmstadt, Hessian, Germany) in conjunction with a tandem mass spectrometry (MS/MS) device (QTRAP 5500, SCIEX). The LC separation was carried out on a Waters Acquity UPLC HSS T3 C18 column (1.8 μm, 2.1 mm × 100 mm) with a flow rate of 0.4 mL/min at 40 °C. The sample injection volume was 2 μL, mobile phase A was water (0.1% formic acid), and mobile phase B was acetonitrile (0.1% formic acid). The gradient program was mobile phase B 5% at 0 min, 90% at 11.0–12.0 min, 5% at 12.1–14.0 min.
The temperature of electron-spray ionization was 500 °C, ion spray voltage was 5500 V (positive) and −4500 V (negative), and the pressures of ion source gas I, gas II, and curtain gas were 55, 60, and 25 psi, respectively. The collision-activated dissociation parameter was set to high. In the triple quadrupole mass analyzer, each ion pair was scanned and detected according to the optimized declustering potential and collision energy.
2.5. Qualitative and quantitative metabolite analyses
Quantitative analysis of metabolites followed the procedure reported by Chen et al. (2013). First, high quality, highly reproducible and non redundant metadata signals were filtered and obtained. Then, the metadata was annotated based on the fragmentation pattern (delivered by MS/MS), the retention time, and the accurate m/z value (delivered by MS) of each metabolite. By comparing these information with the standards (MWDB database, Metware Biotechnology Co., Ltd., Wuhan, China), metabolites were identified.
The multiple reaction monitoring mode (MRM) of triple quadrupole mass spectrometry was used for the quantification of egg yolk metabolites. Briefly, the precursor ions of the target metabolites were screened at MS1 to exclude interference from other ions. Subsequently, after the precursor ions were fragmented by the collision cell, the characteristic fragment ions were screened in MS2 to exclude non-target ion interference, thereby improving quantitative accuracy and repeatability. The signal intensity of the characteristic ions was obtained in the detector. After obtaining the mass spectrometry data of egg yolk metabolites, peak area integration was performed based on the mass spectrum peaks of all substances. The mass spectrum peak area integration of the same metabolite in different samples was corrected by the internal standard. Finally, metabolite qualitative information and corrected quantitative abundance (counts per second, cps) were exported by the Analyst (1.6.3, SCIEX). The comparison of metabolite abundance of egg yolks treated with different heating intensities was based on the ion current intensity in mass spectrometry (cps).
Principal component analysis (PCA) was performed to evaluate the overall difference between the egg yolk lipidomes. Orthogonal partial least squares discriminant analysis (OPLS-DA) was used to screen differentially abundant metabolites between different samples. Discrimination of significant differences in metabolite abundance based on p-values (p < 0.05), fold change (FC < 0.67, or FC > 1.50), and variable importance in projection (VIP > 1).
2.6. Statistical analysis
The statistical analysis of histogram and violin plot was performed using GraphPad Prism 7.00 (GraphPad Software Inc., San Diego, CA, US) by one-way analysis of variance, and p < 0.05 were considered significant.
3. Results and discussion
3.1. Overview of egg yolk metabolomes
A total of 797 metabolites were detected in the five groups of egg yolk samples (FEY, HEY, SEY, NEY, and OEY) (Table S2). Among them, 332 “amino acid and its metabolite” metabolites were identified, accounting for 41.66% of the metabolite species in egg yolks. Other major metabolites in egg yolks included 91 kinds of “organic acid and its derivatives”, 69 kinds of “nucleotide and its metabolites”, 55 kinds of “glycerophospholipids (GPs)”, 50 kinds of “heterocyclic compounds”, 49 kinds of “fatty acyls”, 44 kinds of “carbohydrate and its metabolites”, 32 kinds of “benzene and substituted derivatives”, and 31 kinds of “alcohols and amines”. Quantitative comparisons based on metabolite abundance (cps) showed that the total cumulative abundance of metabolites was highest in OEY and significantly different compared to that in other groups (Fig. 1A). Abundance-based calculations showed that “amino acid and its metabolites”, “GPs”, “coenzymes and vitamins” were the three metabolite categories with the highest abundance, and their cumulative abundance reached 75.61%–81.47% of the total metabolite abundance in egg yolks. “Amino acids and its derivatives” are small molecular compounds derived from egg yolk proteins, and “GPs” are the water-soluble lipid subcategories of yolk lipids, so they have the highest abundance in egg yolk metabolome. In addition, the rich “coenzyme and vitamins” in egg yolk is necessary to ensure the development of chicken embryo. Therefore, the results of metabolome analysis was consistent with the nutrient content of egg yolk.
Fig. 1.
Metabolomic analysis of boiled egg yolks under different heating intensities. A, comparison of metabolite categories between the five egg yolks; B, PCA plots of metabolomes; C–H, OPLS-DA plots of the pairwise comparisons. FEY, fresh egg yolk; HEY, hot spring egg yolk; SEY, soft-boiled egg yolk; NEY, normal-boiled egg yolk; OEY, over-boiled egg yolk.
3.2. Overall differences in yolk metabolomes under different heating intensities
The overall differences in the metabolite profiles among egg yolks under different heating intensities were first assessed by principal component analysis (PCA). Results showed that the three replicate samples within an experiment were relatively clustered (Fig. 1B), indicating that our metabolomic analysis had high stability and good reproducibility (Leng et al., 2021; Sebzalli and Wang, 2001). There was some overlap in the distribution of the five groups of yolk samples on the PCA score plots. However, FEY was clearly separated from HEY, NEY, and OEY, indicating that the egg yolk metabolome changed significantly after boiling. Taking FEY as a reference, the distance between the boiled egg yolk group and the FEY group on PC1 was positively correlated with the heat intensity of boiling. In particular, OEY was far away from the other groups, suggesting that the OEY metabolome had the greatest difference from the other groups. These results suggest that a PCA of egg yolk metabolomic data can be used to discriminate boiled egg yolks with large differences in heat intensity.
