Abstract
Oocyte quality is the limiting factor in female fertility. It is well known that maternal nutrition plays a role in reproductive function, and manipulating nutrition to improve fertility in livestock has been common practice in the past, particularly with respect to negative energy balance in cattle. A deficiency in nicotinamide adenine dinucleotide (NAD+) production has been associated with increased incidences of miscarriage and congenital defects in humans and mice, while elevating NAD+ through dietary supplements in aged subjects improved oocyte quality and embryo development. NAD+ is consumed by Sirtuins and poly-ADP-ribose polymerases (PARPs) within the cell and thus need constant replenishment in order to maintain various cellular functions. Sirtuins and PARPs play important roles in oocyte maturation and embryo development, and their activation may prove beneficial to in vitro embryo production and livestock breeding programs. This review examines the roles of NAD+, Sirtuins and PARPs in aspects of fertility, providing insights into the potential use of NAD+-elevating treatments in livestock breeding and embryo production programs.
Keywords: Fertility, Nicotinamide adenine dinucleotide (NAD+), Oocyte quality, poly-ADP-ribose polymerases (PARPs), Sirtuins
Introduction
Acquisition of oocyte developmental competence is essential to female fertility, with the follicular environment responsible for supporting the maturing oocyte. Oocyte developmental competence, or oocyte quality, refers to the ability of an oocyte to form an embryo that can develop to term. The link between oocyte quality and embryo viability has been shown in numerous species including sheep [1, 2], humans [3], cattle [4] and mice [5]. A poor follicular environment, such as that in dairy cows experiencing negative energy balance [6], leads to decreased oocyte developmental competence, which can prove detrimental in breeding programs across all livestock species.
Maternal nutrition has been shown to alter the follicular environment, impacting oocyte quality and subsequent embryo development in cattle [7, 8] and sheep [9, 10]. It is common for livestock to receive nutritional supplements to enhance their reproductive performance, particularly due to the increased time and cost associated with breeding or inseminating herds, pregnancy complications and recurrent pregnancy loss. Common ingredients in livestock nutritional supplements include fatty acids, amino acids, vitamins and minerals, with each supplement exerting different effects on reproductive function [11,12,13,14,15,16,17,18]. Vitamins in particular have been found to have beneficial effects on processes fundamental to fertility [19,20,21,22,23,24,25,26] with recent research indicating that maternal deficiency in nicotinamide adenine dinucleotide (NAD+) production is linked with an increase in spontaneous abortion and congenital defects in mice and women [27]. The study by Shi et al. [27] uncovered genetic mutations in genes encoding NAD+ biosynthesis in women that gave birth to babies with congenital defects. Mutant knockout (KO) mice fed a niacin free diet and displaying a similar disruption in NAD+ biosynthesis also exhibited higher rates of miscarriage and congenital malformations in surviving embryos, in which these effects were mitigated by supplementing maternal diets with niacin [27]. This finding highlights the importance of NAD+-elevating agents, such as niacin, to oocyte maturation and as a result, has stimulated research to improve female fertility in a range of species [28,29,30,31,32,33,34].
Sirtuins (SIRTs) and poly-ADP-ribose polymerases (PARPs), which are NAD+-dependent enzymes, have been implicated in cellular processes that are critical to fertility and reproductive function [16, 29, 30, 35,36,37,38,39]. As such, the activation of SIRTs and PARPs using NAD+-elevating agents may prove beneficial in livestock breeding programs and in the refinement of in vitro embryo production programs in which oocytes typically have a lower developmental competence than their in vivo counterparts [40]. This review will discuss the production of NAD+ in cells, its role in the activation of SIRTs and PARPs and their subsequent effects on fertility with a focus on livestock, drawing on studies in mice and humans where studies in livestock are absent.
Nicotinamide Adenine Dinucleotide (NAD+)
NAD+ is a key cellular metabolite derived from dietary niacin (vitamin B3) [41], which plays an important role in redox metabolism in the mitochondria [42]. NAD+ can appear in one of two forms: its oxidised form, NAD+, or its reduced form, NADH. In its oxidised form, NAD has the ability to accept a hydride ion, and thus is reduced, forming NADH. NAD+ can be synthesised through two means: through catalytic conversion of niacin [42] or synthesised from the amino acid tryptophan (Trp) [43,44,45,46]. Although NAD+ is not consumed in redox metabolism, it is consumed in ADP-ribose reactions, acting as a substrate for cyclic-ADP ribose (cADPr) and nicotinic acid adenine dinucleotide (NaAD) phosphate synthesis [47, 48]. These reactions in the cell are important in mediating DNA repair and controlling cellular aging [49]. NAD+ can also be generated through transfer of hydrogen from NADH to NADP+ via the enzyme nucleotide transhydrogenase (NNT) [50] in mitochondria with impaired function, thereby increasing the NAD+ pools in compromised cells. Therefore, there are numerous mechanisms and pathways in place to help preserve the pools of NAD+ in cells, particularly when major pathways are compromised, highlighting its importance in energy production and control over cellular aging. Nevertheless, the majority of cellular NAD+ in mammals is still currently sourced from dietary niacin [51,52,53].
NAD has a very short half-life of approximately 1–2 hours in cells [54]. Therefore, rapid cellular replenishment of NAD is required in order to maintain sufficient cellular pools. Reactions resulting in the consumption of NAD+ necessitate NAD+ resynthesis via one of three biochemical pathways that utilise molecules containing a pyridine base [49, 51]. The salvage and Preiss-Handler pathways involve the absorption and digestion of dietary niacin, while de novo synthesis generates NAD+ by enzymatic conversion of the amino acid tryptophan (Fig. 1). The Preiss-Handler and salvage pathways are more commonly utilised for the generation of NAD+, due to the large amount of tryptophan required to obtain NAD+; compared with niacin, 60 times more tryptophan is needed to yield the equivalent amount of NAD+ [55]. Dietary nicotinic acid (NA) is shuttled through the Preiss-Handler pathway and converted to NAD+, while nicotinamide (NAM) is shuttled into the salvage pathway (Fig. 1). Due to the inefficient generation of NAD+ from tryptophan, the use of tryptophan supplementation alone is not sufficient to maintain adequate cellular pools of NAD+ in mammals [56]. Consequently, under conditions where NAD+ pools are at risk of depletion, reliance on tryptophan for the production of NAD+ is not feasible. While the salvage pathway generally functions as a separate pathway to the Preiss-Handler pathway for NAD+ synthesis, the two have previously been shown to overlap. Nicotinamidase, an enzyme present in gut bacteria, can convert NAM to NA in the intestinal lumen before being absorbed through the intestinal wall [57]. This may represent a crucial finding whereby NAD+ homeostasis in cells is maintained, and at concentrations where NAM is inhibitory to other vital cellular proteins that control cellular aging and energy production, it can be converted into another form to be recycled and reused, effectively preserving the cellular pools of NAD+.
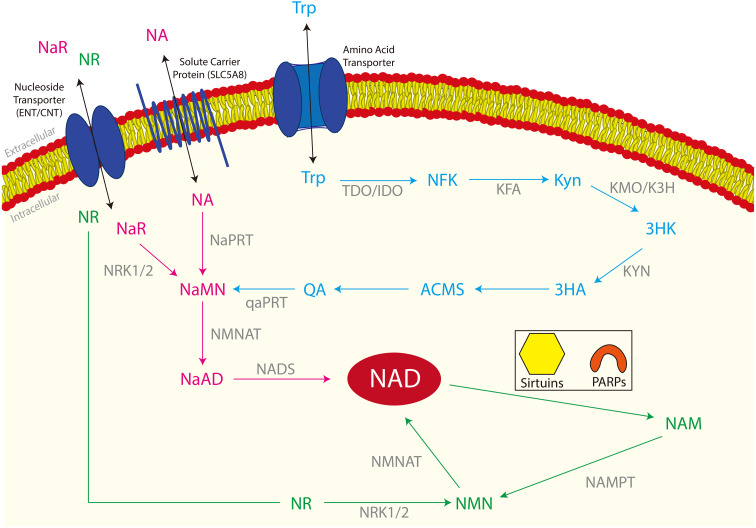
Fig. 1.
