Abstract
Objective
Metformin has attracted more attention from researchers for its newly discovered antitumor effects. A meta-analysis was performed to reveal the efficacy of metformin on overall survival (OS) and recurrence-free survival (RFS) for HCC patients with type 2 diabetes mellitus (T2DM) after curative treatment.
Methods
Databases including PubMed, the Cochrane Library, Web of Science, CNKI, Wangfang, and Weipu Database up until 31 May 2022 were searched for relevant studies. STATA 13.0 was used to perform the meta-analysis.
Results
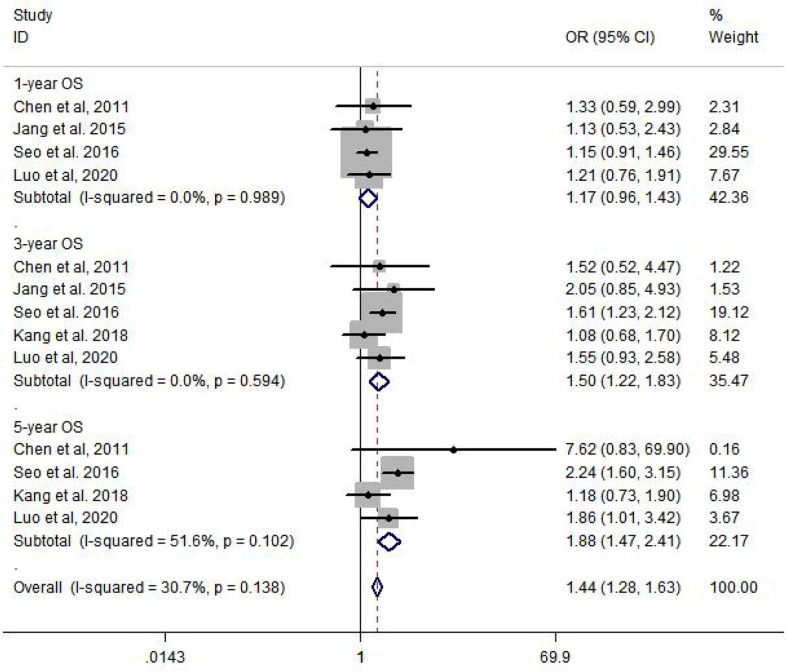
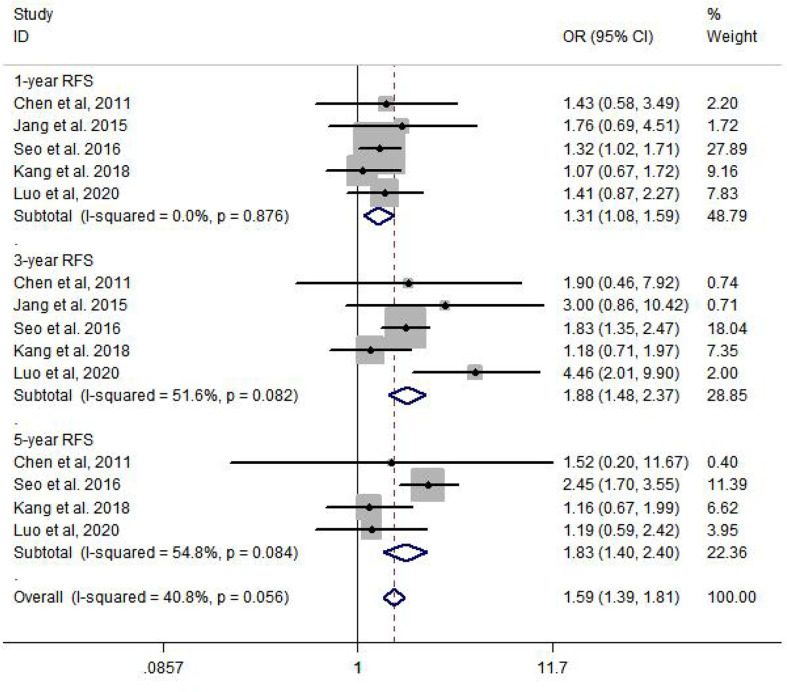
A total of six studies involving 5,936 patients were included in our study. The results from the current study revealed that metformin usage can significantly prolong the 3-year [odds ratio (OR) = 1.50, 95% confidence interval (CI): 1.22–1.83, p = 0.000] and 5-year (OR = 1.88, 95% CI: 1.47–2.41, p = 0.000) OS and decrease the 1-year (OR = 1.31, 95% CI: 1.08–1.59, p = 0.007), 3-year (OR = 1.88, 95% CI: 1.48–2.37, p = 0.000), and 5-year (OR = 1.83, 95% CI: 1.40–2.40, p = 0.000) recurrence rates.
