Abstract
Surgical resection remains a mainstay in the treatment of malignant solid tumors. However, the use of neoadjuvant treatments, including chemotherapy, radiotherapy, phototherapy, and immunotherapy, either alone or in combination, as a preoperative intervention regimen, have attracted increasing attention in the last decade. Early randomized, controlled trials in some tumor settings have not shown a significant difference between the survival rates in long-term neoadjuvant therapy and adjuvant therapy. However, this has not hampered the increasing use of neoadjuvant treatments in clinical practice, due to its evident downstaging of primary tumors to delineate the surgical margin, tailoring systemic therapy response as a clinical tool to optimize subsequent therapeutic regimens, and decreasing the need for surgery, with its potential for increased morbidity. The recent expansion of nanotechnology-based nanomedicine and related medical technologies provides a new approach to address the current challenges of neoadjuvant therapy for preoperative therapeutics. This review not only summarizes how nanomedicine plays an important role in a range of neoadjuvant therapeutic modalities, but also highlights the potential use of nanomedicine as neoadjuvant therapy in preclinical and clinic settings for tumor management.
Keywords: Neoadjuvant treatment, Preoperative preparation, Surgical resection, Nanotechnology
Graphical abstract
Highlights
-
•
Neoadjuvant treatment, as one of the most important tumors preoperative inventions has attracted increasing attentions.
-
•
Nanotechnology exhibits tremendoussuperiority in neoadjuvant treatment due to its specific advantages in drug delivery.
-
•
This review systematically summarizes the application of nanomedicine in neoadjuvant therapy and looks at its trends.
1. Introduction
Despite advances in theranostic technology for oncology, conventional surgical resection is still a mainstay in the treatment of most solid tumors. However, new surgical approaches, including multimodal-imaging–guided accurate surgical resection [1,2], laparoscopic and robotic dominated surgery with minimal invasion [3], and improved preoperative preparation and careful postoperative nursing care [[4], [5], [6]], are constantly improving the outcome and efficiency of surgical treatment. Preoperative treatment (also termed neoadjuvant therapy), defined as a therapeutic course of treatment before surgery is performed [5], has attracted increasing attention. Preoperative treatment has many advantages, including downstaging of the primary tumor to reduce the burden of local and regional disease, delineating the tumor margins for ease of resection and reduced over-excision of adjacent health tissue, and tailoring systemic therapy response as a clinical tool to optimize follow up therapy (adjuvant therapy) [7,8]. However, some early randomized trials showed that the addition of neoadjuvant chemotherapy (NACT) to the therapeutic regimen did not lead to significant differences in survival or long-term outcome compared to conventional adjuvant therapy, despite using the same therapeutic agents in breast cancer patients [9]. Similarly, long-term survival remains largely unchanged in rectal cancer patients who received standard neoadjuvant chemoradiotherapy (nCRT) [10].
Recently, a larger randomized clinical trial found that compared to either capecitabine or gemcitabine alone, the addition of bevacizumab, a monoclonal antibody which targets vascular endothelial growth factor, to the neoadjuvant therapy regimen could significantly increase the complete pathological response rate without any obvious toxic side effects [11]. These exciting results suggest that targeted therapy will be more effective in neoadjuvant treatment. Analogically, the targeted delivery of neoadjuvant therapeutics agents to tumors would elicit similar neoadjuvant therapeutic effect. Consequently, nanotechnology-based nanomedicine was exploited for neoadjuvant treatment due to its ability to target cargos to specific sites, such as tumors [12]. Nanomedicine has several advantages, including safe and effective targeted drug delivery, controlled cargo release and facile multi-modal theranostic integration with few side effects. Further, the use of nanoparticles in medicine has been extensively researched with multiple applications in the diagnosis and treatment of cancer [[12], [13], [14]]. However, the application of nanomedicine as neoadjuvant requires more research. Recently, some clinical trials have adopted universal nanomedicine approaches, such as nanoparticle albumin-bound paclitaxel [[15], [16], [17]] and liposome-encapsulated doxorubicin [18,19], into their neoadjuvant regimen. These studies have achieved some significant outcomes, including increasing the proportion of patients achieving a pathological complete response, while verifying the safety and efficacy of the preoperative, neoadjuvant treatment formulations used. The success of these studies has increased interest in research devoted to the application of nanomedicine in neoadjuvant treatment [20,21]. The rapid advance of nanotechnology-based nanomedicine and its importance in medical practice may further promote the clinical use of neoadjuvant treatment.
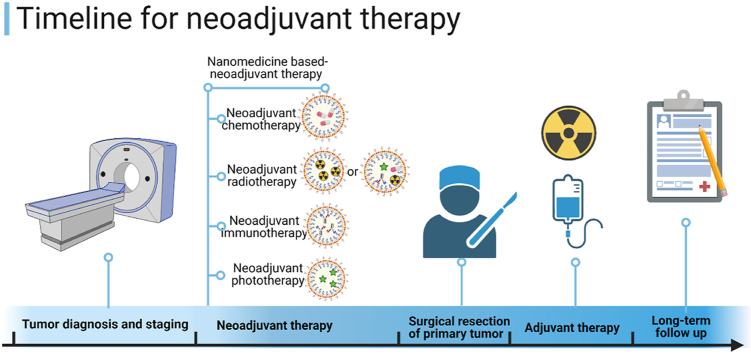
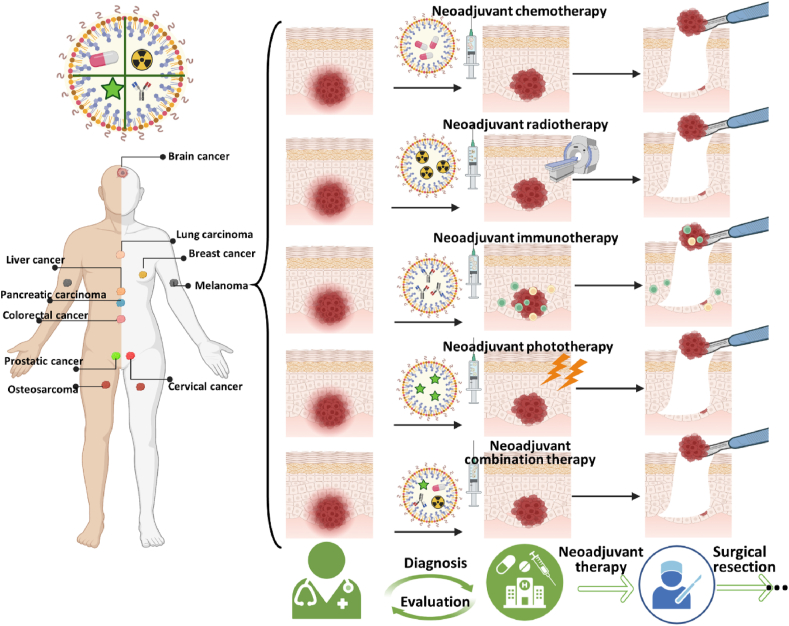
Currently, there are no comprehensive reviews which address the development of nanomedicine-based neoadjuvant therapy. Therefore, we will briefly describe several neoadjuvant therapy modalities, including neoadjuvant use of chemotherapy, radiotherapy, and phototherapy, either alone or in combination. We will then summarize the application of nanomedicine as a neoadjuvant therapy in both preclinical and clinical practices, and discuss its potential future applications (Fig. 1).
