Abstract
Background
Improved patient and limb survival rates have led to an increased interest in the functional outcome and return to sports of patients undergoing megaprosthetic reconstruction in musculoskeletal oncology. This study evaluates the functional outcome and postoperatively performed level of sports in patients undergoing proximal humeral replacement (PHR) following resection of a primary bone sarcoma and identifies potential beneficial and limiting factors.
Patients and methods
Between 2007 and 2020, a total of 606 patients underwent resection of a primary bone sarcoma and reconstruction with a single-design modular implant. For 112 (18%) patients, the location of the tumour was the proximal humerus. Exclusion criteria were death (n = 65), patients living overseas (n = 8), and subsequent amputation (n = 1), leaving 38 patients for evaluation, of whom 32 were available for the study (13 women, median age 42 years). Clinical data regarding oncological and surgical treatment as well as subsequent complications were obtained from the patients’ electronic medical records. Functional outcome was determined using the Musculoskeletal Tumor Society Score (MSTS) and Toronto Extremity Salvage Score (TESS) as well as the Subjective Shoulder Value (SSV). Return to sports was assessed using the Tegner Activity Score (TS) and the modified Weighted Activity Score (WAS).
Results
At the last follow-up after a median of 30 months (IQR 22–58), median MSTS was 18 (IQR 12–24), median TESS was 80% (IQR 69–87), median SSV was 35% (IQR 10–58), median TS was 5 (IQR 4–6) and median WAS was 5 (IQR 0–10). Preservation of the axillary nerve, a reverse shoulder reconstruction and a WAS of > 10 prior to surgery were associated with better functional outcome and return to sports activity scores.
Conclusion
Following PHR, good to excellent functional outcomes are possible, and patients regularly return to participate in sports activities—most commonly in low-impact types of sports, but some individuals are even able to participate in high-impact sports activities.
Level of evidence
IV.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10195-022-00678-z.
Keywords: Megaprosthesis, Humerus, Bone sarcoma, Return to sport, Functional outcome
Introduction
The humerus is the third most common site of primary malignant bone tumours [1, 2]. Treatment of these entities is usually defined by multimodal study protocols, and surgical treatment commonly requires excision of the tumour with wide surgical margins [3]. A common mode of reconstruction is to use a modular, megaprosthetic replacement with a proximal humerus replacement [4, 5]. Over the past decades, advances in oncological and surgical treatment have resulted in improved limb and patient survival rates [6]. These achievements have subsequently led to an increased interest in patients’ postoperative function and return to sports activities and the potential factors associated with a successful return to sports activities—especially considering that mostly young patients are affected by primary bone sarcomas [7–10]. However, while sports activities in sarcoma patients who have undergone megaprosthetic reconstruction have previously been studied for lower extremity tumours, there is a paucity of studies on upper extremity reconstructions, particularly studies applying homogeneous inclusion criteria and larger patient cohorts [11, 12]. Furthermore, patient- and procedure-related factors associated with outcome are widely unknown. Thus, the aims of the present study were (1) to determine the functional outcome and (2) to evaluate the postoperatively achieved level of sports using standardized scoring systems in a large cohort of patients who had undergone proximal humeral replacement (PHR) following resection of a primary bone sarcoma, and (3) to identify the associated beneficial and limiting factors.
Patients and methods
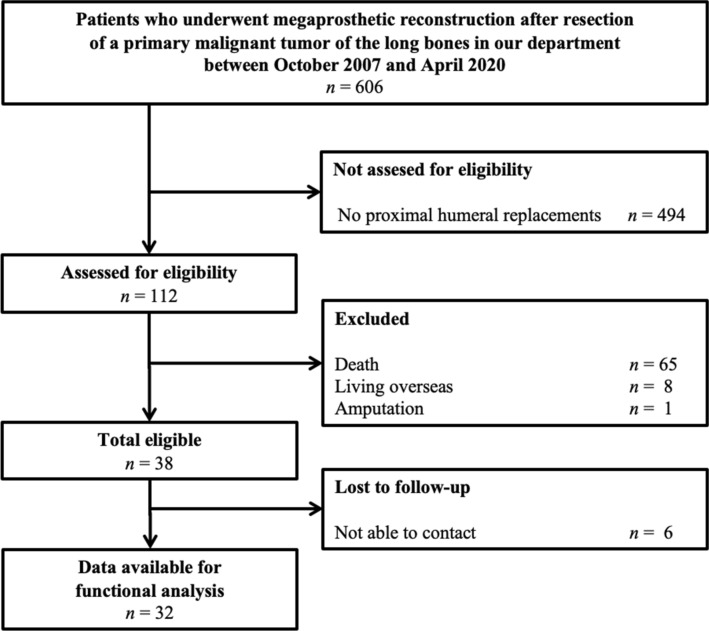
Between October 2007 and April 2020, a total of 606 patients underwent resection of a primary bone sarcoma of the long bones and subsequent reconstruction with a single-design modular universal tumour and revision system (MUTARS, Implantcast, Buxtehude, Germany) in our department in a tertiary university hospital. For 112 (18%) of these patients, the location of the tumour was the proximal humerus. Exclusion criteria for the present study were death (n = 65), patients living overseas (n = 8), and subsequent amputation (n = 1), leaving 38 patients. Six patients could not be contacted for the study, leaving a final cohort of 32 patients (Fig. 1, Table 1).
