Abstract
In mammals, the corpus luteum (CL) is a transient organ that secretes progesterone (P4). In the absence of pregnancy, the CL undergoes regression (luteolysis), which is a crucial preparation step for the next estrous cycle. Luteolysis, initiated by uterine prostaglandin F2α (PGF) in cattle, is usually divided into two phases, namely functional luteolysis characterized by a decline in P4 concentration and structural luteolysis characterized by the elimination of luteal tissues from the ovary. Programmed cell death (PCD) of luteal cells, including luteal steroidogenic cells (LSCs) and luteal endothelial cells (LECs), plays a crucial role in structural luteolysis. The main types of PCD are caspase-dependent apoptosis (type 1), autophagic cell death (ACD) via the autophagy-related gene (ATG) family (type 2), and receptor-interacting protein kinase (RIPK)-dependent programmed necrosis (necroptosis, type 3). However, these PCD signaling pathways are not completely independent and interact with each other. Over the past several decades, most studies on luteolysis have focused on apoptosis as the principal mode of bovine luteal cell death. Recently, ATG family members were reported to be expressed in bovine CL, and their levels increased during luteolysis. Furthermore, the expression of RIPKs, which are crucial mediators of necroptosis, is reported to increase in bovine CL during luteolysis and is upregulated by pro-inflammatory cytokines in bovine LSCs and LECs. Therefore, apoptosis, ACD, and necroptosis may contribute to bovine CL regression. In this article, we present the recent findings regarding the mechanisms of the three main types of PCD and the contribution of these mechanisms to luteolysis.
Keywords: Apoptosis, Autophagic cell death, Corpus luteum, Necroptosis, Programmed cell death
Introduction
The corpus luteum (CL) is a transient organ that secretes progesterone (P4), which contributes to the establishment and maintenance of pregnancy [1]. In the absence of embryos, the CL undergoes regression, and this is essential for normal cyclicity and allows the development and ovulation of new follicles. Luteolysis in ruminants is stimulated by prostaglandin F2α (PGF), which is mainly secreted by the uterine endometrium [2]. Moreover, several cytokines, including tumor necrosis factor-α (TNF) and interferon-γ (IFNG), as well as nitric oxide (NO), act as mediators or activators of the luteolytic cascade in cattle [3,4,5].
Luteolysis involves a decline in the secretory function of the CL, a reduction in P4 production (functional luteolysis), and tissue degradation and elimination by cell death (structural luteolysis) [6, 7]. Caspase-dependent apoptosis in cells that form the CL, such as luteal steroidogenic cells (LSC) and luteal endothelial cells (LEC), is hypothesized to be the predominant pathway for cell death during luteolysis in several species, including cattle [6, 7]. A large number of factors, such as pro-inflammatory cytokines, TNF, IFNG and FAS ligand (FASL), and NO [8,9,10,11,12,13], have been implicated in structural and functional luteolysis in cattle. Thus, many studies have reported the relationship between apoptosis and luteolysis in cells in the CL. In addition to apoptosis, the regulatory mechanisms and the roles of the other types of programmed cell death (PCD) in the mammalian body have been revealed in recent years. Although in cattle, these PCD types may be involved in structural luteolysis, the details of the relationship between these PCD types and luteal regression are not well understood.
Programmed cell death was first mentioned by Lockshin and Williams [14], followed by its classification into three main types: type 1, caspase-dependent apoptosis; type 2, autophagic cell death (ACD); and type 3, programmed necrosis and necroptosis [15, 16]. Recently, several new types of PCD such as pyroptosis and ferroptosis have been termed; however, no evidence has been found regarding their involvement in structural luteolysis in ruminants and other animal species.
Type 1 PCD plays a substantial role in mammalian luteolysis. In addition, ACD contributes to luteolysis in humans [17, 18], rats [19, 20], goats [21], pigs [22], and cattle [23, 24]. Necroptosis also induces luteolysis in humans [25, 26], pigs [27], and cattle [28]. In this article, we reviewed recent findings regarding the mechanisms of these three types of PCD and their contributions to luteolysis in mammals, including cattle.