The three NAD+ biosynthetic pathways in cells. The De novo synthesis pathway is shown in blue, the Preiss-Handler pathway is shown in pink and the Salvage pathway is shown in green. All enzymes involved in the three pathways are shown in grey. De novo synthesis: Tryptophan (Trp) enters the cell through amino acid transporter proteins where Trp is converted to N-formylkynurenine (NFK). NFK is then converted to Kynurenine (Kyn) then to 3-hydroxykynurenine (3HK). 3HK is converted to 3-hydroxyanthranilate (3HA) and alanine (Ala), then to α-amino-β-carboxymuconate-ε-semialdehyde (ACMS) before yielding quinolinic acid/quinolate (QA). QA is then converted to nicotinic acid mononucleotide (NaMN), the second metabolite of the Preiss-Handler pathway. The Preiss-Handler pathway: Nicotinic acid riboside (NaR) and nicotinic acid (NA) enter the cell through transporters. NaR is converted to NA within the cell. NA is then converted to nicotinic acid mononucleotide (NaMN), then to nicotinic acid adenine dinucleotide (NaAD) before catalysis to nicotinamide adenine dinucleotide (NAD+). NAD+ is then consumed by Sirtuins and poly-ADP-ribose polymerases (PARPs) and nicotinamide (NAM) is released. The salvage pathway: Nicotinamide riboside (NR) enters the cell through nucleoside transporter proteins where it is converted to nicotinamide mononucleotide (NMN). NMN is then converted to nicotinamide adenine dinucleotide (NAD+) which is consumed by Sirtuins and poly-ADP-ribose polymerases (PARPs), releasing nicotinamide (NAM). NAM then re-enters the salvage pathway, being converted to NMN. Enzymes: tryptophan-2,3-dioxygenase (TDO); indoleamine-2,3-dioxygenase (IDO); N-formylkynurenine formamidase (KFA); kynurenine 3-monoxygenase (KMO); kynurenine 3-hydroxylase (K3H); kynurenase (KYN); quinolate phosphoribosyltransferase (qaPRT); NRK1/2 – nicotinamide riboside kinase 1/2; NaPRT – nicotinic acid phosphoribosyltransferase; NMNAT – nicotinamide mononucleotide adenylyltransferase; NADS – nicotinamide adenine dinucleotide synthase; NAMPT – nicotinamide phosphoribosyltransferase.
Roles of NAD+ in the Cell
The role of NAD+ as an electron acceptor has been recognised since the 1960s, when it was observed to facilitate electron transfer through the mitochondrial electron transport chain [58]. However, NAD+ has more recently been implicated in various other biological processes including, but not limited to, inflammation, stress responses and aging [59,60,61]. In its oxidised form, NAD+ accepts electrons from various cellular electron donors, shuttling them through the electron transport chain, to generate energy for use in anabolic reactions. NAD+ also functions as a substrate for three different families of enzymes; cyclic adenosine-diphosphate-ribose (cADPr) synthases, adenosine-diphosphate-ribosyl transferases (also known as poly-ADP-ribose polymerases, or PARPs) and NAD+-dependent deacetylases (Sirtuins) [47]. The consumption of NAD+ by PARPs, cADPR synthases and Sirtuins results in the activation of these proteins and enzymes, subsequently requiring the regeneration of NAD+ to preserve the cellular pools needed for other cell functions.
Poly-ADP-ribose polymerases (PARPs) are a sub-family of the ADP-ribosyltransferase family of proteins that consume NAD within the cell [62], producing NAM and an ADP-ribose moiety [63, 64]. ADP-ribosylation is a post-translational modification whereby PARPs catalyse the transfer of ADP-ribose from NAM to lysine residues, or the carboxyl group of glutamine and asparagine on target proteins [64, 65]. There are currently seventeen known ADP-ribosyltransferase proteins, six of these are known to possess poly-ADP-ribosylation activity, including PARP1–4, PARP5a and PARP5b [64], while a further 7 PARP proteins possess mono-ADP-ribosylation activity, including PARP3, PARP4, PARP6, PARP10 and PARPs14–16 [66]. Each PARP protein contains its own distinct structural domain and cellular localisation, giving rise to specific activities and functions within the cell [64, 66, 67].
PARPs have long been implicated in cell division, transcriptional regulation, DNA repair, mitochondrial function and stress responses [64, 68,69,70]. PARPs 1–3 have been involved in DNA repair and DNA damage signalling, modulation of chromatin structure and the cell cycle checkpoint, and induction of apoptosis [71]. Recently, PARP1 has also been implicated in regulating ribosomal synthesis and mRNA processing, translation and stability [72]. While PARPs catalyse the addition of ADP-ribose to multiple target proteins, they also supply NMNAT enzymes [73] which are enzymes involved in the synthesis of NAD+. There are three NMNAT enzymes (NMNAT 1–3), with each localised to a different site within the cell. NMNAT1 is localised to the nucleus, while NMNAT2 and NMNAT3 are localised to the cytoplasm and golgi, and the mitochondria, respectively [74]. As a result, PARP proteins function to maintain genome integrity as well as maintain the cellular pools of NAD.
Cyclic adenosine-diphosphate-ribose is a cyclical form of ADP-ribose which is produced when NAD+ is consumed by PARPs and acts as a second messenger in calcium signalling [75]. It is formed from the catalysis of NAD+ by the actions of cluster of differentiation 38 (CD38), alternatively known as cyclic ADP-ribose hydrolase. Adenosine-diphosphate-ribosyl cyclase is responsible for converting ADP-ribose to its cyclic form, however, the N1-glycosylic linkage is quite unstable, so cADPR readily converts back to ADP-ribose [76]. In sea urchin gametes, cADPR production increases just prior to a rise in calcium release from intracellular stores [77], suggestive of a role in fertilisation.
In addition to its role in the activation of PARPs and cADPr, NAD+ is also consumed by sirtuin proteins. Sirtuins are a family of NAD+-dependent deacetylases, and are often collectively referred to as class III histone deacetylases (HDACs). Not all sirtuin proteins partake in the deacetylation of histones but have other enzyme activities within the cell. There are seven mammalian sirtuin proteins (SIRT1 to SIRT7), with SIRT1 being the first identified in mammals and the most studied in any species to date [78]. It is well known that SIRT1 in mammals is the equivalent of Sir2 in yeast, and both proteins have similar function with regards to longevity and circadian rhythm [79, 80]. Sirtuins are known to increase lifespan in both yeast [81, 82], and in lower organisms such as Caenhorhabditis elegans and Drosophila [80, 83]. In comparison, mammalian SIRT1 has not been linked to extended lifespan, but rather is involved in preventing premature cellular aging in stress and disease states [84]. The Sirtuins have more recently been implicated in energy production and fertility, which will be discussed in further detail. The last decade has seen a substantial increase in the number of studies investigating the roles of SIRTs in various biochemical pathways and their impact on health and disease. Each of the seven Sirtuins have different subcellular location, target molecules and effects. SIRT1, SIRT6 and SIRT7 are localised to the nucleus [85, 86] and their primary role is that of gene transcription regulation. SIRT3–5 are encoded by nuclear genes [87] but are localised to the mitochondria [85, 88] and play roles in energy production. Lastly, SIRT2 is localised to the cytoplasm, where its role is targeted to proteins and protein expression, although nuclear proteins also become targets of SIRT2 during the mitotic phase of the cell cycle [87, 89]. The role of Sirtuins in oocyte maturation and embryo development is gaining increasing attention, but there are limited studies in species other than mice.
Role of Sirtuins in Reproductive Functions
Sirtuins and their roles in promoting DNA repair, maintaining genomic integrity and mitigating cellular aging have been well characterised in Caenorhabditis elegans, yeast and mice. However, there is a paucity of studies investigating the role of Sirtuins in reproductive functions, particularly in livestock species. SIRT1, 2 and 3 are the most studied of the Sirtuin family of proteins from a fertility perspective (reviewed in Tatone et al. [84]; summarised in Table 1). All seven SIRT genes have been detected in mouse ovaries [90] and in mature oocytes at the metaphase II stage of meiosis, with protein levels decreasing following the first embryonic cleavage, suggesting they are stored during oogenesis [91, 92] and play an important role during early embryo development.