Fig. 1.
STROBE study flow diagram
Table 1.
Demographics and oncological and surgical details
| Variable | n (%) |
|---|---|
| Gender | |
| Female | 13 (41) |
| Male | 19 (59) |
| Tumour entity | |
| Osteosarcoma | 13 (41) |
| Chondrosarcoma | 10 (31) |
| Ewing sarcoma | 6 (19) |
| Pleomorphic sarcoma of the bone | 1 (3) |
| Myxofibrosarcoma | 1 (3) |
| Fibromyxosarcoma | 1 (3) |
| Type of tumour resection | |
| Intra-articular | 22 (69) |
| Extra-articular | 10 (31) |
| Type of shoulder reconstruction | |
| Anatomic | 20 (62) |
| Reverse | 12 (38) |
Surgical technique
All patients underwent a planned wide tumour resection with histopathological confirmation of surgical margins. In order to achieve these wide surgical margins, the axillary nerve had to be resected or could be preserved. In patients with tumour infiltration of the glenohumeral joint, an extraarticular tumour resection was performed. For soft-tissue reconstruction, an attachment tube (Trevira, Implantcast, Buxtehude, Germany) was used [13, 14]. As of 2006, silver-coated implants were available and used in all patients [15]. As of 2010, implants for reverse shoulder reconstruction were available and used in patients in whom the axillary nerve and a sufficient portion of the deltoid could be preserved [11–13].
Data collection
Clinical data regarding the oncological and surgical treatment as well as subsequent complications were obtained from the patients’ electronic medical records. Functional outcome and pre- and post-operatively performed levels of sports were evaluated using standardized scoring systems.
Assessment of functional outcome and classification of complications
The functional outcome was determined using the Musculoskeletal Tumor Society Score (MSTS), the Toronto Extremity Salvage Score (TESS) and the Subjective Shoulder Value (SSV). MSTS and TESS are commonly used in tumour orthopaedics to assess the functional outcome following limb-sparing surgery, and there are specifically designed versions of each for upper and lower extremities, respectively [16, 17]. The upper extremity version of the MSTS includes six questions on dexterity, pain, emotional acceptance, function, hand positioning and the ability to lift objects with the affected arm [17]. Each question is scored on a scale from 0 (very limited) to 5 (no restriction), with a maximum score of 30 points [17]. The upper extremity version of the TESS includes 29 questions, each scored on a scale from 1 (impossible to do) to 5 (not at all difficult), on everyday upper extremity tasks such as cutting vegetables or doing household chores [16]. The maximum score is 145 points, which is converted to a percentage to allow easier comparability [16]. Furthermore, we obtained the SSV, where a patient rates the function of the operated shoulder (as a percentage) compared to the contralateral shoulder [18].
The performed level of sports activities was assessed using the Tegner Activity Score (TS) and the modified Weighted Activity Score (WAS). For the TS, the patient states his highest level of performed sports on a scale from 1 (cannot move) to 10 (participates in competitive contact sports—national elite level) [19, 20]. The WAS is an individual performance score covering the frequency, duration and type of performed sports (low, medium or high impact) [21]. The score is obtained by multiplying the frequency (per week), duration (in hours) and weighted points based on the respective impact of the performed sports activity—e.g. a sports activity with a low load (e.g. swimming) equals a factor of 1 and a sports activity with a high load (e.g. soccer) equals a factor of 3 (Table 2) [21–23]. If more than one activity was performed, the respective scores for each individual activity were added to the final WAS [21, 22]. WASs from 0 to 10 indicate low-activity patients and WASs higher than 10 indicate high-activity patients.
Table 2.
Impact of performed sports activities according to Healy et al. [21]
| Impact | Type of sports activity |
|---|---|
| Low | Nordic walking, dancing, diving, aqua gymnastic, swimming, hiking, cycling, fishing, bowling, sailing |
| Medium | Skiing, canoeing, badminton, golf |
| High | American football, soccer, tennis, triathlon, judo, basketball, jogging |
Endoprosthetic complications were classified according to Henderson et al. as soft tissue failure (type 1), aseptic loosening (type 2), structural failure (type 3), infection (type 4) and tumour progression (type 5) [24].
Statistical analysis
Statistical analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). The duration of follow-up was calculated from the date of surgery to the date of the event or the last documented contact with the patient as of December 2021. The data distribution was determined with the Kolmogorov–Smirnov test. Non-parametric analyses were performed with the Mann–Whitney U test, and parametric analyses were performed with Student’s t test. All p values were two-sided and a p value of less than 0.05 was considered statistically significant.
Results
Results from a total of 32 patients (84% follow-up rate, 13 women) with a median age of 42 years (IQR 24–56) on the day of surgery and a median BMI of 25 (IQR 22–27) were available for analysis after a median follow-up of 30 months (IQR 22–58).
Functional outcome
The median MSTS before diagnosis was 30 (IQR 28–30) and 18 (IQR 12–24) at final follow-up. The median TESS before diagnosis was 100% (145/145, IQR 100–100) and 80% (116/145, IQR 69–87) at final follow-up. The median SSV at final follow-up was 35% (IQR 10–58).
