Abstract
The systematics of the Cricetid genus Neodon have long been fraught with uncertainty due to sampling issues and a lack of comprehensive datasets. To gain better insights into the phylogeny and evolution of Neodon, we systematically sampled Neodon across the Hengduan and Himalayan Mountains, which cover most of its range in China. Analyses of skulls, teeth, and bacular structures revealed 15 distinct patterns corresponding to 15 species of Neodon. In addition to morphological analyses, we generated a high-quality reference genome for the mountain vole and generated whole-genome sequencing data for 47 samples. Phylogenomic analyses supported the recognition of six new species, revealing a long-term underestimation of Neodon diversity. We further identified positively selected genes potentially related to high-elevation adaptation. Together, our results illuminate how climate change caused the plateau to become the centre of Neodon origin and diversification and how mountain voles have adapted to the hypoxic high-altitude plateau environment.
Subject terms: Phylogenetics, Taxonomy, Evolutionary genetics
Morphological and molecular phylogenetic analyses reveal six new species of mountain vole from China and climate drivers of mountain vole diversification.
Introduction
Voles constitute one of the youngest groups of rodents. Mountain voles, belonging to genus Neodon (Rodentia: Cricetidae), which occur only in the Tibetan-Himalayan region (THR) (Fig. 1)1, originated less than seven million years ago (Mya)2,3 owing to the orogenesis of the Hengduan Mountains4–6. The THR, comprising of the Himalayas, Hengduan Mountains and the Qinghai-Xizang (Tibet) Plateau (QTP), consists of a series of parallel alpine ridges and deep river valleys and thus shows dramatic ecological stratification and environmental heterogeneity5,7,8. The topographical complexity and vastness of the territory constrains fieldwork, which subsequently affects biodiversity estimations. It also affects the testing of hypotheses on species interactions, especially for species with limited dispersal abilities. There is accumulating evidence that the QTP is the centre of origin for many organisms with specific biogeographical relationships to other Palaearctic regions4,6,9–15. Thus, systematic sampling with comprehensive analyses in the THR (Fig. 1) can provide critical clues about how geology and climate together drive evolution, biogeography and adaptation.
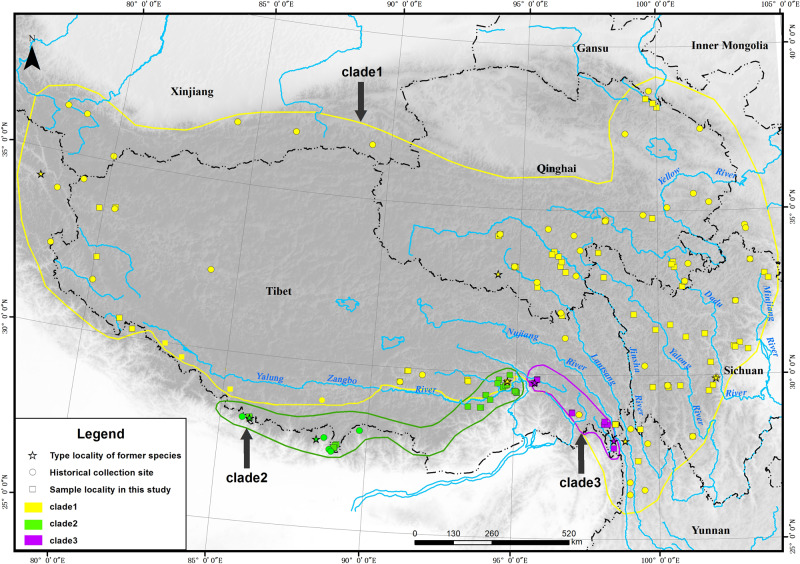
Fig. 1. Distribution of Neodon used in this study.
The approximate extent of occurrence of each clade is shown with coloured lines (Clade 1: yellow; Clade 2: green, Clade 3: purple, refer to Fig. 3 for clade information). Stars show the type localities of the described species. Circles show the historical collection sites. Squares show the distribution of newly collected specimens.
Previous studies of Neodon have been limited by sampling issues. Genus Neodon was erected by Horsfield in 1841, with four species (N. sikimensis, N. irene, N. forresti and N. juldaschi);16 however, there has been a long-running taxonomic debate involving the phylogenetic position of Neodon, as a subgenus of either Microtus17–19 or Pitymys20–22. Recent morphological and molecular evidence confirmed the monophyly of Neodon1,23,24 and revealed that several Microtus-like species once assigned to Microtus actually belong to Neodon1,24,25. Thus, Neodon includes far more than four species.
To gain better insight into the diversity, adaptation and evolution of Neodon, as well as the roles played by the QTP and climate changes in driving diversification and evolution, we used a collection of more than 2000 specimens collected over the past 20 years from throughout its distribution, especially from the Himalayas. The sampling activities covered tens of thousands of square kilometres, and we analysed the morphology of 235 samples herein (Supplementary Data 1). In addition to morphological and geographic data, we provide a high-quality Neodon reference genome obtained using 10X Genomics technology26, along with 1X–15X whole-genome sequencing (WGS) data for each morphologically distinct taxon (Supplementary Data 2). This extensive dataset allowed the identification and description of six new species of Neodon. Our analyses showed that rapid climate change, complex topography and founder events resulting from dispersal were the key factors driving Neodon diversification and evolution. In addition, our de novo genome assembly revealed the genetic basis of the adaptations of mountain voles to high-latitude environments, characterised by pressures such as hypoxia, high UV radiation and low temperatures.
Results
Morphological evidence of six unidentified lineages of Neodon
We analysed 235 specimens of Neodon to examine the possible existence of new species of Neodon based on morphological evidence. We also generated WGS data for 48 specimens to explore their genetic divergence and potential taxonomic status (Supplementary Data 1 and 2).
Initial observations of skulls, teeth and bacular structures showed 15 distinct patterns (Fig. 2 and Supplementary Fig. 1), each representing a putative species of Neodon. This included eight described species1,24, one previously evaluated group with unclear status and six tentatively unidentified taxa. We recorded the characteristics of the genitalia and bacular structures for males; and dental, external and cranial measurements for both sexes (detailed abbreviations of the measurements are provided in Supplementary Data 3) and further conducted morphological comparisons of these 15 putative species. The morphology of the glans penis provided useful clues about the affinities of microtine species and the differences in the characters of the glans penis clearly distinguished all putative species (Fig. 2). The pairwise Euclidean distances of dental measurements (e.g., the number of closed triangles on the first lower molar) also distinguished 15 patterns (Supplementary Fig. 2 and Supplementary Data 4). In addition, principal component analysis (PCA) (Supplementary Fig. 3) and subsequent two-sided t-tests or Wilcoxon rank-sum tests (Supplementary Fig. 4) of 17 statistical measurements of external and cranial characteristics of 95 intact adults (Supplementary Data 5–7) also resolved all 15 putative species.
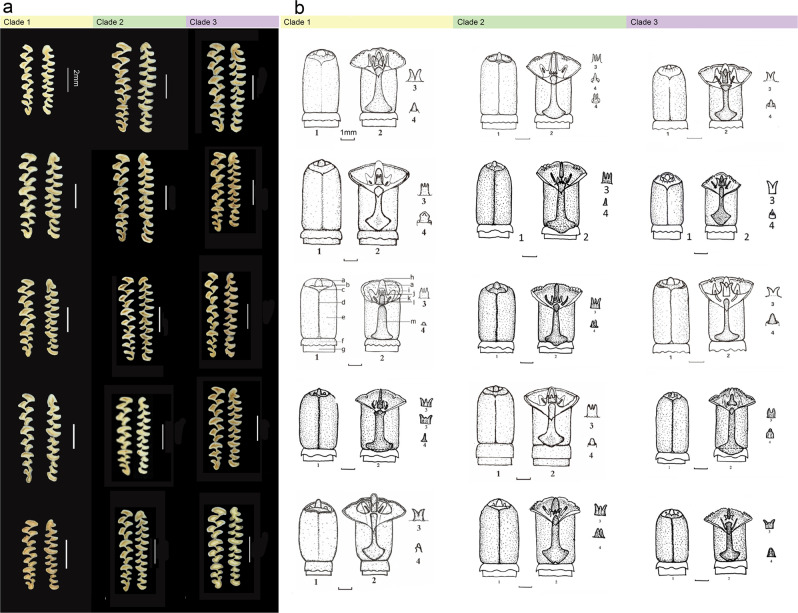
Fig. 2. Comparison of morphological features.