Table 1. Summary of the proposed roles of Sirtuins in female mammalian reproductive functions.
| Sirtuin | Proposed Role/Reported Finding | Species | References |
|---|---|---|---|
| SIRT1 | Promotes granulosa cell differentiation | Pig | [4, 33, 35,36,37,38, 93,94,95,96,97,98, 108,109,110] |
| Improves oocyte quality | Pig, Cow, Mouse | ||
| Reduces spindle defects | Pig | ||
| Promotes chromosome compaction | Cow | ||
| Promotes fertilisation | Cow | ||
| Promotes follicle dormancy | Mouse | ||
| Preserves primordial follicle pool | Mouse | ||
| Delays sexual maturity | Mouse | ||
| Promotes foetal development | Mouse | ||
| Promotes mammary gland development | Mouse | ||
| SIRT2 | Germinal vesicle progression | Mouse | [103,104,105,106, 111, 124] |
| Promotes mitochondrial biogenesis in oocytes | Mouse | ||
| Promotes spindle assembly in oocytes | Cow | ||
| Improves chromosome alignment in oocytes | Cow | ||
| Reduces apoptosis | Cow, Pig | ||
| Improves mitochondrial function in oocytes | Cow, Pig | ||
| Reduces ROS production in oocytes | Cow, Pig | ||
| Enhances morula and blastocyst formation | Pig | ||
| Increases total blastocyst cell number | Pig | ||
| SIRT3 | Promotes mitochondrial biogenesis in oocytes | Mouse | [103,104,105,106,107] |
| Reduces apoptosis in oocytes and follicular cells | Cow, Pig | ||
| Improves mitochondrial function in oocytes | Cow, Pig | ||
| Reduces ROS production in oocytes | Cow, Pig | ||
| Regulates hormone production | Human | ||
| SIRT4 | Improves metabolic function in oocytes | Mouse, Cow | [98, 99] |
| Promotes meiotic progression | Mouse, Cow | ||
| SIRT5 | Controls ovarian reserve | Human | [130] |
| SIRT6 | Improves spindle morphology in oocytes | Mouse | [90, 102, 131] |
| Improves chromosome alignment in oocytes | Mouse | ||
| Preserves primordial follicle pool | Mouse | ||
| SIRT7 | Controls ovarian reserve | Mouse | [100, 132] |
| Promotes meiotic progression | Mouse | ||
| Improves spindle formation in oocytes | Mouse | ||
| Improves chromosome alignment in oocytes | Mouse | ||
During in vitro maturation (IVM), the activation of SIRT1 improves oocyte quality and embryo development in mice [37], pigs [6, 35] and cattle [36, 38, 39]. Furthermore, porcine granulosa cells transfected with SIRT1 transcripts exhibit an increased expression of proliferative markers, which suggests that SIRT1 plays a role in terminal differentiation of granulosa cell luteinisation during oocyte maturation [93, 94]. SIRT1 mRNA levels increase during chromosome condensation in bovine germinal vesicle oocytes [95, 96] while activation of SIRT1 ameliorates spindle defects and chromosome misalignment in aged porcine oocytes [97], which suggests that SIRT1 is involved in spindle assembly and chromosome alignment. The improvement in mitochondrial function during IVM of both bovine [98] and murine [99] oocytes as a result of SIRT1 activation suggests the effects on spindle assembly and chromosome alignment is the work of improved mitochondrial bioenergetics. SIRT7 has been localised to chromosomes from the premetaphase I stage in mouse oocytes [100, 101] with SIRT4 localised to the spindle region [101], also suggesting that these SIRTs play important roles in spindle assembly and chromosome alignment, likely through actin polymerisation during meiosis [100]. Inhibition of SIRT2, SIRT3 and SIRT6 impairs spindle organisation and chromosome alignment in bovine, porcine and murine oocytes during maturation [102,103,104], increases reactive oxygen species (ROS) production, mitochondrial dysfunction, and apoptosis in porcine and bovine oocytes [103,104,105], and prevented mitochondrial biogenesis in mice [106]. Furthermore, in addition to its role in mitochondrial function during oocyte maturation, SIRT3 regulates the production of steroidogenic hormones important during follicle growth and oocyte maturation [107]. Collectively, these data provide support for the role of SIRTs in maintaining the structural and functional integrity of the oocyte during its growth and development, though some SIRTs do not appear to be as crucial as others for the maturation of oocytes.
In contrast to the number of studies showing the importance of SIRTs during oocyte growth and development, few studies have documented the effects of SIRTs during embryo development. Inhibition of SIRT1 leads to abnormal fertilisation of bovine oocytes [98], and an increase in postnatal mortality and compromised foetal development in mice [108,109,110]. Inhibition of SIRTs in preimplantation pig embryos cultured in vitro resulted in lower expression of porcine SIRT2 mRNA transcripts, which in turn reduced the incidence of morula and blastocyst formation and the total cell count of blastocysts [111]. Additionally, a decrease in mitochondrial biogenesis resulted in a reduction in embryo quality and an increase in spontaneous abortions which was linked to SIRT3 inhibition [106]. Interestingly, the deletion of Sirt1 in actively growing oocytes had no effect in young female mice, although 50% of females without oocyte Sirt1 displayed signs of premature sterility attributed to a reduction in oocyte developmental competence [112]. In contrast, the activation of SIRT1 led to an increase in embryo implantation in oxidative stress induced mice and an increase in superoxide dismutase activity [113].
It is evident that SIRTs play a very important role in the acquisition of oocyte developmental competence and during subsequent embryo development. However, studies investigating the activation of SIRTs through elevation of NAD+ levels are scarce. Also, the vast majority of reports are in mice, and little is known regarding the requirement of Sirtuins in oocyte growth and development in livestock. Studies examining NAD+ biosynthesis and activation of SIRTs are needed to provide further insights into the acquisition of oocyte quality and the requirements for embryo development, especially in livestock species.
NAD, SIRTs and PARPs in the Oocyte
The beneficial effects of NAD+ on oocyte maturation have been reported in various species. The addition of NAD+ to IVM media in conjunction with other tricarboxylic acid cycle metabolites increased the percentage of denuded bovine oocytes reaching the metaphase II stage of meiosis [114, 115], and in Xenopus laevis oocytes, NAD+ turnover rapidly increased following fertilisation and continued to increase during early embryonic development [116]. This comes as no surprise considering the energy demand of the oocyte is drastically increased upon fertilisation. Furthermore, bovine oocytes exhibited no changes in NAD+ activity during IVM, though NADP enzymatic activity increased dramatically in cumulus cells [115]. Consequently, due to the maintenance of meiotic arrest during oocyte development and therefore a lack of cellular activity in comparison to other cell types, NAD+ activity may remain at minimal levels so that it can accumulate in the oocyte in preparation for fertilisation and embryonic development. The first study to quantify NAD+ in oocytes using mass spectrometry revealed that mouse oocytes contain between 6-10 fmol of NAD+ [117]. In another study, the reported NAD+ content of mouse oocytes was substantially higher; when supplemented with NA, the NAD+ content of oocytes from young and aged mice measured 2.1 and 1.8 pmol per oocyte, respectively, which was also markedly higher than the 0.8 pmol of NAD+ in aged mouse oocytes which had not been supplemented with NA [34]. Bertoldo et al. [29] also showed that the NAD+ content of aged mouse oocytes was much lower than the NAD+ content in oocytes from young mice. These results indicate that NAD+ is present in the oocyte and important for oocyte quality, with NAD+ concentrations decreasing with advancing maternal age.
Effects of NAD-elevating treatments on oocyte quality
Following the report that a deficiency of NAD+ can result in birth defects and spontaneous miscarriage in women and mice [27], studies involving treatments that elevate NAD+ in oocytes has gained increasing attention (summarised in Table 2). The most direct strategy for rapidly increasing the concentration of NAD+ in oocytes is to supplement the maternal diet with NAD+ precursors. Oocytes from larger follicles only show a minimal response to such dietary treatments, while oocytes from smaller follicles and those from aged females appear to show the greatest response.