Return to sports activities
The median TS prior to diagnosis was 6 (IQR 6–7) and 5 (IQR 4–6) at final follow-up. The median WAS before diagnosis was 10 (IQR 5–21) and 5 (IQR 0–10) at final follow-up.
Prior to surgery, 91% (29/32) of the patients reported that they were active in sports activities. Participants engaged particularly frequently in fitness/gymnastics (16%, 5/32), cycling (13%, 4/32) and swimming (13%, 4/32). Three patients preoperatively performed sports at the competitive tournament level (defined as TS > 8), namely dancing, soccer and American football, respectively.
At the time of the last follow-up, 69% (22/32) of the patients participated in at least one low-impact sports activity, with cycling (25%, 8/32), walking (19%, 6/32) and fitness/gymnastics (16%, 5/32) being the three most frequent types of performed sports (Tables 3, 4). Of the three patients who preoperatively participated in a sport at the competitive tournament level, two patients returned to their sports at the tournament level: the first at dancing (prior diagnosis TS = 10, at last follow-up TS = 8; Fig. 2a, b, Additional file 1: Video S1) and the second at playing soccer (prior diagnosis TS = 9, at last follow-up TS = 7). The third patient postoperatively switched sports from American football to cycling. The previously performed sports activities of basketball, badminton, judo, fishing and American football were no longer performed postoperatively by any patient (Table 4).
Table 3.
Sports activities performed by each patient prior to diagnosis and at the time of last follow-up
| Patient | Prior to diagnosis | At the last follow-up |
|---|---|---|
| 1 | Tennis, cycling, jogging | Tennis, cycling, jogging |
| 2 | Tennis, cycling, hiking, swimming, skiing | – |
| 3 | – | – |
| 4 | Tennis, bowling | Cycling, walking |
| 5 | – | – |
| 6 | Cycling, jogging | Cycling, walking |
| 7 | Fitness | Cycling, fitness |
| 8 | Cycling, Nordic walking, walking, aqua sport | Nordic walking, jogging, swimming, walking |
| 9 | American football, cycling, fitness | Cycling, walking |
| 10 | Soccer, badminton | Cycling, fitness |
| 11 | Diving | – |
| 12 | Nordic walking, gymnastics, swimming | Gymnastics, aqua gymnastics, swimming |
| 13 | – | – |
| 14 | Fishing | – |
| 15 | Tennis, skiing | Tennis, skiing, golf |
| 16 | Swimming | – |
| 17 | Cycling | – |
| 18 | Horse riding | Walking |
| 19 | Triathlon | Cycling, sailing |
| 20 | Soccer | Soccer |
| 21 | Soccer, canoeing, cycling | Cycling |
| 22 | Horse riding | – |
| 23 | Swimming, fitness | Swimming, fitness, hiking, diving |
| 24 | Canoeing, judo | Canoeing, jogging |
| 25 | Dancing | Dancing |
| 26 | – | Hiking |
| 27 | – | – |
| 28 | Dancing | Dancing |
| 29 | Fitness | Fitness |
| 30 | – | Cycling |
| 31 | Diving | Diving |
| 32 | Basketball, dancing | Dancing |
Table 4.
Sports activities performed prior to diagnosis and at the last follow-up, ranked by frequency, as given in brackets
| Prior diagnosis | At the last follow-up |
|---|---|
| Cycling (7) | Cycling (9) |
| Gymnastics/fitness (5) | Walking (6) |
| Swimming (4) | Gymnastics/fitness (5) |
| Tennis (4) | Dancing (3) |
| Soccer (3) | Swimming (3) |
| Dancing (3) | Jogging (3) |
| Nordic walking (2) | Hiking (2) |
| Skiing (2) | Tennis (2) |
| Walking (2) | Diving (2) |
| Diving (2) | Nordic walking (1) |
| Canoeing (2) | Skiing (1) |
| Triathlon (1) | Aqua sport (1) |
| Jogging (1) | Canoeing (1) |
| Aqua sport (1) | Sailing (1) |
| American football (1) | Golf (1) |
| Badminton (1) | Soccer (1) |
| Fishing (1) | |
| Bowling (1) | |
| Judo (1) | |
| Basketball (1) |
Sports activities that are not performed anymore postoperatively are shown in italics
Fig. 2.