To obtain a better understanding of egg yolk metabolome differences under different heating intensities, the metabolomic data were further subjected to orthogonal partial least squares-discriminant analysis (OPLS-DA) (Arendse et al., 2018; Kang et al., 2022). The OPLS-DA score plots of the pairwise comparison between FEY and the boiled egg yolks (HEY, SEY, NET, and OEY) were shown in Fig. 1 C–F. The metabolomes of each group of samples were significantly separated in pairwise comparisons, suggesting that the OPLS-DA clearly distinguishes the FEY from the boiled egg yolks. The R2X(cum)-value of the OPLS-DA analysis of FEY and HEY was 0.521, meaning that 52.1% of the variation in the egg yolk metabolome dataset could be modeled. In addition, an excellent goodness-of-fit of 99.9% (R2Y(cum) = 0.999, p < 0.005) and goodness-of-prediction of 66.5% predictability (Q2 (cum) = 0.665, p = 0.235) for the discrimination of yolk metabolite profiles (FEY vs HEY) were determined. Metabolome differences between FEY and SEY were also analyzed by OPLS-DA (Fig. 1D), and the R2Y(cum)-value and Q2(cum)-value were 1.000 (p < 0.005) and 0.734 (p < 0.005), respectively. Additionally, the Q2(cum)-values of the comparisons of FEY vs NEY and FEY vs OEY were 0.767 (p < 0.005) and 0.893 (p < 0.005), respectively (Fig. 1E and F). OPLS-DA results showed that the metabolomes of both semi-solidified egg yolks cooked at lower heating intensity (SEY) and overcooked egg yolks at higher heating intensity (OEY) were significantly changed compared with egg yolks that had just completely solidified (NEY) (Fig. 1G and H). The Q2(cum)-values of the comparisons of NEY vs SEY and NEY vs OEY were 0.73 (p < 0.005) and 0.853 (p < 0.005), respectively. These OPLS-DA results indicated that the model could evaluate differences in metabolome between FEY and heated egg yolks.
3.3. Differentially abundant metabolites (DAMs) in egg yolk under different heating intensities
The DAMs were screened based on the variable importance in projection (VIP ≥1) and fold change ≥1.5 (or ≤ 0.67). Compared with FEY, 35, 50, 80, and 108 DAMs were screened in the HEY, SEY, NEY, and OEY, respectively (Fig. 2A). The total number of DAMs in egg yolk increased with increases in the heating intensity of boiling, implying a correlation between heating intensity and changes in the egg yolk metabolome. There were 13 common DAMs between the FEY and the four boiled egg yolks (Fig. 2B, Table S3). Among them, only barbituric acid showed a higher abundance in FEY, 12 other DAMs all showed higher abundance in the four types of boiled egg yolks. Notably, Hyp-Trp, calcitriol, DL-aminopimelic acid, and free fatty acids(15:1) (FFA(15:1)) were not detected in FEY but were identified in the four boiled egg yolk. These four DAMs can be used as potential markers to discriminate boiled egg yolk from FEY. Compared with FEY, there were 9, 15, 20, and 49 unique DAMs in HEY, SEY, NEY, and OEY, respectively, suggesting that boiling at different heating intensities would bring about unique changes in egg yolk metabolites.
Fig. 2.
Differential abundance metabolites (DAMs) in boiled egg yolks. A, the number of DAMs in boiled egg yolks; B, Venn diagram of DAMs; C–F, the top five differential DAMs with the largest variable importance in projection (VIP) value in the pairwise comparisons. FEY, fresh egg yolk; HEY, hot spring egg yolk; SEY, soft-boiled egg yolk; NEY, normal-boiled egg yolk; OEY, over-boiled egg yolk.
Continued changes in the species, content, and structure of food composition are the molecular basis for changes in food texture, color, aroma, and digestive properties during thermal processing. Thermal processing has different effects on the sensory and nutritional qualities of food arising from different heating intensities (heating temperature, heating rate, and heating time) (Li et al., 2016; Liu et al., 2022b). For example, the composition and nutritional properties of milk would be altered during heating, including lactose degradation, protein denaturation, and lipid hydrolysis (Zhu et al., 2021). Furthermore, lactulose is a specific product during the heat treatment of milk and an important indicator to evaluate the degree of sterilization and storage time of milk (Sakkas et al., 2014). In daily home cooking, eggs are heated and cooked in various ways, including boiling, frying, baking and frying, etc. Ogura et al. (2020) compared the differences in metabolites between microwave cooked (600 W for 10 min) egg yolks and raw egg yolks and found that most egg yolk metabolites (67 species) showed no significant change in abundance after microwave heating, except for the increase cysteine and decrease N-acetylaspartate. In this study, benefiting from the separation efficiency of UPLC and the identification accuracy of MS/MS, the number of metabolites identified and quantified in egg yolks was greatly increased, and more metabolite changes in the boiled egg yolks were detected.
3.4. The representative DAMs
The five DAMs that contributed most to the differences in metabolomes (highest VIP values) in HEY compared with FEY were Tyr-Pro, Thr-Leu-Ile, 5-hydroxy-L-tryptophan, FFA(15:1), and LPC(0:0/18:3) (Fig. 2C). LPC (0:0/18:3) was identified in FEY but not in HEY, and the other four metabolites were newly generated metabolites in HEY but not identified in FEY. Therefore, the newly generated protein hydrolysates (peptides and amino acids) were significantly altered in the metabolome of HEY compared to that of FEY. With increasing heating intensity, the five representative DAMs with the highest VIP values in SEY/FEY were asterina-330, indole-3-acetamide, FFA(15:1), aminolevulinic acid, and Hyp-Trp (Fig. 2D). Changes in amino acids and their derivatives were representative of SEY compared to FEY, including the production of asterina-330 and Hyp-Trp and the disappearance of indole-3-acetamide and aminolevulinic acid in SEY. Asn-Ala, p-hydroxymandelic acid, Ile-Leu, 2-methoxybenzoic acid, and FFA(15:1) were the five DAMs with the highest VIP values in NEY/FEY (Fig. 2E), demonstrating the diversity of metabolome changes in NEY compared to FEY. The five representative DAMs in the metabolome of OEY compared to that of FEY were 5-hydroxy-L-tryptophan, FFA(15:1), LPC(22:2/0:0), DL-aminopimelic acid, and andasterina-330. These five representative DAMs all showed higher abundance in OEY, showing that larger changes in the yolk metabolome are brought about by overheating (Fig. 2F).