Fig. 1.
Scheme of nanotechnology-assisted preoperative therapeutic strategies, including neoadjuvant chemotherapy, neoadjuvant radiotherapy, neoadjuvant immunotherapy, and neoadjuvant phototherapy, as singular as well as combination therapies. Created with permission by BioRender.com.
2. Nanotechnology in neoadjuvant chemotherapy
The deployment of nanotechnology and its extensive application to NACT may overcome the intrinsic theoretical and clinical limitations of NACT, opening up new areas for preoperative drug management. The following sections will focus on the superiority of nanotechnology-based anti-tumor drugs as NACT:
2.1. Using nanotechnology to combine multiple chemotherapeutic agents in a single cart
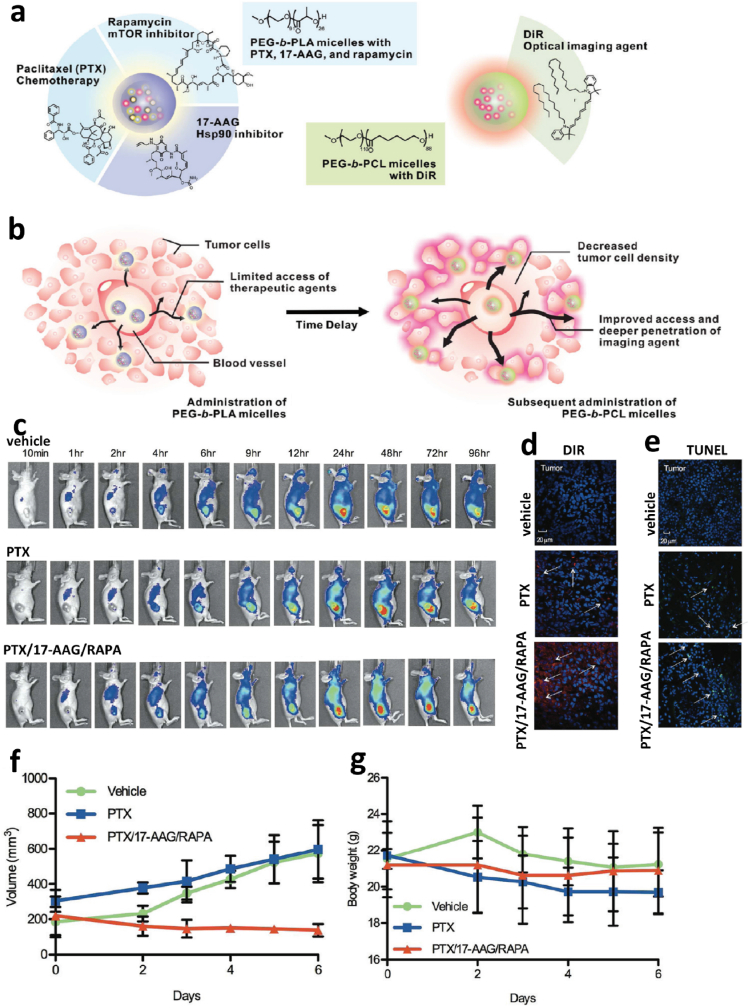
Combining multiple chemotherapeutic agents is the most common regimen in NACT. For example, combining taxanes and anthracyclines is standard for NACT for high-risk breast cancer [22], combining epirubicin/cyclophosphamide (EC) with sequential weekly paclitaxel, followed by 5-FU/epirubicin/cyclophosphamide (FEC) [23], and sequentially adding preoperative docetaxel to doxorubicin and cyclophosphamide for breast cancer [24,25]. From a pharmacological point of view, these combinations and/or sequential administrations aim to create a favorable condition prior to surgical resection of the tumor. NACT directly kills tumor cells and modifies the tumor microenvironment by downstaging the primary tumor, reducing tumor size, outlining the lesion margins, tailoring the following adjuvant chemotherapy, and eradicating micro-metastatic lesions, which may be present but are undetectable [26]. However, meta-analysis of multiple randomized studies indicated that NACT does not increase overall survival of breast cancer patients when compared with the same chemotherapy regimen administered as adjuvant chemotherapy [9,27]. However, some other benefits emerged with NACT, including the opportunity for breast-conserving surgery, reducing axillary lymph-node dissection in breast cancer cases, tailoring systemic adjuvant therapy tactics, and understanding the complete pathological response [1]. Multiple medical and biological factors, such as the tumor stage and pathology grade, tumor heterogeneity, and the differential expression of markers on tumor cells, may account for these paradoxical phenomena. Notably, these contradictions may also derive from differences in the pharmacokinetics of individual chemotherapeutics. Due to the different physicochemical properties, each chemotherapeutic agent used in NACT has a unique pharmacodynamic profile with respect to its absorption, tissue distribution, metabolism, and excretion. Thus, the composition and ratio of each chemotherapeutic agent in the primary tumor may deviate from expectations. From a pharmacokinetic point of view, the integration of all NACT agents into a single delivery vehicle may bring benefits to NACT. The rapid advance of nanotechnology provides an excellent drug delivery platform to achieve this for NACT. On one hand, different chemotherapeutic agents are readily loaded or grafted to nanoparticles (termed nanomedicine) in a specific ratio by optimizing several factors, including their feed, loading condition through hydrophobic interaction, electrostatic interactions, physical restraint, and covalent binding. On the other hand, by designing and constructing an appropriate nano-carrier, such as tumor microenvironment-responsive nanomedicine and stimuli-responsive nanoparticles, it is possible to realize the precise and spatiotemporal release of carried cargos (controlled release), thus potentiating the therapeutic efficacy and minimizing side effects. At present, polymeric micelles are the main kind of nano-carriers investigated for drug delivery and several are being used in clinical trials [28,29]. One of representative polymers is Poly(ethylene glycol)-block-poly(D,l-lactic acid) (PEG-b-PLA), approved by the FDA as a pharmaceutical excipient [30], is widely used as a multiple anti-tumor drug nano-container. For instance, poorly water-soluble anti-cancer drugs paclitaxel (PTX), 17-allylamino-17-demethoxygeldanamycin (17-AAG), and rapamycin (RAPA) are readily co-loaded into the hydrophobic core of PEG-b-PLA micelles in defined proportions through optimization of the loading conditions [31]. The 3-in-1 PEG-b-PLA micelles exhibited strong synergistic anti-tumor activity in vitro and was well-tolerated in FVB albino mice, providing a novel and simple approach for tumor combinational therapy [31]. In preclinical practice, the 3-in-1 polymeric micelles were used as a novel neoadjuvant cancer therapy approach in a LS180 human colon xenograft model, where a single intravenous injection reduced tumor volume 1.6-fold without significant change in body weight. Further, this would provide new approach for the use of near-infrared (NIR) region optical imaging for intraoperative surgical guidance in oncology (Fig. 2) [32].