a Comparison of tooth rows. b Comparison of glans penes. Numbered views are 1: glans; 2: midventral cut view; 3: urethral lappet; 4: dorsal papilla. Lettered structural features in a1 and a2 are a. distal baculum; b. outer crater; c. inner crater; d. ventral groove; e. glans; f. prepuce; g. penis body; h. station of dorsal papilla; i. lateral baculum (cartilage); j. urethral lappet; k. lateral baculum (bony part); l. distal baculum (bony part); and m. proximal baculum. The taxa are (from top to bottom) Neodon leucurus, N. fuscus, N. linzhiensis, N. forresti, N. irene from Clade 1, N. nyalamensis, N. sikimensis, unidentified taxon 1 (from Nanyi township, Milin County), unidentified taxon 2 (from Shergyla Mountains, Linzhi county), unidentified taxon 3 (from Motuo County, south of the Namchabarwa Mountains) from Clade 2, N. medogensis, unidentified taxon 4 (from Ridong village, Bershula Mountains, Chayu County), N. clarkei, unidentified taxon 5 (from Bomi County) and unidentified taxon 6 (from Chibagou National Nature Reserve, Chayu County) from Clade 3 (refer to Fig. 3 for clade information).
Molecular evidence for all lineages of Neodon
We generated a total of 241 Gb of 10X Genomics linked-reads (67.56X) for one specimen of Neodon sampled from north of the Yarlung Zangbo River on Shergyla Mountain (unidentified taxon 2), and produced a genome assembly with a total length of 2.25 Gb and a scaffold N50 of 10.85 Mb. In addition, we obtained a total of 620.45 Gb of reads for an additional 47 samples, with the amount of data generated from each sample ranging from 2.21 Gb to 43.38 Gb (Supplementary Data 2). A total of 4951 full-length single-copy orthologous gene groups were annotated in our reference genome using BUSCO27. The “nuclear gene set” obtained for all lineages after the removal of low-confidence genes, consisted of a total of 4624 coding genes, with an average, maximal and minimal lengths of 1885 nt, 23,046 nt and 222 nt, respectively (Supplementary Fig. 5).
We obtained complete mitogenomes for sequenced specimens and calculated Kimura 2-parameter genetic distances for mitochondrial protein-coding genes (Supplementary Fig. 6 and Supplementary Data 8–10). The results for cox1 and cytb, the two most-widely used barcoding genes in mammals, showed an average interspecific genetic distances of 11.00% for cox1 and 11.30% for cytb. Species delimitation methods (bayesian implementation of Poisson Tree Processes (bPTP), automatic barcode gap discovery (ABGD) and BPP) based on mitochondrial or nuclear datasets recognised the same 15 species, including all six undescribed morphological lineages (Supplementary Figs. 7–10). Furthermore, the species delimitation results identified a split within N. sikimensis (Supplementary Fig. 7), and these specimens showed the greatest intraspecific genetic distances (average of 5.23% for cox1 and 5.20% for cytb). However, the specimens of N. sikimensis, which were collected in the same region at the same time, did not differ significantly in their morphology. This likely indicated the cooccurrence of divergent mitogenomes, as reported in the Asian elephant28 and other species. Further work can explore the possibility of unabated gene flow (one species) or restricted gene flow (two species).
Phylogenetic analysis corroborates taxonomic status
The analyses generated eight phylogenetic trees, among which two were based on barcoding genes (Supplementary Fig. 11), two were based on 13 mitochondrial coding genes (Supplementary Fig. 7), and four were based on 4,624 nuclear genes (Supplementary Fig. 10). All nuclear trees generated by coalescent and concatenation approaches shared the same topology, with only small-scale incongruences being identified between the nuclear trees and the other four mitochondrial gene trees. We used the nuclear ASTRAL III tree as the species tree in downstream analyses (Supplementary Fig. 10a). The phylogenetic analysis recovered Neodon as sister to Lasiopodomys, sharing a common ancestor with Microtus and Alexandromys, and the species of Neodon formed three major clades.
Description of six new species
Multiple resources supported the recognition of six new morphological species. Thus we described these species as follows (Expanded description in Supplementary Note 1, 2). All type series specimens have been deposited with the Sichuan Academy of Forestry:
Neodon namchabarwaensis Liu SY., Zhou CR., Murphy WR. & Liu SL., sp. nov. (unidentified taxon 1)
Holotype
Adult female, field number XZGB0818009, collected by Liao Rui on 10 August 2008. Specimen preserved as a skin, cleaned skull, and tissues. Skull, dentition and mandible in Supplementary Fig. 1a.
Type locality
Nanyi township, Milin County, south of Xizang, China, 29.17889° E, 94.15113° N, elevation 3160 m a.s.l.
Paratypes
Five specimens topotypes (3♂♂, 2♀♀), field numbers: XZGB0817007♂, XZGB0818006♀, XZGB0818010♂, XZGB0828001♀, XZGB09N235♂;
Distribution
Known from south of the Yarlung Zangbo River, north of the Namchabarwa Mountains. The lowest elevation is 3130 m a.s.l.
Etymology
Species named for the famous Namcha Barwa Mountain, the highest mountain in this region where the new species occurs.
Diagnosis
Medium body, average length 114.9 mm (adult); average hind foot length 20.1 mm. Average tail length 46.4 mm, approximately 40.4% of HBL. First lower molar with 3 closed triangles in front of the posterior transverse space, 6 inner and 5 outer angles. 1st upper molar with 4 inner and 3 outer angles. 2nd upper molar with 3 inner and 3 outer angles. 3rd upper molar with 4 inner and 3 outer angles.
Neodon shergylaensis Liu SY., Zhou CR., Murphy WR. & Liu SL., sp. nov. (unidentified taxon 2)
Holotype
Adult male, field number XZGB09N195, collected by Liao Rui and Liu Yang on 30 May 2009. Specimen preserved as a skin, cleaned skull, penis and tissues. Skull, dentition, and mandible are in Supplementary Fig. 1b.
Type locality
Shergyla Mountains of Linzhi county, southeast of Xizang, China, 29.62368° E, 94.66174° N, elevation 4500 m.
Paratypes
6 specimens (1♂, 5♀♀), field numbers: LZRAP01013♂, LZRAP01020♀, LZRAP01014♀, LZRAP01019♀, XZGB09N197♀, GB0815001J♀
Distribution
Known from north of the Yarlung Zangbo River at over 3160 m a.s.l. both sides of Shergyla Mountains and Niyang River.
Etymology
The species is named for its type locality. This region supports a high biodiversity.
Diagnosis
Medium body, average length 115.7 mm (adult); average hind foot length 19.5 mm. Average tail length 42.7 mm, approximately 37% of HBL. The first lower molar with 3 closed triangles in front of the posterior transverse space, 6 inner and 4 outer angles in 63% specimens, 6 inner and 5 outer angles in 37% specimens. 1st upper molar with 3 inner and 3 outer angles. 2nd upper molar with 3 inner and 3 outer angles. 3rd upper molar with 4 inner and 3 outer angles.
Neodon liaoruii Liu SY., Zhou CR., Meng GL. & Liu SL., sp. nov. (unidentified taxon 3)
Holotype
Adult male, field number XZ11117, collected by Liao Rui on 1 November 2011. Specimen preserved as a skin, cleaned skull, penis and tissues. Skull, dentition and mandible in Supplementary Fig. 1c.
Type locality
Motuo County, south of Xizang, China, 29.47028° E, 94.984° N, elevation 3260 m.
Paratypes
Ten specimens (4♂♂, 6♀♀), field numbers: MT11036♀, MT11066♀, MT11067♀, MT11109♂, MT11118♂, MT11120♂, MT11122♀, MT11142♂, MT11143♀, MT11144♀.
Distribution
Known from south of the Namchabarwa Mountains. Lowest elevation 2660 m a.s.l.
Etymology
Species epithet is a patronym for the collector, Mr. Liao Rui. He made an important contribution to our collecting specimens.