Table 2. Summary of the effects of various NAD+ metabolite supplements on aspects of female mammalian fertility.
| Supplement | Reported Finding | Species | References |
|---|---|---|---|
| NA in vitro | Enhanced cumulus expansion | Mouse | [31,32,33,34, 118, 119, 126] |
| Granulosa cell proliferation | Mouse | ||
| Elevation of NAD+ in oocytes | Mouse | ||
| Reduction in ROS in oocytes | Mouse, Pig | ||
| Reduced spindle and chromosome defects | Mouse, Pig | ||
| Improved oocyte maturation | Cow | ||
| Enhanced cleavage rates | Cow, Pig | ||
| Enhanced blastocyst formation | Cow, Pig | ||
| Improved glutathione levels | Pig | ||
| Higher blastocyst cell number | Pig | ||
| Enhanced gene expression related to apoptosis, energy metabolism and lipid metabolism in oocytes and cumulus cells | Pig | ||
| NA in vivo | Reduced congenital defects in NAD+ KO | Mouse | [27, 33] |
| Reduced rates of miscarriage | Mouse | ||
| Enhanced cumulus expansion | Mouse | ||
| Granulosa cell proliferation | Mouse | ||
| NMN in vitro | Enhanced spindle assembly | Mouse | [28, 29, 30, 31] |
| Increased cleavage rates | Mouse | ||
| Improved blastocyst formation | Mouse | ||
| Higher blastocyst cell number | Mouse | ||
| Increased NAD+ levels in oocytes | Mouse | ||
| No difference in oocyte maturation or embryo development | Pig | ||
| NMN in vivo | Increased number of preantral follicles | Mouse | [29, 30] |
| Enhanced embryo development | Mouse | ||
| Enhanced spindle assembly | Mouse | ||
| Increased live birth rates | Mouse | ||
| NaR in vitro | Improved blastocyst formation | Mouse | [29] |
| NR in vitro | Improved blastocyst formation | Mouse | [29] |
| NaMN in vitro | Improved blastocyst formation | Mouse | [29] |
| NAM in vitro | Delayed GVBD | Mouse | [31, 92, 104, 124, 125] |
| Reduced oocyte fragmentation | Mouse | ||
| Improved microtubule structure | Mouse | ||
| Reduced spindle elongation | Mouse | ||
| Reduced cumulus expansion at high doses | Pig | ||
| Increased spindle defects at high doses | Pig | ||
| Reduced blastocyst formation at high doses | Pig | ||
| Lower blastocyst cell number at high doses | Pig | ||
| Increased embryo cleavage at low doses | Pig | ||
| Higher blastocyst cell number at low doses | Pig | ||
NA supplementation has been found to significantly increase cumulus expansion and granulosa cell proliferation during in vitro follicle culture [33], and supplementing bovine oocytes and embryos with NA during IVM and IVF improved the rates of oocyte maturation through polar body extrusion, embryo cleavage, and blastocyst formation [118, 119]. Furthermore, porcine oocytes supplemented with NA in vitro had greater blastocyst formation rates and an increased blastocyst cell number compared with unsupplemented oocytes [31]. Even at doses of NA that were too low to influence oocyte maturation rates in mice, the production of ROS, and chromosome and spindle defects were markedly diminished [34], which further supports the notion that NAD+ sequesters in the oocyte during maturation in preparation for fertilisation and embryonic development.
Nicotinamide mononucleotide (NMN) is the final metabolite in the salvage pathway before conversion to NAD+. When mice are fed a high fat diet, there is a reduction in the transcript levels of Gdf9, an oocyte secreted factor implicated in the acquisition of oocyte developmental competence [120]. In these mice, dietary supplementation with NMN increased the number of large preantral follicles [28], and in aged mice, supplementation of NMN both in vivo and in vitro restored spindle assembly, increased cleavage and blastocyst formation rates and blastocyst cell number, and also increased live birth rates [29]. Furthermore, when NMN was microinjected into mouse oocytes it restored spindle assembly and rescued asymmetrical division during meiosis [121]. An increase in fertilisation rates during IVF in addition to blastocyst formation rates may also be achieved via intraperitoneal administration of NMN to aged mice [30]. On the other hand, our studies have shown that supplementing oocyte maturation media with NMN did not improve oocyte maturation or embryo development in the pig [31]. These species differences may result from variations in the type of energy substrate utilised during oocyte maturation; glucose is more readily utilised by pig oocytes in vitro while mice rely more heavily on pyruvate [122, 123]. Alternatively, NMN may not be a potent mediator of oocyte quality and embryo development in the pig as in the mouse. As such, further studies are required to determine whether NMN is a viable supplement for improving oocyte quality and embryo development in other species.
In one in vitro study, the salvage pathway was inhibited in order to elucidate the action of NMN. Supplementation with alternative NAD+ metabolites (nicotinamide riboside; NR, nicotinic acid riboside; NaR and nicotinic acid mononucleotide; NaMN) in salvage pathway-inhibited aged mouse oocytes rescued blastocyst formation rates [29]. This finding indicates that metabolites in the Preiss-Handler pathway and those downstream of the inhibitor were able to rescue aspects of fertility in mice. It should be noted that this is the only study to date to examine the effects of NR, NaR and NaMN on oocyte quality in any species, so follow up studies are required to confirm these results.
While the beneficial effects of NA, NMN, NR, NaR and NaMN on oocyte quality are unarguable, the use of NAM to improve oocyte quality has returned inconsistent results. A delay in germinal vesicle breakdown (GVBD) was observed when mouse oocytes were supplemented with NAM [124], while aged mouse oocytes exhibited a reduction in the rate of oocyte fragmentation, a decrease in the incidence of abnormal microtubule structures and a reduction in spindle elongation in response to NAM treatment [125]. Additionally, porcine oocytes treated with low concentrations of NAM in vitro had higher cleavage rates following artificial activation [31] while bovine oocytes treated with low concentrations of NAM during IVM exhibited higher cleavage rates, blastocyst hatching rates and higher blastocyst total cell numbers following IVF [126]. Conversely, porcine oocytes treated with high concentrations of NAM exhibited an inhibition of cumulus expansion and an increased incidence of spindle defects compared with porcine oocytes that were not treated with NAM [105]. Porcine embryos cultured with higher concentrations of NAM attained the blastocyst stage at a lower rate and formed blastocysts with fewer cells compared with untreated embryos [92]. The apparent detrimental effects on porcine oocyte quality may stem from the higher concentration of NAM utilised in these studies. It has previously been shown that NAM acts as a non-competitive SIRT inhibitor at higher concentrations [105, 124], which could explain the reduction in oocyte quality observed in some studies. Fertility studies investigating the effects of the metabolite NaAD are yet to be undertaken in any species, yet is regarded as the most sensitive biomarker of NAD+ metabolism [127].
Inhibition of NAD+ biosynthesis on the function of SIRTs and PARPs in oocytes and embryos
While studies on the effects of SIRTs and PARPs in oocytes and embryos are increasing, few studies have implemented the use of NAD+ metabolites to increase the cellular pools of NAD+ and measure downstream effects on SIRTS and PARPs. Primarily, studies use specific inhibitors to knock down protein levels and gene expression to elucidate their effects on cell function rather than promoting their function through increased NAD+ concentrations. The effect of NAM utilisation as a non-competitive Sirtuin inhibitor is the most documented of all NAD+ metabolites. Here we discuss the findings of studies in which NAD+ synthesis was inhibited to assess the effects on SIRTs and PARPs in the oocyte and developing embryo.
Supplementing NAM at high concentrations in mouse oocytes during IVM caused delays in GVBD in a manner reflective of SIRT2 inhibition [124], suggesting that NAM at high concentrations inhibits SIRT2. Furthermore, high concentrations of NAM (5 mM) during IVM of porcine oocytes resulted in a significant reduction in polar body extrusion rates, a disruption in actin cap formation, abnormal cortical granule distribution and impaired spindle assembly [105] compared with control oocytes, indicating that SIRTs are vital during oocyte maturation. The use of NAM during in vitro embryo culture of porcine embryos at inhibitory concentrations significantly reduced the levels of Sirt3 mRNA compared with untreated oocytes, while Sirt1 and Sirt2 mRNA levels tended to be lower, leading to reduced blastocyst formation rates [92]. On the other hand, bovine embryos exhibited an increase in SIRT1 levels when oocytes were treated with low concentrations of NAM during IVM [126]. Recent studies have shown that NAD+ and ATP levels are increased, and DNA damage and ROS are reduced, when aged mice are supplemented with NMN either through their drinking water [29] or via intraperitoneal injection [30]. These findings suggest that the elevation of NAD+ through NMN supplementation restores Sirtuin function.