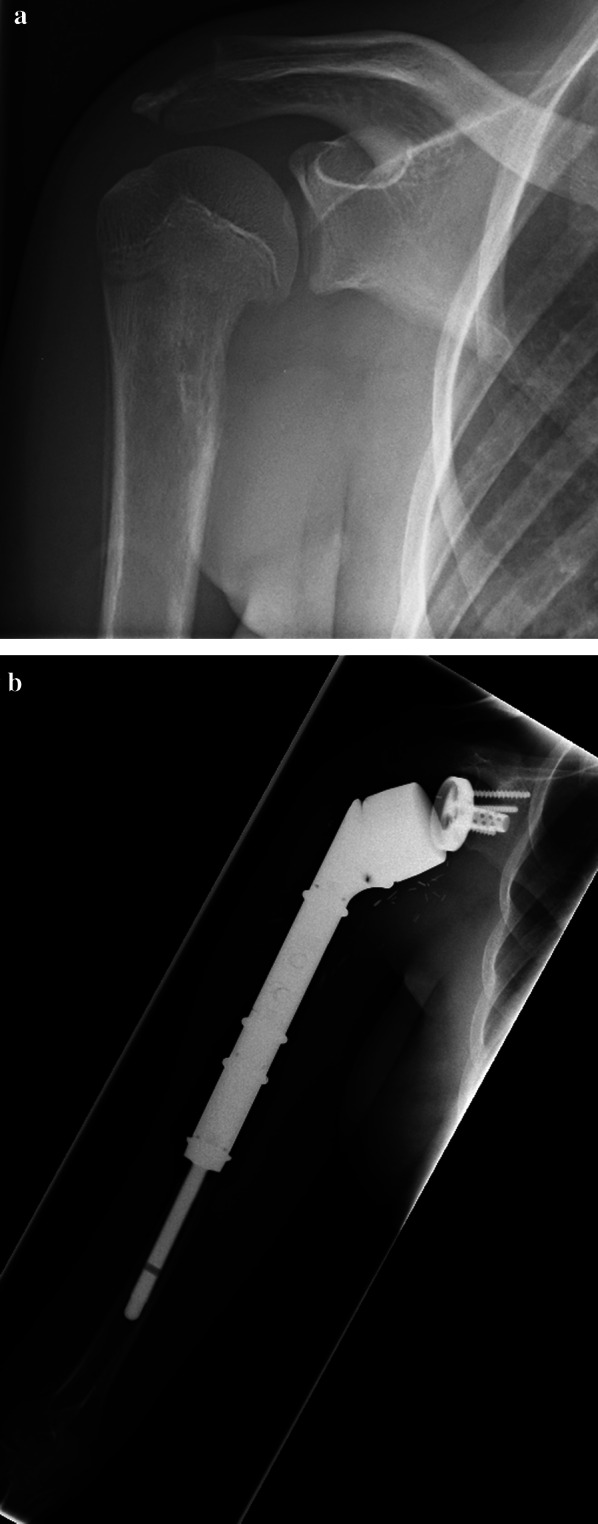
a Osteosarcoma of the right proximal humerus in an 18-year-old female patient; b radiographic imaging 40 months following PHR
Complications
A total of five complications as defined by Henderson et al. were observed in our cohort, with the most common reason for revision surgery being a periprosthetic infection (Henderson type 4, n = 3). Furthermore, there was one soft tissue failure (Henderson type 1) and one aseptic loosening (Henderson type 2) [24].
Beneficial and limiting factors
Patients in whom preservation of the axillary nerve was possible had overall higher postoperative functional outcome scores (MSTS and TESS) and achieved higher WASs overall than patients in whom the axillary nerve had to be resected in order to achieve wide surgical margins (Table 5).
Table 5.
Association of demographic and clinical factors with postoperative outcome scores
| Factors | Yes | No | p value |
|---|---|---|---|
| Male | MSTS: 18 (IQR 12–24) | MSTS: 18 (IQR 11–24) | p = 0.270 |
| TESS: 83% (IQR 70–88) | TESS: 77% (IQR 64–83) | p = 0.596 | |
| TS: 5 (IQR 4–6) | TS: 5 (IQR 4–6) | p = 0.850 | |
| WAS: 6 (IQR 2–10) | WAS: 5 (IQR 0–13) | p = 0.650 | |
| BMI (< 30) | MSTS: 16 (IQR 12–20) | MSTS: 18 (IQR 12–24) | p = 0.734 |
| TESS: 79% (IQR 76–82) | TESS: 80% (IQR 67–86) | p = 0.907 | |
| TS: 2 (IQR 0–4) | TS: 5 (IQR 4–6) | p = 0.081 | |
| WAS: 3 (IQR 0–6) | WAS: 5 (IQR 2–10) | p = 0.532 | |
| Intraarticular tumour resection | MSTS: 19 (IQR 12–25) | MSTS: 17 (IQR 13–20) | p = 0.795 |
| TESS: 81% (IQR 59–87) | TESS: 78% (IQR 71–86) | p = 0.952 | |
| TS: 5 (IQR 4–6) | TS: 5 (IQR 3–6) | p = 0.920 | |
| WAS: 6 (IQR 2–14) | WAS: 2 (IQR 0–5) | p = 0.070 | |
| Preservation of the axillary nerve | MSTS: 21 (IQR 14–26) | MSTS: 14 (IQR 11–19) | p = 0.027 |
| TESS: 84% (IQR 70–93) | TESS: 76% (IQR 47–82) | p = 0.049 | |
| TS: 5 (IQR 5–6) | TS: 4 (IQR 3–6) | p = 0.193 | |
| WAS: 11 (IQR 5–18) | WAS: 2 (IQR 0–4) | p < 0.001 | |
| Reverse shoulder reconstruction | MSTS: 21 (IQR 17–26) | MSTS: 15 (IQR 12–22) | p = 0.116 |
| TESS: 83% (IQR 78–88) | TESS: 76% (IQR 61–85) | p = 0.158 | |
| TS: 5 (IQR 4–6) | TS: 5 (IQR 3–6) | p = 0.578 | |
| WAS: 7 (IQR 5–12) | WAS: 3 (IQR 0–7) | p = 0.032 | |
| Radiotherapy | MSTS: 18 (IQR 11–26) | MSTS: 15 (IQR 12–24) | P = 0.593 |
| TESS: 84% (IQR 69–93) | TESS: 79% (IQR 65–85) | p = 0.404 | |
| TS: 6 (IQR 5–7) | TS: 5 (IQR 3–6) | p = 0.124 | |
| WAS: 10 (IQR 4–17) | WAS: 4 (IQR 0–7) | p = 0.037 | |
| Chemotherapy | MSTS: 18 (IQR 15–24) | MSTS: 16 (IQR 12–24) | p = 0.464 |
| TESS: 83% (IQR 71–92) | TESS: 77% (IQR 62–83) | p = 0.168 | |
| TS: 5 (IQR 4–6) | TS: 6 (IQR 4–6) | p = 0.985 | |
| WAS: 6 (IQR 2–10) | WAS: 4 (IQR 0–11) | p = 0.464 | |
| Revision surgery | MSTS: 12 (IQR 11–22) | MSTS: 18 (IQR 13–24) | p = 0.389 |
| TESS: 76% (IQR 45–87) | TESS: 80% (IQR 70–87) | p = 0.479 | |
| TS: 4 (IQR 1–6) | TS: 5 (IQR 4–6) | p = 0.241 | |
| WAS: 2 (IQR 0–6) | WAS: 6 (IQR 2–11) | p = 0.166 | |
| High level of sports prior to diagnosisa | MSTS: 18 (IQR 12–21) | MSTS: 18 (IQR 12–26) | p = 0.722 |
| TESS: 81% (IQR 66–93) | TESS: 79% (IQR 68–84) | p = 0.512 | |
| TS: 6 (IQR 3–6) | TS: 6 (IQR 4–6) | p = 0.722 | |
| WAS: 24 (IQR 2–34) | WAS: 6 (IQR 0–10) | p = 0.023 |
Statistically significant p values are shown in bold
MSTS Musculoskeletal Tumor Society Score, TESS Toronto Extremity Salvage Score, TS Tegner Activity Score, WAS weighted activity score