Diagnosis
Relatively large body, average length 116.8 mm (adult); average hind foot length 21.1 mm. Avarage tail length 59.3 mm, ~50.8% of HBL. First lower molar with 3 closed triangles in front of the posterior transverse space, 6 inner and 5 outer angles. 1st upper molar with 3 inner and 3 outer angles. 2nd upper molar with 2 inner and 3 outer angles in 66% specimens, and 3 inner and 3 outer angles in another 34% specimens. 3rd upper molar with 4 inner and 3 outer angles in 61% specimens, and 3 inner and 3 outer angles in another 39% specimens.
Neodon bershulaensis Liu SY., Zhou CR., Liu Y. & Liu SL., sp. nov. (unidentified taxon 4)
Holotype
Adult male, field number XZ11010, collected by Liao Rui on 3 Mach 2011. Specimen preserved as a skin, cleaned skull, penis and tissues. Skull, dentition, and mandible in Supplementary Fig. 1d.
Type locality
Ridong village, Bershula Mountains, Chayu County, southeast of Xizang, China. 98.12407° E, 28.58392° N, elevation 3750 m a.s.l.
Paratypes
3 intact adult specimens, field number: CHYRD-03♀, CHYRD-04♂, CSD3825♂.
Distribution
Known from the type locality only, Ridong village, Chayu County, southeast of Xizang.
Etymology
Species epithet for the famous Bershula Mountains, where type locality, Ridong is at its foot.
Diagnosis
Medium body, average length 107 mm (adult); hind feet length 18–20 mm (average 19 mm). Average tail length 51.5 mm, 48.1% of HBL. First lower molar with 5 closed triangles in front of the posterior transverse space, 6 inner and 4 outer angles. 1st upper molar with 4 inner and 3 outer angles in 70% specimens; other 30% with 3 inner and 3 outer angles. 2nd upper molar with 3 inner and 3 outer angles. 3rd upper molar with 4 inner and 3 outer angles.
Neodon bomiensis Liu SY., Zhou CR., Meng GL. & Liu SL., sp. nov. (unidentified taxon 5)
Holotype
Adult male, field number XZ13015, collected by Liao Rui on 31 October 2013. Specimen preserved as a skin, cleaned skull, penis and tissues. Skull, dentition and mandible in Supplementary Fig. 1e.
Type locality
Bomi County, southeast of Xizang, China, 95.9575816° E, 29.82959° N, elevation 2900 m a.s.l.
Paratypes
2 intact adults specimens, field numbers: MT11304♀, XZ13016♀
Distribution
Known only from the type locality, Bomi County, southeast of Xizang.
Etymology
Species epithet derived from the county where type series collected.
Diagnosis
Medium body, average length 111.75 mm (adult); hind feet length 18–19 mm (average 18.75 mm). Tail length 53–56 mm (average 53.75 mm), approximately 48.1% of HBL. First lower molar with 4 closed triangles in front of the posterior transverse space, 6 inner and 4 outer angles in 60% specimens; other 40% with 5 inner and 4 outer angles. 1st upper molar with 4 closed triangles after the anterior transverse space, forming 3 inner and 3 outer angles. 2nd upper molar with 3 inner and 3 outer angles, and the last inner angle much small. 3rd upper molar with 3 inner and 3 outer angles.
Neodon chayuensis Liu SY., Zhou CR., Liu Y., Tang MK. & Liu SL., sp. nov. (unidentified taxon 6)
Holotype
Adult female, field number CY37, collected by Liu Yang on 8 October 2007. Specimen preserved as a skin, cleaned skull and tissues. Skull, dentition, and mandible in Supplementary Fig. 1f.
Type locality
Chibagou National Nature Reserve, Chayu County, southeast of Xizang, China, 96. 98858° E, 28. 85716° N, elevation 2960 m a.s.l.
Paratypes
Three intact adults specimens (1♂, 2♀♀), field numbers: CY35♀, CY44♂, CY45♀.
Distribution
Known from Cibagou National Nature Reserve, Chayu County, southeast of Xizang.
Etymology
Species epithet derived from the county where type series were collected.
Diagnosis
Medium body, average length 106.6 mm (adult); hind feet length 19–21 mm. Average tail length 47.9 mm, approximately 45% of HBL. Tooth row sturdy. First lower molar with 4 closed triangles in front of the posterior transverse space, but more or less confluent each other in many specimens, this tooth with 6 inner and 5 outer angles in 55% specimens; other 45% specimens with 6 inner and 4 outer angles. 1st upper molar with 4 inner and 3 outer angles in 67% specimens, another 33% with 3 inner and 3 outer angles. 2nd upper molar with 3 inner and 3 outer angles. 3rd upper molar with 3 inner and 3 outer angles.
Historical biogeography of mountain voles
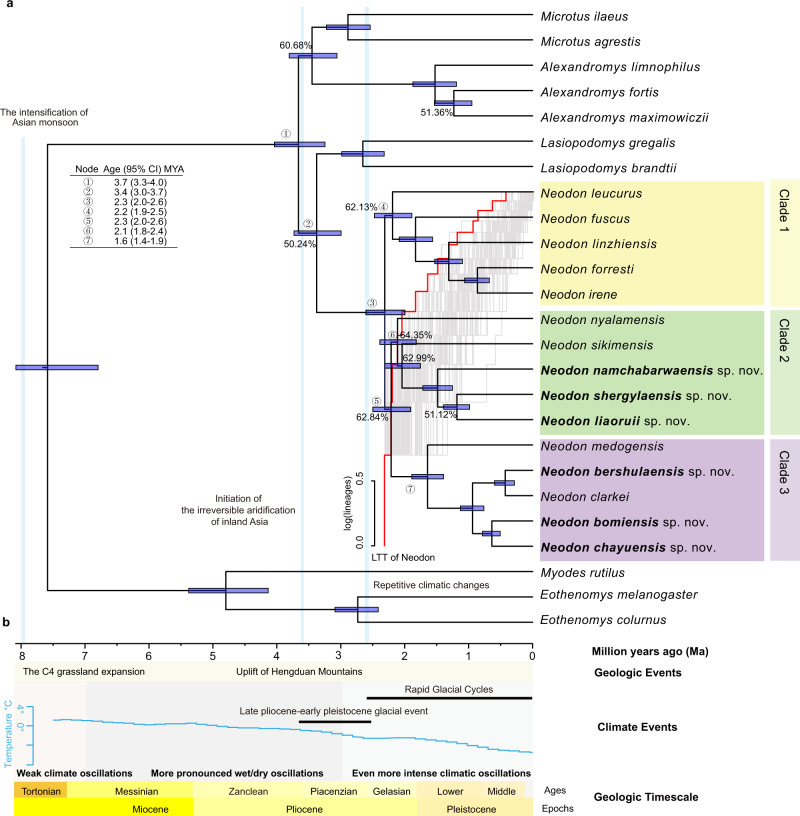
The divergence of Neodon and Lasiopodomys at ca. 3.4 Mya (3.7–3.0 Mya) coincided with the onset of the Late Pliocene-early Pleistocene glacial event, and most lineages of Neodon have diverged over the past 3 million years (Fig. 3). The newly identified species belonged mainly to Clades 2 and 3. The short branch lengths of the inner nodes revealed that Neodon experienced an explosive radiation during the late Neogene and that its speciation occurred very quickly (Fig. 3). Both the lineage-through-time plot and gamma statistic (γ < 0, p < 0.05) suggested a deceleration of the rate of speciation after the initial burst (Fig. 3 and Supplementary Fig. 12). The best-fitting model was dispersal-extinction cladogenesis with a long-distance J parameter (DEC + J) based on BioGeoBEARS analysis (Supplementary Data 11). The most recent common ancestor of Neodon was dated to approximately 2.6 Mya and it was located on the QTP with the highest probability (Fig. 3). Within the next two million years, glacial cycles and climate oscillations increased and caused more uncertainty in the environment (Fig. 3b). The ancestor of each clade appeared to have dispersed to plateaus, such as those in the Himalayas and Hengduan Mountains. Speciation involved different niches within the plateau and surrounding mountains, suggesting that the QTP is the centre of origin of mountain voles (Fig. 4). Our phylogeny demonstrates that the evolution of mountain voles has involved several critical geographical and climate events.
Fig. 3. Divergence time tree, diversification patterns, the frequency of the non-main topologies and photos of Neodon.
a Divergence time-tree with the Astral branch supports values of the nuclear gene tree are shown near the branches. New species denoted in bold. Clades of Neodon indicated by shading with different colours on the tree (Clade 1: yellow; Clade 2: green; Clade 3: purple). The blue rectangles at the nodes represent 95% confidence intervals of the corresponding estimated divergence times. Branches with high non-main topology occurrence frequencies (>50%) are marked with the content number. Log-lineage-through-time (LTT) plots for Neodon estimated from the time-calibrated phylogeny (red curve), and the semilucent red dashed line indicates the null distribution under a Yule process. b The divergence time, geologic timescale, geologic events and climate events are shown at the bottom of the figure.