Similar to the investigation of SIRTs in oocyte maturation and embryo development, most studies rely on the inhibition of PARPs to demonstrate their effects. Inhibition of PARP1 during porcine IVM reduced cumulus expansion, embryo development and total blastocyst cell number, and increased apoptosis [111]. In mouse embryos, PARP inhibition induced developmental arrest and adversely affected spindle formation and tubulin polymerisation [128]. ADP-ribose polymers, which are formed via catalysis of NAD+ by PARPs and subsequent release of ADP-ribose and NAM [73], were found to be localised to the chromatin of oocytes during chromatin remodelling throughout meiosis and specifically in anaphase chromosomes in the zygote [129]. In addition, inhibition of PARPs during mouse in vitro embryo culture accelerated pronuclear formation, increased embryo fragmentation, and inhibited blastocyst formation [129], likely due to the inability of ADP-ribose polymers to localise to the chromosomes during embryo development.
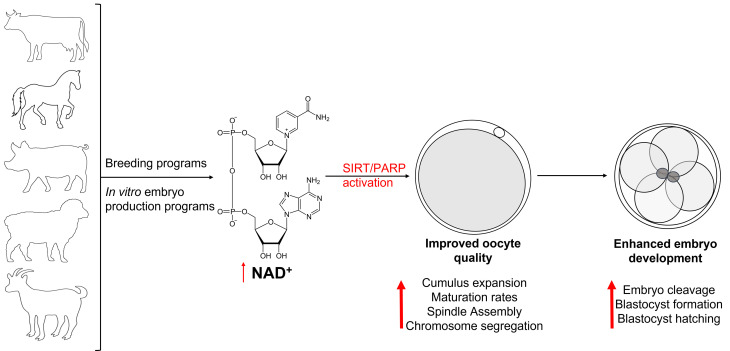
It has becoming increasingly clear that SIRTs and PARPs play essential roles during oocyte maturation and embryo development in a range of species. However, evidence for the direct effects of treatment with NAD+ precursors on the activation of these proteins and their expression throughout oocyte maturation and embryo development remains scarce, particularly during states of stress and in aging. Further studies investigating the effects of boosting NAD+ synthesis in oocytes and embryos both in vitro and in vivo, and the effect on activation of SIRTs and PARPs is warranted to provide stronger evidence of the beneficial effects on oocyte quality and embryo development in all species (Fig. 2).
Fig. 2.
Proposed beneficial effects of elevating NAD+ in breeding and embryo production programs.
Conclusions
In this review we have discussed the production of NAD+ within the cell and its role in activating SIRT and PARP proteins. Studies have demonstrated potential links between the activation of SIRTs on improvements in various reproductive parameters in a number of species, although indirectly. Elevating cellular levels of NAD+ through supplementation with NA, NAM, NMN and NaMN has been found to improve cumulus cell function and mitochondrial function, thereby improving oocyte quality and embryo development. While studies investigating the effects of NAD+ precursor supplementation on SIRT and PARP activation in the maturing oocyte and embryo are scarce, these treatments have enormous potential for improving female reproductive efficiency in all species, and therefore warrant further investigation.
Conflict of interests
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to thank the Australian Research Council for supplying the funding to complete this research (LP160100824).
References
- 1.Papadopoulos S, Lonergan P, Gath V, Quinn KM, Evans AC, O’Callaghan D, Bolan MP. Effect of diet quantity and urea supplementation on oocyte and embryo quality in sheep. Theriogenology 2001; 55: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 2.Grazul-Bilska AT, Vonnahme KA, Bilski JJ, Borowczyk E, Soni D, Mikkelson B, Johnson ML, Reynolds LP, Redmer DA, Caton JS. Expression of gap junctional connexin proteins in ovine fetal ovaries: effects of maternal diet. Domest Anim Endocrinol 2011; 41: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigala J, Sifer C, Dewailly D, Robin G, Bruyneel A, Ramdane N, Lefebvre-Khalil V, Mitchell V, Decanter C. Is polycystic ovarian morphology related to a poor oocyte quality after controlled ovarian hyperstimulation for intracytoplasmic sperm injection? Results from a prospective, comparative study. Fertil Steril 2015; 103: 112–118. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Wang J, Zhang Z, Yi J, He C, Wang F, Tian X, Yang M, Song Y, He P, Liu G. Resveratrol compares with melatonin in improving in vitro porcine oocyte maturation under heat stress. J Anim Sci Biotechnol 2016; 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoldo MJ, Caldwell ASL, Riepsamen AH, Lin D, Gonzalez MB, Robker RL, Ledger WL, Gilchrist RB, Handelsman DJ, Walters KA. A hyperandrogenic environment causes intrinsic defects that are detrimental to follicular dynamics in a PCOS mouse model. Endocrinology 2019; 160: 699–715. [DOI] [PubMed] [Google Scholar]
- 6.De Bie J, Langbeen A, Verlaet AAJ, Florizoone F, Immig I, Hermans N, Fransen E, Bols PEJ, Leroy JLMR. The effect of a negative energy balance status on β-carotene availability in serum and follicular fluid of nonlactating dairy cows. J Dairy Sci 2016; 99: 5808–5819. [DOI] [PubMed] [Google Scholar]
- 7.Sinclair KD, Kuran M, Gebbie FE, Webb R, McEvoy TG. Nitrogen metabolism and fertility in cattle: II. Development of oocytes recovered from heifers offered diets differing in their rate of nitrogen release in the rumen. J Anim Sci 2000; 78: 2670–2680. [DOI] [PubMed] [Google Scholar]
- 8.Mattos R, Staples CR, Arteche A, Wiltbank MC, Diaz FJ, Jenkins TC, Thatcher WW. The effects of feeding fish oil on uterine secretion of PGF2alpha, milk composition, and metabolic status of periparturient Holstein cows. J Dairy Sci 2004; 87: 921–932. [DOI] [PubMed] [Google Scholar]
- 9.Fahey J, Boland M, O’Callaghan D. The effects of dietary urea on embryo development in superovulated donor ewes and on early embryo survival and development in recipient ewes. Anim Sci 2001; 72: 395–400. [Google Scholar]
- 10.Rhoads ML, Rhoads RP, Gilbert RO, Toole R, Butler WR. Detrimental effects of high plasma urea nitrogen levels on viability of embryos from lactating dairy cows. Anim Reprod Sci 2006; 91: 1–10. [DOI] [PubMed] [Google Scholar]
- 11.Anbari K, Schultz RM. Effect of sodium and betaine in culture media on development and relative rates of protein synthesis in preimplantation mouse embryos in vitro. Mol Reprod Dev 1993; 35: 24–28. [DOI] [PubMed] [Google Scholar]
- 12.Biggers JD, Lawitts JA, Lechene CP. The protective action of betaine on the deleterious effects of NaCl on preimplantation mouse embryos in vitro. Mol Reprod Dev 1993; 34: 380–390. [DOI] [PubMed] [Google Scholar]
- 13.Mattos R, Staples CR, Thatcher WW. Effects of dietary fatty acids on reproduction in ruminants. Rev Reprod 2000; 5: 38–45. [DOI] [PubMed] [Google Scholar]
- 14.Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 2007; 77: 190–201. [DOI] [PubMed] [Google Scholar]
- 15.Santos JE, Bilby TR, Thatcher WW, Staples CR, Silvestre FT. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reprod Domest Anim 2008; 43(Suppl 2): 23–30. [DOI] [PubMed] [Google Scholar]
- 16.Thatcher W, Santos JE, Staples CR. Dietary manipulations to improve embryonic survival in cattle. Theriogenology 2011; 76: 1619–1631. [DOI] [PubMed] [Google Scholar]
- 17.Waters SM, Coyne GS, Kenny DA, MacHugh DE, Morris DG. Dietary n-3 polyunsaturated fatty acid supplementation alters the expression of genes involved in the control of fertility in the bovine uterine endometrium. Physiol Genomics 2012; 44: 878–888. [DOI] [PubMed] [Google Scholar]
- 18.Xiong X, Lan D, Li J, Lin Y, Li M. Selenium supplementation during in vitro maturation enhances meiosis and developmental capacity of yak oocytes. Anim Sci J 2018; 89: 298–306. [DOI] [PubMed] [Google Scholar]
- 19.Eberhardt DM, Will WA, Godkin JD. Retinol administration to superovulated ewes improves in vitro embryonic viability. Biol Reprod 1999; 60: 1483–1487. [DOI] [PubMed] [Google Scholar]
- 20.Hübner U, Alwan A, Jouma M, Tabbaa M, Schorr H, Herrmann W. Low serum vitamin B12 is associated with recurrent pregnancy loss in Syrian women. Clin Chem Lab Med 2008; 46: 1265–1269. [DOI] [PubMed] [Google Scholar]