Apoptosis
Apoptotic pathways
Programmed cell death, characterized by DNA fragmentation and cell shrinkage, was first defined as apoptosis by Kerr et al. [29]. Apoptosis is triggered by two main signaling pathways, namely the death receptor and mitochondrial pathways [30, 31]. A series of cysteine proteases, caspases (CASPs), are the most common activators of PCD and are induced by the binding of death ligands to death receptors [32, 33]. The death receptor pathway, also known as the extrinsic apoptotic pathway, is initiated by extracellular ligands, including FASL and TNF, that interact with cell surface receptors such as FAS and TNF receptors (TNFRs), to initiate cell death signaling through CASP8 and CASP3, resulting in DNA fragmentation and cell degeneration [30, 33]. These typical forms of apoptosis were first characterized in bovine CL tissues at the luteal regression stage [6, 34]. Notably, during the last few decades, type 1 PCD was considered the principal cause of structural luteolysis. Apoptosis in cultured bovine LSCs and LECs is induced by inflammatory cytokines, TNF and IFNG, and a typical death ligand, FASL, secreted by immune cells, such as T lymphocytes or macrophages, that infiltrate the bovine CL during spontaneous luteolysis [8, 10, 13, 35]. Furthermore, the levels of these immune cells increased in the bovine CL 6 h after PGF injection on day 10 of the estrous cycle [35], suggesting that these cytokines may increase during both spontaneous and PGF-induced luteolysis and stimulate structural luteolysis by inducing apoptosis. In contrast, cellular FLICE-like inhibitory protein (cFLIP), known as an intracellular apoptosis inhibitor, inhibits the apoptotic pathway by blocking the formation of the death-inducing signaling complex (DISC), which is essential for activating the death receptor pathway [36]. Cellular FLIP is expressed in bovine CL tissues throughout the estrous cycle, and its expression decreases during luteal regression [37], suggesting that cFLIP maintains luteal function by inhibiting apoptosis in the bovine CL until luteolysis initiation.
The mitochondrial pathway, also known as the intrinsic apoptotic cascade, is another form of the apoptotic pathway. This pathway is regulated by members of the BCL2-protein family. The ratio of BCL2, which protects against cell death, to BAX, a pro-apoptotic protein, determines cell fate [31]. The death receptor and mitochondrial pathways of apoptosis are not completely different from each other; they share some aspects of signaling. Both are characterized by CASP activations [32]. In bovine CL tissues, compared with anti-apoptotic protein BCL2 expression, pro-apoptotic protein BAX expression increases during both spontaneous and PGF-induced luteolysis [7, 38]. These findings suggest that death receptor and mitochondrial pathways work together to induce rapid apoptosis in bovine LSCs and LECs during luteolysis.
Extracellular regulators of apoptosis
In bovine LSCs and LECs, TNF, IFNG, and FASL are well-known inducers of apoptosis. In contrast to these death ligands, several steroids and prostaglandins are known to inhibit TNF+IFNG-induced apoptosis in cultured LSCs and LECs [8, 11, 39, 40]. Thus, pro-apoptotic factors, such as TNF, IFNG, and FASL, and anti-apoptotic factors, such as P4, cortisol, and prostaglandins, are expressed in bovine CL and possibly regulate luteal functions, including the regulation of apoptosis in LSCs and LECs, depending on the estrous cycle phase.
Inhibitors of NO synthesis can suppress PGF-induced luteolysis in vivo [4], suggesting that NO secreted by neutrophils and LECs mediates the luteolytic action of PGF [4, 41, 42]. NO induces apoptosis in cultured bovine LSCs [12]. Moreover, reactive oxygen species (ROS) induce apoptosis by stimulating p53 and BAX mRNA expression in bovine LSCs [43]. Furthermore, hypoxia, which may occur in bovine CL during luteolysis by the disappearance of capillaries [44], is reported to inhibit P4 synthesis [45] and induce apoptosis in bovine LSCs [46]. Apoptosis in LSC and LEC is regulated by a balance between intracellular and extracellular apoptosis-related factors. In addition, P4 secretion decreases before apoptosis under hypoxic conditions in bovine LSCs [46], suggesting that functional luteolysis is followed by rapid apoptosis of LSCs and structural luteolysis in cattle.
Autophagic Cell Death
Contribution of ACD in luteolysis
Autophagy, first described by Lockshin and Zakeri, was originally believed to be a mechanism that promotes survival during malnutrition; however, ACD is now believed to be involved in PCD [47]. Furthermore, ACD is involved in inducing cell death and excreting damaged intracellular proteins [48, 49]. Generally, autophagy, including ACD, is characterized by an intracellular dual membrane called the autophagosome [50, 51], and is regulated by autophagy-related genes (ATG) and their associated proteins in mammals [52]. Furthermore, microtubule-associated protein light chain 3 (LC3), a critical marker for ACD, is present in autophagosomes [53], and the primary components of autophagosomes are lysosome-associated membrane protein-1 (LAMP1) and -2 (LAMP2) [54]. Moreover, Beclin-1 (BECN1) can induce ACD, and this ACD-inducing activity of BECN1 is suppressed by the anti-apoptotic protein BCL2 [55], suggesting possible interaction between the apoptotic and ACD pathways. Mammalian targeting of rapamycin (mTOR) inhibits the initiation of autophagy and formation of autophagosome [56]. Furthermore, cFLIP, which is an intracellular apoptosis inhibitor, also suppresses ACD by preventing ATG3 from binding and processing LC3 [57]. Although the possibility that ACD participates in luteal regression in guinea pigs was mentioned in a morphological study in 1978 [58], reports on the role of ACD in luteolysis are limited. However, recent studies suggest that ACD contributes to luteolysis in mammals.