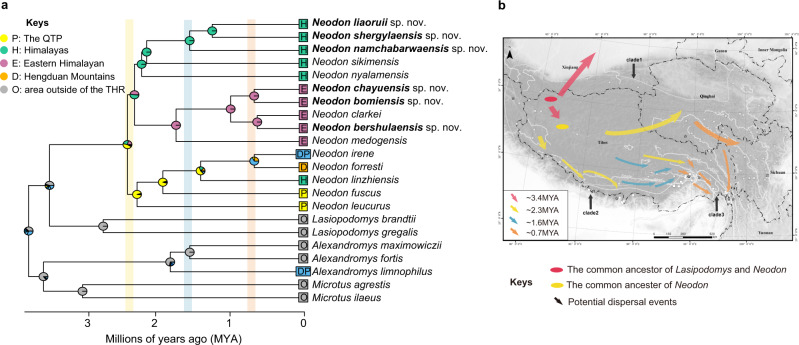
Fig. 4. Ancestral range estimation of Neodon.
a Ancestral range estimation based on the best-fitting model DEC + J implemented in BioGeoBEARS. The relative probabilities of each ancestral reconstruction are indicated with pie charts at each node. New species denoted in bold. Transparent bars represent the timelines following the coding described in b. b Estimated origin and dispersal of extant Neodon.
Positively selected genes
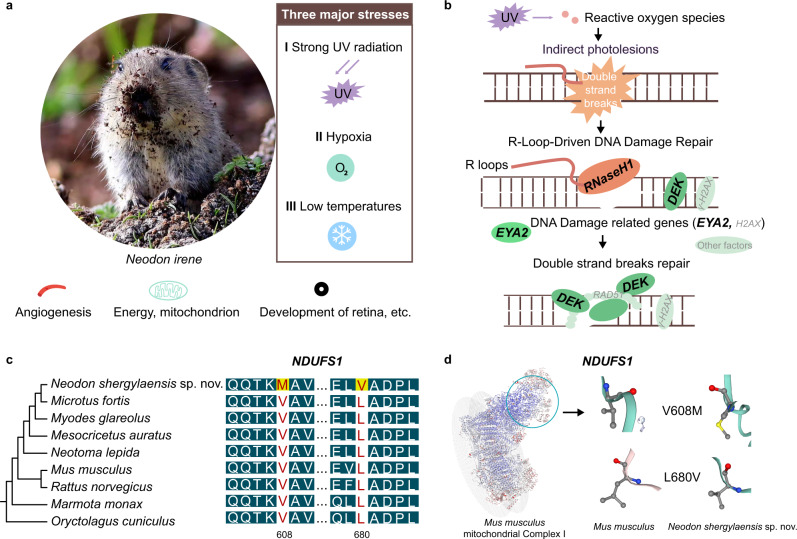
High-elevation mammals show many adaptations to hypoxia, low temperatures and high levels of ultraviolet radiation15,29. Hence, we investigated the plateau adaptation of mountain voles based on 6,678 high-confidence orthologous genes of Neodon shergylaensis sp. nov. (our de novo genome) and eight other low-elevation Glires taxa (Fig. 5). We identified 127 positively selected genes (PSGs) (Supplementary Data 12) using the PAML branch-site model30. These genes were associated with physiological processes that may contribute to adaptations such as DNA repair (RNASEH1, EYA2, DEK)31–33, eye development (RAB25, WSCD2, MYO7A, MEGF11, RPE65), skin and fur (KRTAP5-1, KRTAP4-6), energy production or mitochondrion development (NDUFS, MPZL3, TRAK1, REEP1)34,35, angiogenesis (MST1, PHACTR1, CHRM2)36, and the somatosensory system37 (IGF2BP2, RHBDF1). Furthermore, KEGG and GO enrichment analyses of the PSGs revealed “Retinol metabolism” as a significant process (Supplementary Data 13, 14), which suggested that mountain voles have evolved specific mechanisms to protect their eyes and skins from the risks of UV-induced tumours or other diseases38. Genes that function in energy production, angiogenesis and the somatosensory system (Fig. 5 and Supplementary Data 13–15) may have contributed to adaptations by increasing oxygen delivery via adaptive vasodilation39–41 and low-temperature resistance42.
Fig. 5. Gene functional adaptations in Neodon species.
a The three major environmental stresses of high-elevation areas and the adaptive traits that the voles may have developed (Photograph by Shaoying Liu). b The repair of DNA double-strand breaks damage, involving the three PSGs of RNaseH1, EYA2 and DEK, is a classic DNA repair pathway for UV-induced DNA damage. c Visualisation of two positively selected gene sites in the NDUFS1 gene, which represents one of the PSGs related to mitochondrial function. d Mitochondrial complex I and positive selection sites affect the protein structure of NDUFS1.
Discussion
The morphological traits of the penes and dentition, and external and cranial characters are critical for classifying species in tribe Arvicolini1,13 and vary among species of Neodon. For example, features of the glans penis and baculum offer the most valuable clues for identification (Fig. 2), while the number of closed triangles on the first lower molar, which has been used to characterise diversity in Neodon13,43,44, does not explain the full spectrum of morphological diversity. All specimens with three closed triangles in the first lower molar (distributed in southern Tibet) were once regarded as N. sikimensis1,24, but our results reveal that at least five species share the same feature, including N. sikimensis, N. nyalamensis, N. liaoruii sp. nov., N. shergylaensis sp. nov. and N. namchabarwaensis sp. nov. It is possible to identify the species of Neodon by combining external measurements and other morphological traits of the upper and lower molars, such as the number of inner and outer angles.
Climate oscillations and geographical events appear to have played crucial roles in Neodon speciation. For instance, global cooling and the irreversible aridification of inland Asia coincided with the divergence of Neodon and Lasiopodomys: the divergence of the common ancestor species of the two groups occurred in the late Pliocene (~3.4 Mya), when the Earth’s surface was generally cooled (Fig. 3b). Orogenesis in the THR imposed a large barrier effect on the ancient southwestern monsoons, which directly resulted in the South Branch Westerly Jetstream and further promoted the transformation of the frigid and rough environment on the plateau surface into a humid environment45,46. The common ancestor of Lasiopodomys and Neodon appears to have differentiated as it adapted to different climates. One lineage adapted to drought conditions and grassland environments appears to have retreated to the arid north plateau and evolved into the Lasiopodomys, while another branch, adapted to humid environments, occupied the humid plateau surface and evolved into Neodon (Fig. 4b, magenta arrows). Then, as the last Ice Age began at ~2.6 Mya47, the climate cooled and forced the ancestral population of Neodon to migrate to the southern or eastern edge of the THR and then return to the inner THR after the ice sheet receded, which resulted in the speciation of many Tibetan species48.
A further inspection of the geographic distributions and barriers of those species showed that the lower Himalayan foothills, Hengduan Mountains and Yalung Zangbo River played vital roles in Neodon speciation. The two most widely distributed species of Neodon, N. leucurus and N. fuscus, were located at the basal position of the Neodon species tree (Clade 1, Fig. 3 and Supplementary Figs. 13 and 14), confirming that Neodon originated on the QTP. The species in Clade 2 included two described and three newly discovered taxa distributed mainly in the Himalayas (Fig. 3 and Supplementary Fig. 15). Neodon sikimensis and N. nyalamensis specimens collected near their type localities formed a root lineage with other members of Clade 2. The three new taxa occured around the Yalung Zangbo River, Namchabarwa Mountains and Duoxiongla Peak, which were not previously explored. The species of Clade 3 live in the eastern THR (including the Hengduan Mountains and the eastern margins of the QTP), where mountain ranges or single summits could have served as glacial isolated island refugia (Fig. 3, Supplementary Fig. 16)4,49. Furthermore, the Yarlung Zangbo River and Palung Zangbo Ancient River Channel appear to have formed physical barriers that facilitated the genetic divergence between N. nyalamensis, N. medogensis and other species (Supplementary Figs. 14–16).