Fig. 2.
Three-in-one nano-micelle-mediated tumor priming, tumor optical imaging and neoadjuvant chemotherapy. a. Scheme of 3-in-1 PEG-b-PLA nanoparticles containing PTX/17-AAG/RAPA, and diagram of DIR containing PEC-b-PCL micelles. b. Schematic illustration of PTX/17-AAG/RAPA nano-micelle-mediated tumor priming and subsequently promoted NIR optical imaging of tumor by DiR loaded PEC-b-PCL micelles. c. In vivo NIR imaging of tumor bearing mice after administering DiR-containing PEG-b-PCL micelles following vehicle, PTX, and PTX/17-AAG/RAPA injection for 48 h. d. Intratumoral distribution of DiR-containing PEG-b-PCL micelles in different treatment groups. e. Apoptotic cells in tumor tissue of different treatment groups. f. Tumor growth curve in different treatment groups. g. Mice body weight in different treatment groups [32]. Copyright 2011. American Chemical.
2.2. Targeting drug delivery to primary and micro-lesions
One of the primary aims of NACT is tumor shrinkage with clear margins to enable surgical resection, which requires the chemotherapeutics to be precisely targeted to the primary tumor lesion. However, most chemotherapeutic agents are limited by their pharmacodynamic distribution, so that only a small proportion reaches the tumor, especially for the tumor at early stages. In a large random clinical trial [11], it found that the addition of bevacizumab [11], a typical monoclonal antibody targeted agent, to the regimen of NACT for breast cancer could significantly increase the pathological complete response (PCR) (28.2% without bevacizumab vs. 34.5% with bevacizumab: p = 0.02). However, the addition of capecitabine or gemcitabine to the regimen did not significantly increase the PCR but increased the toxic side effects. These give us a clue that the deliberate addition of chemotherapeutics with high interaction between the chemotherapeutics and tumor cell could make more sense than adding a single agent. In this sense, nanoparticle-based chemotherapy (nanomedicine) would be more effective in NACT, based on the obvious advantages in drug delivery through the enhanced permeability and retention effect (EPR), passive targeting delivery, ligand/high-affinity moieties mediated active targeting delivery, and stimuli-responsive controlled drug delivery and release [22]. Nanomedicine is a significant part of recently conducted preclinical research and clinical practices in NACT. One of the typical representative nanomedicines is pegylated liposomal doxorubicin (PLDox). PLDox was the first FDA approved nano-formulation for cancer chemotherapy in 1995, including advanced ovarian and metastatic breast cancer. A prominent feature of PLDox is the surface PEGylation, which provides a hydrophilic layer and an opsonin-like function, thus decreasing the nonspecific removal by macrophages and increasing its stability in the circulatory system (also termed stealth function). In another multicenter phase Ⅱ study, it was found that the combination of PLDox (Caelyx®) and paclitaxel as NACT generated an active response in locally advanced breast cancer. Notably, 54% of patients had a partial response and 17% of patients had a complete response, with some manageable cutaneous toxicity [33]. Following this, a series of multicenter clinical trial studies further demonstrated that the addition of PLDox to the NACT regimen could also produce equivalent or improved pathological complete response rates compared to free doxorubicin or epirubicin, while significantly lowering the chemotherapy associated side-effects, such as neutropenia, vomiting and alopecia, and especially anthracycline-associated cardiac toxicity [34,35]. Another retrospective study found that the two-weekly regimens of albumin-bound paclitaxel (nab-P) followed by four cycles of dose-dense epirubicin and cyclophosphamide did not realize better pathological complete response than the solvent-based paclitaxel (sb-P) group across all patients. However, patients exhibited significantly higher pathological complete response rates in triple-negative breast cancer, indicating a two-weekly nab-p as NACT would be a promising choice for triple-negative breast cancer patients [15]. These advantages can be partially attributed to the passive targeting and distribution of chemotherapeutics mediated by nanoparticles in the primary tumor.
Although passive targeting is the primary foundation for most nanomedicine applied in clinical trials and practice as NACT, in recent years, more and more attention is being paid to active targeting by nanomedicines for NACT. Cai and co-authors developed a cisplatin (CDDP) delivery system (HA-TiO2), endowed with pH-responsive drug release and active targeting system to potentiate its application as NACT for ovarian cancer [36]. On one hand, hyaluronic acid (HA), modified on the surface of titanium dioxide nanoparticles (TiO2 NPs), can allow HA-TiO2 to actively interact with the CD44 receptor, which is highly expressed on ovarian cancer, triggering internalization of the nanoparticles into tumor cells through receptor-mediated internalization. On the other hand, CDDP loaded onto TiO2 NPs could respond to pH gradients (decreasing from pH 7.4 in the circulatory system to pH 5.0 in lysosomes) by rapidly releasing its cargo due to weakening interactions with the TiO2 NP [36]. These two remarkable features could maximize the anti-tumor effect of HA-TiO2 and minimize its potential toxicity in NACT. Apart from HA, many other ligands (including antibodies, aptamers and peptides) could be developed as targeting motifs to mediate the nanomedicine distribution. For example, by using a B5 peptide, which has high affinity for the low-density lipoprotein receptor-related protein-1 (LRP-1), Lee et al. developed a novel nanoparticle-based theranostic agent targeting LRP-1 for enhanced neoadjuvant radiotherapy in colorectal cancer [37]. The theranostic nanoparticles were constituted with human serum albumin nanoparticles (HAS), 5-FU as the chemo-radiotherapy agent and Cy7 fluorophore for diagnostic imaging. Importantly, the B5 peptide, which had high affinity with LRP-1, a radio-resistant marker protein of colorectal cancer (CRC), was decorated on the surface of the nanoparticles. This decoration made the nanoparticles accurately and actively target the designated protein for diagnostic imaging and eradicated the radio-resistant CRC through NACT [37].