aDefined as a WAS prior to diagnosis of > 10
Regarding the postoperatively performed level of sports, patients with a reverse shoulder reconstruction as well as patients who performed sports at a high level prior to diagnosis (WAS > 10) achieved higher WASs than their respective counterparts (Table 5).
With the numbers available, gender, obesity (body mass index > 30), handedness (surgery on the dominant vs non-dominant extremity), type of tumour resection (intraarticular vs extraarticular), chemotherapy and revision surgery during follow-up were not associated with higher/lower functional outcomes or higher/lower level of sports activity performed postoperatively (Table 5).
Prior to diagnosis, a total of ten patients performed sports activities involving the upper extremity. Postoperatively, five of those patients returned to upper-extremity-demanding types of sports, and the remaining five patients switched to alternative sports activities with more focus on the lower extremity (Table 6).
Table 6.
Demographics and clinical and surgical details of patients who performed sports activities involving the upper extremity prior to diagnosis, and the respective postoperatively performed sports activities and outcome scores
| Patient | Demographic and clinical details | Surgical details | Sports activities involving the upper extremity | Outcome scores | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age and gender | Entity | Type of resection | Type of shoulder reconstruction | Preservation of the axillary nerve | Prior to diagnosis | After surgery | MSTS | TESS (%) | TS | WAS | |
| 1 | 74f | Chondrosarcoma | Intra | Reverse | Yes | Tennis | Tennis | 21 | 82 | 6 | 28 |
| 2 | 53m | Chondrosarcoma | Intra | Reverse | Yes | Tennis | Tennis, golf | 27 | 93 | 6 | 23 |
| 3 | 28m | Osteosarcoma | Extra | Anatomic | Yes | Canoeing | Canoeing | 28 | 98 | 5 | 8 |
| 4 | 19f | Ewing sarcoma | Intra | Reverse | Yes | Dancing | Dancing | 26 | 94 | 5 | 20 |
| 5 | 21f | Osteosarcoma | Intra | Reverse | Yes | Dancing | Dancing | 18 | 80 | 5 | 5 |
| 6 | 73f | Chondrosarcoma | Extra | Anatomic | Yes | Tennis | None | 12 | 59 | 2 | 0 |
| 7 | 72m | Chondrosarcoma | Intra | Reverse | Yes | Tennis | Cycling | 12 | 63 | 1 | 8 |
| 8 | 59m | Ewing sarcoma | Intra | Reverse | No | American football | Cycling | 11 | 82 | 5 | 6 |
| 9 | 58m | Ewing sarcoma | Intra | Reverse | Yes | Soccer, badminton | Cycling | 17 | 85 | 3 | 11 |
| 10 | 33m | Osteosarcoma | Extra | Anatomic | No | Canoeing | Cycling | 18 | 72 | 3 | 2 |
m male, f female, intra intraarticular, extra extraarticular, MSTS Musculoskeletal Tumor Society Score, TESS Toronto Extremity Salvage Score, TS Tegner Activity Score, WAS weighted activity score
Discussion
The main findings of our study are: (1) good to excellent functional outcomes are possible following PHR after resection of a primary bone sarcoma; (2) postoperatively, patients generally perform sports activities with a low to medium physical demand, although individual patients are able to return to high-impact sports activities; and (3) preservation of the axillary nerve, a reverse shoulder reconstruction and higher levels of performed sports activities prior to diagnosis (WAS > 10) seem to be associated with a higher postoperatively achieved level of sports.