Regulatory mechanisms of ACD in the mammalian corpus luteum
Recently, a study reported that autophagy-related factors, such as ATG3, ATG7, and LC3, were more highly expressed at the late luteal stage than at the mid-luteal stage in bovine CL [23], indicating that ACD could be involved in structural luteolysis in cattle. Furthermore, this study indicated that P4 secretion was significantly lower at the late luteal stage than at the mid-luteal stage, suggesting that ACD is involved in both functional and structural luteolysis [23].
However, the detailed regulatory mechanisms of ACD in bovine CL remain unclear. Nevertheless, TNF induces autophagy in Ewing sarcoma cells [59], and hypoxia triggers ACD [60]. Thus, both TNF and hypoxia can trigger structural luteolysis and cell death mechanisms in bovine CL. Therefore, these factors may contribute to acute structural luteolysis in cattle by inducing apoptosis and ACD. Furthermore, ACD and apoptosis occur in goat CL tissues at the regressing luteal stage [61], and ACD-like PCD occurs in the human CL [17]. Moreover, the expression levels of BECN1, LC3-II, and LAMP1 increase during luteolysis in pigs [22], and LC3-II expression increases with the progression of luteolysis in rats [19]. These findings strongly suggest that ACD participates in luteolysis and apoptosis in various mammals, including cattle. In contrast, in mouse and human LSCs, BECN1 is necessary for steroid synthesis [62, 63], suggesting that autophagy plays a critical role in maintaining luteal function. Thus, autophagy probably contributes not only to luteolysis but also maintains luteal function, such as steroid synthesis. Further studies are necessary to clarify the mechanism by which autophagy balances the life cycle and death of luteal cells.
Necroptosis
Necroptosis in ovarian cells
Necrosis is another cell death mechanism that is independent of caspases. Generally, necrosis is considered an accidental and undesirable cell death. Furthermore, it occurs in an unregulated manner and is triggered by extreme conditions. However, necrosis can be regulated by intracellular mechanisms, leading to programmed necrosis and necroptosis [64, 65]. The necroptotic pathway is activated when apoptosis is blocked by CASP inhibitors such as zVAD-FMK [64, 66, 67]. Receptor-interacting protein kinase (RIPK) 1 and RIPK3 function as sensors of cellular stress [68], and they are essential kinases that mediate necroptosis in experiments with the RIPK1 activity inhibitor necrostatin-1 [67, 69, 70]. Although necrosis plays the most predominant role in the death of granulosa cells in the atretic follicle of ewes [71, 72], no information was available on whether ovarian cells exhibited programmed necrosis or necroptosis until Blohberger et al. reported necroptosis and its role in the human ovary [25]. They demonstrated that necroptosis occurs in human granulosa and luteal cells and contributes to follicular atresia and luteolysis [25]. Necroptosis probably plays a substantial role in the regulation of primate folliculogenesis [73] and luteolysis [26]. Furthermore, necroptosis is an alternative mechanism responsible for the death of bovine granulosa and theca cells [74]. These findings suggest that necroptosis is a critical factor in the regulation of ovarian function. Recently, we demonstrated that both RIPK1 and RIPK3 are expressed in bovine CL throughout the estrous cycle and their expression levels increase during luteolysis [28], suggesting that RIPK-dependent necroptosis participates in structural luteolysis in cattle. This study was the first to reveal the role of necroptosis in luteolysis in domestic animals. Notably, this study indicated that necroptosis-inducing treatment did not affect P4 secretion in bovine LSCs [28], suggesting that necroptosis is not involved in functional luteolysis in cattle. These findings indicate that the relationship between PCD, including necroptosis, and functional luteolysis is not well understood, and further studies are needed.