It is worth noting that FFA(15:1) was the representative DAM in all four comparisons of boiled egg yolks with FEY. FFA(15:1) was not detected in FEY but was identified with high abundance (1.0–1.6 × 107 cps) in boiled egg yolks. Its abundance increased with increases in the heating intensity of boiling, although there were no significant differences among the four boiled egg yolks. Odd-carbon fatty acids are usually present in low abundance in some raw foods. It was reported that FFA(15:0) and FFA(15:1) in egg yolk accounted for 0.04% and 0.06% of total fatty acids, respectively. Furthermore, in the lipidomic analysis of egg yolk, FFA(15:0) and 21 species of lipid molecules containing (15:0) or (15:1) were identified with low to medium abundance (He et al., 2023). This study identified FFA(15:1) as a potential characteristic metabolite of boiled egg yolks; a possible explanation is that lipid molecules containing (15:1) are hydrolyzed/degraded during the boiling process to release FFA(15:1). Nevertheless, the changes in the species and abundance of odd-carbon fatty acids in stored or processed egg yolks are a concern that warrants further study.
3.5. Maillard reactions during egg yolk boiling
In the thermal processing of foods, Maillard reactions are an important series of reaction processes that alter their color, taste, and smell (Starowicz and Zielinski, 2019). Therefore, we investigated the changes in the abundance of free amino acids and reducing sugars in egg yolks associated with the onset of the Maillard reaction during the boiling.
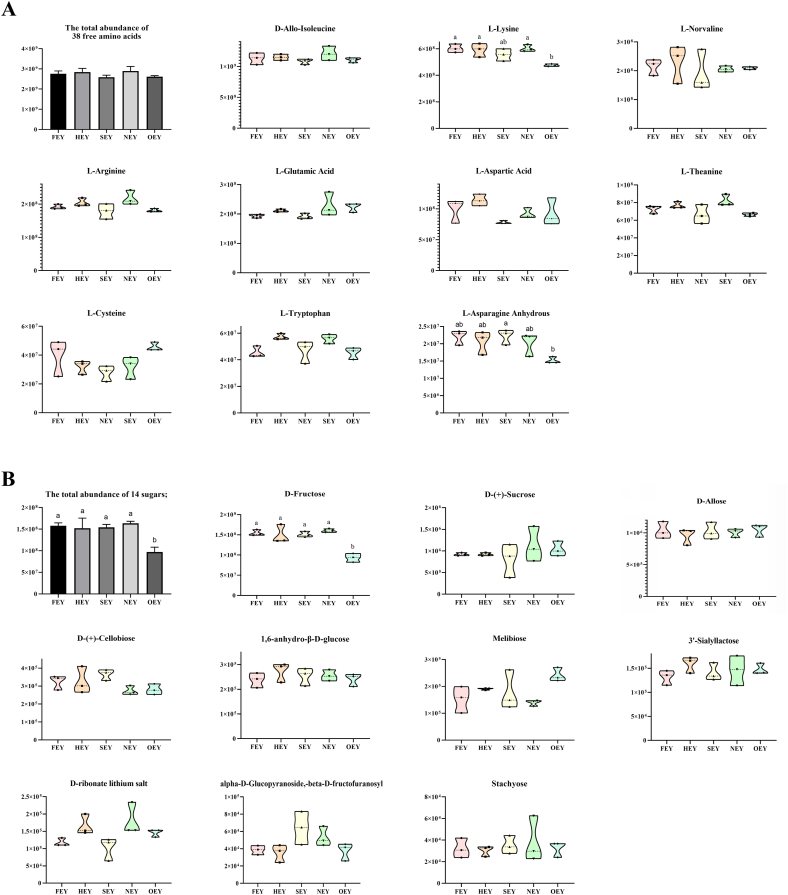
A total of 38 free amino acids were identified in the metabolomic analysis of egg yolk (Table S4). The total abundance of free amino acids was not significantly changed in the five groups of egg yolks. However, the abundance of some amino acids changed significantly. The top-10 most abundant free amino acids received special attention, as their abundance accounted for over 94% (94.29%–94.85%) of the total free amino acids in egg yolks. The results showed that their abundances fluctuated nonsignificantly with increasing boiling heat intensity, except for that of L-lysine, which was significantly lower (reduced by 16.61%–26.66%, p < 0.05) in OEY compared to that in other egg yolks (Fig. 3A). In addition to free amino acids, 14 reducing sugar metabolites were screened (Table S5). Remarkably, the abundance of D-fructose accounted for 96.49–98.00% of the total abundance of reducing sugar metabolites (Fig. 3B). The abundance of D-fructose did not change significantly during boiling at moderate heating intensity but decreased significantly in OEY, by 58.91%–70.84%.
Fig. 3.
Changes in the abundance of free amino acids (A) and sugars (B) in boiled egg yolks. FEY, fresh egg yolk; HEY, hot spring egg yolk; SEY, soft-boiled egg yolk; NEY, normal-boiled egg yolk; OEY, over-boiled egg yolk. Significant differences (p < 0.05) between groups are indicated by different letters (a, b).