Conclusion
Metformin treatment significantly prolongs the OS and decreases the recurrence rate for HCC patients with T2DM after curative HCC therapy.
Keywords: hepatocellular carcinoma, diabetes mellitus, metformin, overall survival, recurrence-free survival
1. Background
Metformin, an oral hypoglycemic agent, has become one of the first-line drugs of choice for the treatment of type 2 diabetes mellitus (T2DM) due to its low cost and high efficacy (1). Recently, metformin has attracted more attention from researchers for its newly discovered antitumor effects (2). Growing lines of evidence have shown that metformin can inhibit the progression of cancer, especially in hepatocellular carcinoma (HCC) (3). As we all know, HCC, an aggressive digestive system cancer associated with increasing mortality and morbidity, remains a major global challenge in the field of anticancer treatment (4). However, surgical liver resection, radiofrequency ablation, and liver transplantation have become curative therapy approaches for HCC patients with a 5-year survival rate of 30%–70% (5). There are no effective drugs to prolong their survival outcomes.
T2DM is characterized by metabolic disorder, and the liver plays an important role in glucose metabolism (6). Most HCC patients suffer from a glucose metabolism disorder (7). Recently, more studies have proven that T2DM was associated with a higher risk of HCC and metformin treatment can decrease the risk of HCC for many types of cancers including HCC for patients with T2DM (8). Previous studies have suggested that metformin can prolong overall survival (OS) for cancer patients with DM, which acted as an antitumor drug (9). However, a few studies have shown that metformin increased the OS outcomes of HCC patients with T2DM (10). Therefore, we conducted a meta-analysis with the aim of revealing the efficacy of metformin on OS and recurrence-free survival (RFS) for HCC patients with T2DM after curative treatment.
2. Materials and methods
2.1. Search strategy
Databases including PubMed, the Cochrane Library, Web of Science, CNKI, Wangfang, and Weipu Database up until 31 May 2022 were searched for relevant studies. The search terms used were as follows: “hepatocellular carcinoma” OR “liver cancer” OR “liver tumor” OR “HCC”, “type 2 diabetes mellitus” OR “diabetes mellitus” OR “T2DM” OR “DM”, “metformin” OR “dimethylguanylguanidine”. Random words and Mesh words were combined in study search. Languages were limited in Chinese and English.
2.2. Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) HCC patients with T2DM who were treated with metformin after curative therapy including surgical liver resection and radiofrequency ablation; (2) patients who received metformin for more than 12 months in any dose; (3) all of the randomized controlled trials (RCTs) and case–control studies were included; and (4) survival outcomes including OS and RFS were reported in studies.
Exclusion criteria were as follows: (1) abstracts, review, case reports, editorials, and letters; (2) without sufficient data after contacting the corresponding authors of studies; and (3) duplicate publications.
2.3. Data extraction
The data were extracted from eligible studies by two reviewers, including (1) the baseline of included studies, such as author information, the year of publication, country, gender (male/female), metformin usage, mean age, sample size, tumor size (≤5 cm/>5 cm), Child–Pugh grading (A/B/C/D), BCLC (0/A/B/C/D), and type of curative therapy for HCC; and (2) survival outcomes: OS and RFS.
2.4. Quality assessment of included studies
The quality of included studies was assessed according to the Newcastle–Ottawa scale (NOS) (11): (1) the selection of cohorts; (2) comparability of cohorts; and (3) the exposure or outcome of the participant. Each item was regarded as 0–4 points. Finally, the total score of each study represented the overall result of quality assessment. Studies with more than seven points were considered as high quality.