Regulatory mechanisms of necroptosis
Death ligands, such as TNF and FASL, induce apoptosis as well as necroptosis in a number of tissues [75,76,77]. In death ligand-induced necroptosis, RIPKs are deubiquitinated by intracellular factors such as cylindromatosis (CYLD). Moreover, RIPKs and a pseudokinase, mixed lineage kinase-domain protein (MLKL), form a death-inducing signaling complex termed the necrosome [78]. Phosphorylated MLKL probably executes necroptosis [79,80,81,82]. Many intracellular proteins, including RIPKs, CYLD, and MLKL, induce necroptosis. Following this general mechanism of necroptosis regulation, we investigated the regulatory mechanisms of RIPKs expressed in bovine LSCs and LECs in vitro [28, 83] and during PGF-induced luteolysis in vivo [84, 85]. First, we demonstrated that TNF in combination with IFNG, but not PGF or NO, stimulates RIPK expression [28]. By contrast, PGF, NO, TNF, and IFNG induce RIPK expression in bovine LECs [83]. These findings suggest that necroptosis can occur in both bovine LSCs and LECs; however, different necroptotic pathways and signaling orders exist within each cell.
Cellular FLIP could be a candidate intracellular inhibitor of death ligand-receptor system-induced RIPK activation and necroptosis. The CASP8-cFLIP complex inhibits apoptosis as well as RIPK-dependent necroptosis [86, 87]. As previously mentioned, cFLIP also inhibits apoptosis and ACD [36, 57], indicating that cFLIP inhibits all three types of PCD. Thus, cFLIP plays a substantial role in determining the fate of CL cells. Furthermore, one of our previous studies indicated that cFLIP was expressed throughout the estrous cycle in bovine CL tissues and decreased during the regressing luteal stage [37]. Thus, RIPK expression and the occurrence of necroptosis are probably inhibited by cFLIP in bovine CL from the early to late luteal stages.
Although the typical luteolytic factor, PGF, did not affect RIPK expression and necroptosis in cultured bovine LSCs [28], in vivo studies indicated that PGF treatment induced the expression of RIPKs, CYLD, and MLKL in bovine CL [84, 85]. The effects of PGF on luteal function are very complex, and the luteolytic effect of PGF does not induce apoptosis directly in LSCs and LECs but depends on the cell composition, contact, and number of intraluteal mediators [88].
Conclusion
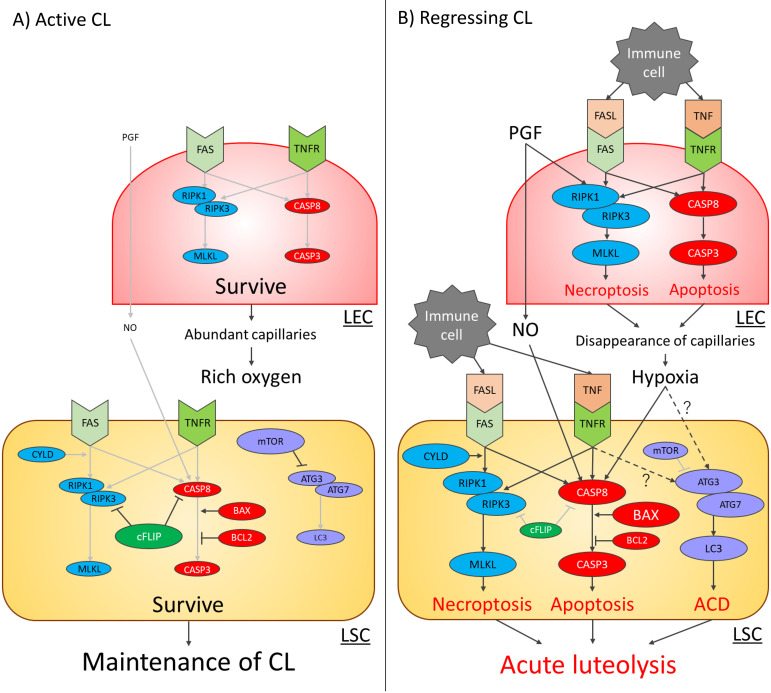
In this article, we reviewed the mechanisms of the three PCD types and their contributions to ovarian function, mainly luteolysis, in mammals, including cattle. The PCD pathways interact with each other and share some aspects of signaling [89]. Figure 1 depicts the schema of PCD signals in bovine LECs and LSCs with active and regressing CL. Further studies will contribute to an in-depth understanding of the regulatory mechanisms of ovarian function in mammals.
Fig. 1.
Programmed cell death (PCD) signaling pathways in bovine luteal endothelial cells (LECs) and luteal steroidogenic cells (LSCs) during the active CL (A) and regressing CL (B) stages. Red, purple, and light blue circles in the cells indicate apoptosis-related, autophagic cell death (ACD)-related, and necroptosis-related factors, respectively. In the active CL stage (A), the expression of extracellular PCD stimulators, including pro-inflammatory cytokines, immune cell-derived death ligands, and nitric oxide, is low. Thus, the expression of each PCD signal-related factor is also low, and these signals are weak (light gray arrows) in LECs and LSCs. Finally, both types of cells survive to maintain the CL. In the regressing CL stage (B), the expression of each PCD signal-related factor is stimulated by extracellular PCD stimulators and conditions of hypoxia. Therefore, each PCD signal becomes strong (black arrows), and PCD is induced. The details of the effects of the TNF-TNF receptor (TNFR) system and conditions of hypoxia on ACD-related signals (dotted arrows) are not well understood. Finally, interaction between various PCD results in structural luteolysis and the elimination of the active CL from the ovary.