2.3. “Tumor priming” and tumor microenvironment regulation
Partly due to the heterogeneity of tumors and the complexity of the tumor microenvironment, it is a difficult challenge to completely eradiate the tumor mass and tumor micro-lesions and prevent recurrence. The anatomical structure and pathophysiology of solid tumors could partially account for the intratumor barriers that hinder the delivery of therapeutic agents, including nanoparticles, to the tumor and their penetration depth within the tumor mass [32,38]. Firstly, the poor and discrepant intratumoral vascularization and uneven vessel distribution in tumor results in a heterogeneous distribution due to leakage of agents. The high intratumoral interstitial fluid pressure (IFP) could diminish EPR leaded leakage of nanoparticles from the blood vessels to the tumor interstitial region by diffusion [38]. Moreover, the abundant stromal cells and the dense extra-tumor matrix constitute a physical barrier to resist the diffusion of nanoparticles [39]. In terms of these issues, Au et al. proposed a new concept of tumor priming to improve the delivery and efficacy of nanomedicine in solid tumors [40]. Its key concept was that pretreatment with an optimized apoptosis-inducing agent during the appropriate time window could expand the interstitial space of tumor, increasing vessel diameter and blood perfusion, and thus enhance the delivery and anti-tumor efficacy of nanomedicine [40], which would create conditions for further surgery and other treatments. These tumor priming inducers could be chemotherapeutics, such as PTX [40,41], cyclophosphamide [42] or Bevacizumab [43]. As a different approach to the usual tumor priming regimen (free drug and a single administration), we recently developed a series of novel tumor targeting and priming strategies through metronomic application of nanoparticles actively targeted to the tumor neovasculature [[44], [45], [46], [47], [48]]. The tumor priming regulator PTX was loaded in PEGylated polylactide nanoparticles decorated with a tumor vascular endothelial cell targeting peptide F56, to formulate F56-PTX-NP. One of the prominent characterizations of F56-PTX-NP was that it could be metronomically administrated at a low dose of PTX, with negligible side effects for tumor priming, including lowering IFP, enhancing pO2 and increasing vascular perfusion of doxorubicin, and thereby significantly prolonging tumor vasculature normalization (by at least 9 days) compared to other antiangiogenic agents [44]. This could provide a longer window of opportunity for subsequent treatment. Similarly, Hyunah Cho and Glen S. Kwon also employed a nanomedicine strategy as NACT for tumor priming prior to optical imaging guiding operations [32].
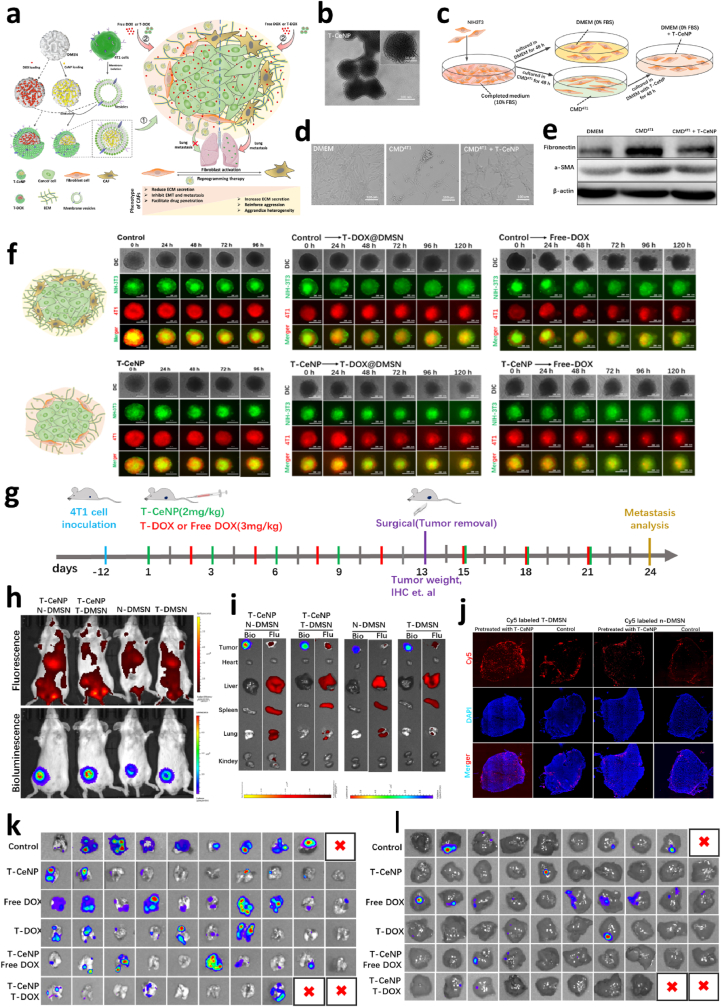
Apart from regulating the tumor mechanical environment, another important target of tumor priming by nanotechnology-based NACT are tumor stroma cells, including cancer associated fibroblasts (CAF), tumor-associated macrophages (TAM), immune-associated cells (such as Tregs and Bregs) and cancer stem cells (CSC) [[49], [50], [51]]. Taking CAF as an example, CAFs are the most abundant stromal cells in the TME in most tumors and can exert significant influence on the progression of tumor by regulating the phenotype of the stromal layer [52]. Therefore, re-programming tumor-promoting CAFs back to tumor suppressor fibroblasts is an emerging paradigm for combating primary tumors and their metastases [53]. We recently developed a new kind of biomimetic nanomedicine coupled with NCAT to remodel CAFs to quiescent fibroblasts, which can suppress the primary and metastatic tumors of breast cancer [20]. Thereof, we adopted a cancer cell membrane to camouflage cerium oxide nanoparticle (CeNP)-loaded dendritic mesoporous organosilica nanoparticles to develop a novel homologous nanomedicine (T-CeNP) as the additive agent of NACT (Fig. 3). It was found that T-CeNP could actively target breast cancer and reprogram CAF to quiescent fibroblast through its transdifferentiation function. The combination application of T-CeNP with chemotherapy as NACT or preventive usage alone could not only decrease the size of primary breast cancer but also inhibit the post-surgery lung metastasis and live metastasis [20].