- 21.Kwong WY, Adamiak SJ, Gwynn A, Singh R, Sinclair KD. Endogenous folates and single-carbon metabolism in the ovarian follicle, oocyte and pre-implantation embryo. Reproduction 2010; 139: 705–715. [DOI] [PubMed] [Google Scholar]
- 22.Muthayya S, Dwarkanath P, Mhaskar M, Mhaskar R, Thomas A, Duggan C, Fawzi WW, Bhat S, Vaz M, Kurpad A. The relationship of neonatal serum vitamin B12 status with birth weight. Asia Pac J Clin Nutr 2006; 15: 538–543. [PubMed] [Google Scholar]
- 23.Muthayya S, Kurpad AV, Duggan CP, Bosch RJ, Dwarkanath P, Mhaskar A, Mhaskar R, Thomas A, Vaz M, Bhat S, Fawzi WW. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr 2006; 60: 791–801. [DOI] [PubMed] [Google Scholar]
- 24.O’Neill C. Endogenous folic acid is essential for normal development of preimplantation embryos. Hum Reprod 1998; 13: 1312–1316. [DOI] [PubMed] [Google Scholar]
- 25.Ratan SK, Rattan KN, Pandey RM, Singhal S, Kharab S, Bala M, Singh V, Jhanwar A. Evaluation of the levels of folate, vitamin B12, homocysteine and fluoride in the parents and the affected neonates with neural tube defect and their matched controls. Pediatr Surg Int 2008; 24: 803–808. [DOI] [PubMed] [Google Scholar]
- 26.Reznikoff-Etiévant MF, Zittoun J, Vaylet C, Pernet P, Milliez J. Low Vitamin B(12) level as a risk factor for very early recurrent abortion. Eur J Obstet Gynecol Reprod Biol 2002; 104: 156–159. [DOI] [PubMed] [Google Scholar]
- 27.Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, Lim CK, Szot JO, Ip E, Hughes JN, Sugimoto K, Humphreys DT, McInerney-Leo AM, Leo PJ, Maghzal GJ, Halliday J, Smith J, Colley A, Mark PR, Collins F, Sillence DO, Winlaw DS, Ho JWK, Guillemin GJ, Brown MA, Kikuchi K, Thomas PQ, Stocker R, Giannoulatou E, Chapman G, Duncan EL, Sparrow DB, Dunwoodie SL. NAD deficiency, congenital malformations, and niacin supplementation. N Engl J Med 2017; 377: 544–552. [DOI] [PubMed] [Google Scholar]
- 28.Bertoldo MJ, Uddin GM, Youngson NA, Agapiou D, Walters KA, Sinclair DA, Morris MJ, Gilchrist RB. Multigenerational obesity-induced perturbations in oocyte-secreted factor signalling can be ameliorated by exercise and nicotinamide mononucleotide. Hum Reprod Open 2018; 2018: hoy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertoldo MJ, Listijono DR, Ho WJ, Riepsamen AH, Goss DM, Richani D, Jin XL, Mahbub S, Campbell JM, Habibalahi A, Loh WN, Youngson NA, Maniam J, Wong ASA, Selesniemi K, Bustamante S, Li C, Zhao Y, Marinova MB, Kim L-J, Lau L, Wu RM, Mikolaizak AS, Araki T, Le Couteur DG, Turner N, Morris MJ, Walters KA, Goldys E, O’Neill C, Gilchrist RB, Sinclair DA, Homer HA, Wu LE. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep 2020; 30: 1670–1681.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao Y, Cui Z, Gao Q, Rui R, Xiong B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Reports 2020; 32: 107987. [DOI] [PubMed] [Google Scholar]
- 31.Pollard CL, Gibb Z, Hawdon A, Swegen A, Grupen CG. Supplementing media with NAD+ precursors enhances the in vitro maturation of porcine oocytes. J Reprod Dev 2021; 67: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollard CL, Younan A, Swegen A, Gibb Z, Grupen CG. Insights into the NAD+ biosynthesis pathways involved during meiotic maturation and spindle formation in porcine oocytes. J Reprod Dev 2022; 68: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Sun M, Yu L, Wang Y, Yao Y, Wang D. Niacin inhibits apoptosis and rescues premature ovarian failure. Cell Physiol Biochem 2018; 50: 2060–2070. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Hu F, Zeng J, Han L, Qiu D, Wang H, Ge J, Ying X, Wang Q. NMNAT2-mediated NAD+ generation is essential for quality control of aged oocytes. Aging Cell 2019; 18: e12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itami N, Shirasuna K, Kuwayama T, Iwata H. Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology 2015; 83: 1360–1367. [DOI] [PubMed] [Google Scholar]
- 36.Khan I, Kim SW, Lee KL, Song SH, Mesalam A, Chowdhury MMR, Uddin Z, Park KH, Kong IK. Polydatin improves the developmental competence of bovine embryos in vitro via induction of sirtuin 1 (Sirt1). Reprod Fertil Dev 2017; 29: 2011–2020. [DOI] [PubMed] [Google Scholar]
- 37.Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L. Resveratrol protects against age-associated infertility in mice. Hum Reprod 2013; 28: 707–717. [DOI] [PubMed] [Google Scholar]
- 38.Takeo S, Sato D, Kimura K, Monji Y, Kuwayama T, Kawahara-Miki R, Iwata H. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J Reprod Dev 2014; 60: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F, Tian X, Zhang L, He C, Ji P, Li Y, Tan D, Liu G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil Steril 2014; 101: 577–586. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder AC, Eppig JJ. The developmental capacity of mouse oocytes that matured spontaneously in vitro is normal. Dev Biol 1984; 102: 493–497. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh S, George S, Roy U, Ramachandran D, Kolthur-Seetharam U. NAD: a master regulator of transcription. Biochim Biophys Acta 2010; 1799: 681–693. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, McReynolds MR, Goncalves JF, Shu M, Dhondt I, Braeckman BP, Lange SE, Kho K, Detwiler AC, Pacella MJ, Hanna-Rose W. Comparative metabolomic profiling reveals that dysregulated glycolysis stemming from lack of salvage NAD+ biosynthesis impairs reproductive development in Caenorhabditis elegans. J Biol Chem 2015; 290: 26163–26179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bender DA, Olufunwa R. Utilization of tryptophan, nicotinamide and nicotinic acid as precursors for nicotinamide nucleotide synthesis in isolated rat liver cells. Br J Nutr 1988; 59: 279–287. [DOI] [PubMed] [Google Scholar]
- 44.Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Adv Enzymol Relat Areas Mol Biol 1999; 73: 135–182: xi. [DOI] [PubMed] [Google Scholar]
- 45.Kurnasov O, Goral V, Colabroy K, Gerdes S, Anantha S, Osterman A, Begley TP. NAD biosynthesis: identification of the tryptophan to quinolinate pathway in bacteria. Chem Biol 2003; 10: 1195–1204. [DOI] [PubMed] [Google Scholar]
- 46.Magni G, Orsomando G, Raffelli N, Ruggieri S. Enzymology of mammalian NAD metabolism in health and disease. Front Biosci 2008; 13: 6135–6154. [DOI] [PubMed] [Google Scholar]
- 47.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci 2007; 32: 12–19. [DOI] [PubMed] [Google Scholar]
- 48.Pollak N, Niere M, Ziegler M. NAD kinase levels control the NADPH concentration in human cells. J Biol Chem 2007; 282: 33562–33571. [DOI] [PubMed] [Google Scholar]
- 49.de Figueiredo LF, Gossmann TI, Ziegler M, Schuster S. Pathway analysis of NAD+ metabolism. Biochem J 2011; 439: 341–348. [DOI] [PubMed] [Google Scholar]
- 50.Olgun A. Converting NADH to NAD+ by nicotinamide nucleotide transhydrogenase as a novel strategy against mitochondrial pathologies during aging. Biogerontology 2009; 10: 531–534. [DOI] [PubMed] [Google Scholar]
- 51.Preiss J, Handler P. Enzymatic synthesis of nicotinamide mononucleotide. J Biol Chem 1957; 225: 759–770. [PubMed] [Google Scholar]
- 52.French JB, Cen Y, Vrablik TL, Xu P, Allen E, Hanna-Rose W, Sauve AA. Characterization of nicotinamidases: steady state kinetic parameters, classwide inhibition by nicotinaldehydes, and catalytic mechanism. Biochemistry 2010; 49: 10421–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srivastava S. Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders. Clin Transl Med 2016; 5: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rechsteiner M, Hillyard D, Olivera BM. Turnover at nicotinamide adenine dinucleotide in cultures of human cells. J Cell Physiol 1976; 88: 207–217. [DOI] [PubMed] [Google Scholar]