Conflict of interests
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank the Society for Reproduction and Development for granting us the Young Investigator Award and giving us the opportunity to write this review. We also thank Dr. Naoki Takenouchi and Dr. Miki Sakatani for their kind advice and suggestions on our study. Finally, we thank our colleagues and staff at the Kyushu Okinawa Agricultural Research Center, NARO, Polish Academy of Sciences, and Okayama University for their contributions and assistance with the research. DJS was supported by a grant from the National Science Center (No. UMO-2018/29/B/NZ9/00391).
This publication was supported by JSPS KAKENHI (22HP2009).
References
- 1.Hansel W, Blair RM. Bovine corpus luteum: a historic overview and implications for future research. Theriogenology 1996; 45: 1267–1294. [DOI] [PubMed] [Google Scholar]
- 2.McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev 1999; 79: 263–323. [DOI] [PubMed] [Google Scholar]
- 3.Skarzynski DJ, Bah MM, Deptula KM, Woclawek-Potocka I, Korzekwa A, Shibaya M, Pilawski W, Okuda K. Roles of tumor necrosis factor-α of the estrous cycle in cattle: an in vivo study. Biol Reprod 2003; 69: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 4.Skarzynski DJ, Jaroszewski JJ, Bah MM, Deptula KM, Barszczewska B, Gawronska B, Hansel W. Administration of a nitric oxide synthase inhibitor counteracts prostaglandin F2-induced luteolysis in cattle. Biol Reprod 2003; 68: 1674–1681. [DOI] [PubMed] [Google Scholar]
- 5.Korzekwa A, Murakami S, Wocławek-Potocka I, Bah MM, Okuda K, Skarzynski DJ. The influence of tumor necrosis factor α (TNF) on the secretory function of bovine corpus luteum: TNF and its receptors expression during the estrous cycle. Reprod Biol 2008; 8: 245–262. [DOI] [PubMed] [Google Scholar]
- 6.Juengel JL, Garverick HA, Johnson AL, Youngquist RS, Smith MF. Apoptosis during luteal regression in cattle. Endocrinology 1993; 132: 249–254. [DOI] [PubMed] [Google Scholar]
- 7.Rueda BR, Tilly KI, Botros IW, Jolly PD, Hansen TR, Hoyer PB, Tilly JL. Increased bax and interleukin-1beta-converting enzyme messenger ribonucleic acid levels coincide with apoptosis in the bovine corpus luteum during structural regression. Biol Reprod 1997; 56: 186–193. [DOI] [PubMed] [Google Scholar]
- 8.Friedman A, Weiss S, Levy N, Meidan R. Role of tumor necrosis factor alpha and its type I receptor in luteal regression: induction of programmed cell death in bovine corpus luteum-derived endothelial cells. Biol Reprod 2000; 63: 1905–1912. [DOI] [PubMed] [Google Scholar]
- 9.Petroff MG, Petroff BK, Pate JL. Mechanisms of cytokine-induced death of cultured bovine luteal cells. Reproduction 2001; 121: 753–760. [PubMed] [Google Scholar]
- 10.Taniguchi H, Yokomizo Y, Okuda K. Fas-Fas ligand system mediates luteal cell death in bovine corpus luteum. Biol Reprod 2002; 66: 754–759. [DOI] [PubMed] [Google Scholar]
- 11.Okuda K, Korzekwa A, Shibaya M, Murakami S, Nishimura R, Tsubouchi M, Woclawek-Potocka I, Skarzynski DJ. Progesterone is a suppressor of apoptosis in bovine luteal cells. Biol Reprod 2004; 71: 2065–2071. [DOI] [PubMed] [Google Scholar]
- 12.Korzekwa AJ, Okuda K, Woclawek-Potocka I, Murakami S, Skarzynski DJ. Nitric oxide induces apoptosis in bovine luteal cells. J Reprod Dev 2006; 52: 353–361. [DOI] [PubMed] [Google Scholar]
- 13.Hojo T, Oda A, Lee SH, Acosta TJ, Okuda K. Effects of tumor necrosis factor α and Interferon γ on the viability and mRNA expression of TNF receptor type I in endothelial cells from the bovine corpus luteum. J Reprod Dev 2010; 56: 515–519. [DOI] [PubMed] [Google Scholar]
- 14.Lockshin RA, Williams CM. Programmed cell death—I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. J Insect Physiol 1965; 11: 123–133. [DOI] [PubMed] [Google Scholar]
- 15.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990; 181: 195–213. [DOI] [PubMed] [Google Scholar]
- 16.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1: 112–119. [DOI] [PubMed] [Google Scholar]
- 17.Del Canto F, Sierralta W, Kohen P, Muñoz A, Strauss JF, 3rd, Devoto L. Features of natural and gonadotropin-releasing hormone antagonist-induced corpus luteum regression and effects of in vivo human chorionic gonadotropin. J Clin Endocrinol Metab 2007; 92: 4436–4443. [DOI] [PubMed] [Google Scholar]