Fig. 3.
Nanomedicine-dominated neoadjuvant-therapy-mediated tumor microenvironment remodeling and metastasis suppression. a. Scheme of the synthesis of T-CeNP and the remodeling of the tumor microenvironment. b. The TEM of T-CeNP. c. Diagrammatic illustration of T-CeNP-dominated cancer-associated fibroblast reprogramming. d. Representative morphological images of fibroblasts, cancer-associated fibroblasts, and reprogrammed fibroblasts. e. Validation of fibroblasts, cancer-associated fibroblasts, and reprogrammed fibroblasts by western blotting. f. T-CeNP-mediated cancer-associated fibroblast reprogramming promoted growth suppression of tumor spheroid in 3D cocultures of fibroblast and tumor cells. g. Diagram illustrating the therapy regimen of neoadjuvant therapy, surgery, and therapeutic evaluation. h. In vivo fluorescence imaging of nanoparticle distribution in tumors with or without neoadjuvant treatment. i. Ex vivo fluorescence imaging of nanoparticle distribution with or without neoadjuvant treatment. j. Fluorescence images of different nanoparticles in frozen tumor sections with or without neoadjuvant treatment. k. Ex vivo bioluminescence imaging for micrometastases in the lung of different treatment groups. l. Ex vivo bioluminescence imaging for micrometastases in the liver of different treatment groups. The red X represents animals that died during tumor removal surgery [20]. Copyright 2021, John Wiley and Sons.
TAM, as the main community of tumor-associated immune cells in TME, is a kind of immunosuppressive cell [54] which can hinder the infiltration and activation of T cells and minimize the anti-tumor efficacy of immune checkpoint blockade [54,55]. Much evidence shows a positive correlation between enrichment for TAM and tumor recurrence after surgery, and TAM reprogramming and/or depletion would elicit positive effects on preventing tumor recurrence after surgery [56,57]. It was found that in human breast cancer parenchyma, CD68+, but not stabilin-1+, TAMs negatively correlated with lymphatic metastasis after NACT [58]. The average score of CD68 expression in patients with a complete response to NACT in the coarse fibrous stroma of breast cancer was lower than that in patients with a partial response [58], which means that NACT could elicit different effects on TAM function depending on their location and local TME. Li et al. developed a biocompatible alginate-based hydrogel encapsulating Pexidartinib (PLX) loaded nanoparticles and anti-PD-1-conjugated platelets to target and deplete TAM. This formulation may facilitate the infiltration of T cells and block the PD-1 receptors, re-activating the T cells and thus enhancing post-surgery treatment of recurrent tumors [56].
3. Nanotechnology in neoadjuvant radiotherapy
Similar to NACT, the goal of neoadjuvant radiotherapy is also to minimize the primary tumor and clear the margins prior to surgical resection. It has been demonstrated that both short- and long-course pre-operative radiotherapy confers benefits regarding local recurrence. It is worth noting that some patients could completely respond to radiotherapy, but were included in surveillance programs without undergoing surgery. A fraction of patients responded poorly to radiotherapy but suffered radiotherapy-related toxicity, negatively impacting patients' health and quality of life [59]. Therefore, to minimize radiotherapy-associated side effects and improve patients’ outcomes, it is imperative to consider the sensitivity of patients to radiotherapy and develop general radiotherapy strategies. The role of neoadjuvant radiotherapy, especially nanotechnology-based neoadjuvant radiotherapy, in the management of cancer, will be discussed in the following section:
3.1. Neoadjuvant radiotherapy in cancer management
Radiation therapy, an important component of neoadjuvant treatment, is advocated by the European Society of Medical Oncology (ESMO) when the setting of the tumor may compromise the surgical outcome, including advanced disease (>T3), lymph node involvement on imaging and pre-operative disease (circumferential resection margin) [60]. For example, in the management of rectal cancer, if the anal sphincter is involved, successful neoadjuvant therapy may be able to downsize a tumor and provide a safe resection margin for surgery, increasing the chance of preserving the anal sphincter and maintaining anal continence [61]. Neoadjuvant treatment may be completely effective in certain cases, with the tumor replaced by fibrous tissue after a course of radiation therapy. Generally, whether a patient should be treated with neoadjuvant therapy is dependent upon the stage of the tumor. For instance, a thorough medical history, physical examination, digital rectal examination, and radiographic examination are essential for determining the stage of rectal cancer [62]. An unavoidable problem of radiotherapy, including neoadjuvant radiotherapy, are side effects (complications and toxicities). Sauer et al. documented all instances of toxicity in their cohort of patients [63]. Neoadjuvant patients experienced 27% toxicity in the acute phase, and 12% experienced diarrhea. On the basis of long-term data for the same cohort, toxicity was reported at a rate of 14.4%, including 4% of those undergoing neoadjuvant treatment that developed a stricture at the site of the anastomosis. In 2017, the Trans-Tasman Oncology Group assessed the differences in immediate postoperative outcomes between patients receiving short-course versus long-course neoadjuvant therapy [64]. This study found that patients undergoing short-course radiation therapy had an increased risk of toxic events. The adverse events included proctitis (0% vs 3.7%, P = 0.016) and diarrhea (1.3% vs 14.2%, P < 0.001).