The distributions and phylogenetic positions of the species suggest that Pleistocene glacial periods drove dispersal from the inner QTP to refugia in the Himalayas, Hengduan Mountains and montane regions isolated by intervening valleys4,49. The complex topographical features led to the geographic isolation of biota. An overall positive correlation existed between genetic distances and geographic distances, but several geographically close populations did not follow this pattern (Supplementary Figs. 14–16). The pattern likely matches with sky island refugia distribution located along the dispersal route. Furthermore, the dispersal route of Neodon closely corresponded to with those of other species, such as the woolly rhinoceros50, dipodids11 and pikas15. A strong negative correlation (R2 = 0.93, P value < 0.01) was identified between the frequency of the non-main topologies (the two alternative topologies) and inner-node branch length (Supplementary Fig. 12), indicating that potentially high levels of incomplete linage sorting or gene flow played a role in the initial rapid species radiation (Supplementary Fig. 12c). This result implies that genetic drift drives most molecular changes in lineages of Neodon and that the species still share similar ecological niches but present different morphologies.
The genome resources will provide better insight into the adaptation and evolution of Neodon and additional THR species. The analyses revealed 127 orthologous genes that have undergone positive selection. Gene function analyses revealed involvement in multiple physiological processes that facilitate survival on the QTP. These genetic signatures of adaptation provide important insights for understanding of how the genomes have changed during high-elevation adaptation. Nevertheless, the confirmation of adaptation awaits functional testing.
Morphological, genetic and biogeographic evidence allows the exploration of the history of Neodon’s evolution. Our results offer a more complete understanding of speciation and biogeographic events and further highlight how mountain voles adapted to new environments of the THR during climate events. Most of the newly described species came from localities that were not sampled previously. Thus, similar sampling and integrative molecular and morphological analyses of relatively sedentary species, such as small mammals, reptiles and amphibians, are necessary to accurately document the biodiversity of the THR. Similar investigations of other small mammals will likely reveal greater diversity and ultimately identify the common driver(s) of patterns in the THR ecosystem, and further revealing the evolutionary history of these animals and elucidating the genetic basis of their adaptation toward extreme ecological preferences.
Methods
Sample information
We analysed up to 235 specimens of Neodon (Supplementary Data 1). All samples were obtained following the Guidelines of the American Society of Mammalogists51 and the laws and regulations of China for the implementation of the protection of terrestrial wild animals (State Council Decree 1992). Voucher specimens were deposited in the Sichuan Academy of Forestry, Chengdu, China.
Morphological analyses
External measurements were recorded in the field from all freshly captured specimens at an accuracy of 0.5 mm, and the cranial and dental characteristics of all specimens were measured using a Vernier calliper with an accuracy of 0.02 mm in the lab (Supplementary Data 3)1. The morphometric variation in 17 non-sex-related measurements of adult specimens was analysed using PCA in SPSS v17.0 (SPSS Inc., Chicago, IL, USA). We employed Kaiser-Meyer-Olkin52 and Bartlett’s tests53 to check the fitness of the PCA, followed by Tukey’s test54. Independent-samples two-sided t-tests or Wilcoxon rank-sum tests55 were also performed to check the differences between the taxon pairs after PCA. Plots were generated using R corrplot56, ggplot257 and Python3.
High-throughput sequencing and genome assembly
We extracted the genomic DNA of each specimen from muscle tissues using a Gentra Puregene Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol and then generated >10 Gb of data for most morphologically distinct species and ~3 Gb of data (~1X) for the other specimens of the same species. In addition, we sequenced Neodon shergylaensis sp. nov. using 10X technology to obtain ~241 Gb of data (~86X). Low-quality reads were removed if they met one or more of the following criteria: (1) an N-content of more than 10%; (2) the presence of adaptor contamination (reads overlapping more than 50% with the adaptor sequence, with a maximal 1 bp mismatches to the adaptor sequence); or (3) more than 30% of the read length below Q30 (Supplementary Data 2).
A total of 1,612.36 million paired-dnd reads were generated with 10X technology was generated for Neodon shergylaensis sp. nov., and the reference genome was assembled using SuperNova v2.1.158, with a preset genome size of 2.50 Gb and a weighted mean molecule size of 18.39 kb. One of the two pseudohaplotypes generated using SuperNova with “--maxreads = ‘all’ --accept-extreme-coverage, --style = pseudohap2” was used to obtain the core gene set using BUSCO v3.0.127. In addition, the assembled genome was annotated using MAKER2 v2.31.1059,60 (control files can be found in Supplementary Data 16) for further evolutionary analysis. We also assembled and annotated the mitogenome of each sample using MitoZ61 with ~3 Gb (~1X) of filtered data61.
Gene dataset construction
A read-mapping-then-consensus-calling pipeline was used to obtain orthologous nuclear genes for each sample. For this purpose, (1) we obtained complete and single-copy orthologues of the N. shergylaensis sp. nov. genome using BUSCO v3.0.127 with a database of the Euarchontoglires group (6192 genes, v2), and we removed orthologues with high homologue to each other (BLASTn v2.6.0+ with e value < 1e-5)62 to avoid mapping uncertainty in the subsequent steps, deleted orthologues with internal stop codons, extracted corresponding genomic regions for the remaining qualified orthologues with custom Python scripts and then used the residual data as a reference for the resequenced samples. (2) We mapped the WGS data of each sample onto the reference using BWA-MEM v0.7.1763 with the default parameters, the corresponding genes were acquired using the consensus calling function in BCFtools v1.864 (detailed in Supplementary Note 1) and then selected high-quality coding sequences (no internal stop codons, ‘N’ content below 20%, present in more than 50% of samples) for subsequent analyses. (3) To obtain gene alignments, we applied MAFFT v7.31365 to generate the multiple protein sequence alignment for each qualified orthologue and then obtained the corresponding gene alignments based on the protein alignments using PAL2NAL66 for mitochondrial genes and TrimAl v1.4.rev2267 with the parameters “-backtrans -automated1” for nuclear genes. All multiple sequence alignments are provided in the Supplementary Data 17.
To evaluate the potential bias of the reference-mapping-based (“mapping-derived”) method, we obtained the orthologues of six other high-coverage sequencing samples using a de novo assembly method. We compared the exon datasets obtained from the “mapping-derived” method and the “de novo-derived” methods, and calculated the mismatches between them. We additionally subsampled the clean read dataset and added three more filtering parameters for the mapping-derived method for comparison. The details are provided in Supplementary Note 1 (Supplementary Fig. 17 and Supplementary Data 18 and 19). The “mapping-derived” gene dataset was used in subsequent analyses.
Species delimitation
We calculated the Kimura 2-parameter genetic distances between lineages for each gene using the dist.dna function in R ape v1.1-168 and explored the correlation between genetic distance and geographic distance. Geographic distances between different sampling sites were calculated with the geopy.distance.geodesic function in Python geopy v2.0.0 package69 (https://github.com/geopy/geopy). Pearson correlation coefficients were calculated with the cor function in R and plotted using ggpubr70. In addition to morphological identification, we conducted species delimitation analysis using the clustering-based method bPTP71 based on both the mitochondrial and nuclear trees. We also applied the similarity-based ABGD72 and the multispecies coalescent (MSC) model-based BPP v4.3.873 analysis using only the mitochondrial data. We also applied both the A1074,75 and A1176 analyses implemented in BPP to the 31 Neodon specimens using mitochondrial data. Specimens of each morphological species were grouped into the same population, including 1 to 5 specimens of each morphological species. Species delimitation were performed with a user-specified guide tree in the A10 analysis, while species delimitation and species tree inference were jointly calculated in the A11 analysis (detailed in Supplementary Note 1).
Phylogenetic inference
Both coalescent and concatenation methods were used to infer phylogenetic trees. We inferred the best maximum likelihood trees using RAxML v8.2.1277 with the GTR + GAMMA model from 20 independent tree searches and 500 bootstrap replicates for each gene, and then obtained the final species tree using ASTRAL-III78 based on the multispecies coalescent model with the bootstrap support of each node being estimated by the multilocus resampling method79. SVDquartets (parameters of “eval Quartets = 1e + 6 bootstrap = standard”) implemented in PAUP v4.0a16780,81 was also utilised to estimate the species tree with the same dataset to validate the results. Additionally, we concatenated the gene alignments to generate a “supergene” alignment for each species and obtained species trees using IQ-tree v1.6.1282, RAxML or MrBayes83. The inferred phylogenetic tree comprised two gene sets - the “mitochondrial Gene Set” and the “nuclear Gene Set”. All trees inferred from the nuclear dataset showed the same topology, and we thus re-estimated the branch lengths of the final species tree in units of substitutions per site using ExaML v3.0.2184.