- 18.Gaytán M, Morales C, Sánchez-Criado JE, Gaytán F. Immunolocalization of beclin 1, a bcl-2-binding, autophagy-related protein, in the human ovary: possible relation to life span of corpus luteum. Cell Tissue Res 2008; 331: 509–517. [DOI] [PubMed] [Google Scholar]
- 19.Choi J, Jo M, Lee E, Choi D. The role of autophagy in corpus luteum regression in the rat. Biol Reprod 2011; 85: 465–472. [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Jo M, Lee E, Choi D. ERK1/2 is involved in luteal cell autophagy regulation during corpus luteum regression via an mTOR-independent pathway. Mol Hum Reprod 2014; 20: 972–980. [DOI] [PubMed] [Google Scholar]
- 21.Wen X, Liu L, Li S, Lin P, Chen H, Zhou D, Tang K, Wang A, Jin Y. Prostaglandin F2α induces goat corpus luteum regression via endoplasmic reticulum stress and autophagy. Front Physiol 2020; 11: 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grzesiak M, Michalik A, Rak A, Knapczyk-Stwora K, Pieczonka A. The expression of autophagy-related proteins within the corpus luteum lifespan in pigs. Domest Anim Endocrinol 2018; 64: 9–16. [DOI] [PubMed] [Google Scholar]
- 23.Aboelenain M, Kawahara M, Balboula AZ, Montasser A-M, Zaabel SM, Okuda K, Takahashi M. Status of autophagy, lysosome activity and apoptosis during corpus luteum regression in cattle. J Reprod Dev 2015; 61: 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Przygrodzka E, Monaco CF, Plewes MR, Li G, Wood JR, Cupp AS, Davis JS. Protein kinase A and 5′ AMP-activated protein kinase signaling pathways exert opposite effects on induction of autophagy in luteal cells. Front Cell Dev Biol 2021; 9: 723563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blohberger J, Kunz L, Einwang D, Berg U, Berg D, Ojeda SR, Dissen GA, Fröhlich T, Arnold GJ, Soreq H, Lara H, Mayerhofer A. Readthrough acetylcholinesterase (AChE-R) and regulated necrosis: pharmacological targets for the regulation of ovarian functions? Cell Death Dis 2015; 6: e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagnjuk K, Stöckl JB, Fröhlich T, Arnold GJ, Behr R, Berg U, Berg D, Kunz L, Bishop C, Xu J, Mayerhofer A. Necroptosis in primate luteolysis: a role for ceramide. Cell Death Dis 2019; 5: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makowczenko KG, Jastrzebski JP, Paukszto L, Dobrzyn K, Kiezun M, Smolinska N, Kaminski T. Chemerin impact on alternative mRNA transcription in the porcine luteal cells. Cells 2022; 11: 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hojo T, Siemieniuch MJ, Lukasik K, Piotrowska-Tomala KK, Jonczyk AW, Okuda K, Skarzynski DJ. Programmed necrosis - a new mechanism of steroidogenic luteal cell death and elimination during luteolysis in cows. Sci Rep 2016; 6: 38211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata S. Apoptosis by death factor. Cell 1997; 88: 355–365. [DOI] [PubMed] [Google Scholar]
- 31.Antonsson B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res 2001; 306: 347–361. [DOI] [PubMed] [Google Scholar]
- 32.Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997; 326: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281: 1312–1316. [DOI] [PubMed] [Google Scholar]
- 34.Zheng J, Fricke PM, Reynolds LP, Redmer DA. Evaluation of growth, cell proliferation, and cell death in bovine corpora lutea throughout the estrous cycle. Biol Reprod 1994; 51: 623–632. [DOI] [PubMed] [Google Scholar]
- 35.Penny LA, Armstrong D, Bramley TA, Webb R, Collins RA, Watson ED. Immune cells and cytokine production in the bovine corpus luteum throughout the oestrous cycle and after induced luteolysis. J Reprod Fertil 1999; 115: 87–96. [DOI] [PubMed] [Google Scholar]
- 36.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature 1997; 388: 190–195. [DOI] [PubMed] [Google Scholar]
- 37.Hojo T, Al-Zi’abi MO, Komiyama J, Manabe N, Acosta TJ, Okuda K. Expression and localization of cFLIP, an anti-apoptotic factor, in the bovine corpus luteum. J Reprod Dev 2010; 56: 230–235. [DOI] [PubMed] [Google Scholar]
- 38.Yadav VK, Lakshmi G, Medhamurthy R. Prostaglandin F2alpha-mediated activation of apoptotic signaling cascades in the corpus luteum during apoptosis: involvement of caspase-activated DNase. J Biol Chem 2005; 280: 10357–10367. [DOI] [PubMed] [Google Scholar]