2.5. Data analysis
STATA 13.0 was used to perform the meta-analysis. Statistic heterogeneity between included studies was assessed by I 2. If I 2 was less than 50%, a fixed-effects model was conducted to calculate the combined estimates. Otherwise, the estimates were combined by a random-effects model. Odds ratios (ORs) and its 95% confidence intervals (CIs) were used to compare outcome variants between the metformin and non-metformin groups. Sensitivity analyses were also performed as a possible evaluation of the existing heterogeneity.
3. Results
3.1. Literature screening
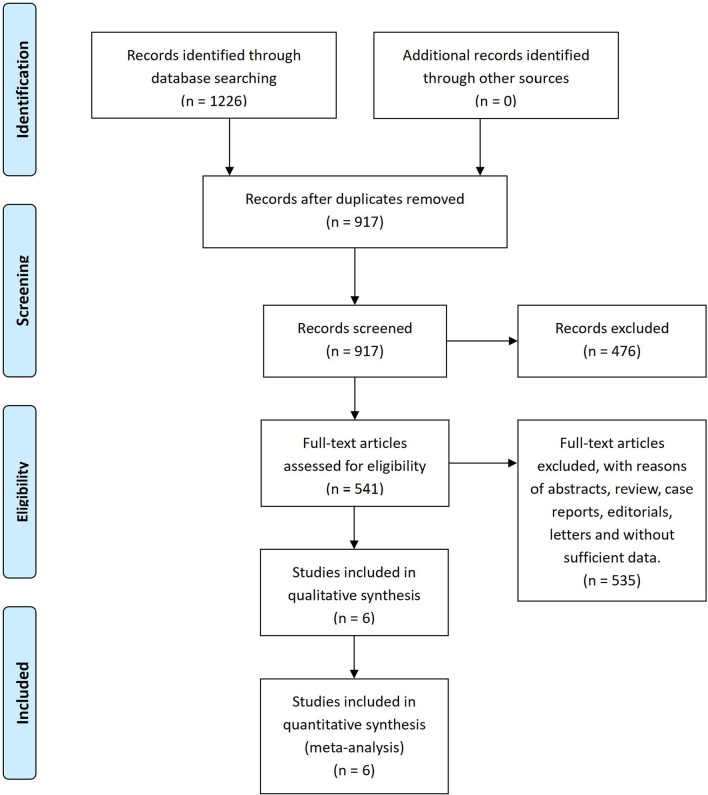
After the systematic literature search, 1,226 papers were found from databases by using Mesh and specific search words. A total of 309 duplicates were removed by Endnote X7 and 917 articles were confirmed by screening titles and abstracts. Then, 541 records were assessed in full text for their eligibility. Finally, a total of six studies (12–17) involving 5,936 patients were included in this meta-analysis for qualitative synthesis ( Figure 1 ).
Figure 1.
PRISMA flow diagram of the literature search and selection.
3.2. The characteristics of the included studies
Among the included studies, three were from China and South Korea. A total of 2,313 HCC patients with T2DM were treated with metformin for more than 12 months and they were regarded as the metformin group. Otherwise, 3,623 patients were treated with other anti-hyperglycemic agents or metformin for less than 12 months, and they were regarded as the non-metformin group. Curative therapy approaches included hepatic resection, radiofrequency ablation, and stereotactic body radiotherapy. The baseline of the included studies is shown in Table 1 . Quality assessment of the included studies shows that each of the six included studies had a score of more than eight in NOS, which suggests the high methodological quality of each eligibility study.
Table 1.
