Abstract
We have recently found that strain B31 infection-immune rabbits are completely protected against homologous challenge with large numbers (>106) of host-adapted Borrelia burgdorferi (HAB) (E. S. Shang, C. I. Champion, X. Wu, J. T. Skare, D. B. Blanco, J. N. Miller, and M. A. Lovett, Infect. Immun. 68:4189–4199, 2000). In this study, we have extended these findings to determine whether B31 strain infection-immune rabbits are also protected against heterologous HAB challenge. Infection-immune rabbits challenged with large numbers (>106) of homologous HAB strain B31 were completely protected from erythema migrans (EM) and skin and disseminated infection. In contrast, infection-immune rabbits challenged with heterologous HAB strains N40 and Sh-2-82 were completely susceptible to EM and skin and disseminated infection; challenge with strain 297 also resulted in EM and infection of the skin and viscera, but clearance of infection occurred 3 weeks postchallenge. These findings confirm that immunity elicited in rabbits by B31 strain infection confers complete protection against large-dose homologous HAB challenge but not against a heterologous strain.
Lyme disease in humans, caused by tick transmission of the spirochete Borrelia burgdorferi, is known to result from different strains of B. burgdorferi sensu stricto and sensu lato found in different geographic regions of the world. While outer surface protein A (OspA) of B. burgdorferi, a surface protein expressed during tick adaptation, is currently being used for human vaccination (39, 43), it has been shown to possess antigenic heterogeneity among many strains (26, 29, 35, 48). Moreover, it is now recognized that OspA is downregulated during mammalian infection and that its mechanism of protective immunity works by inhibiting B. burgdorferi growth in the tick following a blood meal from a vaccinated individual (15, 37). Furthermore, organisms that have become host adapted and no longer express OspA are not susceptible to killing by OspA-specific antibodies (7). Thus, the search for additional cross-protective immunogens has attracted considerable interest for future adjuncts to the OspA vaccine.
Several experimental animal models for the study of Lyme disease pathogenesis and immunity have been utilized, including the rat (8), hamster (25), mouse (5), rabbit (18, 47), guinea pig (41), dog (10, 21), and monkey (33). With the exception of rabbits, mammalian infection with B. burgdorferi results in chronic infection in these animals (3, 6, 16, 31, 34). In the mouse model, Barthold has demonstrated that chronic infection elicits complete protection against homologous challenge and partial protection against heterologous challenge using cultivated organisms (4). However, protection studies in mice challenged with organisms acquired from infected tissues which no longer express OspA, called host adapted by Barthold and which we now refer to as host-adapted Borrelia (HAB), show only partial protection against homologous challenge and little to no protection against heterologous challenge (4). Recently, Hansen and coworkers have demonstrated that immunization of mice with decorin-binding protein A (DbpA) from strain 297, a surface protein which has been implicated in spirochetal adhesion and which is upregulated during mammalian infection, protected against homologous challenge with in vitro-cultivated B. burgdorferi but not against heterologous challenge (23). Further, mice immunized with DbpA were only partially protected against homologous HAB challenge (11). Thus, in experimental mouse protection studies, only limited partial protection has been achievable when using host-adapted organisms for homologous and heterologous challenge.
We have previously demonstrated that the rabbit model of Lyme disease has unique features relevant to the immunobiology of B. burgdorferi, including the highly reproducible development of erythema migrans (EM) (18). Of particular significance, infected rabbits clear the infection after several months, resulting in complete protection against challenge reinfection using large numbers (>107) of cultivated organisms (18). Passive immunization of rabbits with infection-immune rabbit serum results in complete protection against homologous challenge with cultivated organisms (13), suggesting that antibody is the major factor in this protection. We have also previously reported that infection-immune rabbits were resistent to challenge using numbers of organisms that were manyfold greater than that which could be demonstrated following immunization with either OspA (19, 38) or outer membrane vesicles derived from cultivated, virulent B. burgdorferi (38). We have also recently found that B31 infection-immune rabbits are completely protected against homologous HAB challenge using greater than 106 organisms (38).