- 55.Fukuwatari T, Ohta M, Kimtjra N, Sasaki R, Shibata K. Conversion ratio of tryptophan to niacin in Japanese women fed a purified diet conforming to the Japanese Dietary Reference Intakes. J Nutr Sci Vitaminol (Tokyo) 2004; 50: 385–391. [DOI] [PubMed] [Google Scholar]
- 56.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 2008; 28: 115–130. [DOI] [PubMed] [Google Scholar]
- 57.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci 2004; 61: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 1967; 103: 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol 2003; 15: 241–246. [DOI] [PubMed] [Google Scholar]
- 60.Mendelsohn AR, Larrick JW. The NAD+/PARP1/SIRT1 axis in aging. Rejuvenation Res 2017; 20: 244–247. [DOI] [PubMed] [Google Scholar]
- 61.Moon P, Minhas P. Teasing apart NAD+ metabolism in inflammation: commentary on Zhou et al. (2016). Br J Pharmacol 173: 2352-2368. Br J Pharmacol 2017; 174: 281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hottiger MO, Hassa PO, Lüscher B, Schüler H, Koch-Nolte F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem Sci 2010; 35: 208–219. [DOI] [PubMed] [Google Scholar]
- 63.Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci 2008; 13: 3046–3082. [DOI] [PubMed] [Google Scholar]
- 64.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol 2012; 13: 411–424. [DOI] [PubMed] [Google Scholar]
- 65.Daniels CM, Ong SE, Leung AK. The promise of proteomics for the study of ADP-ribosylation. Mol Cell 2015; 58: 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vyas S, Matic I, Uchima L, Rood J, Zaja R, Hay RT, Ahel I, Chang P. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nat Commun 2014; 5: 4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vyas S, Chesarone-Cataldo M, Todorova T, Huang YH, Chang P. A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nat Commun 2013; 4: 2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 2011; 13: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bai P, Canto C, Brunyánszki A, Huber A, Szántó M, Cen Y, Yamamoto H, Houten SM, Kiss B, Oudart H, Gergely P, Menissier-de Murcia J, Schreiber V, Sauve AA, Auwerx J. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab 2011; 13: 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Devalaraja-Narashimha K, Padanilam BJ. PARP1 deficiency exacerbates diet-induced obesity in mice. J Endocrinol 2010; 205: 243–252. [DOI] [PubMed] [Google Scholar]
- 71.Sousa FG, Matuo R, Soares DG, Escargueil AE, Henriques JA, Larsen AK, Saffi J. PARPs and the DNA damage response. Carcinogenesis 2012; 33: 1433–1440. [DOI] [PubMed] [Google Scholar]
- 72.Ryu KW, Kim DS, Kraus WL. New facets in the regulation of gene expression by ADP-ribosylation and poly(ADP-ribose) polymerases. Chem Rev 2015; 115: 2453–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupte R, Liu Z, Kraus WL. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev 2017; 31: 101–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem 2005; 280: 36334–36341. [DOI] [PubMed] [Google Scholar]
- 75.Okamoto H. Cyclic ADP-ribose-mediated insulin secretion and Reg, regenerating gene. J Mol Med (Berl) 1999; 77: 74–78. [DOI] [PubMed] [Google Scholar]
- 76.Lee HC, Zocchi E, Guida L, Franco L, Benatti U, De Flora A. Production and hydrolysis of cyclic ADP-ribose at the outer surface of human erythrocytes. Biochem Biophys Res Commun 1993; 191: 639–645. [DOI] [PubMed] [Google Scholar]
- 77.Kuroda R, Kontani K, Kanda Y, Katada T, Nakano T, Satoh Y, Suzuki N, Kuroda H. Increase of cGMP, cADP-ribose and inositol 1,4,5-trisphosphate preceding Ca(2+) transients in fertilization of sea urchin eggs. Development 2001; 128: 4405–4414. [DOI] [PubMed] [Google Scholar]
- 78.Iside C, Scafuro M, Nebbioso A, Altucci L. SIRT1 activation by natural phytochemicals: an overview. Front Pharmacol 2020; 11: 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jung-Hynes B, Schmit TL, Reagan-Shaw SR, Siddiqui IA, Mukhtar H, Ahmad N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J Pineal Res 2011; 50: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001; 410: 227–230. [DOI] [PubMed] [Google Scholar]
- 81.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 1999; 13: 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Imai S, Johnson FB, Marciniak RA, McVey M, Park PU, Guarente L. Sir2: an NAD-dependent histone deacetylase that connects chromatin silencing, metabolism, and aging. Cold Spring Harb Symp Quant Biol 2000; 65: 297–302. [DOI] [PubMed] [Google Scholar]
- 83.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA 2004; 101: 15998–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tatone C, Di Emidio G, Barbonetti A, Carta G, Luciano AM, Falone S, Amicarelli F. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update 2018; 24: 267–289. [DOI] [PubMed] [Google Scholar]
- 85.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell 2005; 16: 4623–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 2007; 282: 6823–6832. [DOI] [PubMed] [Google Scholar]
- 87.Guarente L, Mostoslavsky R, Kazantsev A. Introductory Review on Sirtuins in Biology and Disease. 2018, MIT, Cambridge, MA, United States: Academic Press. [Google Scholar]
- 88.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 2008; 28: 6384–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.North BJ, Verdin E. Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS One 2007; 2: e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okamoto N, Kawamura K, Kawamura N, Nishijima C, Ishizuka B, Suzuki N, Hirata K. Effects of maternal aging on expression of Sirtuin genes in ovulated oocyte and cumulus cells. J Mamm Ova Res 2013; 30: 24–29. [Google Scholar]
- 91.Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest 2010; 120: 2817–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kwak SS, Cheong SA, Yoon JD, Jeon Y, Hyun SH. Expression patterns of sirtuin genes in porcine preimplantation embryos and effects of sirtuin inhibitors on in vitro embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 2012; 78: 1597–1610. [DOI] [PubMed] [Google Scholar]
- 93.Pavlová S, Klucska K, Vašíček D, Ryban L, Harrath AH, Alwasel SH, Sirotkin AV. The involvement of SIRT1 and transcription factor NF-κB (p50/p65) in regulation of porcine ovarian cell function. Anim Reprod Sci 2013; 140: 180–188. [DOI] [PubMed] [Google Scholar]
- 94.Sirotkin AV, Dekanová P, Harrath AH, Alwasel SH, Vašíček D. Interrelationships between sirtuin 1 and transcription factors p53 and NF-κB (p50/p65) in the control of ovarian cell apoptosis and proliferation. Cell Tissue Res 2014; 358: 627–632. [DOI] [PubMed] [Google Scholar]
- 95.Labrecque R, Lodde V, Dieci C, Tessaro I, Luciano AM, Sirard MA. Chromatin remodelling and histone m RNA accumulation in bovine germinal vesicle oocytes. Mol Reprod Dev 2015; 82: 450–462. [DOI] [PubMed] [Google Scholar]
- 96.Lodde V, Luciano AM, Franciosi F, Labrecque R, Sirard MA. Accumulation of chromatin remodelling enzyme and histone transcripts in bovine oocytes. Results Probl Cell Differ 2017; 63: 223–255. [DOI] [PubMed] [Google Scholar]
- 97.Ma R, Zhang Y, Zhang L, Han J, Rui R. Sirt1 protects pig oocyte against in vitro aging. Anim Sci J 2015; 86: 826–832. [DOI] [PubMed] [Google Scholar]
- 98.Takeo S, Kawahara-Miki R, Goto H, Cao F, Kimura K, Monji Y, Kuwayama T, Iwata H. Age-associated changes in gene expression and developmental competence of bovine oocytes, and a possible countermeasure against age-associated events. Mol Reprod Dev 2013; 80: 508–521. [DOI] [PubMed] [Google Scholar]