The characteristics of the included studies.
| Study ID | Country | Gender (male/female) | Metformin usage | Age (mean, years) | Sample size | Tumor size (≤5 cm/>5 cm) | Child–Pugh grading (A/B/C/D) | BCLC (0/A/B/C/D) | Curative therapy for HCC |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al., (14) | China | 21/32 | Metformin, 750 mg/day | 64.2–67.4 | 21 | 10/11 | 10.2 ± 4.5 | NR | Radiofrequency ablation |
| Sulfonylurea + insulin | 32 | 13/19 | 9.7 ± 3.4 | NR | Radiofrequency ablation | ||||
| Jang et al., (13) | South Korea | 57/19 | Metformin, 1,000 mg/day | NR | 19 | 13/6 | A: 18, B: 1 | A: 12, B: 7 | Stereotactic body radiotherapy |
| Sulfonylurea + thiazolidinedione + others, dose NR | 57 | 37/20 | A: 37, B: 20 | A: 36, B: 21 | Stereotactic body radiotherapy | ||||
| Seo et al., (12) | South Korea | 598/153 | Metformin + sulfonylurea + thiazolidinedione, dose NR | 60 | 533 | NR | NR | NR | Hepatic resection |
| Sulfonylurea + thiazolidinedione, dose NR | 218 | NR | NR | NR | Hepatic resection | ||||
| Chan et al., 2016 (16) | China | 3,346/1,264 | Metformin, dose NR | 64 | 1,632 | NR | NR | NR | Hepatic resection |
| Other antidiabetic drugs | 2,978 | NR | NR | NR | Hepatic resection | ||||
| Kang et al., (15) | South Korea | 214/56 | Metformin, dose NR | 60.8 | 45 | 45/0 | A:45 | NR | Hepatic resection |
| Other antidiabetic drugs | 225 | 225/0 | A:225 | NR | Hepatic resection | ||||
| Luo et al., (17) | China | 163/13 | Metformin + biguanides/sulfonylureas/insulin, dose NR | 54.95 ± 8.31 | 63 | NR | NR | A+B: 63 | Radical resection |
| Biguanides/sulfonylureas/insulin, dose NR | 53.77 ± 8.88 | 113 | NR | NR | A+B: 113 | Radical resection |
NR, not reported; BCLC, Barcelona Clinic Liver Cancer Stage.
3.3. Results of meta-analysis
3.3.1. Overall survival
Among the six included studies, there is no statistic heterogeneity between studies that reported 1-year OS and 3-year OS. The results of meta-analysis show that there is a significant difference in 3-year OS (OR = 1.50, 95% CI: 1.22–1.83, p = 0.000) and 5-year OS (OR = 1.88, 95% CI: 1.47–2.41, p = 0.000), compared to HCC patients with T2DM who were not treated with metformin ( Figure 2 ).
Figure 2.
Meta-analysis of overall survival.
3.3.2. Recurrence-free survival
Compared to the non-metformin group, patients from the metformin group after curative therapy have a significantly longer RFS after 1 year (OR = 1.31, 95% CI: 1.08–1.59, p = 0.007), 3 years (OR = 1.88, 95% CI: 1.48–2.37, p = 0.000), and 5 years (OR = 1.83, 95% CI: 1.40–2.40, p = 0.000) ( Figure 3 ).
Figure 3.
Meta-analysis of RFS.
3.3.3. Subgroup analysis
Subgroup analysis was performed by country and curative therapy approaches in OS and RFS. Compared to HCC patients with T2DM treated without metformin, the metformin group has a higher 3-year OS (OR = 1.49, 95% CI: 1.19–1.86) and 5-year OS (OR = 1.84, 95% CI: 1.40–2.41) among South Korean patients and a higher 5-year OS (OR = 1.86, 95% CI: 1.01–3.42) among Chinese patients. For curative therapy, hepatic resection can prolong the 3-year OS (OR = 1.47, 95% CI: 1.19–1.82) and 5-year OS (OR = 1.84, 95% CI: 1.43–2.36) ( Table 2 ).
Table 2.
Subgroup analysis of OS (OR, 95% CI) .
| Subgroup | 1-year | 3-year | 5-year |
|---|---|---|---|
| Country | |||
| China | 1.23 (0.83–1.84) | 1.54 (0.97–2.45) | 1.86 (1.01–3.42) |
| South Korea | 1.15 (0.92–1.44) | 1.49 (1.19–1.86) | 1.84 (1.40–2.41) |
| Curative therapy | |||
| RFA | 1.33 (0.59–2.99) | 1.52 (0.52–4.47) | |
| SBRT | 1.13 (0.53–2.43) | 2.05 (0.85–4.93) | |
| Hepatic resection | 1.17 (0.94–1.44) | 1.47 (1.19–1.82) | 1.84 (1.43–2.36) |
RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy.