The good to excellent functional outcomes of our cohort are in line with the findings of previous authors [25–29]. For PHR with an allograft prosthesis composite, Abdeen et al. reported a mean MSTS of 26 (IQR 14–27) in a cohort of 36 patients with a median age of 23 years who underwent this procedure after resection of a primary bone sarcoma, failure of a reconstruction following a previous tumour resection, and resection of a local recurrence [27]. The longest follow-up for PHR was reported by Kumar et al., who reported a mean MSTS of 24 [standard deviation (SD) 3.8] and a mean TESS of 72% (SD 23.2) after a mean follow-up of 9 years (range 2–20 years) in 30 patients with a median age of 34 years who underwent PHR for resection of various bone tumours, including primary sarcomas, metastases and giant cell tumours [26]. Regarding the indication for PHR, Böhler et al. described a homogeneous cohort of 49 patients with a mean age of 18 years (IQR 17–21) who all underwent PHR following the resection of an osteosarcoma but subsequent reconstruction with different types of prostheses, reporting a mean MSTS of 24 (21–26) [28]. Similar to our findings, and despite the heterogeneity of the implants used, Böhler et al. found that preservation of the axillary nerve and the deltoid muscle were associated with better functional outcomes [28]. Houdek et al. described a cohort of 83 patients with a median age of 57 years (SD 18) who underwent PHR with endoprostheses (56 patients) or allograft-prosthesis composites (27 patients) following the intra-articular resection of a metastasis or a primary bone sarcoma and subsequent reconstruction with a reverse shoulder reconstruction (30 patients) or a hemiarthroplasty (53 patients) [29]. Houdek et al. found that patients with a reverse shoulder reconstruction had better functional results regarding the MSTS (22 vs 19) and improved range of motion compared to patients with a hemiarthroplasty [29]. However, with varying indications for PHR (such as resection for a primary bone sarcoma or for metastases or as a revision surgery following a previously failed endoprosthetic reconstruction in non-oncologic patients) and the different prosthesis systems used, the reported patient cohorts become heterogeneous, diluting the conclusion and relevance for specific groups of patients—such as patients undergoing PHR for resection of a primary bone sarcoma with a modular megaprosthesis.
Postoperatively, patients in our cohort mainly performed low- to medium-impact types of sports activities. Matching results were reported by Lang et al., who described a cohort of 18 patients undergoing PHR for bone sarcomas, with 14 patients performing low-impact sports activities and only 2 patients participating in medium- or high-impact sports activities after surgery [23]. Furthermore, Lang et al. also observed a postoperative switch from preoperatively performed sports activities that are demanding for the upper extremity to sports activities that are more demanding for the lower extremity [23]. However, contrary to the findings of Lang et al., we found that five of ten patients in our cohort continued to perform upper-extremity-demanding types of sports, partially compensating for functional limitations by using the non-affected upper extremity (Additional file 2: Video S2, Additional file 3: Video S3) [23].
In order to guide patients’ and surgeons’ expectations, it appears desirable to identify factors that might influence the postoperative functional outcome and the ability to do sports. In the present study, a preserved axillary nerve, a reverse shoulder reconstruction and a higher level of sports activity (WAS > 10) prior to diagnosis were associated with better results. These findings are in line with previous studies, who have also highlighted the functional importance of the deltoid muscle and the axillary nerve; however, in order to achieve wide margins, it can become necessary to resect these structures, which is paramount for the oncological outcome [30, 31]. Furthermore, reverse shoulder reconstruction with a proximal humerus megaprosthesis has previously been shown to be associated with good postoperative functional outcomes in non-oncologic as well as oncologic patient cohorts [29, 32]. While Lang et al. found no beneficial effect of a reverse shoulder reconstruction on the postoperatively performed level of sports in their cohort, Guven et al. described good to excellent functional outcomes and no endoprosthetic complications in ten patients following PHR with a reverse shoulder reconstruction after resection of a bone tumour [25, 32]. The latter finding is in line with our findings and appears plausible considering the improved range of motion described for reverse megaprosthetic PHR [33]. Regarding the level of sports performed prior to diagnosis, Hobusch et al. could not find a correlation with the achieved level of sports in 16 patients undergoing proximal femoral replacement for bone sarcomas [7]. As, for obvious reasons, upper and lower extremity megaprosthetic reconstructions are not comparable when it comes to a return to sport activities, we hypothesize that patients who return to a sports activity that is demanding for the upper extremity might benefit from previous proficiency when it comes to developing compensatory mechanisms or switching dexterity. However, as this study is the first to describe this aspect, future studies are needed to confirm or refute this association.
