Abstract
An interleukin-4 (IL-4)-dependent Th2 response allows wild-type mice to survive infection with the parasite Schistosoma mansoni. In the absence of IL-4, infected mice mount a Th1-like proinflammatory response, develop severe disease, and succumb. Neither the Th1 response nor morbidity is IL-12 dependent in this system.
The immune response to Schistosoma mansoni infection is characterized by elevated production of type 2 cytokines, including interleukin-4 (IL-4), IL-5, IL-10, and IL-13 (4, 9, 15). In the absence of IL-4, spleen cells from schistosome-infected mice produce lower levels of type 2 cytokines (IL-5, IL-10, and IL-13) and higher levels of inflammatory mediators, including gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and nitric oxide (NO) (2, 5, 9, 15). Concurrent with this altered immune response, infected IL-4−/− mice, unlike wild-type (WT) animals, develop severe disease and die (2). The dramatic weight loss that precedes death in this model suggests a role for inflammatory mediators, such as TNF-α and associated molecules (IFN-γ and NO), in the disease (3, 6, 11, 20). Supporting this view that severe disease is immunologically mediated, treatment with anti-TNF monoclonal antibody, IL-4, anti-CD4 monoclonal antibody, or the peroxynitrite scavenger uric acid was found to delay mortality (2; A. C. La Flamme et al., submitted for publication). Of the inflammatory mediators examined that could be responsible for the exacerbated disease in infected IL-4−/− mice, it is NO and its downstream product, peroxynitrite, that are most tightly correlated with disease severity (2; La Flamme et al., submitted).
The production of NO by cells such as macrophages and endothelial cells is promoted by IFN-γ. Since in many instances IL-12 is essential for IFN-γ production, we analyzed the role of IL-12 in the development of severe schistosomiasis that occurs in the absence of IL-4, reasoning that in the absence of IL-12, IFN-γ and thus NO and peroxynitrite production would be minimized. We hypothesized that this would result in an alleviation of the disease and prolonged survival.
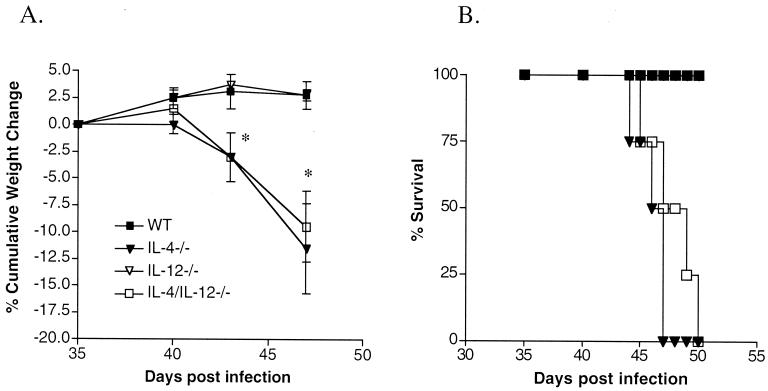
For these studies, we utilized C57BL/6 IL-4/IL-12p35−/− mice generated by Manfred Kopf by interbreeding IL-4−/− B6 mice (10) with IL-12p35−/− B6 mice (12) to produce homozygous double knockouts. These mice and the IL-4−/− and IL-12−/− mice were bred at Cornell University. C57Bl/6 (WT) mice were purchased from Taconic Farms, Germantown, N.Y. Mice were infected percutaneously with approximately 70 S. mansoni cercariae (NMRI strain). From day 35 of infection, mice were weighed using a spring balance (Forestry Suppliers, Jackson, Miss.), and once one or more of the infected IL-4−/− mice had lost ∼20% of their body weight the experiment was terminated (2). Differences in weight changes in the infected mice were assessed using nonparametric statistical analysis by the Wilcoxon signed rank test. We found that the infected IL-4/IL-12−/− mice developed a severe disease that was clinically indistinguishable from that seen in infected IL-4−/− mice; this was defined by marked cachexia followed by death (Fig. 1A and B). Disease development in schistosome-infected IL-12−/− mice mirrored that in WT mice (Fig. 1).
FIG. 1.
Morbidity and mortality in infected WT, IL-4−/−, IL-12−/−, and IL-4/IL-12−/− mice. (A) Time course of cumulative weight changes in infected animals following percutaneous exposure to ∼70 cercariae. Changes in body weight are expressed as means ± standard errors of the means. There were five mice per group. Cumulative weight changes in IL-4−/− and IL-4/IL-12−/− mice were significantly different from those in WT and IL-12−/− mice (∗, P < 0.05). Results are representative of two separate experiments. (B) Mortality over time in infected animals following percutaneous exposure to ∼90 cercariae. There were five mice per group. Mortality was significantly higher in IL-4−/− and IL-4/IL-12−/− mice than in WT and IL-12−/− mice (∗, P < 0.05).