In this study, we have tested whether the high degree of homologous protective immunity that occurs following infection in rabbits also extends to heterologous challenge using HAB. HAB organisms were also used for challenge in order to assess protective immunity against Borrelia antigens other than OspA, which is present in both tick-infecting and cultivated organisms. The results show that B31 infection-immune rabbits are completely protected against homologous challenge with HAB but not heterologous challenge with HAB strains N40 and Sh-2-82. While B31 infection-immune rabbits challenged with HAB strain 297 also developed EM and skin infection, these animals completely cleared the infection 3 weeks postchallenge, as assessed by the absence of organisms in skin and internal tissues cultured in BSKII medium. These findings indicate that the high degree of complete protective immunity against high-dose homologous HAB challenge does not extend to heterologous HAB strain challenge.
Homologous and heterologous challenge of B. burgdorferi B31 infection-immune rabbits with HAB.
To generate B. burgdorferi B31 infection-immune rabbits, 20 New Zealand White rabbits were inoculated intradermally at eight sites with 107 in vitro-cultivated B. burgdorferi strain B31 passage 1 for a total of 8 × 107 organisms per rabbit, as previously described (38). All rabbits developed typical EM lesions and were shown to have culture-positive skin infection in BSKII medium at 7 days postinoculation and clearance of skin infection 4 months later, as previously reported (18). Infection-immune rabbits were challenged 5 months after the initial inoculation as described below.
In order to generate HAB for homologous and heterologous implant challenge, a single donor rabbit was inoculated with 107 in vitro-cultivated B. burgdorferi of either strain B31, N40, 297, or SH2-82 at eight sites, yielding a total of 8 × 107 organisms/rabbit as described above. Rabbits inoculated with each strain developed typical EM lesions at each site 7 days postinoculation. At this time point, skin punch biopsies of EM were obtained for implantation into recipient rabbits or for culture in BSKII medium and quick frozen in dry ice-ethanol for quantitative PCR analysis. For challenge, the 20 B. burgdorferi B31 infection-immune rabbits and 20 naive control rabbits were bled and divided into four groups of five rabbits so that each group of five naive and five infection-immune rabbits were implant challenged with one of the four HAB strains of B. burgdorferi. For skin implantation, donor and recipient rabbits were first anesthetized with ketamine and xylazine as previously described (19). Each 5-mm skin punch from a donor rabbit (subsequently shown to be culture positive) was dissected into five 1-mm2 pieces. Each of four sites per rabbit was implanted subcutaneously with five of these 1-mm2 pieces (38). At 7 and 21 days following implant challenge, the rabbits were bled and punch biopsies were taken near the site of challenge for culture in BSKII medium as previously described (18). All rabbits were sacrificed 3 weeks following challenge with the exception of two naive rabbits from each group. The skin, right and left popliteal lymph nodes, right and left stifle joint tissues, and spinal cord were cultured in BSKII medium with antibiotics as previously described (18). All cultures were examined once a week for a total of 5 weeks for growth of B. burgdorferi.
All naive rabbits challenged with each of the four B. burgdorferi HAB strains (five rabbits/strain) developed typical EM lesions, had culture-positive skin biopsies at 7 days and 3 weeks postchallenge, and had disseminated infection based on positive BSKII cultures (Table 1). By comparison, all five B31 infection-immune rabbits challenged with HAB strain B31 were completely protected from EM and from skin and disseminated infection (Table 1), corroborating our recent findings (38). In contrast, B31 infection-immune rabbits challenged with HAB strains N40 and Sh-2-82 developed typical EM as well as skin and disseminated infection, similar to the naive controls. Interestingly, while B31 infection-immune rabbits challenged with HAB strain 297 also developed EM lesions and had skin infection 7 days postchallenge, none of the rabbits showed evidence of skin or disseminated infection 3 weeks postchallenge. Thus, while HAB of strain 297 infected the B31 strain-immune animals, the rapid resolution of this infection suggests that some cross-immunity may be present. This rapid resolution of infection has not been observed previously in other animal models following heterologous challenge.
TABLE 1.
Homologous and heterologous HAB challenge of B31 infection-immune rabbitsa
| Rabbit group
|
Challenge strain | No. with EM lesions/total no. | No. with positive cultures/no. tested
|
||
|---|---|---|---|---|---|
| Skin
|
Tissue, 3 wk | ||||
| 8 days | 3 wk | ||||
| B31 immune | B31 | 0/5 | 0/5 | 0/5 | 0/5 |
| N40 | 5/5 | 5/5 | 5/5 | 5/5 | |
| 297 | 5/5 | 5/5 | 0/5 | 0/5 | |
| Sh2-82 | 5/5 | 5/5 | 5/5 | 3/5 | |
| Naive | B31 | 5/5 | 5/5 | 5/5 | 3/3 |
| N40 | 5/5 | 5/5 | 5/5 | 3/3 | |
| 297 | 5/5 | 5/5 | 5/5 | 3/3 | |
| Sh-282 | 5/5 | 5/5 | 5/5 | 3/3 | |
Rabbits were challenged with four EM skin biopsies containing HAB. Rabbits were scored positive if any one of spinal cord, joint, or node tissues was culture positive.