3.2. Applications of nanotechnology in neoadjuvant radiotherapy
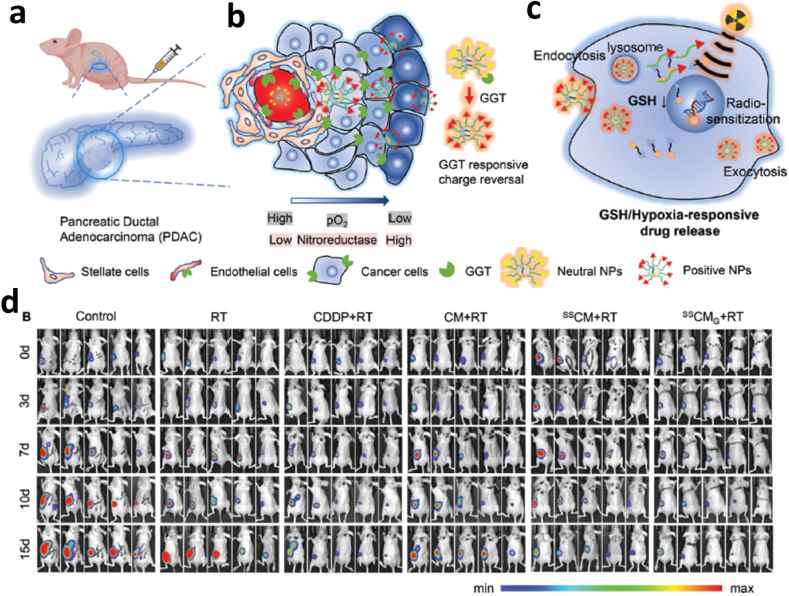
Nanotechnology-based contrast agents for radiotherapy and magnetic resonance imaging (MRI), PET, and CT, have gained attention in oncology due to their unique advantages in targeted radiotherapy, including a tailored distribution in tumor, radiation sensitization and their role as delivery vehicles [65]. The initial research on nanotechnology-based radiotherapy mainly focused on the design of nanoparticle-based contrast agents for increased accumulation in tumor, and some current research tentatively investigated the use of nanotechnology-mediated radiotherapy to modulate the tumor microenvironment for improved resection and treatment [66]. In some early phase I and phase I/II clinical trials, the tolerability and efficacy of nanoparticle-based radiotherapy were evaluated and validated [[67], [68], [69], [70]]. Additional basic research and clinical trials further demonstrated the efficacy of nanomedicine-based radiotherapy in radioresistant cancers with tolerable toxicity, including sarcoma [71], head and neck squamous cell carcinoma [72], non-small cell lung cancer [73], and breast cancer [74]. Also, some pre-clinical research was preformed simultaneously to broaden the application of nanoparticles in neoadjuvant radiotherapy, especially preoperative chemoradiotherapy (CRT), mainly relying on the superiority of nanomedicine to deliver drugs to the tumor [[75], [76], [77]]. For example, to overcome the stromal and hypoxic tumor microenvironment and preoperative radioresistance in pancreatic ductal adenocarcinoma (PDAC) and increase the R0 resection rate and long-term survivor after CRT, Xiao Hu et al. developed a γ-glutamyl transpeptidase (GGT)/glutathione (GSH)/hypoxia triple-responsive prodrug nanosensitizers SSCMG NPs [78] (Fig. 4). SSCMG NPs were composed of a hypoxic radiosensitization prodrug CM and a triple-responsive nanoparticle prepared using SSG polymer, and which had the following advantages: (1) avoiding systemic toxicity of CM derived from its premature release in circulation system; (2) augmenting tumor penetration via GGT-activated charge reversal; (3) enhancing the radiotherapy efficacy due to the GSH-responsive release of CM and its hypoxia-induced chemoradio-sensitization activity. The results indicate that SSCMG NPs could shrink the subcutaneous and orthotopic PDAC after neoadjuvant CRT, verifying the potential of nanotechnology in neoadjuvant radiotherapy.
Fig. 4.
Deep-penetrating triple-responsive prodrug nanosensitizer Actuates Efficient CRT in PDAC a. Efficient PDAC tumor accumulation, b. GGT-triggered tumor penetration, c. The potential mechanism of GSH/hypoxia dual-responsive drug release and hypoxic chemo-radio sensitization, and d. Representative in vivo bioluminescence images of orthotopic PDAC models treated as indicated [78]. Copyright 2022, John Wiley and Sons.
Another representative of nanotechnology-based neoadjuvant radiotherapy is the application of gold nanoparticles. 177Lu-labeled nano-seed brachytherapy was reported by Yook et al. as a neoadjuvant treatment for locally-advanced breast cancers [79]. This novel neoadjuvant brachytherapy was developed using 30-nm gold nanoparticles (AuNP), with their surfaces modified with PEG chains to link DOTA for complexing with the β-particle emitter 177Lu, and panitumumab for targeting to epidermal growth factor receptors (EGFR) (177Lu-T-AuNP). It was found that these gold nano-seeds, when injected intravenously, could inhibit the growth of breast cancer tumors in CD-1 athymic mice and prolong the overall survival, with no adverse effects at high radiation doses.
Another promising and encouraging nanotechnology-based neoadjuvant radiotherapy is NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle used in preoperative treatment. In a phase I clinical trial, its optimized recommended dose was determined at 10% of baseline tumor volume for preoperative treatment in adult patients with locally-advanced soft tissue sarcoma [80]. In this setting, there was a clear increase in median tumor shrinkage (22% increase), with manageable toxicity after external beam radiotherapy. In a multi-center, phase II-III randomized trial of patients with locally advanced soft-tissue sarcoma, addition of NBTXR3 to the regime of neoadjuvant radiotherapy and its activation by radiotherapy could significantly increase the rate of pathological complete response compared with radiotherapy alone (16% vs 8%), without altering radiotherapy-related adverse effects [81]. All these positive pre-clinical and clinical results, especially with NBTXR3, further verify the efficacy and feasibility of nanomedicine-based neoadjuvant radiotherapy.
4. Nanotechnology in neoadjuvant immunotherapy
4.1. Immune checkpoint inhibitors for neoadjuvant immunotherapy
Immunotherapeutics, especially immune checkpoint inhibitor mediated immunotherapy, has demonstrated its clinical efficiency in treating different types of cancers [82,83]. A reasonable follow-up setting is the extensive application of immunotherapeutics as neoadjuvant therapies, by targeting the interaction between PD-1 and its ligands PD-L1/PD-L2 or CTLA-4 pathway using immune checkpoint inhibitors prior to curative surgery [84,85]. The potential benefits of neoadjuvant immunotherapy include: (1) eliminating micrometastases and suppressing recurrence by boosting anti-tumor immunity; (2) tailoring the effectiveness of the immunotherapy regimen while the disease is present in the patient; (3) reducing the tumor size, and delineating the tumor margin for surgery and decreasing surgical morbidity; (4) diminishing surgery-induced immunosuppressive effects, such as the secretion of glucocorticoids into the bloodstream, and the subsequent inhibitory effect on T cell proliferation and promotion of apoptosis of naïve T cells [[84], [85], [86]].