Limitations
We acknowledge several limitations to our study. (1) The study follows a retrospective study design and includes only a small number of patients, which may not fully represent the risk factors. However, to the best of our knowledge, our study represents one of the largest and most homogeneous cohorts of patients who underwent PHR with a single-design megaprosthesis at a single institution, reflecting the rarity of these entities and this procedure. (2) Some patients could not be contacted and, despite the follow-up rate of 84%, it is possible that patients lost to follow-up may have poorer outcomes. Therefore, the results presented here may be considered high-end estimates. (3) Patients included in this study are considered to have been free of disease over the long term, so functional results and the performed level of sports might be biased, as patients who died during follow-up might have had lower functional outcome scores, participated in lower levels of sports, or even performed no sports activity at all. Again, this makes the results presented here likely to be a high-end estimate.
Conclusion
A good to excellent functional outcome is possible after PHR following sarcoma resection. While low-impact activities appear to be more common, some individuals are able to participate in high-impact sports activities. Preservation of the axillary nerve, a reverse shoulder reconstruction and a higher level of sports prior to surgery were associated with better functional outcome scores and a higher level of postoperative sports activity.
Supplementary Information
Additional file 1: Video S1. An initially 18-year-old female patient taking part in a professional dancing competition 5 years after PHR following resection of an osteosarcoma
Additional file 2: Video S2. An initially 53-year-old male patient playing golf 20 years after PHR following resection of an Ewing sarcoma
Additional file 3: Video S3. An initially 27-year-old male patient kayaking 10 years after PHR following resection of a chondrosarcoma
Acknowledgements
Not applicable.
Abbreviations
- IQR
Interquartile range
- MSTS
Musculoskeletal Tumor Society Score
- MUTARS
Modular universal tumour and revision system
- PHR
Proximal humeral replacement
- SD
Standard deviation
- SSV
Subjective Shoulder Value
- TESS
Toronto Extremity Salvage Score
- TS
Tegner Activity Score
- WAS
Weighted Activity Score
Author contributions
ME, CT, GG and KNS designed the study and collected the data. ME, CT and KNS were responsible for data management, data analysis and the preparation of figures. ME, CT and KNS wrote the manuscript. GG, ND, SK and CR helped with data analysis and with the editing of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge support from the Open Access Fund of the University of Münster.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Approval was obtained from the ethical commission of the Ärztekammer Westfalen-Lippe, Münster, Germany (reference number: 2018-199-f-S).
Consent for publication
Written informed consent was obtained from all patients.
Competing interests
GG certifies that he holds a patent for the silver coating of a metallic prosthesis and receives payments from Implantcast GmbH (Buxtehude, Germany). CT and ND certify that they have received travel expenses from Implantcast GmbH (Buxtehude, Germany). All other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Moritz Ellerbrock, Email: moritz.ellerbrock@ukmuenster.de.
Christoph Theil, Email: christoph.theil@ukmuenster.de.
Georg Gosheger, Email: georg.gosheger@ukmuenster.de.
Niklas Deventer, Email: niklas.deventer@ukmuenster.de.
Sebastian Klingebiel, Email: sebastian.klingebiel@ukmuenster.de.
Carolin Rickert, Email: carolin.rickert@ukmuenster.de.
Kristian Nikolaus Schneider, Email: kristian.schneider@ukmuenster.de.
References
- 1.Devaney K. Dahlin’s bone tumors. General aspects and data on 11,087 cases. Am J Surg Pathol. 1996;20:1298. doi: 10.1097/00000478-199610000-00019. [DOI] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Strauss SJ, Frezza AM, Abecassis N, et al. Bone sarcomas: ESMO–EURACAN–GENTURIS–ERNPaedCan clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:1520–1536. doi: 10.1016/j.annonc.2021.08.1995. [DOI] [PubMed] [Google Scholar]
- 4.Potter BK, Adams SC, Pitcher JD, et al. Proximal humerus reconstructions for tumors. Clin Orthop Relat Res. 2009;467:1035–1041. doi: 10.1007/s11999-008-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balke M, Ahrens H, Streitbürger A, et al. Treatment of bone and soft tissue sarcomas. Recent Results Cancer Res. 2009 doi: 10.1007/978-3-540-77960-5_4. [DOI] [Google Scholar]
- 6.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. Am J Clin Oncol. 2015;33:3029–3035. doi: 10.1200/jco.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobusch GM, Bollmann J, Puchner SE, et al. What sport activity levels are achieved in patients after resection and endoprosthetic reconstruction for a proximal femur bone sarcoma? Clin Orthop Relat Res. 2017;475:817–826. doi: 10.1007/s11999-016-4790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajasekaran RB, Ashford R, Stevenson JD, et al. Reconstruction after resection of a proximal humeral tumour: what challenges remain? Bone Joint J. 2022;104-B:3–5. doi: 10.1302/0301-620x.104b1.bjj-2021-1410. [DOI] [PubMed] [Google Scholar]