Since disease severity is related to infection intensity, we examined whether the morbidity observed in infected IL-4/IL-12−/− mice was due to the survival of a greater percentage of the infectious inoculum in these mice than in IL-4−/− or WT mice. Mice were infected with equal numbers of cercariae, and worms and eggs were recovered and quantitated as previously described (2, 19). Analysis of variance (ANOVA) was used to evaluate differences in worm and egg burdens. We found no significant differences in either worm burden (ANOVA, P = 0.67) or hepatic egg load (ANOVA, P = 0.83) among the different groups (Table 1) and therefore conclude that the severe morbidity observed in mice lacking IL-4 or IL-4 and IL-12 was not due to increased susceptibility to S. mansoni infection per se.
TABLE 1.
S. mansoni parasite burden and pathology in micea
| Mouse genotype | Mean no. of worm pairs/mouse ± SEM | Mean no. of eggs/liver ± SEM | Mean granuloma diamb (μm) ± SEM |
|---|---|---|---|
| WT | 11 ± 1.2 | 17,797 ± 2,696 | 216 ± 15.7 |
| IL-4−/− | 12 ± 1.9 | 22,160 ± 3,954 | 220 ± 12.0 |
| IL-12−/− | 14 ± 1.8 | 19,604 ± 2,590 | 198 ± 8.2 |
| IL4/12−/− | 12 ± 1.6 | 19,178 ± 3,076 | 211 ± 21.0 |
Mice were infected with approximately 70 cercariae each, and there were five animals per group.
Measurements were obtained by using an ocular micrometer to determine the diameter of the granulomatous lesion around a single egg containing a mature embryo. Twenty granulomas were measured per liver.
During infection, liver-trapped parasite eggs elicit granulomatous lesions. The size of these granulomas, which alter normal tissue architecture, become fibrotic, and eventually lead to organ malfunctions, has been correlated with disease severity. To determine whether the morbidity observed in infected IL4/12−/− mice was due to differences in granuloma size, we compared the diameters of the lesions around trapped eggs in the different groups. Frontal liver lobes were processed for histological examination of lesions, and granulomas were imaged under a Nikon Optiphot microscope and captured with a Sony 3CCD color video camera (Sony, Teaneck, N.J.). Granuloma size was measured using NIH/SCION IMAGE (Scion, Frederick, Md.) (data not shown) and an ocular micrometer (Table 1); remaining hepatic tissue was used to assess egg burden. No significant differences in granuloma diameter were recorded (ANOVA, P = 0.76) (Table 1).
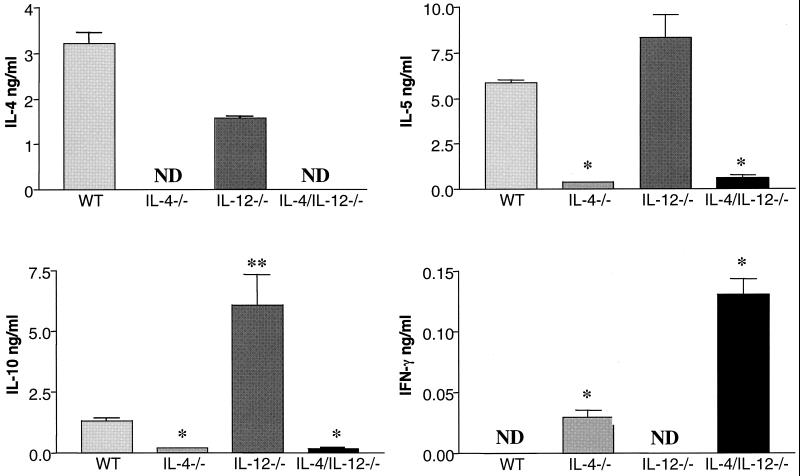
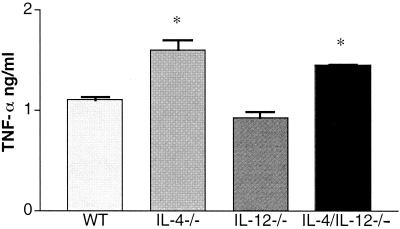
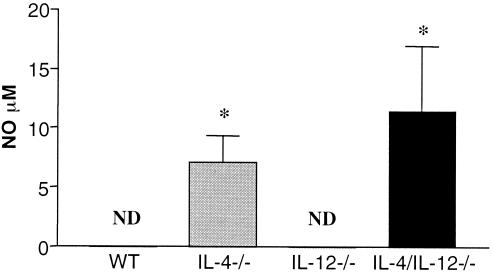
Infected IL-4−/− mice mount an antigen (Ag)-specific immune response deficient in type 2 cytokines and biased towards type 1 cytokine production (2, 5, 9, 15). To determine whether IL-12 contributes to the development of the type 1 response in infected IL-4−/− mice, Ag-specific immune responses were measured in infected IL-4/IL-12−/− animals. Single-cell suspensions of splenocytes were prepared as previously described (2). Cells used for cytokine assays were incubated either alone, with 50 μg of soluble egg antigen (SEA) per ml (1), or with 50 μg of SEA per ml plus 5 μg of lipopolysaccharide (LPS) per ml (2) (Sigma) in 96-well flat-bottom plates (Falcon, Lincoln Park, N.J.) at 37°C and 5% CO2. Cell culture supernatants were collected at 72 h and stored at −20°C until cytokine analysis was performed. Reagents for enzyme-linked immunosorbent assays were either prepared as previously described (IL-4 and IL-5) (21) or purchased from Pharmingen, San Diego, Calif. (IFN-γ and IL-10) or Genzyme, Boston, Mass. (TNF-α). Differences in cytokine and NO levels between groups were evaluated, using Student's t test. Levels of cytokine production by WT and IL-12−/− mice were similar, with the exception that infected IL-12−/− mice produced significantly