The significant reduction in L-lysine and D-fructose in OEY is presumed to be caused by Maillard reactions. These results were in line with the general law of Maillard reactions: basic amino acids and free monosaccharides are easily involved in these reactions. The reaction of L-lysine and D-fructose readily produces advanced glycation end products (AGEs) such as N-ε-carboxymethyl-lysine and N-ε-carboxyethyl-lysine, as well as intermediate products such as glyoxal and methylglyoxal (Xu et al., 2022). Temperature and time of heating are the most important and interdependent factors affecting Maillard reactions, where increasing temperature leads to an increase in molecular kinetic energy, collision frequency, and Maillard conjugation formation rate (Lee et al., 2017). Specifically, the significant decrease of the abundance of L-lysine and D-fructose in boiled egg yolk was found only when the cooking time was extended to 1 h. In addition, the food composition determines the specific reaction details and reaction products (volatile odorants and colorants) of the Maillard reactions. It has been reported that milk are temperature-sensitive due to the high amount of L-lysine-rich proteins and reducing sugars, and the flavour and nutritional value of dairy products would be altered due to the heat induced Maillard reaction (Naranjo et al., 2013). The detection of Maillard reaction products, such as 5-hydroxymethylfurfural and furansine, is important for the quality control of milk and dairy products (Van Den Oever and Mayer, 2021). As for meat, active amino groups of proteins would react with reducing sugars during cooking and thermal processing, and include many important aromatic chemicals, including pyrazines, oxazoles, thiophenes, thiazoles and other heterocyclic compounds (Arihara et al., 2021). Due to the different nutritional composition, the Maillard reaction of heated egg yolks will be significantly different from that of milk and meat. However, the characteristics and effects of Maillard reactions in egg yolk during heat processing have not received enough attention. The data from this study suggested that the primary amino and carbonyl compounds involved in Maillard reactions in OEY should be L-lysine and D-fructose, respectively. This finding provides an important basis for in-depth characterization of the Maillard reactions in egg yolk.
3.6. Protein hydrolysis during egg yolk boiling
Many dipeptides and tripeptides were found in the metabolome of egg yolks (Table S6). Among them, there were 200 dipeptides, and the five most abundant dipeptides included: Val-Thr, Pro-Glu, Ala-Pro, Gly-Lys, and Cys-Pro. There were 6 kinds of tripeptides, with Gly-Gly-Phe, Phe-Ala-Ser, and Thr-Leu-Ile having the highest abundance. Boiling had no significant effect on the total abundance of dipeptides and tripeptides in egg yolks. However, among the top-10 abundance dipeptides, the abundances of Val-Thr and Cys-Pro decreased significantly in SEY and OEY compared to those in FEY, while excessive heating resulted in a significant increase in the abundance of cyclo(Glu-Glu) in OEY (Fig. 4). Notably, 2 of the top-5 most abundant peptides were found to be significantly decreased in OEY compared to FEY, but the abundance of total peptides increased by 4.75% (p = 0.9780). The reason was that a large number of low-medium abundance dipeptides are significantly elevated after boiling. In particular, the abundance of 8 dipeptides among the 13 common DAMs were increased in the comparison of boiled egg yolks with FEY (Table S3). Among them, the abundance of five representative dipeptides increased several times: Leu-Asp (FEY: HEY: SEY: NEY: OEY = 1 : 4.26: 3.77 : 6.39: 4.45), Glu-Ile (1 : 2.38: 3.32 : 3.79: 3.66), Val-Glu (1 : 1.95: 2.36 : 3.07: 2.05), Val-Asp (1 : 5.89: 5.21 : 10.10: 5.76), Hyp-Trp (ND: 1 : 1.04: 2.45 : 17.99). These results suggested that the hydrolysis of egg yolk proteins might occur during the boiling process. Some endogenous proteases and peptidases, such as cathepsin D, cathepsin E-A-like, and carboxypeptidase D, etc., could exert their hydrolytic activity during the warm-up stage of boiling (Mann and Mann, 2008; Wang et al., 2022; Zheng et al., 2018). The results of chicken egg proteomics suggested that endogenous proteases in egg yolk can be well inhibited by a protease-inhibitor homeostatic system at room temperature (Geng et al., 2018; Xiao et al., 2020b). However, at the incubation temperature (37–38 °C), this protease-inhibitor homeostatic system is biased towards the protease side, and the hydrolysis of egg yolk protein is initiated to provide nutrition for chicken embryo development (Meng et al., 2022; Zhu et al., 2019). These findings suggested that the protease-inhibitor homeostatic system is temperature-sensitive. In the boiling of eggs, the increased temperature would also destabilize the protease-inhibitor homeostatic system, resulting in mild hydrolysis of egg yolk proteins.
Fig. 4.
Changes in the abundance of dipeptides and tripeptides in boiled egg yolks. FEY, fresh egg yolk; HEY, hot spring egg yolk; SEY, soft-boiled egg yolk; NEY, normal-boiled egg yolk; OEY, over-boiled egg yolk. Significant differences (p < 0.05) between groups are indicated by different letters (a–c).
Thermal degradation is another potential pathway for yolk protein degradation during boiling. The abundance of cysteine in this study was found to be slightly increased (by 13.19%) in OEY compared to FEY (p = 0.8605), whereas the abundance of cystine decreased in OEY by 293.80% compared to that in FEY (p < 0.0001) (Table S2). These results suggested that overheating may cause cystine to be broken down to cysteine in yolk proteins. Similarly, cysteine content was significantly elevated in microwave-cooked egg yolks compared to raw egg yolks (Ogura et al., 2020). In addition, when comparing the edible quality of liquid egg cooked by different methods, the content of cysteine was significantly higher in baked and fried liquid egg compared to raw and steamed liquid egg (Tang et al., 2021). These studies collectively suggested that disulfide bonds in yolk proteins would be broken under high-intensity thermal processing.
The low-to-medium abundance dipeptides or tripeptides in boiled egg yolks warrant further study because of their potential bioactivities and health benefits. Egg yolk peptides have been shown to have various activities, such as antibacterial, antioxidant, hypotensive, hypoglycemic, and immunomodulatory functions. For example, in an extensive description of the peptidomic repertoire of the hen egg yolk plasma, Arena and Scaloni (2018) noted that 628 peptides were identified as protein hydrolysates derived from larger protein fractions under the physiological action of chicken chymotrypsin-like and pepsin-like enzymes, 198 of which were associated with antihypertensive, antimicrobial, anticancer, and antiviral activities. Marcet et al. (2022) hydrolyzed the lipoproteins remaining after phosvitin extraction from egg yolk granular fraction using trypsin and assessed the biological activity of the resulting peptides by computer analysis. The results showed that the hydrolysis of isolated lipovitellins by trypsin produced hydrolysis products containing 26% of peptides with predicted antihypertensive activity. In addition, enzymatic digestion of phosvitin pretreated with high temperature and mild pressure can yield various phosphopeptides, among which phosvitin phosphopeptides can be used as metal supplements to prevent osteoporosis or anemia, anti-cavity agents and antioxidants to slow down the skin aging process, and to improve food quality and safety by inhibiting oxidative changes and preventing microbial growth in food during storage (Huang et al., 2019).