QPCR of donor skin implants.
In order to demonstrate that differences in protection between the strains were not due to variable numbers of HAB in the skin punches used for implant, quantitative PCR (QPCR) was performed as previously described on the donor skin punches using B. burgdorferi gyrase B (gyrB) as the target DNA and a nonhomologous internal standard (PCR MIMIC) as the competitor (38). To first determine if the gene copy number of gyrB was the same in B. burgdorferi strains B31, N40, 297, and Sh-282, QPCR was performed on DNA extracted from 108 in vitro-cultivated B. burgdorferi of each strain enumerated by dark-field microscopy. As shown in Table 2, each strain of 108 organisms was found to have 1.25 × 109 to 2.5 × 109 copies of gyrB. The discrepancy between the number of organisms and the gyrB copy number determined by QPCR is likely due to multiple copies of the chromosome (38). Although a recent study using PCR analysis of cultured B. burgdorferi showed that the chromosomal gene recA was consistent with a single recA per bacterium (32), our findings are more consistent with those for Borrelia hermsii, in that organisms isolated from mice have 13 to 18 chromosome copies per cell (27). Thus, the comparison of the number of organisms determined by QPCR to that enumerated by dark-field microscopy (Table 2) indicates that in vitro-cultivated B. burgdorferi has 12 to 25 chromosome copies per cell. Our findings also demonstrate that the four B. burgdorferi strains have relatively comparable copy numbers of gyrB, validating the use of this gene to determine relative copy numbers of organisms in each strain present in infected tissue.
TABLE 2.
Quantitation of HAB challenge inocula by PCRa
| Inoculum | Strain | No. of gyrB copies |
|---|---|---|
| In vitro cultivated Borrelia | B31 | 2.5 × 109 |
| N40 | 1.25 × 109 | |
| 297 | 2.5 × 109 | |
| Sh-2-82 | 2.5 × 109 | |
| HAB | B31 | 7.3 × 106 |
| N40 | 1.71 × 107 | |
| 297 | 9.96 × 106 | |
| Sh-2-82 | 3.6 × 106 |
In vitro-cultivated Borrelia (108) were enumerated by dark field; HAB were quantified using a 5-mm skin punch biopsy from a 7-day-infected rabbit used for HAB challenge. QPCR was performed as previously described (38) using B. burgdorferi gyrB as the target DNA and a nonhomologous internal standard as the competitor. Average number of gyrB gene copies per sample is based on duplicate QPCR.
QPCR using gyrB was then performed on DNA extracted from each of two donor skin punches taken from different EM sites. All donor skin implants had relatively comparable copy numbers of gyrB, ranging from 3.6 × 106 to 1.71 × 107 copies per 5-mm skin biopsy (Table 2). The exact number of copies of the chromosome in host-adapted organisms is unknown at this time but is not likely to be greater than 25 copies/cell. These results demonstrate that any differences in protection against the different B. burgdorferi strains were not due to variable numbers of HAB used for implant challenge.
Antigenic comparison of cultivated B. burgdorferi strains by two-dimensional Western blot analysis of Triton X-100-solubilized outer membrane proteins.
In order to determine if antigenic differences in outer membrane proteins between the different strains could account for the lack of HAB heterologous cross-protection, two-dimensional Western blot analysis using B31 strain-immune rabbit serum was performed. In vitro-cultivated B. burgdorferi strains B31, N40, 297, and SH-2-82 (4 × 108 organisms) were incubated in 2% Triton X-100 for 2 h at 4°C, which has been shown previously to solubilize the spirochetal outer membrane and enrich for outer membrane-associated proteins (14). Following detergent treatment, samples were centrifuged at 20,000 × g for 15 min to remove protoplasmic cylinders, and the outer membrane-enriched soluble supernatants were precipitated with 10% trichloroacetic acid, washed with acetone, and suspended in isoelectric focusing sample buffer as previously described (40). The solubilized proteins were separated by two-dimensional nonequilibrium pH gel electrophoresis and transferred to a polyvinylidene difluoride (Immobilon P) membrane as previously described (40). The blots from each strain were probed with pooled prechallenge immune rabbit serum from the 20 B31 infection-immune rabbits at a dilution of 1:1,000, and antibody-antigen interactions were identified with the enhanced chemiluminescence system (Amersham Corp., Arlington Heights, Ill.) as previously described (40).