Subgroup analysis of RFS showed that there is a significant difference in South Korea in 1-year (OR = 1.28, 95% CI: 1.03–1.60), 3-year (OR = 1.68, 95% CI: 1.30–2.16), and 5-year (OR = 1.98, 95% CI: 1.47–2.66) RFS and hepatic resection in 1-year (OR = 1.28, 95% CI: 1.05–1.58), 3-year (OR = 1.85, 95% CI: 1.45–2.35), and 5-year (OR = 1.84, 95% CI: 1.40–2.41) RFS ( Table 3 ).
Table 3.
Subgroup analysis of RFS (OR, 95% CI).
| Subgroup | 1-year | 3-year | 5-year |
|---|---|---|---|
| Country | |||
| China | 1.41 (0.93–2.15) | 3.77 (1.90–7.48) | 1.23 (0.63–2.39) |
| South Korea | 1.28 (1.03–1.60) | 1.68 (1.30–2.16) | 1.98 (1.47–2.66) |
| Curative therapy | |||
| RFA | 1.43 (0.58–3.49) | 1.90 (0.46–7.92) | 1.52 (0.20–11.67) |
| SBRT | 1.76 (0.69–4.51) | 3.00 (0.86–10.42) | |
| Hepatic resection | 1.28 (1.05–1.58) | 1.85 (1.45–2.35) | 1.84 (1.40–2.41) |
RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy.
4. Discussion
HCC is regarded as the third leading cause of cancer-related deaths worldwide (18). A better survival outcome has been observed only in patients with early-stage HCC who received curative therapy (19). However, most HCC patients are diagnosed in the advanced stages of the disease and undergo non-curative treatment including targeted therapy, transhepatic arterial chemoembolization, and traditional Chinese medicine, which were not satisfactory given the high incidence of progression and recurrence (20). T2DM has been proven to be a risk factor for the development of HCC, which promotes the progression and recurrence of HCC after comprehensive treatment (21). Therefore, whether metformin usage is beneficial to HCC patients with T2DM after curative therapy needs to be further evaluated. Thus, we performed a meta-analysis to provide evidence on the beneficial effects of metformin on the prognosis of HCC patients with T2DM after curative therapy.
In our study, a total of six studies involving 5,936 patients were included to determine the 1-year, 3-year, and 5-year OS and RFS rates. The results from the current study revealed that metformin usage can significantly prolong the 3-year (OR = 1.50, 95% CI: 1.22–1.83, p = 0.000) and 5-year (OR = 1.88, 95% CI: 1.47–2.41, p = 0.000) OS and decrease the 1-year (OR = 1.31, 95% CI: 1.08–1.59, p = 0.007), 3-year (OR = 1.88, 95% CI: 1.48–2.37, p = 0.000), and 5-year (OR = 1.83, 95% CI: 1.40–2.40, p = 0.000) recurrence rate. Subgroup analysis was performed by country and curative therapy approaches in OS and RFS, which showed that patients who received hepatic resection may have a longer survival time and a lower recurrence rate.
A previous study has revealed that metformin treatment can inhibit HCC cell proliferation and decrease the risk of HCC development in T2DM patients (22). We also found that metformin not only can prolong the OS but also can decrease the recurrence rate for these patients (23). Furthermore, metformin can inhibit energetic metabolism including blood glucose, which may influence the proliferation and migration of HCC cells (24). Moreover, metformin can control the development of T2DM, thereby repairing the liver injury from DM and HCC (25). Sun et al. (26) reported that metformin enhances the antitumor effect by inhibiting growth and invasion and by inducing apoptosis and autophagy in HCC through the PI3K/AKT/mTOR pathway. Another study also showed that metformin activates the Hippo signaling pathway to regulate IL-22-mediated HCC progression (27). All of the above may contribute to increase the OS for HCC patients with T2DM.