3.7. Phospholipid hydrolysis during egg yolk boiling
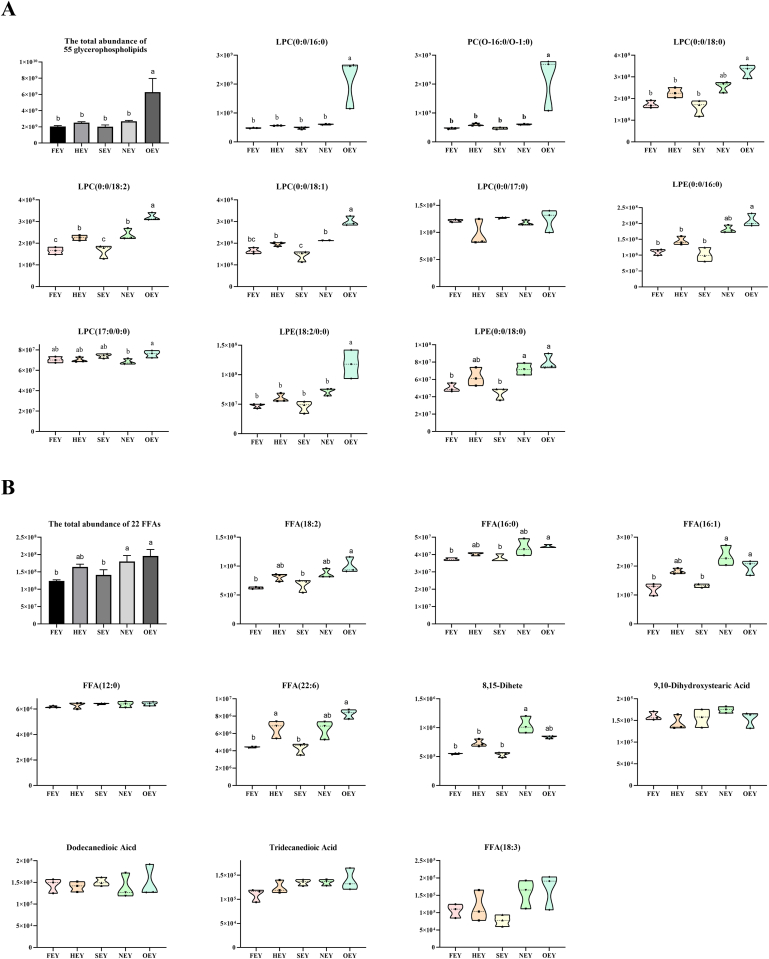
The egg yolk metabolome is rich in hydrophilic small molecule compounds, so the solvent used to extract total metabolites during sample pretreatment in this study was water-methanol (70:30, V:V). Therefore, only a small number of egg yolk lipids were identified (He et al., 2023), including 55 kinds of “glycerophospholipids” (mainly lysophospholipids) and 49 kinds of “fatty acyls” (mainly including 22 FFAs and 18 acylcarnitines). The total abundance of glycerophospholipids in OEY was substantially increased by 206.75% compared to that in FEY (Fig. 5A; Table S7). Among the top-10 “glycerophospholipids” metabolites with the highest abundance, 9 of them showed a significantly higher abundance in OEY compared to that in FEY. Meanwhile, the total abundance of 22 FFAs increased by 57.83% in OEY compared to that in FEY (Table S8). Representatively, FFA(18:2), FFA(16:1), and FFA(22:6) increased significantly by 60.83%, 62.41%, and 87.29%, respectively (Fig. 5B).
Fig. 5.
Changes in the abundance of glycerophospholipids (A) and FFAs (B) in boiled egg yolks. FFAs: free fatty acids; FEY, fresh egg yolk; HEY, hot spring egg yolk; SEY, soft-boiled egg yolk; NEY, normal-boiled egg yolk; OEY, over-boiled egg yolk. Significant differences (p < 0.05) between groups are indicated by different letters (a–c).
In egg yolk, the endogenous enzymes related to lipid hydrolysis mainly include acid lipases, neutral lipases, and phospholipases. Therefore, phospholipid hydrolysis would be accelerated during the temperature ramp-up period of the boiling process, resulting in a substantial increase in the abundance of FFAs and lysophospholipids. Additionally, the breakdown of lipid molecules induced by high-intensity heat treatment might be another reason why lysophospholipids and FFA abundances were substantially increased in OEY. Similar thermal processing-induced hydrolysis of phospholipids has also been found in other food materials. For example, it was reported that the FFA content in herring oil obtained from herring by-products under 60–90 °C tended to decrease and then increased, with 70 °C being the inflection point (Šližytė et al., 2014). Zhang et al. (2022) applied ultra-performance liquid chromatography-electrospray ionization mass spectrometry (UPLC-ESI-MS) to compare the lipidomic profile of chicken breasts heated to different temperatures and found that a series of characteristic unsaturated phospholipids decreased significantly after heating, while characteristic lysophospholipids, which were mainly derived from hydrolysis of phospholipids, increased significantly with increasing heating temperatures. In addition, the high-pressure thermal treatment of pork significantly enhanced the lipid hydrolysis and oxidation of intramuscular lipids, leading to the increased degradation of polyunsaturated fatty acids (PUFA) in phospholipids or the production of free fatty acids (FFA) (Huang et al., 2015).