- 99.Di Emidio G, Falone S, Vitti M, D’Alessandro AM, Vento M, Di Pietro C, Amicarelli F, Tatone C. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod 2014; 29: 2006–2017. [DOI] [PubMed] [Google Scholar]
- 100.Gao M, Li X, He Y, Han L, Qiu D, Ling L, Liu H, Liu J, Gu L. SIRT7 functions in redox homeostasis and cytoskeletal organization during oocyte maturation. FASEB J 2018; fj201800078RR. [DOI] [PubMed] [Google Scholar]
- 101.Zeng J, Jiang M, Wu X, Diao F, Qiu D, Hou X, Wang H, Li L, Li C, Ge J, Liu J, Ou X, Wang Q. SIRT4 is essential for metabolic control and meiotic structure during mouse oocyte maturation. Aging Cell 2018; 17: e12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Han L, Ge J, Zhang L, Ma R, Hou X, Li B, Moley K, Wang Q. Sirt6 depletion causes spindle defects and chromosome misalignment during meiosis of mouse oocyte. Sci Rep 2015; 5: 15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu D, Wu L, Jiang X, Yang L, Cheng J, Chen H, Hua R, Geng G, Yang L, Li Q. SIRT2 inhibition results in meiotic arrest, mitochondrial dysfunction, and disturbance of redox homeostasis during bovine oocyte maturation. Int J Mol Sci 2019; 20: E1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang L, Hou X, Ma R, Moley K, Schedl T, Wang Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J 2014; 28: 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang L, Ma R, Hu J, Ding X, Xu Y. Sirtuin inhibition adversely affects porcine oocyte meiosis. PLoS One 2015; 10: e0132941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao HC, Ding T, Ren Y, Li TJ, Li R, Fan Y, Yan J, Zhao Y, Li M, Yu Y, Qiao J. Role of Sirt3 in mitochondrial biogenesis and developmental competence of human in vitro matured oocytes. Hum Reprod 2016; 31: 607–622. [DOI] [PubMed] [Google Scholar]
- 107.Fu H, Wada-Hiraike O, Hirano M, Kawamura Y, Sakurabashi A, Shirane A, Morita Y, Isono W, Oishi H, Koga K, Oda K, Kawana K, Yano T, Kurihara H, Osuga Y, Fujii T. SIRT3 positively regulates the expression of folliculogenesis- and luteinization-related genes and progesterone secretion by manipulating oxidative stress in human luteinized granulosa cells. Endocrinology 2014; 155: 3079–3087. [DOI] [PubMed] [Google Scholar]
- 108.McBurney MW, Clark-Knowles KV, Caron AZ, Gray DA. SIRT1 is a highly networked protein that mediates the adaptation to chronic physiological stress. Genes Cancer 2013; 4: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA, Liang F. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci 2007; 27: 2606–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coussens M, Maresh JG, Yanagimachi R, Maeda G, Allsopp R. Sirt1 deficiency attenuates spermatogenesis and germ cell function. PLoS One 2008; 3: e1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim DH, Lee HR, Kim MG, Lee JS, Jin SJ, Lee HT. The effect of poly(ADP-ribosyl)ation inhibition on the porcine cumulus-oocyte complex during in vitro maturation. Biochem Biophys Res Commun 2017; 483: 752–758. [DOI] [PubMed] [Google Scholar]
- 112.Iljas JD, Wei Z, Homer HA. Sirt1 sustains female fertility by slowing age-related decline in oocyte quality required for post-fertilization embryo development. Aging Cell 2020; 19: e13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aksu K, Golal E, Aslan MA, Ustunel I, Acar N. The investigation of the role of sirtuin-1 on embryo implantation in oxidative stress-induced mice. J Assist Reprod Genet 2021; 38: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Effect of lactate dehydrogenase activity and isoenzyme localization in bovine oocytes and utilization of oxidative substrates on in vitro maturation. Theriogenology 1999; 51: 541–550. [DOI] [PubMed] [Google Scholar]
- 115.Cetica P, Pintos L, Dalvit G, Beconi M. Involvement of enzymes of amino acid metabolism and tricarboxylic acid cycle in bovine oocyte maturation in vitro. Reproduction 2003; 126: 753–763. [PubMed] [Google Scholar]
- 116.Williams GT, Ford CC, Shall S. NAD-glycohydrolase activity in Xenopus laevis oocytes and early embryos. Biochem Biophys Res Commun 1982; 108: 36–41. [DOI] [PubMed] [Google Scholar]
- 117.Bustamante S, Jayasena T, Richani D, Gilchrist RB, Wu LE, Sinclair DA, Sachdev PS, Braidy N. Quantifying the cellular NAD+ metabolome using a tandem liquid chromatography mass spectrometry approach. Metabolomics 2017; 14: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Almubarak AM, Kim E, Yu IJ, Jeon Y. Supplementation with Niacin during in vitro maturation improves the quality of porcine embryos. Theriogenology 2021; 169: 36–46. [DOI] [PubMed] [Google Scholar]
- 119.Kafi M, Ashrafi M, Azari M, Jandarroodi B, Abouhamzeh B, Asl AR. Niacin improves maturation and cryo-tolerance of bovine in vitro matured oocytes: An experimental study. Int J Reprod Biomed 2019; 17: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol 2006; 296: 514–521. [DOI] [PubMed] [Google Scholar]
- 121.Wei Z, Greaney J, Loh WN, Homer HA. Nampt-mediated spindle sizing secures a post-anaphase increase in spindle speed required for extreme asymmetry. Nat Commun 2020; 11: 3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krisher RL, Brad AM, Herrick JR, Sparman ML, Swain JE. A comparative analysis of metabolism and viability in porcine oocytes during in vitro maturation. Anim Reprod Sci 2007; 98: 72–96. [DOI] [PubMed] [Google Scholar]
- 123.Wen J, Wang GL, Yuan HJ, Zhang J, Xie HL, Gong S, Han X, Tan JH. Effects of glucose metabolism pathways on nuclear and cytoplasmic maturation of pig oocytes. Sci Rep 2020; 10: 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Riepsamen A, Wu L, Lau L, Listijono D, Ledger W, Sinclair D, Homer H. Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes. PLoS One 2015; 10: e0126194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee AR, Kishigami S, Amano T, Matsumoto K, Wakayama T, Hosoi Y. Nicotinamide: a class III HDACi delays in vitro aging of mouse oocytes. J Reprod Dev 2013; 59: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.El Sheikh M, Mesalam AA, Idrees M, Sidrat T, Mesalam A, Lee KL, Kong IK. Nicotinamide supplementation during the in vitro maturation of oocytes improves the developmental competence of preimplantation embryos: potential link to SIRT1/AKT signaling. Cells 2020; 9: E1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 2016; 7: 12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Osada T, Ogino H, Hino T, Ichinose S, Nakamura K, Omori A, Noce T, Masutani M. PolyADP-ribosylation is required for pronuclear fusion during postfertilization in mice. PLoS One 2010; 5: e12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Imamura T, Neildez TM, Thenevin C, Paldi A. Essential role for poly (ADP-ribosyl)ation in mouse preimplantation development. BMC Mol Biol 2004; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pacella-Ince L, Zander-Fox DL, Lane M. Mitochondrial SIRT3 and its target glutamate dehydrogenase are altered in follicular cells of women with reduced ovarian reserve or advanced maternal age. Hum Reprod 2014; 29: 1490–1499. [DOI] [PubMed] [Google Scholar]
- 131.Liu WJ, Zhang XM, Wang N, Zhou XL, Fu YC, Luo LL. Calorie restriction inhibits ovarian follicle development and follicle loss through activating SIRT1 signaling in mice. Eur J Med Res 2015; 20: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vazquez BN, Blengini CS, Hernandez Y, Serrano L, Schindler K. SIRT7 promotes chromosome synapsis during prophase I of female meiosis. Chromosoma 2019; 128: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]