Divergence time estimation
We estimated the divergence times of lineages of Neodon based on the second codon sites of nuclear genes using MCMCTree, a Bayesian relaxed clock method implemented in the PAML v4.9 h package30. For estimation, an approximate likelihood calculation of the ‘REV’ (GTR, model = 7) model was applied, and multiple fossil calibration points taken from records in the Palaeobiology Database (Accessed 2018 Dec 12)85 and the timetree database86 were included, as follows: (a) the root age was set as 7.9 Mya, as supported by the occurrence of Promimomys in the fossil record;87 (b) the splits of Lasiopodomys and Neodon, Eothenomys and Myodes were calibrated as <0.53 Mya based on the earliest occurrence of the oldest fossil record of Myodes from the Paleobiology Database and another fossil record of Promimomys;87 (c) the split data of Eothenomys was set as 2.7–5.3 Mya based on the fossil calibration point of Eothenomys (3.6–2.6 Mya);88,89 and (d) the split data of Microtus and Alexandromys was dated between 0.6 and 3.5 Mya based on previous studies90,91. The minimum boundary was supported by the earliest occurrence of Allophaiomys in the fossil record in the database. BaseML first estimated a prior substitution rate, and MCMCTree then generated the Gradient and Hessian matrices with following settings: ‘correlated rates clock’ (clock = 3), overall substitution rate (rgene gamma) set of G (1, 12.0), and rate drift parameter (sigma2 gamma) set of G (1, 4.5). Next, we conducted two independent MCMC runs with different random seed numbers and a burn-in of 500,000 iterations to check for convergence. Each run was sampled every 1000 iterations until 500,000 samples had accumulated. We also applied Tracer v1.7.192 to examine the convergence of the MCMC analysis.
Evolutionary and biogeographic analyses
We scanned for the presence of incomplete lineage sorting (and/or gene flow) spanning the evolution of Neodon with the nuclear dataset using DiscoVista78. We then calculated the correlation between the content of the non-main topologies of gene trees and the inner-node branch length using a linear model and Pearson’s test in R93 and visualised the results using ggplot257. We generated log-lineage through time (LTT) plots for both the time-calibrated phylogeny and 100 simulated trees with the same age and taxonomic richness using Phytools94. We used BioGeoBears v1.1.295 for biogeographic reconstruction based on the species tree (detailed in Supplementary Note 1) and assigned the species to one or two of the following biogeographical regions according to their distributions, were the Tsangpo River and the Mekong-Salween rivers divide were used as the borders for E-H and H-D, respectively: P (the QTP); E (Eastern Himalayan Mountains); H (Himalayas); D (Hengduan Mountains); O (area outside of the THR).
Putative molecular adaptation
Genomes of the low-elevation species Microtus fortis, Oryctolagus cuniculus, Mus musculus, Rattus norvegicus, Myodes glareolus, Mesocricetus auratus, Neotoma lepida and Marmota monax were downloaded from the Ensembl database (release 101) and used as background taxa in the evolutionary rate analysis. We obtained an orthologous gene set using a BLAST reciprocal best hits (RBH) method96, and we then obtained multiple sequence alignments using the same method described above. Next, we retained genes present in all taxa that met the following criteria for analysis using CODEML implemented in PAML v4.9j:30 (1) mapping to human gene with at least 50% coverage; (2) lacking frameshift indels in coding sequences; and (3) lacking premature stop codons.
Genes with Bonferroni-adjusted P values ≤ 0.05 in the evolutionary rate analysis were treated as candidates that have undergone positive selection. To minimise the influence of alignment errors, positively selected sites were removed when: (1) they appeared as a gap in >2 species; (2) showed >2 forms of nonsynonymous substitutions; and (3) showed probability scores generated by Bayes Empirical Baye analysis (PAML) < 0.9. We further manually checked each PSG to avoid potential false-positives caused by low-quality alignment. The gene functions of the PSGs were annotated using the SwissProt and MGI databases, and KOBAS v3.097 was applied to perform KEGG and GO enrichment annotation. Protein structure was predicted using SWISS-MODEL98.
Statistics and reproducibility
The morphometric variation in non-sex-related measurements of adult specimens was analysed using PCA in SPSS v17.0. We employed Kaiser-Meyer-Olkin and Bartlett’s tests to check the fitness of the PCA, followed by Tukey’s test. Independent-samples two-sided t tests or Wilcoxon rank-sum tests were also performed to check the differences between the taxon pairs after PCA. The significant positively selected genes were confirmed using Bonferroni test. Reproducibility was confirmed by performing analyses with independent replicates (for morphological analyses), five hundred bootstrap replicates or different coalescent and concatenation approaches as described in the Methods section.
Nomenclatural Acts
This published work and the nomenclatural acts it contains have been registered in ZooBank, the proposed online registration system for the International Code of Zoological Nomenclature (ICZN). The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:794808AA-EA46-4E86-B482-9983214688BB.
The LSID for Neodon namchabarwaensis Liu SY., Zhou CR., Murphy WR. & Liu SL., sp. nov. is: urn:lsid:zoobank.org:act:8B19E76E-2E5F-452E-A94B-0824DB45CB30
The LSID for Neodon shergylaensis Liu SY., Zhou CR., Murphy WR. & Liu SL., sp. nov. is: urn:lsid:zoobank.org:act:811C522A-2B13-48EE-A8B4-3B758E3EB129
The LSID for Neodon liaoruii Liu SY., Zhou CR., Meng GL. & Liu SL., sp. nov. is: urn:lsid:zoobank.org:act:D4E07979-F92F-4825-BA6B-BB5B6881F9FD
The LSID for Neodon bershulaensis Liu SY., Zhou CR., Liu Y. & Liu SL., sp. nov. is: urn:lsid:zoobank.org:act:A72C6927-1269-4E65-8183-6F48D86F06E9
The LSID for Neodon bomiensis Liu SY., Zhou CR., Meng GL. & Liu SL., sp. nov. is: urn:lsid:zoobank.org:act:445E9955-1D43-41E9-AE51-71A3CDFDB28D
The LSID for Neodon chayuensis Liu SY., Zhou CR., Liu Y., Tang MK. & Liu SL., sp. nov. is: urn:lsid:zoobank.org:act:0F26DDC2-C279-4DE6-AAF2-B1E4C9917B6F
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Supplementary Information - clean version
Description of Additional Supplementary Files
Acknowledgements
We thank Rui Liao for assistance in collecting specimens in the field. Special thanks to Yinjuan Mao and Junhua Bai for drawing the figures. This work was supported by the National Natural Science Foundation of China (31470110, 31970399) and Survey of Wildlife Resources in Key Areas of Tibet (ZL202203601). This work was also supported by China National GeneBank (CNGB).
Author contributions
Sy.L. and Sl.L. conceived the study. Sy.L. and Y.L. collected the samples. C.Z., G.M., T.W., M.T. and C.Y. performed bioinformatics and computational analyses of the data. R.W.M., Z.F., T.Z. and Y.Z. provided advice on the data analyses. Sy.L. and C.Z. drafted the manuscript. All authors contributed to the final version of the manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Luciano Matzkin and Luke R. Grinham.
Data availability
Data that support our findings have been deposited in the NCBI database under BioProject PRJNA564473 (ncbi.nlm.nih.gov/bioproject/?term= PRJNA564473) and CNGB Nucleotide Sequence Archive (CNSA) under the accession number CNP0000173 (https://db.cngb.org/search/project/CNP0000173). Source data for figures can be found in Supplementary Data 20.
Code availability
All analyses were performed using open source software tools, and the detailed parameters for each tool are shown in the relevant methods. Custom scripts are archived at GitHub (https://github.com/linzhi2013/busco_process_scripts).
Ethical approval
All authors have agreed to all manuscript contents, the author list and its order and the author contribution statements. All samples were obtained following the Guidelines of the American Society of Mammalogists and the laws and regulations of China for the implementation of the protection of terrestrial wild animals. Collecting protocols and research project were approved by the Ethics Committee of the Sichuan Academy of Forestry and the Institutional of Review Board on Bioethics and Biosafety of BGI (NO. FT17005).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shaoying Liu, Chengran Zhou.