higher levels of IL-10 than did WT mice (Fig. 2). The levels of cytokines produced by splenocytes from infected IL-4/IL-12−/− mice were, in type and quantity, comparable to those produced by infected IL-4−/− animals (Fig. 2). Specifically, there was a significant decrease in type 2 cytokine (IL-5 and IL-10) levels and an increase in IFN-γ and TNF-α levels in response to Ag or Ag and LPS in all IL-4-deficient mice (Fig. 2 and 3). The finding that IFN-γ levels were higher in the IL-4/IL-12−/− culture supernatants than in the IL-4−/− samples was not consistently observed in all experiments. Nitrite levels measured using the Griess reaction (7) indicated that NO levels were higher in the culture supernatants of Ag-stimulated splenocytes from infected IL-4−/− and IL-4/IL-12−/− mice than from WT and IL-12−/− samples (Fig. 4).
FIG. 2.
Cytokine production by spleen cells from infected mice. Spleen cells from WT, IL-4−/−, IL-12−/−, and IL-4/IL-12−/− mice, exposed to ∼70 cercariae 47 days earlier, were assayed individually. Spleen cells were stimulated in vitro with SEA, and levels of IL-4, IL-5, IL-10, and IFN-γ were measured in culture supernatants by enzyme-linked immunosorbent assay. ∗, significant differences (P < 0.05) between cytokine production in IL-4−/− and IL-4/IL-12−/− mice and cytokine production in WT and IL-12−/− mice. ∗∗, significant increase (P < 0.05) in IL-10 production by IL-12−/− mice than by WT mice. ND, not detected. There were five animals per group. Data are means ± standard errors of the means and are representative of three separate experiments.
FIG. 3.
TNF-α production by spleen cells from infected WT, IL-4−/−, IL-12−/−, and IL-4/IL-12−/− mice. Mice had been infected with ∼70 cercariae 47 days earlier, and spleen cells were stimulated in vitro with SEA and LPS. IL-4−/− and IL-4/IL-12−/− mice produced significantly more (∗, P < 0.05) TNF-α than did WT and IL-12−/− mice. There were five mice per group. Data are means ± standard errors of the means and are representative of two separate experiments.
FIG. 4.
NO production by spleen cells from infected WT, IL-4−/−, IL-12−/−, and IL-4/IL-12−/− mice. In vitro nitrite levels in supernatants of spleen cell cultures from mice exposed to ∼70 cercariae 47 days earlier were measured following stimulation with SEA. IL-4−/− and IL-4/IL-12−/− mice had significantly higher (∗, P < 0.05) NO levels than did WT and IL-12−/− mice. There were five mice per group. Data are means ± standard errors of the means and are representative of three separate experiments.
Based on these data, we conclude that the enhanced production of the inflammatory mediators IFN-γ, TNF-α, and NO and the development of severe disease in schistosome-infected IL-4−/− mice are IL-12 independent. Indeed, the absence of IL-12 had no discernable effect on immune response or infection outcome. In the absence of IL-4, a Th1-like response developed in an IL-12-independent manner, and in the absence of IL-12 alone, the Th2 response was indistinguishable from that in WT mice. The IL-12-independent development of IFN-γ-producing immune responses is uncommon but not unprecedented (8, 13, 18). Although the IL-12p40 homodimer may, in some circumstances, act as an IL-12 agonist to stimulate IFN-γ production (17), splenocytes from IL-12p40−/− mice produced levels of IFN-γ comparable to those of WT and IL-12p35−/− mice after anti-CD3 stimulation (data not shown), indicating that in this setting, IL-12p40 did not act to promote IFN-γ production. Because disruption of the IL-12p35 gene did not alter IFN-γ production, it was not possible for us to directly address the role of IFN-γ in the development of severe disease that occurs in the absence of IL-4. This issue will be addressed in the future, using IL-4/IFN-γ−/− animals.