3.8. Changes in vitamin abundance during egg yolk cooking
Egg yolks are rich in vitamins, and a total of 19 vitamins were identified in this study, including 4 kinds of vitamin A derivatives, 13 kinds of B vitamins, 1 kind of vitamin C (L-ascorbate), and 1 kind of vitamin D derivative (calcitriol) (Table S9). Quantitative results showed that boiling had no significant effect on the total abundance of vitamins. However, there were various changes in the abundance of different vitamins. Among them, the abundance of the four derivatives of vitamin A increased with increasing boiling heat intensity (Fig. 6A). Vitamin A and its derivatives are insensitive to heating, due to their multiple unsaturated double bonds. Theoretically, boiling is more likely to cause their loss in the yolk; however, vitamin A and its derivatives are fat-soluble and tightly complexed with egg yolk lipids, which are mainly found in the form of lipoproteins. That is, the protein-lipid-lipophilic compounds in the egg yolk are assembled into tightly bound complexes. During boiling, egg yolk proteins are denatured, resulting in a structural disassembly or reorganization of the protein-lipid-lipophilic compound complex. This will lead to an easier extraction and detection of lipophilic compounds (such as vitamin A and its derivatives) in the metabolome analysis. In addition, fat-soluble calcitriol was not identified in FEY but was found in boiled egg yolks (Fig. 6B), possibly also due to a heat-induced dissociation of protein-lipid-lipophilic compounds.
Fig. 6.
Changes in the abundance of vitamins in boiled egg yolks. A, vitamin A derivatives; B, calcitriol; C, vitamin B5 and its derivatives; D, riboflavin; E, biotin. FEY, fresh egg yolk; HEY, hot spring egg yolk; SEY, soft-boiled egg yolk; NEY, normal-boiled egg yolk; OEY, over-boiled egg yolk; “nd”: not detected. Significant differences (p < 0.05) between groups are indicated by different letters (a, b).
Pantothenate, a widely existing water-soluble vitamin, was the most abundant vitamin in egg yolks. The present study identified pantothenate and its 2 derivatives in egg yolks. Their cumulative abundance fluctuated but did not differ significantly with increasing boiling heat intensity (Fig. 6C). Riboflavin is another highly abundant water-soluble vitamin in egg yolks. The abundance of riboflavin in egg yolks detected based on metabolome analysis was underestimated because it exists in egg yolks in a bound form with riboflavin-binding protein. Riboflavin-binding-protein (RBP) has been reported to have a high affinity for riboflavin. Wasylewski (2000) determined various parameters of the thermodynamic folding of egg white RBP molecules using differential scanning calorimetry. The results showed that the thermal stability of RBP protein is high, and the denaturation temperature of RBP molecule without a cofactor was 60.8 °C. The binding of riboflavin would enhance its thermal stability, and the denaturation temperature could be directly increased to 72.8 °C. Interestingly, the detected abundance of riboflavin was significantly increased with increasing boiling heat intensity in HEY, NEY, and OEY compared to FEY (Fig. 6D). A possible reason was that the heat treatment dissociated more riboflavin from the riboflavin-binding protein as the protein was denatured. Similarly, the abundance of biotin was 68.94% higher in OEY than in FEY (Fig. 6E), also possibly due to thermal denaturation of avidin-binding proteins present in egg yolk. A total of 4 kinds of vitamins B3 and their derivatives were identified in this study, with OEY having the highest cumulative abundance and SEY the smallest, with significant differences between them (P < 0.05). There was no significant difference between the rest of the groups (P > 0.05). The abundance of other water-soluble vitamins (vitamin C, vitamin B1, 2 vitamin B6 derivatives, and vitamin B13) in egg yolk did not change significantly after boiling.
Significant changes in vitamins in eggs were observed when using other cooking methods. A study has shown that vitamin A and vitamin E abundances were highest in fried liquid egg samples and lowest in baked liquid egg samples, probably because the longer heating time and higher heating temperature of baking lead to the oxidative damage of vitamin A and vitamin E (Tang et al., 2021). However, the effect of boiling on the abundance of vitamins in egg yolks was slight. On the one hand, the maximum temperature is limited below the boiling point of water (<100 °C); on the other hand, the yolk is surrounded by eggshell and egg white, which is isolated from oxygen. Therefore, the vitamins in egg yolk can be better retained. The increased abundance of heat-sensitive fat-soluble vitamins (A and D) in egg yolks after boiling deserves further investigation. Thermally-induced depolymerization and remodeling of the complex of protein-lipid-lipophilic compounds is a potential underlying mechanism, which requires future in-depth exploration.
4. Conclusion
Quantitative metabolomics based on LC-MS/MS was used to analyze the metabolites in fresh and boiled egg yolks. It was found that “amino acids and its metabolites”, “GPs”, “coenzyme and vitamins” were the three major metabolite categories in egg yolk. In the comparison of yolk metabolome with different boiling heat intensities, 162 DAMs were screened from the 797 quantitated metabolites. Hyp-Trp, calcitriol, DL-aminopimelic acid, and FFA(15:1) would be potential markers for distinguishing cooked egg yolks. Maillard reactions should occurr in the OEY due to the substantial reduction in the abundance of L-lysine and D-fructose. The partial hydrolysis of egg yolk protein was believed to occur in the boiled egg yolks due to the significant increase in the abundance of a large number of low-medium abundance dipeptides. The significantly increased abundance of lysophospholipids and free fatty acids suggested the hydrolysis of phospholipids in boiled egg yolks. In addition, boiling possibly altered the complex structure of protein-lipid-lipophilic compounds in egg yolk and increased the detectable abundance of fat-soluble vitamins. These changes in egg yolk metabolites obtained through high-throughput metabolome analysis provide new insights into the safety (potential Maillard reactions) and nutrition (changes in peptides, phospholipids and vitamins) of boiled egg yolk.
Funding
This research was supported by the National Natural Science Foundation of China (32072236).
CRediT authorship contribution statement
Jinghui Wang: Investigation, Data curation, Writing – original draft. Wei Luo: Investigation, Data curation, Writing – original draft. Yan Chen: Data curation. Qionglian Zhang: Methodology, Writing – review & editing. Putri Widyanti Harlina: Methodology, Writing – review & editing. Jinqiu Wang: Writing – original draft, Supervision. Fang Geng: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.100409.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
Data availability
Data will be made available on request.