- 39.Komiyama J, Nishimura R, Lee HY, Sakumoto R, Tetsuka M, Acosta TJ, Skarzynski DJ, Okuda K. Cortisol is a suppressor of apoptosis in bovine corpus luteum. Biol Reprod 2008; 78: 888–895. [DOI] [PubMed] [Google Scholar]
- 40.Bowolaksono A, Nishimura R, Hojo T, Sakumoto R, Acosta TJ, Okuda K. Anti-apoptotic roles of prostaglandin E2 and F2α in bovine luteal steroidogenic cells. Biol Reprod 2008; 79: 310–317. [DOI] [PubMed] [Google Scholar]
- 41.Paape MJ, Bannerman DD, Zhao X, Lee JW. The bovine neutrophil: Structure and function in blood and milk. Vet Res 2003; 34: 597–627. [DOI] [PubMed] [Google Scholar]
- 42.Klipper E, Gilboa T, Levy N, Kisliouk T, Spanel-Borowski K, Meidan R. Characterization of endothelin-1 and nitric oxide generating systems in corpus luteum-derived endothelial cells. Reproduction 2004; 128: 463–473. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Sakamoto K. Reactive oxygen species up-regulates cyclooxygenase-2, p53, and Bax mRNA expression in bovine luteal cells. Biochem Biophys Res Commun 2001; 284: 203–210. [DOI] [PubMed] [Google Scholar]
- 44.Hojo T, Al-Zi’abi MO, Skarzynski DJ, Acosta TJ, Okuda K. Changes in the vasculature of bovine corpus luteum during the estrous cycle and prostaglandin F2α-induced luteolysis. J Reprod Dev 2009; 55: 512–517. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura R, Sakumoto R, Tatsukawa Y, Acosta TJ, Okuda K. Oxygen concentration is an important factor for modulating progesterone synthesis in bovine corpus luteum. Endocrinology 2006; 147: 4273–4280. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura R, Komiyama J, Tasaki Y, Acosta TJ, Okuda K. Hypoxia promotes luteal cell death in bovine corpus luteum. Biol Reprod 2008; 78: 529–536. [DOI] [PubMed] [Google Scholar]
- 47.Lockshin RA, Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol 2004; 36: 2405–2419. [DOI] [PubMed] [Google Scholar]
- 48.Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis 2014; 19: 555–566. [DOI] [PubMed] [Google Scholar]
- 49.Oral O, Akkoc Y, Bayraktar O, Gozuacik D. Physiological and pathological significance of the molecular cross-talk between autophagy and apoptosis. Histol Histopathol 2016; 31: 479–498. [DOI] [PubMed] [Google Scholar]
- 50.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 290: 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernard A, Klionsky DJ. Autophagosome formation: tracing the source. Dev Cell 2013; 25: 116–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010; 140: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19: 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eskelinen EL. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 2006; 27: 495–502. [DOI] [PubMed] [Google Scholar]
- 55.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 2011; 18: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, Cecconi F. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol 2013; 15: 406–416. [DOI] [PubMed] [Google Scholar]
- 57.Lee JS, Li Q, Lee JY, Lee SH, Jeong JH, Lee HR, Chang H, Zhou FC, Gao SJ, Liang C, Jung JU. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol 2009; 11: 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paavola LG. The corpus luteum of the guinea pig. III. Cytochemical studies on the Golgi complex and GERL during normal postpartum regression of luteal cells, emphasizing the origin of lysosomes and autophagic vacuoles. J Cell Biol 1978; 79: 59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Djavaheri-Mergny M, Amelotti M, Mathieu J, Besançon F, Bauvy C, Souquère S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem 2006; 281: 30373–30382. [DOI] [PubMed] [Google Scholar]
- 60.Zhang F, Zhao X, Shen H, Zhang C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int J Mol Med 2016; 37: 1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang YF, Hsu MC, Cheng CH, Tsui KH, Chiu CH. Ultrastructural changes of goat corpus luteum during the estrous cycle. Anim Reprod Sci 2016; 170: 38–50. [DOI] [PubMed] [Google Scholar]