Due to the low number of IFN-γ-producing cells in Ag-stimulated cultures, we have not yet been able to definitively identify the cellular source of Ag-stimulated IFN-γ in mice lacking IL-4, although schistosome-specific CD8 (14, 16) and CD4 (9) cells have been implicated in IFN-γ production during infection. Further studies will be necessary to definitively identify the cellular population which produces IFN-γ following Ag-specific stimulation.
In this study, we have shown that the lack of IL-12 altered neither the course of schistosomiasis nor the accompanying immune response in WT mice. IL-12 was not necessary for the development of the proinflammatory condition seen in schistosome-infected IL-4-deficient mice.
Acknowledgments
We thank Andrew MacDonald for critical review of the manuscript and the laboratory of Cornelia Farnum for technical advice and use of equipment.
This work was supported by National Institutes of Health grant AI32573 (to E.J.P.). E.A.P. is supported by National Research Service award (F32) AI10374; L.R.B. was supported by F32 AI09512 while at Cornell. A.C.L. is supported by F32 AI10151. The Basel Institute was founded by and is supported by Hoffman-La Roche. Schistosome life cycle stages for this work were supplied through NIH-NIAID contract N01-AI-55270.
REFERENCES
- 1.Boros D L, Warren K S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunet L R, Finkelman F D, Cheever A W, Kopf M A, Pearce E J. IL-4 protects against TNF-α mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 3.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15:1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 4.Chiaramonte M G, Donaldson D D, Cheever A W, Wynn T A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Investig. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fallon P G, Richardson E J, McKenzie G J, McKenzie A N. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Martinez C, Costelli P, Lopez-Soriano F J, Argiles J M. Is TNF really involved in cachexia? Cancer Investig. 1997;15:47–54. doi: 10.3109/07357909709018916. [DOI] [PubMed] [Google Scholar]
- 7.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann K F, James S L, Cheever A W, Wynn T A. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J Immunol. 1999;163:927–938. [PubMed] [Google Scholar]
- 9.Jankovic D, Kullberg M C, Noben-Trauth N, Caspar P, Ward J M, Cheever A W, Paul W E, Sher A. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163:337–342. [PubMed] [Google Scholar]
- 10.Kopf M, Le Gros G, Bachmann M, Lamers M C, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 11.Matthys P, Billiau A. Cytokines and cachexia. Nutrition. 1997;13:763–770. doi: 10.1016/s0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 12.Mattner F, Magram J, Ferrante J, Launois P, Di Padova K, Behin R, Gately M K, Louis J A, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 13.Oxenius A, Karrer U, Zinkernagel R M, Hengartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J Immunol. 1999;162:965–973. [PubMed] [Google Scholar]
- 14.Pancre V, Delacre M, Herno J, Auriault C. Schistosomal egg antigen-responsive CD8 T-cell population in Schistosoma mansoni-infected BALB/c mice. Immunology. 1999;98:525–534. doi: 10.1046/j.1365-2567.1999.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce E J, Cheever A, Leonard S, Covalesky M, Fernandez-Botran R, Kohler G, Kopf M. Schistosoma mansoni in IL-4 deficient mice. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 16.Pedras-Vasconcelos J A, Pearce E J. Type 1 CD8+ T cell responses during infection with the helminth Schistosoma mansoni. J Immunol. 1996;157:3046–3053. [PubMed] [Google Scholar]
- 17.Piccotti J R, Chan S Y, Li K, Eichwald E J, Bishop D K. Differential effects of IL-12 receptor blockade with IL-12 p40 homodimer on the induction of CD4+ and CD8+ IFN-gamma-producing cells. J Immunol. 1997;158:643–648. [PubMed] [Google Scholar]
- 18.Schijns V E, Haagmans B L, Wierda C M, Kruithof B, Heijnen I A, Alber G, Horzinek M C. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J Immunol. 1998;160:3958–3964. [PubMed] [Google Scholar]
- 19.Smithers S R, Terry R J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 20.Tisdale M J. Wasting in cancer. J Nutr. 1999;129:243S–246S. doi: 10.1093/jn/129.1.243S. [DOI] [PubMed] [Google Scholar]
- 21.Vella A T, Hulsebosch M D, Pearce E J. Schistosoma mansoni eggs induce antigen-responsive CD44-hi T helper 2 cells and IL-4-secreting CD44-lo cells. Potential for T helper 2 subset differentiation is evident at the precursor level. J Immunol. 1992;149:1714–1722. [PubMed] [Google Scholar]