References
- Arena S., Scaloni A. An extensive description of the peptidomic repertoire of the hen egg yolk plasma. J. Agric. Food Chem. 2018;66(12):3239–3255. doi: 10.1021/acs.jafc.8b01183. [DOI] [PubMed] [Google Scholar]
- Arendse E., Fawole O.A., Magwaza L.S., Nieuwoudt H., Opara U.L. Evaluation of biochemical markers associated with the development of husk scald and the use of diffuse reflectance NIR spectroscopy to predict husk scald in pomegranate fruit. Sci. Hortic. 2018;232:240–249. [Google Scholar]
- Arihara K., Yokoyama I., Ohata M. Bioactivities generated from meat proteins by enzymatic hydrolysis and the Maillard reaction. Meat Sci. 2021;180 doi: 10.1016/j.meatsci.2021.108561. [DOI] [PubMed] [Google Scholar]
- Asensio-Grau A., Peinado I., Heredia A., Andrés A. Effect of cooking methods and intestinal conditions on lipolysis, proteolysis and xanthophylls bioaccessibility of eggs. J. Funct.Foods. 2018;46:579–586. [Google Scholar]
- Chen W., Gong L., Guo Z., Wang W., Zhang H., Liu X., Yu S., Xiong L., Luo J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: application in the study of rice metabolomics. Mol. Plant. 2013;6(6):1769–1780. doi: 10.1093/mp/sst080. [DOI] [PubMed] [Google Scholar]
- Claude M., Lupi R., Bouchaud G., Bodinier M., Brossard C., Denery-Papini S. The thermal aggregation of ovalbumin as large particles decreases its allergenicity for egg allergic patients and in a murine model. Food Chem. 2016;203:136–144. doi: 10.1016/j.foodchem.2016.02.054. [DOI] [PubMed] [Google Scholar]
- Fu J., Zhang L.-L., Li W., Zhang Y., Zhang Y., Liu F., Zou L. Application of metabolomics for revealing the interventional effects of functional foods on metabolic diseases. Food Chem. 2022;367 doi: 10.1016/j.foodchem.2021.130697. [DOI] [PubMed] [Google Scholar]
- Geng F., Xie Y., Wang J., Majumder K., Qiu N., Ma M. N-glycoproteomic analysis of chicken egg yolk. J. Agric. Food Chem. 2018;66(43):11510–11516. doi: 10.1021/acs.jafc.8b04492. [DOI] [PubMed] [Google Scholar]
- Geng F., Xie Y., Wang Y., Wang J. Depolymerization of chicken egg yolk granules induced by high-intensity ultrasound. Food Chem. 2021;354 doi: 10.1016/j.foodchem.2021.129580. [DOI] [PubMed] [Google Scholar]
- Gomaa A., Boye J. Impact of irradiation and thermal processing on the immunochemical detection of milk and egg allergens in foods. Food Res. Int. 2015;74:275–283. doi: 10.1016/j.foodres.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Goto T., Mori H., Shiota S., Tomonaga S. Metabolomics approach reveals the effects of breed and feed on the composition of chicken eggs. Metabolites. 2019;9(10) doi: 10.3390/metabo9100224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Wang J., Wang Y., Wang B., Zhang J., Geng F. Quantitative lipidomic analysis of egg yolk, yolk granule, and yolk plasma. J. Food Compos. Anal. 2023;115 [Google Scholar]
- Huang X., Moon S.H., Lee J., Paik H., Lee E.J., Min B., Ahn D.U. Effective preparation method of phosphopeptides from phosvitin and the analysis of peptide profiles using tandem mass spectrometry. J. Agric. Food Chem. 2019;67(51):14086–14101. doi: 10.1021/acs.jafc.9b05973. [DOI] [PubMed] [Google Scholar]
- Huang Y., Gan Y., Li F., Yan C., Li H., Feng Q. Effects of high pressure in combination with thermal treatment on lipid hydrolysis and oxidation in pork. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2015;63(1):136–143. [Google Scholar]
- Kang C., Zhang Y., Zhang M., Qi J., Zhao W., Gu J., Guo W., Li Y. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chem. 2022;387 doi: 10.1016/j.foodchem.2022.132932. [DOI] [PubMed] [Google Scholar]
- Lee Y.Y., Tang T.K., Phuah E.T., Alitheen N.B., Tan C.P., Lai O.M. New functionalities of Maillard reaction products as emulsifiers and encapsulating agents, and the processing parameters: a brief review. J. Sci. Food Agric. 2017;97(5):1379–1385. doi: 10.1002/jsfa.8124. [DOI] [PubMed] [Google Scholar]
- Leng P., Hu H.-W., Cui A.-H., Tang H.-J., Liu Y.-G. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2021;149 [Google Scholar]
- Li Q., Shi X., Zhao Q., Cui Y., Ouyang J., Xu F. Effect of cooking methods on nutritional quality and volatile compounds of Chinese chestnut (Castanea mollissima Blume) Food Chem. 2016;201:80–86. doi: 10.1016/j.foodchem.2016.01.068. [DOI] [PubMed] [Google Scholar]
- Li R., Sun Z., Zhao Y., Li L., Yang X., Cen J., Chen S., Li C., Wang Y. Application of UHPLC-Q-TOF-MS/MS metabolomics approach to investigate the taste and nutrition changes in tilapia fillets treated with different thermal processing methods. Food Chem. 2021;356 doi: 10.1016/j.foodchem.2021.129737. [DOI] [PubMed] [Google Scholar]
- Liu H.H., Yang Q.L., Guo R., Hu J.W., Tang Q., Qi J.J., Wang J.W., Han C.C., Zhang R.P., Li L. Metabolomics reveals changes in metabolite composition of duck eggs under the impact of long-term storage. J. Sci. Food Agric. 2022;102(11):4647–4656. doi: 10.1002/jsfa.11825. [DOI] [PubMed] [Google Scholar]
- Liu X., Huang S., Chao C., Yu J., Copeland L., Wang S. Changes of starch during thermal processing of foods: current status and future directions. Trends Food Sci. Technol. 2022;119:320–337. [Google Scholar]
- Mann K., Mann M. The chicken egg yolk plasma and granule proteomes. Proteomics. 2008;8(1):178–191. doi: 10.1002/pmic.200700790. [DOI] [PubMed] [Google Scholar]