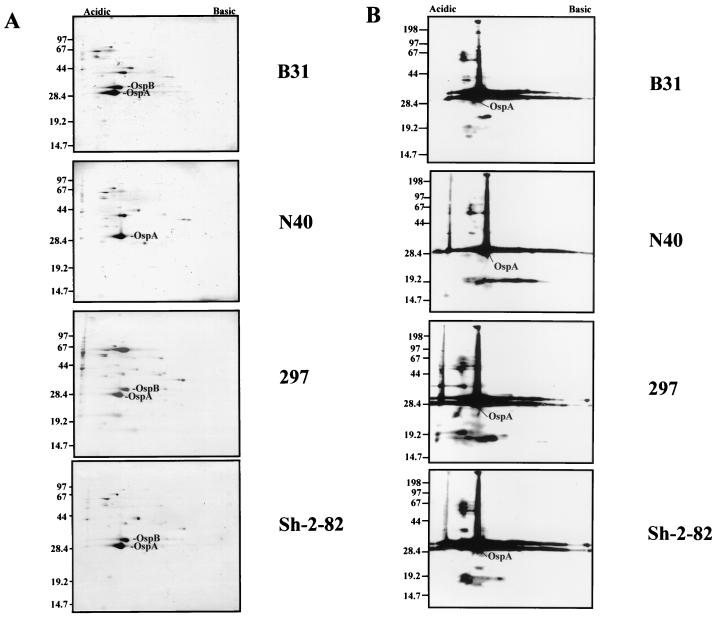
Analysis of the amido black-stained protein profile of each blot showed that all four strains were almost identical with the exception of strain N40, which did not possess the major 32-kDa OspB protein migrating just above OspA, and strain 297, which possessed a prominent 67-kDa protein which is likely albumin contamination (Fig. 1A). Western blot analysis demonstrated that B31 strain-immune rabbit serum reacted strongly with many similar migrating proteins among the different strains, although some differences were apparent which may be relevant to the lack of heterologous cross-protection (Fig. 1B). In addition, a strong anti-OspA reaction was present in Western blots of all four strains (Fig. 1B). Taken together, these findings suggest that many of the highly cross-reactive outer membrane antigens between these different strains cultivated in vitro, including OspA, do not provide cross-immunity when challenged with HAB. This finding for OspA is not surprising given the downregulation of this protein following mammalian host adaptation of B. burgdorferi.
FIG. 1.
Two-dimensional nonequilibrium gel electrophoresis of Triton X-100-solubilized proteins from 4 × 108 B. burgdorferi of strains B31, N40, 297, and Sh-2-82. (A) Amido black stain. (B) Immunoblot in panel A probed with pooled B31 infection-immune prechallenge sera. Molecular size markers are indicated to the left (in kilodaltons). Acidic and basic ends are indicated at the top.