There are normally two mechanisms to account for the anti-tumor effect of immune checkpoint inhibitors. The first mechanism is the reinvigoration of the tumor-specific cytotoxic T lymphocytes present in the tumor microenvironment by the immune checkpoint inhibitors, resulting in the activation and proliferation of T cells. The second mechanism holds the view that the function of immune checkpoint inhibitors is mainly concentrated on the tumor antigens presenting capacity of dendritic cells to T cells, leading to T cells entering the circulation and migrating to the tumor lesion. Immune checkpoint inhibitor-based neoadjuvant immunotherapy has been applied and reported in clinical research. In a phase 1b clinical study, it was found that two cycles of neoadjuvant sintilimab treatment (intravenously, day 1 out of 22) in resectable NSCLC patients (stage IA–IIIB) prior to resection led to an encouraging 40.5% major pathological response [87], demonstrating the feasibility of neoadjuvant immunotherapy. Another clinical study in stage III melanoma patients demonstrated that the use of neoadjuvant immune checkpoint blockade (ICB) in perioperative immune therapy was significantly associated with improved 3-year distant disease-free survival (DDFS) (59% pathologic partial response and 13% pathologic complete response) compared with adjuvant ICB only [88]. As a biomarker of responses to immune checkpoint inhibitor-based immunotherapy, PD-L1/2 was also detected in other tumors, including hepatocellular carcinoma [89], ovarian cancer [90] and colorectal cancer [91], which indicates that immune checkpoint inhibitor-based neoadjuvant immunotherapy may be a universal and promising strategy for immunotherapy.
Although immune checkpoint inhibitors, such as anti–PD-L1 which mainly target intertumoral CD8+ T cell exhaustion, are broadly active and regarded as a “common denominator” for neoadjuvant immunotherapy [92], other targets and methodologies might also elicit durable anti-tumor immune activity during neoadjuvant immunotherapy. These strategies include: (1) targeted depletion of suppressive T regulatory cells (Tregs); (2) reactivating the function of disrupted natural killer cells; (3) enhancing the infiltration and function of antigen-presenting cells (APC) in the tumor; (4) blocking the suppressive signals generated by TAMs, myeloid-derived suppressor cells, and other suppressive factors [93]. Therefore, the performance of neoadjuvant immunotherapy should take full account of the characteristics of the tumor immune microenvironment, the regimen of neoadjuvant immunotherapy and overall tumor prognosis.
4.2. Multiple nanomaterials induce anti-tumor immune responses for cancer immunotherapy
Although surgery, chemotherapy, and radiotherapy are the most common treatments for tumors, tumor relapse and metastasis are two key reasons accounting for therapeutic failure in the clinic [94,95]. To achieve long-term success in cancer treatment (such as survival with tumor) by both decreasing size of primary tumors and reducing metastasis and recurrence, using tumor immunotherapy, which leverages the body's immune system to treat and prevent tumors, has become increasingly attractive [[96], [97], [98]]. However, there are several limitations in immunotherapy, such as the immunosuppressive tumor microenvironment, fluctuating patient responses, and low tumor specificity [99]. Recently, along with the advancement of nanotechnology and its involvement in immunotherapy (termed as nano-immunotherapy), nano-immunotherapy may be the game changer in the battle with tumors, by virtue of its desirable efficiency, safety, and patient compliance [97,100,101]. One of the prominent features of nano-immunotherapy is the specific delivery and/or co-delivery of immunomodulators and therapeutics agents to targeted immune cells to initiate specific immune responses, taking advantage of the superiority of nanotechnology in targeted drug delivery [95,102]. For example, Herck et al. developed a new type of transiently thermo-responsive, polymer-based nanoparticles to deliver amphotericin B (AmpB), an FDA-approved antifungal drug with Toll-like receptor (TLR) agonist activity. This nano-encapsulation technology not only avoids the poor aqueous solubility of AmpB and strongly reduced the cytotoxic effects, but also amplified its TLR-triggering capacity as a vaccine adjuvant, which was mediated through its acid responsive release profile at endosomal pH values [103].
Many nanomaterial-based nanoparticles, including lipid nanoparticles, polymer-based nanoparticles, and inorganic nanoparticles, serve as the delivery platform for immunomodulators, or direct immunomodulation itself can efficiently boost anti-tumor immune responses and provide more precise and effective tumor treatment, while minimizing the side effects [104]. For example, liposomes can be loaded with different peptides, mRNAs and small molecule inhibitors to induce a stronger cellular immune response and retard tumor proliferation [[105], [106], [107], [108], [109]]. Polymer-based micelles (such as polylactic-co-glycolic acid (PLGA) nanoparticles) and hydrogels can also deliver anti-tumor agents, immune checkpoint inhibitors, cytokines or peptides to specific targets to enhance anti-tumor immunotherapy efficacy and inhibit tumor growth [[110], [111], [112]]. In some settings, silica, iron oxide and gold nanoparticles can induce tumor cell apoptosis by recruiting immune cells to activate direct tumor cell killing, initiating immune responses through dendritic cells, and adaptive immune responses through T cells [[113], [114], [115]].
4.3. Nanotechnology-based neoadjuvant immunotherapy
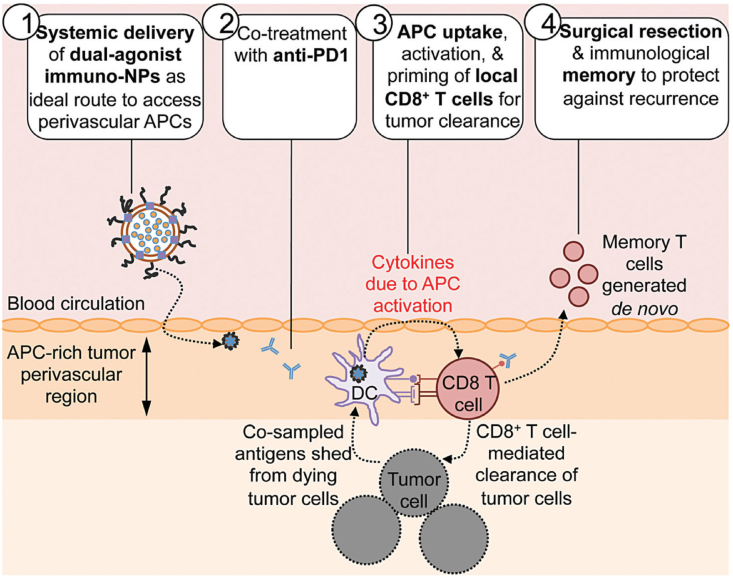
Similar to neoadjuvant immunotherapy, one objective of nanotechnology-based neoadjuvant immunotherapy is attaining a dynamic window for subsequent surgery and to sustain anti-tumor immune defense abilities [116,117]. Consequently, it is critical to optimize the methodology and window, establishing the accurate time, combination, sequence, and delivery for launching anti-tumor immunotherapy [116,118]. The amalgamation of nanotechnology and neoadjuvant immunotherapy yields nanotechnology-based neoadjuvant immunotherapy, which presents a new paradigm and endows a high potential for comprehensive cancer therapy. One of its prominent superiorities is the feasibility of neoadjuvant combination immunotherapy with the help of nanotechnology-nanoplatform [119]. For instance, to activate the otherwise “exhausted” CD8+ T cells and realize the transformation from the “cold” tumor to “hot”, Atukorale et al. developed a novel nanoparticle-based combinational neoadjuvant therapeutic strategy (Fig. 5) [119]. The lipid-based immunostimulatory nanoparticle (immuno-NP) co-encapsulates the STING and TLR4 agonists and delivers them to the tumor. The agonists then reprogram and activate the antigen-presenting cells in the immune suppressive tumor microenvironment, and harness and activate the otherwise “exhausted” CD8+ T cells in combination with the co-administered anti-PD1 immune checkpoint inhibitor. In three aggressive murine tumor models (B16F10 melanoma, and 4T1 and D2.A1 breast cancers), this nanoparticle-based neoadjuvant combination immunotherapy strategy could substantially reduce tumor size (up to 75%), creating favorable conditions for subsequent successful surgical resection. At the same time, it was found that this sequential treatment strategy (nanoparticle-based neoadjuvant combination immunotherapy followed by surgical resection) could also generate robust, protective immunological memory against recurrence, compared with adjuvant combination immunotherapy or surgical resection alone. This impressive outcome can be partly explained by the recruitment of tumor-specific CD8+ T cells, activated by nanoparticle-based neoadjuvant combination immunotherapy, and removal of the immunosuppressive tumor microenvironment by surgical resection [120].