- Marcet I., Delgado J., Díaz N., Rendueles M., Díaz M. Peptides recovery from egg yolk lipovitellins by ultrafiltration and their in silico bioactivity analysis. Food Chem. 2022;379 doi: 10.1016/j.foodchem.2022.132145. [DOI] [PubMed] [Google Scholar]
- Meng Y., Qiu N., Guyonnet V., Mine Y. Unveiling and application of the chicken egg proteome: an overview on a two-decade achievement. Food Chem. 2022;393 doi: 10.1016/j.foodchem.2022.133403. [DOI] [PubMed] [Google Scholar]
- Naranjo G.B., Pereyra Gonzales A.S., Leiva G.E., Malec L.S. The kinetics of Maillard reaction in lactose-hydrolysed milk powder and related systems containing carbohydrate mixtures. Food Chem. 2013;141(4):3790–3795. doi: 10.1016/j.foodchem.2013.06.093. [DOI] [PubMed] [Google Scholar]
- O'Callaghan T.F., O'Donovan M., Murphy J.P., Sugrue K., Tobin J.T., McNamara A.E., Yin X., Sundaramoorthy G., Brennan L. The bovine colostrum and milk metabolome at the onset of lactation as determined by 1H-NMR. Int. Dairy J. 2021;113 [Google Scholar]
- Ogura T., Wakayama M., Ashino Y., Kadowaki R., Sato M., Soga T., Tomita M. Effects of feed crops and boiling on chicken egg yolk and white determined by a metabolome analysis. Food Chem. 2020;327 doi: 10.1016/j.foodchem.2020.127077. [DOI] [PubMed] [Google Scholar]
- Sakkas L., Moutafi A., Moschopoulou E., Moatsou G. Assessment of heat treatment of various types of milk. Food Chem. 2014;159:293–301. doi: 10.1016/j.foodchem.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Sebzalli Y.M., Wang X.Z. Knowledge discovery from process operational data using PCA and fuzzy clustering. Eng. Appl. Artif. Intell. 2001;14(5):607–616. [Google Scholar]
- Šližytė R., Carvajal A.K., Mozuraityte R., Aursand M., Storrø I. Nutritionally rich marine proteins from fresh herring by-products for human consumption. Process Biochem. 2014;49(7):1205–1215. [Google Scholar]
- Starowicz M., Zielinski H. How Maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019;35(8):707–725. [Google Scholar]
- Tang D., Wang R., He X., Chen X., Huo X., Lü X., Shan Y. Comparison of the edible quality of liquid egg with different cooking methods and their antioxidant activity after in vitro digestion. Food Res. Int. 2021;140 doi: 10.1016/j.foodres.2020.110013. [DOI] [PubMed] [Google Scholar]
- Van Den Oever S.P., Mayer H.K. Analytical assessment of the intensity of heat treatment of milk and dairy products. Int. Dairy J. 2021;121 [Google Scholar]
- Wang Y., Wang J., Shi Y., Ye H., Luo W., Geng F. Quantitative proteomic analyses during formation of chicken egg yolk. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131828. [DOI] [PubMed] [Google Scholar]
- Wasylewski M. Binding study of riboflavin-binding protein with riboflavin and its analogues by differential scanning calorimetry. J. Protein Chem. 2000;19(6):523–528. doi: 10.1023/a:1026509717268. [DOI] [PubMed] [Google Scholar]
- Wen X., Geng F., Xu Y., Li X., Liu D., Liu Z., Luo Z., Wang J. Quantitative transcriptomic and metabolomic analyses reveal the changes in Tricholoma matsutake fruiting bodies during cold storage. Food Chem. 2022;381 doi: 10.1016/j.foodchem.2022.132292. [DOI] [PubMed] [Google Scholar]
- Wishart D.S. Metabolomics: applications to food science and nutrition research. Trends Food Sci. Technol. 2008;19(9):482–493. [Google Scholar]
- Xiao J., Wang J., Cheng L., Gao S., Li S., Qiu N., Li H., Peng L., Geng F. A puzzle piece of protein N-glycosylation in chicken egg: N-glycoproteome of chicken egg vitelline membrane. Int. J. Biol. Macromol. 2020;164:3125–3132. doi: 10.1016/j.ijbiomac.2020.08.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N., Zhao Y., Yao Y., Wu N., Xu M., Du H., Tu Y. Biological activities of egg yolk lipids: a review. J. Agric. Food Chem. 2020;68(7):1948–1957. doi: 10.1021/acs.jafc.9b06616. [DOI] [PubMed] [Google Scholar]
- Xu X., Xue T., Jiang Q., Fan D., Wang M., Zhao Y. Inhibitory effects of some hydrocolloids on the formation of NƐ-(carboxymethyl) lysine and NƐ-(carboxyethyl) lysine in chemical models and fish patties. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2022;162 [Google Scholar]
- Yu Z., Yin Y., Zhao W., Chen F., Liu J. Application and bioactive properties of proteins and peptides derived from hen eggs: opportunities and challenges. J. Sci. Food Agric. 2014;94(14):2839–2845. doi: 10.1002/jsfa.6670. [DOI] [PubMed] [Google Scholar]
- Zhang M., Xie D., Wang D., Xu W., Zhang C., Li P., Sun C. Lipidomic profile changes of yellow-feathered chicken meat during thermal processing based on UPLC-ESI-MS approach. Food Chem. 2022;399 doi: 10.1016/j.foodchem.2022.133977. [DOI] [PubMed] [Google Scholar]
- Zheng H., Li H., Tan W., Xu C., Jia L., Wang D., Li Z., Sun G., Kang X., Yan F., Liu X. Oestrogen regulates the expression of cathepsin E-A-like gene through ERΒ in liver of chicken (Gallus gallus) J. Genet. 2018;97(1):145–155. [PubMed] [Google Scholar]
- Zhu D., Kebede B., McComb K., Hayman A., Chen G., Frew R. Milk biomarkers in relation to inherent and external factors based on metabolomics. Trends Food Sci. Technol. 2021;109:51–64. [Google Scholar]
- Zhu F., Qiu N., Sun H., Meng Y., Zhou Y. Integrated proteomic and N-glycoproteomic analyses of chicken egg during embryonic development. J. Agric. Food Chem. 2019;67(42):11675–11683. doi: 10.1021/acs.jafc.9b05133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.