We have recently found that B31 infection-immune rabbits are completely protected against high-dose homologous challenge with HAB, an observation that has not been reported previously in other animal models of Lyme disease (38). Our findings in the rabbit model differ from the results of two recent studies of homologous HAB challenge in the mouse model (4, 11). Actively infected mice which were cured with antibiotics and challenged with ≤1,280 homologous HAB organisms resulted in protection against disseminated infection but only partial protection against skin infection (4). In studies by Cassatt et al., mice immunized with DbpA and challenged with ≤10 homologous B31 and N40 HAB resulted in infection of 3 of 10 and 1 of 10 mice, respectively, indicating only partial protection in both cases (11). Of particular consideration is the small numbers of HAB organisms used for challenge in these studies. The dose of ≤1,280 HAB organisms used for challenge was determined by limiting-dilution QPCR and did not consider the potential for multiple chromosome copy numbers (7). Due to the inability to isolate sufficient numbers of host-adapted organisms, the HAB chromosome copy number is not known at this time. However, given a potential chromosome copy number of 25 per cell, based on the findings in this study, it is conceivable that considerably fewer HAB organisms were used for challenge of these mice. Thus, the small numbers of HAB used for challenge in these mouse studies (7, 11) were 100- to 1,000-fold less than in vitro-cultivated organisms used for standard challenge inoculation (103 to 104 organisms), yet mice were only partially protected against homologous HAB challenge. By comparison, homologous HAB challenge using infected rabbit skin allows for markedly greater numbers of organisms to be used for challenge (5.8 × 105 [Sh-2-82] to 2.7 × 106 [NP40] organisms per rabbit; 1.5 × 105 to 6.8 × 105 organisms at each of four sites). Moreover, infection-immune rabbits challenged with high-dose homologous HAB challenge are completely protected. The number of HAB organisms used for this challenge was at least 10 times greater than the standard challenge of rabbits using 6 × 104 in vitro-cultivated organisms (38). This was also at least 400 times more HAB than used for the challenge of mice. Thus, the relative greater degree of protection in immune rabbits against HAB compared to chronically infected and treated mice may reflect a greater quantitative and/or qualitative response to Borrelia antigens expressed during rabbit infection or, alternatively, the expression during rabbit infection of novel Borrelia antigens which are potent protective immunogens.
We have speculated that proteins specific to or upregulated in HAB are responsible for the high level of homologous protective immunity observed in infection-immune rabbits (38). Several proteins have been previously identified in the mouse model as being upregulated in vivo, including DbpA (11), OspC (37), and OspE and OspF (44). Proteins identified as uniquely expressed in vivo include EppA (12), p35 and p37 (17), the OspE-F homologue p21 (45), the OspF homologues bbk2.1 (2) and pG (46), and the proteins encoded on operon 2.9-71pB (1). While the concept that antigens unique to HAB are essential targets for protective immunity is appealing, many of the molecules currently known to be upregulated or uniquely expressed during mouse infection have not shown great potential as protective immunogens. OspC has been found to be highly variable among strains of B. burgdorferi and not protective against some homologous strains (9, 24). Recent findings also suggest that the protective OspC epitopes are conformation dependent (20). Active and passive immunizations with combined antisera to p35 and p37 were protective following challenge with 100 but not with 10,000 cultivated organisms (17). Recently, it has been reported that mice immunized with recombinant DbpA showed no protection following challenge with infected ticks (22). Thus, it is likely that the B. burgdorferi molecules that confer the high degree of complete protection observed following infection are HAB proteins that as yet have not been identified.
In this study we investigated whether this high degree of protection against large numbers of HAB also extends to heterologous challenge. Although B31 infection-immune rabbits were completely protected against homologous HAB challenge, they were not protected against HAB challenge using the heterologous strains N40, Sh-2-82, and 297. Interestingly, B31 strain-immune animals did show rapid clearance of infection following heterologous challenge with HAB strain 297. This difference following challenge between strain 297 and the other strains was not due to variations in the number of organisms used for challenge or to major differences in antibody recognition of in vitro-expressed membrane antigens. We have recently observed a similar rapid clearance of infection from skin in rabbits immunized with purified outer membrane derived from virulent B31 or B313 strains and challenged with homologous in vitro-cultivated organisms (38). This rapid clearance of infection may represent a degree of partial immunity and is impressive in view of the large numbers of HAB used for the heterologous challenge. However, this potential cross-protection against heterologous HAB challenge using strain 297 must be considered in the context of previous reports showing that cultivated organisms of these two strains are susceptible to killing by antibody raised against the other strain (29). Thus, whether the rapid clearance of infection observed in this study following heterologous HAB challenge is the result of common immunogens on host-adapted organisms, on both host-adapted and cultivated organisms, or on solely cultivated organisms is not known at this time.
Of further interest is the comparison of the strains used for this study. Strains B31, N40 (8), and Sh-2-82 (36) are all Ixodes scapularis isolates from New York State, while strain 297 is a human cerebrospinal fluid isolate from Connecticut (42). Despite isolation from the same species of tick and geographic location, no cross-protection was observed. Strains B31 and N40 have been shown by Mathiesen and coworkers, using genomic macrorestriction neighbor-joining analysis, to be closely related based on their OspA and 23S rDNA gene sequences (30). However, differences between strains B31 and N40 were noted by restriction fragment length polymorphism analysis (30), which in other studies have been associated with differences in human disease manifestations (49). It is therefore conceivable that the lack of protection observed among these strains translates to differences in upregulated HAB antigens that are strain specific.