- 62.Gawriluk TR, Ko C, Hong X, Christenson LK, Rucker EB, 3rd. Beclin-1 deficiency in the murine ovary results in the reduction of progesterone production to promote preterm labor. Proc Natl Acad Sci USA 2014; 111: E4194–E4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gawriluk TR, Rucker EB. BECN1, corpus luteum function, and preterm labor. Autophagy 2015; 11: 183–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008; 135: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11: 700–714. [DOI] [PubMed] [Google Scholar]
- 66.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ 2007; 14: 400–410. [DOI] [PubMed] [Google Scholar]
- 67.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 2008; 4: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell 2009; 138: 229–232. [DOI] [PubMed] [Google Scholar]
- 69.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009; 137: 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 2009; 137: 1100–1111. [DOI] [PubMed] [Google Scholar]
- 71.Rosales-Torres AM, Avalos-Rodríguez A, Vergara-Onofre M, Hernández-Pérez O, Ballesteros LM, García-Macedo R, Ortíz-Navarrete V, Rosado A. Multiparametric study of atresia in ewe antral follicles: histology, flow cytometry, internucleosomal DNA fragmentation, and lysosomal enzyme activities in granulosa cells and follicular fluid. Mol Reprod Dev 2000; 55: 270–281. [DOI] [PubMed] [Google Scholar]
- 72.Alonso-Pozos I, Rosales-Torres AM, Avalos-Rodríguez A, Vergara-Onofre M, Rosado-García A. Mechanism of granulosa cell death during follicular atresia depends on follicular size. Theriogenology 2003; 60: 1071–1081. [DOI] [PubMed] [Google Scholar]
- 73.Du Y, Bagnjuk K, Lawson MS, Xu J, Mayerhofer A. Acetylcholine and necroptosis are players in follicular development in primates. Sci Rep 2018; 8: 6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McEvoy MJ, Sinderewicz E, Creedon L, McAfee M, Jonczyk AW, Piotrowska-Tomala KK, Skarzynski DJ. Death processes in bovine theca and granulosa cells modelled and analysed using a systems biology approach. Int J Mol Sci 2021; 22: 4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 2000; 1: 489–495. [DOI] [PubMed] [Google Scholar]
- 76.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009; 325: 332–336. [DOI] [PubMed] [Google Scholar]
- 77.Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, Vaux DL. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis 2013; 4: e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012; 148: 213–227. [DOI] [PubMed] [Google Scholar]
- 79.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 2014; 16: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai Z, Liu ZG. Execution of RIPK3-regulated necrosis. Mol Cell Oncol 2014; 1: e960759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chan FK, Luz NF, Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol 2015; 33: 79–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He S, Huang S, Shen Z. Biomarkers for the detection of necroptosis. Cell Mol Life Sci 2016; 73: 2177–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hojo T, Piotrowska-Tomala KK, Jonczyk AW, Lukasik K, Jankowska K, Okuda K, Witek KJ, Skarzynski DJ. Receptor interacting protein kinases-dependent necroptosis as a new, potent mechanism for elimination of the endothelial cells during luteolysis in cow. Theriogenology 2019; 128: 193–200. [DOI] [PubMed] [Google Scholar]
- 84.Jonczyk AW, Piotrowska-Tomala KK, Skarzynski DJ. Effects of prostaglandin F2α (PGF2α) on cell-death pathways in the bovine corpus luteum (CL). BMC Vet Res 2019; 15: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jonczyk AW, Piotrowska-Tomala KK, Skarzynski DJ. Comparison of intra-CL injection and peripheral application of prostaglandin F2α analog on luteal blood flow and secretory function of the bovine corpus luteum. Front Vet Sci 2022; 8: 811809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011; 471: 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weinlich R, Dillon CP, Green DR. Ripped to death. Trends Cell Biol 2011; 21: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skarzynski DJ, Piotrowska-Tomala KK, Lukasik K, Galvão A, Farberov S, Zalman Y, Meidan R. Growth and regression in bovine corpora lutea: regulation by local survival and death pathways. Reprod Domest Anim 2013; 48(Suppl 1): 25–37. [DOI] [PubMed] [Google Scholar]
- 89.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 2013; 1833: 3448–3459. [DOI] [PubMed] [Google Scholar]