Contributor Information
Shaoying Liu, Email: shaoyliu@163.com.
Shanlin Liu, Email: shanlin.liu@cau.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-04371-z.
References
- 1.Liu S, et al. Taxonomic position of Chinese voles of the tribe Arvicolini and the description of 2 new species from Xizang, China. J. Mammal. 2017;98:166–182. doi: 10.1093/jmammal/gyw170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lv X, Xia L, Ge D, Wu Y, Yang Q. Climatic niche conservatism and ecological opportunity in the explosive radiation of arvicoline rodents (Arvicolinae, Cricetidae) Evolution. 2016;70:1094–1104. doi: 10.1111/evo.12919. [DOI] [PubMed] [Google Scholar]
- 3.Abramson, N., Lebedev, V., Bannikova, A. & Tesakov, A. Radiation events in the subfamily Arvicolinae (Rodentia): Evidence from nuclear genes. In Doklady Biological Sciences. 458–461 (Springer, 2009). [DOI] [PubMed]
- 4.Mosbrugger, V., Favre, A., Muellner-Riehl, A. N., Päckert, M. & Mulch, A. Cenozoic evolution of geo-biodiversity in the Tibeto-Himalayan region. In Mountains, Climate, and Biodiversity.429–449 (Wiley-Blackwell, 2018).
- 5.Muellner-Riehl AN. Mountains as evolutionary arenas: patterns, emerging approaches, paradigm shifts, and their implications for plant phylogeographic research in the Tibeto-Himalayan region. Front. Plant Sci. 2019;10:195. doi: 10.3389/fpls.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing Y, Ree RH. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc. Natl Acad. Sci. USA. 2017;114:E3444–E3451. doi: 10.1073/pnas.1616063114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 8.Marchese C. Biodiversity hotspots: a shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015;3:297–309. doi: 10.1016/j.gecco.2014.12.008. [DOI] [Google Scholar]
- 9.Tamma K, Ramakrishnan U. Higher speciation and lower extinction rates influence mammal diversity gradients in Asia. BMC Evol. Biol. 2015;15:11. doi: 10.1186/s12862-015-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreau CS, Bell CD. Testing the museum versus cradle tropical biological diversity hypothesis: phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution. 2013;67:2240–2257. doi: 10.1111/evo.12105. [DOI] [PubMed] [Google Scholar]
- 11.Pisano J, et al. Out of Himalaya: the impact of past Asian environmental changes on the evolutionary and biogeographical history of Dipodoidea (Rodentia) J. Biogeogr. 2015;42:856–870. doi: 10.1111/jbi.12476. [DOI] [Google Scholar]
- 12.Weigold, H. Die Biogeographie Tibets Und Seiner Vorländer (Verein Sächsischer Ornithologen, 2005).
- 13.Jia DR, et al. Out of the Qinghai–Tibet Plateau: evidence for the origin and dispersal of Eurasian temperate plants from a phylogeographic study of Hippophaë rhamnoides (Elaeagnaceae) New Phytol. 2012;194:1123–1133. doi: 10.1111/j.1469-8137.2012.04115.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Tseng ZJ, Li Q, Takeuchi GT, Xie G. From ‘third pole’to north pole: a Himalayan origin for the arctic fox. Proc. R. Soc. B Biol. Sci. 2014;281:20140893. doi: 10.1098/rspb.2014.0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, et al. Out of Tibet: genomic perspectives on the evolutionary history of extant pikas. Mol. Biol. Evol. 2020;37:1577–1592. doi: 10.1093/molbev/msaa026. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson BH. Classified catalogue of mammals of Nepal, (corrected to end of 1841, first printed in 1832) J. Asiatic Soc. Bengal. 1841;10:907–916. [Google Scholar]
- 17.Allen, G. M. Mammals of China and Mongolia. Part 2. Central Asiatic Expeditions 1921-1930. Vol. 11, p. 621–1350 (American Museum of Natural History, 1940).
- 18.Carleton, M. D. & Musser, G. G. Systematic studies of oryzomyine rodents (Muridae, Sigmodontinae)-definition and distribution of Oligoryzomys vegetus (Bangs, 1902). Proceedings of the Biological Society of Washington (Biological Society of Washington, 1995).
- 19.Gromov I, Polyakov IY. Mammals: voles (microtinae) Fauna SSSR. 1977;3:1–504. [Google Scholar]
- 20.Corbet, G. B. The mammals of the Palaearctic region: a taxonomic review. British Museum (Natural History) (1978).
- 21.Ellerman, J. R. & Morrison-Scott, T. C. S. Checklist of Palaearctic and Indian Mammals, 1758–1946. (British Museum of Natural History, 1951).
- 22.Ellerman, J. R. The Families and Genera of Living Rodents, Volume III, Part I. (British Museum of Natural History, London, 1949).
- 23.Musser, G. G. & Carleton, M. D. Order rodentia. Mammal Species of the World: A Taxonomic and Geographic Reference2 (2005).
- 24.Liu SY, et al. A new vole from Xizang, China and the molecular phylogeny of the genus Neodon (Cricetidae: Arvicolinae) Zootaxa. 2012;3235:1–22. doi: 10.11646/zootaxa.3235.1.1. [DOI] [Google Scholar]
- 25.Pradhan, N. et al. Further assessment of the Genus Neodon and the description of a new species from Nepal. PLoS ONE14, e0219157 (2019). [DOI] [PMC free article] [PubMed]
- 26.Zheng GXY, et al. Haplotyping germline and cancer genomes with high-throughput linked-read sequencing. Nat. Biotechnol. 2016;34:8. doi: 10.1038/nbt.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 28.Vidya T, Sukumar R, Melnick DJ. Range-wide mtDNA phylogeography yields insights into the origins of Asian elephants. Proc. R. Soc. B: Biol. Sci. 2009;276:893–902. doi: 10.1098/rspb.2008.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storz JF, Scott GR, Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 2010;213:4125–4136. doi: 10.1242/jeb.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen HD, et al. Functions of replication protein A as a sensor of R loops and a regulator of RNaseH1. Mol. Cell. 2017;65:832–847.e834. doi: 10.1016/j.molcel.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith EA, et al. DEK is required for homologous recombination repair of DNA breaks. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep44662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousounis K, et al. Eya2 promotes cell cycle progression by regulating DNA damage response during vertebrate limb regeneration. Elife. 2020;9:e51217. doi: 10.7554/eLife.51217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elkholi R, et al. MDM2 integrates cellular respiration and apoptotic signaling through NDUFS1 and the mitochondrial network. Mol. Cell. 2019;74:452–465.e457. doi: 10.1016/j.molcel.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wikramanayake TC, Nicu C, Chéret J, Czyzyk TA, Paus R. Mitochondrially localized MPZL3 emerges as a signaling hub of mammalian physiology. BioEssays. 2021;43:2100126. doi: 10.1002/bies.202100126. [DOI] [PubMed] [Google Scholar]
- 36.Kim YH, et al. A MST1–FOXO1 cascade establishes endothelial tip cell polarity and facilitates sprouting angiogenesis. Nat. Commun. 2019;10:1–17. doi: 10.1038/s41467-019-08773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai N. The diverse functions of IMP2/IGF2BP2 in metabolism. Trends Endocrinol. Metab. 2020;31:670–679. doi: 10.1016/j.tem.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Johann To Berens P, Molinier J. Formation and recognition of UV-induced DNA damage within genome complexity. Int. J. Mol. Sci. 2020;21:6689. doi: 10.3390/ijms21186689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terman BI, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 1992;187:1579–1586. doi: 10.1016/0006-291X(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Xie P, Opatowsky Y, Schlessinger J. Direct contacts between extracellular membrane-proximal domains are required for VEGF receptor activation and cell signaling. Proc. Natl Acad. Sci. USA. 2010;107:1906–1911. doi: 10.1073/pnas.0914052107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leppänen V-M, et al. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc. Natl Acad. Sci. USA. 2010;107:2425–2430. doi: 10.1073/pnas.0914318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storz JF. High-altitude adaptation: mechanistic insights from integrated genomics and physiology. Mol. Biol. Evol. 2021;38:2677–2691. doi: 10.1093/molbev/msab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng, Z., Cai, G. & Zheng, C. The mammals of Xizang. 1–441 (Science Press, 1986).