In summary, we have corroborated our recent findings showing that infection-immune rabbits are completely protected against high-dose homologous challenge using HAB. However, this high degree of infection-derived protective immunity does not extend to other host-adapted strains used for heterologous challenge. This finding was somewhat surprising given the high degree of protection against high-dose homologous challenge and suggests that strain-specific molecules are the basis for infection-derived immunity. In this regard, we have recently isolated several novel HAB proteins from infected rabbit tissue that we have found to be upregulated relative to the amounts detected in cultivated organisms. We are currently investigating whether these proteins represent strain-specific or common antigens. It is hoped that determining the molecular basis of homologous infection-derived immunity will provide insights into the potential for heterologous cross-protection.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant AI-37312 to J. N. Miller and NIH grant AI-29733 to M. A. Lovett.
We thank Maurice M. Exner and Cheryl Champion for helpful suggestions.
REFERENCES
- 1.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Investig. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 3.Appel M J, Allan S, Jacobson R H, Lauderdale T L, Chang Y F, Shin S J, Thomford J W, Todhunter R J, Summers B A. Experimental Lyme disease in dogs produces arthritis and persistent infection. J Infect Dis. 1993;167:651–664. doi: 10.1093/infdis/167.3.651. [DOI] [PubMed] [Google Scholar]
- 4.Barthold S W. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect Immun. 1999;67:36–42. doi: 10.1128/iai.67.1.36-42.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 6.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold S W, Moody K D, Terwilliger G A, Duray P H, Jacoby R O, Steere A C. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J Infect Dis. 1988;157:842–846. doi: 10.1093/infdis/157.4.842. [DOI] [PubMed] [Google Scholar]
- 9.Bockenstedt L K, Hodzic E, Feng S, Bourrel K W, de Silva A, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess E C. Experimental inoculation of dogs with Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg Ser A Med Microbiol Infect Dis Virol Parasitol. 1986;263:49–54. doi: 10.1016/s0176-6724(86)80102-x. [DOI] [PubMed] [Google Scholar]
- 11.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong C, Wu X Y, Wang Y P, Blanco D R, Lovett M A, Miller J N. Immune serum from rabbits infected with Borrelia burgdorferi B31 confers complete passive protection against homologous challenge. J Spirochetal Tick-borne Dis. 2000;7:3–9. [Google Scholar]
- 14.Cunningham T M, Walker E M, Miller J N, Lovett M A. Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J Bacteriol. 1988;170:5789–5796. doi: 10.1128/jb.170.12.5789-5796.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeSilva A M, Fish D, Burkot T R, Zhang Y, Fikrig E. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect Immun. 1997;65:3146–3150. doi: 10.1128/iai.65.8.3146-3150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duray P H, Johnson R C. The histopathology of experimentally infected hamsters with the Lyme disease spirochete, Borrelia burgdorferi. Proc Soc Exp Biol Med. 1986;181:263–269. doi: 10.3181/00379727-181-42251. [DOI] [PubMed] [Google Scholar]
- 17.Fikrig E, Barthold S W, Sun W, Feng W, Telford S R, 3rd, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 18.Foley D M, Gayek R J, Skare J T, Wagar E A, Champion C I, Blanco D R, Lovett M A, Miller J N. Rabbit model of Lyme borreliosis: erythema migrans, infection-derived immunity, and identification of Borrelia burgdorferi proteins associated with virulence and protective immunity. J Clin Investig. 1995;96:965–975. doi: 10.1172/JCI118144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foley D M, Wang Y P, Wu X Y, Blanco D R, Lovett M A, Miller J N. Acquired resistance to Borrelia burgdorferi infection in the rabbit. Comparison between outer surface protein A vaccine- and infection-derived immunity. J Clin Investig. 1997;99:2030–2035. doi: 10.1172/JCI119371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmore R D, Mbow M L. Conformational nature of the Borrelia burgdorferi B31 outer surface protein C protective epitope. Infect Immun. 1999;67:5463–5469. doi: 10.1128/iai.67.10.5463-5469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene R T, Levine J F, Breitschwerdt E B, Walker R L, Berkhoff H A, Cullen J, Nicholson W L. Clinical and serologic evaluations of induced Borrelia burgdorferi infection in dogs. Am J Vet Res. 1988;49:752–757. [PubMed] [Google Scholar]