- 9.Schneider KN, Bröking JN, Gosheger G, et al. What is the implant survivorship and functional outcome after total humeral replacement in patients with primary bone tumors? Clin Orthop Relat Res. 2021;479:1754–1764. doi: 10.1097/corr.0000000000001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theil C, Schwarze J, Gosheger G, et al. Implant survival, clinical outcome and complications of megaprosthetic reconstructions following sarcoma resection. Cancers. 2022;14:351. doi: 10.3390/cancers14020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobusch GM, Keusch F, Tsuchiya H, et al. What opinions do tumor reconstructive surgeons have about sports activity after megaprosthetic replacement in hip and knee? Results of the MoReSports expert opinion online survey. J Clin Med. 2020;9:3638. doi: 10.3390/jcm9113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlauto HR, Wickman JR, Lazarides AL, et al. Reverse total shoulder arthroplasty for oncologic reconstruction of the proximal humerus: a systematic review. J Shoulder Elbow Surg. 2021;30:e647–e658. doi: 10.1016/j.jse.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Qin H, Cao H, Zhao Y, et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials. 2014;35:9114–9125. doi: 10.1016/j.biomaterials.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Gosheger G, Hillmann A, Lindner N, et al. Soft tissue reconstruction of megaprostheses using a trevira tube. Clin Orthop Relat Res. 2001;393:264–271. doi: 10.1097/00003086-200112000-00030. [DOI] [PubMed] [Google Scholar]
- 15.von HardesEiff JC, Streitbuerger A, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 16.Trost C, Hofer C, Stamm T, et al. Cross-cultural adaption, translation and validation of the Toronto extremity salvage score (TESS) for patients in German-speaking countries. Wien Klin Wochenschr. 2021;133:536–542. doi: 10.1007/s00508-021-01865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. doi: 10.1097/00003086-199301000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the constant score. J Shoulder Elbow Surg. 2007;16:717–721. doi: 10.1016/j.jse.2007.02.123. [DOI] [PubMed] [Google Scholar]
- 19.Gradl G, Postl LK, Lenze U, et al. Long-term functional outcome and quality of life following rotationplasty for treatment of malignant tumors. BMC Musculoskelet Disord. 2015;16:262. doi: 10.1186/s12891-015-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:42–49. doi: 10.1097/00003086-198509000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29:377–388. doi: 10.1177/03635465010290032301. [DOI] [PubMed] [Google Scholar]
- 22.Mont MA, Marker DR, Smith JM, et al. Resurfacing is comparable to total hip arthroplasty at short-term followup. Clin Orthop Relat Res. 2009;467:66–71. doi: 10.1007/s11999-008-0465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang NW, Kasparek MF, Synak L, et al. What sports activity levels are achieved in long-term survivors with modular endoprosthetic humerus reconstruction following primary bone sarcoma resection? Wien Klin Wochenschr. 2021;133:14–20. doi: 10.1007/s00508-020-01779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson ER, Groundland JS, Pala E, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/jbjs.j.00834. [DOI] [PubMed] [Google Scholar]
- 25.Guven MF, Aslan L, Botanlioglu H, et al. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humerus tumors. J Shoulder Elbow Surg. 2016;25:e1–e6. doi: 10.1016/j.jse.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Kumar D, Grimer RJ, Abudu A, et al. Endoprosthetic replacement of the proximal humerus. Long-term results. J Bone Joint Surg Br. 2003;85:717–722. doi: 10.1302/0301-620X.85B5.13838. [DOI] [PubMed] [Google Scholar]
- 27.Abdeen A, Hoang BH, Athanasian EA, et al. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91:2406–2415. doi: 10.2106/jbjs.h.00815. [DOI] [PubMed] [Google Scholar]
- 28.Böhler C, Brönimann S, Kaider A, et al. Surgical and functional outcome after endoprosthetic reconstruction in patients with osteosarcoma of the humerus. Sci Rep. 2018;8:16148. doi: 10.1038/s41598-018-34397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houdek MT, Bukowski BR, Athey AG, et al. Comparison of reconstructive techniques following oncologic intraarticular resection of proximal humerus. J Surg Oncol. 2021;123:133–140. doi: 10.1002/jso.26271. [DOI] [PubMed] [Google Scholar]
- 30.Cladière-Nassif V, Bourdet C, Audard V, et al. Is it safe to preserve the deltoid when resecting the proximal humerus for a primary malignant bone tumour?: a comparative study. Bone Joint J. 2017;99-B:1244–1249. doi: 10.1302/0301-620x.99b9.2016-1317.r1. [DOI] [PubMed] [Google Scholar]
- 31.Wuisman P, Dethloff M, Grünert J, et al. Die Anwendung von massiven Allografts als Rekonstruktionsverfahren nach Resektion von Knochentumoren im Bereich der Extremitäten. Z Orthop. 2008;133:166–175. doi: 10.1055/s-2008-1039432. [DOI] [PubMed] [Google Scholar]
- 32.Grosel TW, Plummer DR, Everhart JS, et al. Reverse total shoulder arthroplasty provides stability and better function than hemiarthroplasty following resection of proximal humerus tumors. J Shoulder Elbow Surg. 2019;28:2147–2152. doi: 10.1016/j.jse.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Trovarelli G, Cappellari A, Angelini A, et al. What is the survival and function of modular reverse total shoulder prostheses in patients undergoing tumor resections in whom an innervated deltoid muscle can be preserved? Clin Orthop Relat Res. 2019;477:2495–2507. doi: 10.1097/corr.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Video S1. An initially 18-year-old female patient taking part in a professional dancing competition 5 years after PHR following resection of an osteosarcoma
Additional file 2: Video S2. An initially 53-year-old male patient playing golf 20 years after PHR following resection of an Ewing sarcoma
Additional file 3: Video S3. An initially 27-year-old male patient kayaking 10 years after PHR following resection of a chondrosarcoma
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.