- 44.Luo Z. Rodentia Part III: Cricetidae. Fauna Sin. Mamm. 2000;6:121–128. [Google Scholar]
- 45.An, Z., John, E. K., Warren, L. P. & Stephen, C. P. Evolution of Asian monsoons and phased uplift of the Himalaya–Tibetan plateau since Late Miocene times. Nature411, 62 (2001). [DOI] [PubMed]
- 46.Guo Z, et al. A major reorganization of Asian climate regime by the Early Miocene. Clim. Discuss. 2008;4:535–584. [Google Scholar]
- 47.Andersen, B. G. & Borns, H. W. The Ice Age World: An Introduction To Quaternary History And Research With Emphasis On North America And Northern Europe During The Last 2.5 Million Years. Vol. 33 (Scandinavian University Press, 1994).
- 48.Meng L, et al. Refugial isolation and range expansions drive the genetic structure of Oxyria sinensis (Polygonaceae) in the Himalaya-Hengduan Mountains. Sci. Rep. 2015;5:10396. doi: 10.1038/srep10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCormack JE, Huang H, Knowles LL, Gillespie R, Clague D. Sky islands. Encycl. Isl. 2009;4:841–843. [Google Scholar]
- 50.Deng T, et al. Out of Tibet: pliocene woolly rhino suggests high-plateau origin of Ice Age megaherbivores. Science. 2011;333:1285–1288. doi: 10.1126/science.1206594. [DOI] [PubMed] [Google Scholar]
- 51.Sikes RS, Gannon WL. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011;92:235–253. doi: 10.1644/10-MAMM-F-355.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaiser HF, Rice J. Little jiffy, mark IV. Educ. Psychol. Meas. 1974;34:111–117. doi: 10.1177/001316447403400115. [DOI] [Google Scholar]
- 53.Bartlett MS. Properties of sufficiency and statistical tests. Proc. R. Soc. Lond. Ser. A-Math. Phys. Sci. 1937;160:268–282. [Google Scholar]
- 54.Abdi H, Williams LJ. Tukey’s honestly significant difference (HSD) test. Encycl. Res. Des. 2010;3:1–5. [Google Scholar]
- 55.Conover, W. J. Practical Nonparametric Statistics. Vol. 350 (John Wiley & Sons, 1999).
- 56.Wei T, et al. Package ‘corrplot’. Statistician. 2017;56:e24. [Google Scholar]
- 57.Wickham, H. ggplot2: Elegant Graphics For Data Analysis. (Springer, 2016).
- 58.Weisenfeld NI, Kumar V, Shah P, Church DM, Jaffe DB. Direct determination of diploid genome sequences. Genome Res. 2017;27:757–767. doi: 10.1101/gr.214874.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinform. 2011;12:1–14. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu S, et al. Ancient and modern genomes unravel the evolutionary history of the rhinoceros family. Cell. 2021;184:4874–4885. e4816. doi: 10.1016/j.cell.2021.07.032. [DOI] [PubMed] [Google Scholar]
- 61.Meng G, Li Y, Yang C, Liu S. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 2019;47:e63–e63. doi: 10.1093/nar/gkz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 63.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:1303.3997 (2013).
- 64.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34:W609–W612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 69.Esmukov, K. GeoPy, <https://github.com/geopy/geopy> (2022).
- 70.Kassambara, A. ggpubr:“ggplot2” based publication ready plots. R package version 0.17 (2018).
- 71.Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29:2869–2876. doi: 10.1093/bioinformatics/btt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, automatic barcode gap discovery for primary species delimitation. Mol. Ecol. 2012;21:1864–1877. doi: 10.1111/j.1365-294X.2011.05239.x. [DOI] [PubMed] [Google Scholar]
- 73.Flouri T, Jiao X, Rannala B, Yang Z. Species tree inference with BPP using genomic sequences and the multispecies coalescent. Mol. Biol. Evol. 2018;35:2585–2593. doi: 10.1093/molbev/msy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Z, Rannala B. Bayesian species delimitation using multilocus sequence data. Proc. Natl Acad. Sci. USA. 2010;107:9264–9269. doi: 10.1073/pnas.0913022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rannala B, Yang Z. Improved reversible jump algorithms for Bayesian species delimitation. Genetics. 2013;194:245–253. doi: 10.1534/genetics.112.149039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Z, Rannala B. Unguided species delimitation using DNA sequence data from multiple loci. Mol. Biol. Evol. 2014;31:3125–3135. doi: 10.1093/molbev/msu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang C, Rabiee M, Sayyari E, Mirarab S. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinform. 2018;19:153. doi: 10.1186/s12859-018-2129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cole TL, et al. Genomic insights into the secondary aquatic transition of penguins. Nat. Commun. 2022;13:1–13. doi: 10.1038/s41467-022-31508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chifman J, Kubatko L. Quartet inference from SNP data under the coalescent model. Bioinformatics. 2014;30:3317–3324. doi: 10.1093/bioinformatics/btu530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swofford, D. L. & Sullivan, J. The Phylogenetic Handbook: A Practical Approach To Dna And Protein Phylogeny, Cáp. Vol. 7, 160–206 (Cambridge University Press, 2003).
- 82.Nguyen L, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evolution. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 84.Kozlov AM, Aberer AJ, Stamatakis A. ExaML version 3: a tool for phylogenomic analyses on supercomputers. Bioinformatics. 2015;31:2577–2579. doi: 10.1093/bioinformatics/btv184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peters SE, McClennen M. The Paleobiology Database application programming interface. Paleobiology. 2016;42:1–7. doi: 10.1017/pab.2015.39. [DOI] [Google Scholar]
- 86.Kumar S, Stecher G, Suleski M, Hedges SB. TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017;34:1812–1819. doi: 10.1093/molbev/msx116. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Zheng S, Wei G. Fossil arvicolines from the Leijiahe section, Lingtai, Gansu Province and current progress of Chinese arvicoline biochronology. Quat. Sci. 2011;31:622–635. [Google Scholar]
- 88.Lv X, et al. Disjunct distribution and distinct intraspecific diversification of Eothenomys melanogaster in South China. BMC Evol. Biol. 2018;18:1–14. doi: 10.1186/s12862-018-1168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kohli BA, Fedorov VB, Waltari E, Cook JA. Phylogeography of a Holarctic rodent (Myodes rutilus): testing high latitude biogeographical hypotheses and the dynamics of range shifts. J. Biogeogr. 2015;42:377–389. doi: 10.1111/jbi.12433. [DOI] [Google Scholar]
- 90.Bannikova AA, et al. Molecular phylogeny and evolution of the Asian lineage of vole genus Microtus (Rodentia: Arvicolinae) inferred from mitochondrial cytochrome b sequence. Biol. J. Linn. Soc. 2010;99:595–613. doi: 10.1111/j.1095-8312.2009.01378.x. [DOI] [Google Scholar]
- 91.Galewski T, et al. The evolutionary radiation of Arvicolinae rodents (voles and lemmings): relative contribution of nuclear and mitochondrial DNA phylogenies. BMC Evol. Biol. 2006;6:1–17. doi: 10.1186/1471-2148-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Field, A., Miles, J. & Field, Z. Discovering Statistics Using R (Sage publications, 2012).
- 94.Revell LJ. phytools: an R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 95.Matzke, N. J. BioGeoBEARS: BioGeography with Bayesian (and likelihood) evolutionary analysis in R Scripts. R. package, version 0. 2. 1, 2013 (2013).
- 96.Yang C, et al. Evolutionary and biomedical insights from a marmoset diploid genome assembly. Nature. 2021;594:227–233. doi: 10.1038/s41586-021-03535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bu D, et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49:W317–W325. doi: 10.1093/nar/gkab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information - clean version
Description of Additional Supplementary Files
Data Availability Statement
Data that support our findings have been deposited in the NCBI database under BioProject PRJNA564473 (ncbi.nlm.nih.gov/bioproject/?term= PRJNA564473) and CNGB Nucleotide Sequence Archive (CNSA) under the accession number CNP0000173 (https://db.cngb.org/search/project/CNP0000173). Source data for figures can be found in Supplementary Data 20.
All analyses were performed using open source software tools, and the detailed parameters for each tool are shown in the relevant methods. Custom scripts are archived at GitHub (https://github.com/linzhi2013/busco_process_scripts).