- 22.Hagman K E, Yang X, Wikel S K, Schoeler G B, Caimano M J, Radolf J D, Norgard M V. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect Immun. 2000;68:4759–4764. doi: 10.1128/iai.68.8.4759-4764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Höök M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jauris-Heipke S, Fuchs R, Motz M, Preac-Mursic V, Schwab E, Soutschek E, Will G, Wilske B. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med Microbiol Immunol. 1993;182:37–50. doi: 10.1007/BF00195949. [DOI] [PubMed] [Google Scholar]
- 25.Johnson R C, Marek N, Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984;20:1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsson M, Noppa L, Barbour A G, Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992;60:1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitten T, Barbour A G. The relapsing fever agent Borrelia hermsii has multiple copies of its chromosome and linear plasmids. Genetics. 1992;132:311–324. doi: 10.1093/genetics/132.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovrich S D, Callister S M, Lim L C, Schell R F. Seroprotective groups among isolates of Borrelia burgdorferi. Infect Immun. 1993;61:4367–4374. doi: 10.1128/iai.61.10.4367-4374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marconi R T, Konkel M E, Garon C F. Variability of osp genes and gene products among species of Lyme disease spirochetes. Infect Immun. 1993;61:2611–2617. doi: 10.1128/iai.61.6.2611-2617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathiesen D A, Oliver J H, Jr, Kolbert C P, Tullson E D, Johnson B J, Campbell G L, Mitchell P D, Reed K D, Telford S R, 3rd, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 31.Moody K D, Barthold S W, Terwilliger G A, Beck D S, Hansen G M, Jacoby R O. Experimental chronic Lyme borreliosis in Lewis rats. Am J Trop Med Hyg. 1990;42:165–174. doi: 10.4269/ajtmh.1990.42.165. [DOI] [PubMed] [Google Scholar]
- 32.Morrison T B, Ma Y, Weis J H, Weis J J. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–992. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philipp M T, Aydintug M K, Bohm R P, Jr, Cogswell F B, Dennis V A, Lanners H N, Lowrie R C, Jr, Roberts E D, Conway M D, Karaçorlu M, et al. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preac Mursic V, Patsouris E, Wilske B, Reinhardt S, Gross B, Mehraein P. Persistence of Borrelia burgdorferi and histopathological alterations in experimentally infected animals: a comparison with histopathological findings in human Lyme disease. Infection. 1990;18:332–341. doi: 10.1007/BF01646399. [DOI] [PubMed] [Google Scholar]
- 35.Rosa P A, Schwan T, Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992;6:3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 36.Schwan T G, Burgdorfer W, Schrumpf M E, Karstens R H. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus) J Clin Microbiol. 1988;26:893–895. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang E S, Champion C I, Wu X Y, Skare J T, Blanco D R, Miller J N, Lovett M A. Comparison of protection in rabbits against host-adapted and cultivated Borrelia burgdorferi following infection-derived immunity or immunization with outer membrane vesicles or outer surface protein A. Infect Immun. 2000;68:4189–4199. doi: 10.1128/iai.68.7.4189-4199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Malawista S E. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease: recombinant outer-surface protein A Lyme disease vaccine study consortium. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 40.Skare J T, Shang E S, Foley D M, Blanco D R, Champion C I, Mirzabekov T, Sokolov Y, Kagan B L, Miller J N, Lovett M A. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Investig. 1995;96:2380–2392. doi: 10.1172/JCI118295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonnesyn S W, Manivel J C, Johnson R C, Goodman J L. A guinea pig model for Lyme disease. Infect Immun. 1993;61:4777–4784. doi: 10.1128/iai.61.11.4777-4784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steere A C, Grodzicki R L, Kornblatt A N, Craft J E, Barbour A G, Burgdorfer W, Schmid G P, Johnson E, Malawista S E. The spirocheteal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 43.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheeler C M, Coleman J L, Habicht G S, Benach J L. Adult Ixodes dammini on rabbits: development of acute inflammation in the skin and immune responses to salivary gland, midgut, and spirochetal components. J Infect Dis. 1989;159:265–273. doi: 10.1093/infdis/159.2.265. [DOI] [PubMed] [Google Scholar]
- 48.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wormser G P, Liveris D, Nowakowski J, Nadelman R B, Cavliere L F, McKenna D, Holmgren D, Schwartz I. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–725. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]