Although a comprehensive and systematic literature search was performed, there are several limitations in the present meta-analysis: (1) The original studies included in our study were retrospective studies, which may lead to the heterogeneity between eligibility studies. Large-scale randomized, placebo-controlled trials are necessary to determine the effect of metformin on HCC patients with T2DM after curative treatment. (2) The provided information on the characteristics of the included studies was incomplete; thus, subgroup analysis cannot be performed thoroughly and comprehensively. (3) Most included studies did not report the dose and duration of metformin, which made it more difficult for us to determine the usage of metformin in clinical practice.
In conclusion, metformin treatment significantly prolongs the OS and decreases the recurrence rate for HCC patients with T2DM after curative HCC therapy. Furthermore, large-scale randomized, placebo-controlled trials are needed to provide us with much more high-quality clinical evidence.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H, et al. Metformin: Is it a drug for all reasons and diseases? Metabolism (2022) 133:155223. doi: 10.1016/j.metabol.2022.155223 [DOI] [PubMed] [Google Scholar]
- 2. Buczyńska A, Sidorkiewicz I, Krętowski AJ, Zbucka-Krętowska M, Adamska A. Metformin intervention-a panacea for cancer treatment? Cancers (Basel) (2022) 14(5):1336. doi: 10.3390/cancers14051336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skuli SJ, Alomari S, Gaitsch H, Bakayoko A, Skuli N, Tyler BM. Metformin and cancer, an ambiguanidous relationship. Pharm (Basel) (2022) 15(5):626. doi: 10.3390/ph15050626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Healy MA, Choti MA. Hepatocellular carcinoma recurrence risk in the context of emerging therapies. Ann Surg Oncol (2022) 29:4030–2. doi: 10.1245/s10434-022-11709-8 [DOI] [PubMed] [Google Scholar]
- 5. Salem R, Tselikas L, De Baere T. Interventional treatment of hepatocellular carcinoma. J Hepatol (2022) 77(4):1205–6. doi: 10.1016/j.jhep.2022.03.037 [DOI] [PubMed] [Google Scholar]
- 6. Barbagallo M, Veronese N, Dominguez LJ. Magnesium in type 2 diabetes mellitus, obesity, and metabolic syndrome. Nutrients (2022) 14(3):714. doi: 10.3390/nu14030714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cucarull B, Tutusaus A, Rider P, Hernáez-Alsina T, Cuño C, García de Frutos P, et al. Hepatocellular carcinoma: Molecular pathogenesis and therapeutic advances. Cancers (Basel) (2022) 14(3):621. doi: 10.3390/cancers14030621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang C, Wan M, Lu Y, Yang X, Yang L, Wang S, et al. Associations between diabetes mellitus and the risk of hepatocellular carcinoma in Asian individuals with hepatitis b and c infection: systematic review and a meta-analysis of cohort studies. Eur J Cancer Prev (2022) 31(2):107–16. doi: 10.1097/CEJ.0000000000000669 [DOI] [PubMed] [Google Scholar]
- 9. Li Q, Xu H, Sui C, Zhang H. Impact of metformin use on risk and mortality of hepatocellular carcinoma in diabetes mellitus. Clin Res Hepatol Gastroenterol (2022) 46(2):101781. doi: 10.1016/j.clinre.2021.101781 [DOI] [PubMed] [Google Scholar]
- 10. Wang K, Zhang K, Zhang X, Chen D, Jiang S. Recent insights of metformin on hepatocellular carcinoma (HCC). Mini Rev Med Chem (2022). doi: 10.2174/1389557522666220623150717 [DOI] [PubMed] [Google Scholar]
- 11. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 12. Seo YS, Kim YJ, Kim MS, Suh KS, Kim SB, Han CJ, et al. Association of metformin use with cancer-specific mortality in hepatocellular carcinoma after curative resection: A nationwide population-based study. Med (Baltimore) (2016) 95(17):e3527. doi: 10.1097/MD.0000000000003527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jang WI, Kim MS, Lim JS, Yoo HJ, Seo YS, Han CJ, et al. Survival advantage associated with metformin usage in hepatocellular carcinoma patients receiving radiotherapy: A propensity score matching analysis. Anticancer Res (2015) 35(9):5047–54. [PubMed] [Google Scholar]
- 14. Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol (2011) 26(5):858–65. doi: 10.1111/j.1440-1746.2011.06664.x [DOI] [PubMed] [Google Scholar]
- 15. Kang WH, Tak E, Hwang S, Song GW, Jwa E, Lee YJ, et al. Metformin-associated chemopreventive effects on recurrence after hepatic resection of hepatocellular carcinoma: From In vitro to a clinical study. Anticancer Res (2018) 38(4):2399–407. doi: 10.21873/anticanres.12490 [DOI] [PubMed] [Google Scholar]
- 16. Chan KM, Kuo CF, Hsu JT, Chiou MJ, Wang YC, Wu TH, et al. Metformin confers risk reduction for developing hepatocellular carcinoma recurrence after liver resection. Liver Int (2017) 37(3):434–41. doi: 10.1111/liv.13280 [DOI] [PubMed] [Google Scholar]
- 17. Luo CS, Lin Y, Zhou WP, Shi J. Survival advantage associated with metformin usage in hepatocellular carcinoma patients with diabetes mellitus receiving radical resection: a propensity score matching analysis. Eur J Gastroenterol Hepatol (2020) 32(8):1030–5. doi: 10.1097/MEG.0000000000001610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen R, Zhao M, An Y, Liu D, Tang Q, Teng G. A prognostic gene signature for hepatocellular carcinoma. Front Oncol (2022) 12:841530. doi: 10.3389/fonc.2022.841530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei F. Neoadjuvant immunotherapy for resectable hepatocellular carcinoma. Lancet Gastroenterol Hepatol (2022) 7(6):504. doi: 10.1016/S2468-1253(22)00083-8 [DOI] [PubMed] [Google Scholar]
- 20. El-Kassas M, Elbadry M. Hepatocellular carcinoma in Africa: Challenges and opportunities. Front Med (Lausanne) (2022) 9:899420. doi: 10.3389/fmed.2022.899420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao JZ, Wang ZG, Yu J, Tao YP, Yang Y, Liu H, et al. Antidiabetic treatment improves prognosis after radical resection in hepatocellular carcinoma patients with diabetes mellitus: a retrospective cohort study from 2000 to 2013. J Gastrointest Oncol (2022) 13(3):1330–9. doi: 10.21037/jgo-22-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yen FS, Huang YH, Hou MC, Hwu CM, Lo YR, Shin SJ, et al. Metformin use and cirrhotic decompensation in patients with type 2 diabetes and liver cirrhosis. Br J Clin Pharmacol (2022) 88(1):311–22. doi: 10.1111/bcp.14970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chavez-Tapia NC, Murúa-Beltrán Gall S, Ordoñez-Vázquez AL, Nuño-Lambarri N, Vidal-Cevallos P, Uribe M. Understanding the role of metabolic syndrome as a risk factor for hepatocellular carcinoma. J Hepatocell Carcinoma (2022) 9:583–93. doi: 10.2147/JHC.S283840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Long MT, Noureddin M, Lim JK. AGA clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology (2022) 163(3):764–74. doi: 10.1053/j.gastro.2022.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miao L, Xu J, Targher G, Byrne CD, Zheng MH. Old and new classes of glucose-lowering agents as treatments for non-alcoholic fatty liver disease: a narrative review. Clin Mol Hepatol (2022) 28(4):725–38. doi: 10.3350/cmh.2022.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun R, Zhai R, Ma C, Miao W. Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Med (2020) 9(3):1141–51. doi: 10.1002/cam4.2723 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Zhao D, Xia L, Geng W, Xu D, Zhong C, Zhang J, et al. Metformin suppresses interleukin-22 induced hepatocellular carcinoma by upregulating hippo signaling pathway. J Gastroenterol Hepatol (2021) 36(12):3469–76. doi: 10.1111/jgh.15674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.