Fig. 5.
Schematic combining systemic dual agonists (STING and TLR4) formulated in immunostimulatory lipid NPs (immuno-NP) with anti-PD1, as a powerful neoadjuvant immunotherapy [119]. Copyright 2009, Royal Society of Chemistry.
Apart from the paradigm of immunostimulatory neoadjuvant combination immunotherapy, nanoparticles can also be used in other neoadjuvant combination immunotherapies, such as neoadjuvant chemoimmunotherapy [121] (Section 6.3), cancer vaccination, and immune checkpoint blockade based combination immunotherapy [122], and TME-regulation based combination immunotherapy [118]. All these paradigms give us a fascinating insight into how combination immunotherapy depends on specific advantages of nanoparticle-based delivery systems, including specific and effective immune cell activation, improved targeted drug/immunomodulator delivery, cell targeting, limiting off systemic and target toxicity [122].
5. Nanotechnology in neoadjuvant phototherapy
5.1. Nano-phototherapy-assisted preoperative delineation of tumor
Surgical resection is a mainstay in the treatment of malignant tumors. As important as tumor diagnosis, the precise localization and clear delineation of the tumor is the prerequisite for precise resection, including consideration of the infiltrated growth nature of most tumor. The preoperative and intraoperative delineation of tumor is directly related to the success of complete tumor resection, minimizing damage to the surrounding healthy tissue and reducing complications and the risk of tumor recurrence. The rapid development of nanotechnology-based imaging provides a great opportunity to delineate the margins of the tumor for precise and complete surgical resection, especially for brain tumor [[123], [124], [125]].
Due to the complexity and critical functions of neurological structures and the invasive growth nature of brain tumors, complete brain tumor resection is one of the greatest challenges in surgery. Gliomas always grow adjacent to and/or infiltrate some crucial neurological structures [126,127], which makes it difficult to distinguish between the brain–tumor boundaries during the operation. Therefore, the choice between surgical resection and cytoreduction must be assessed after comprehensive consideration between postoperative recurrence and nonregenerative nerve injury [128]. To overcome the difficulty in delineating brain tumor margins preoperatively and intraoperatively, Gao et al. exploited the acidic tumor microenvironment and devised a pair of gold nanoprobes, which could not only cross the blood-brain barrier (BBB) and navigate to the brain tumor, but also assemble into bulk aggregates to form 3D spherical nanoclusters [129]. The surface of the Au-NPs was first given azide or alkyne functionality to generate Au-AZ and Au-AK, respectively, followed by conjugating Raman reporters (IR783B) and paramagnetic Chelators (Gd-DTPA) and shielding with a flexible PEG chain. The inward ends of PEG contained the acid-labile hydrazone bond, and their outward ends were modified with angiopep-2 peptide, which endowed the nanoparticles with active targeting to cross the BBB [130]. The difunctional PEG decoration not only endowed the nanoparticles with high colloidal stability and long circulation half-life, but could also respond to the acidic environment by removing the shielding layer to expose the inner azide and alkyne functionality, triggering copper-free cycloaddition and assembly of AuNP. The assembly of the paired nanoprobes would activate the surface-enhanced resonance Raman spectroscopy and MRI signals, which not only could define the location of brain tumor preoperatively (through MRI imaging) with high sensitivity and durability, but also delineate and distinguish the tumor margins to guide the tumor resection through Raman imaging (RI) by portable Raman device. This nanoparticle-based combination of MRI imaging for tumor location preoperatively and RI for guiding surgical resection intraoperatively could improve the outcome of brain-tumor surgery and represents a promising approach for clinical translation. Kircher et al. further developed a unique triple-modality MRI-photoacoustic imaging (PAI)-RI nanoparticle (MPRs) with high sensitivity, specificity and spatial resolution, to allow for more accurate brain tumor resection [125]. The MPRs integrate a gold core, the Raman molecular layer, and Gd3+ ions modification for PAI, RI, and MRI, respectively. The key merits of MPRs include: (i) real-time brain tumor localization in whole brain by magnetic resonance imaging macroscopically, (ii) high spatial and depth resolution and stereo-imaging by using PAI, and (iii) highly specific, distinct, and sensitive tumor margin imaging using RI.
Except for the primary tumor, lymph nodes are also important targets for the design of therapeutic strategies, and prognosis depends on accurate evaluation of lymph nodes metastasis preoperatively and metastatic lymph node resection. To this end, Dong et al. developed a natural cuttlefish melanin nanoprobe by integrating Gd ion-based MRI and near-infrared-II dye imaging for lymphatic location, to allow for assessment and accurate resection of lymph nodes [131]. In addition to Au nanoparticles, by taking full advantage of nanocarriers used in drug/cargo loading and delivery, other nanoparticles, such as Fe3O4 magnetic nanoparticles [123,132], mesoporous silica gadolinium [133], and hydrogel nanoparticles [134], can be used as a multifunctional nanoplatform for integrating optical imaging, fluorescence imaging, PAI, RI, and MRI probes for preoperative and intraoperative tumor imaging.
5.2. Nano-phototherapy mediated preoperative tumor remission
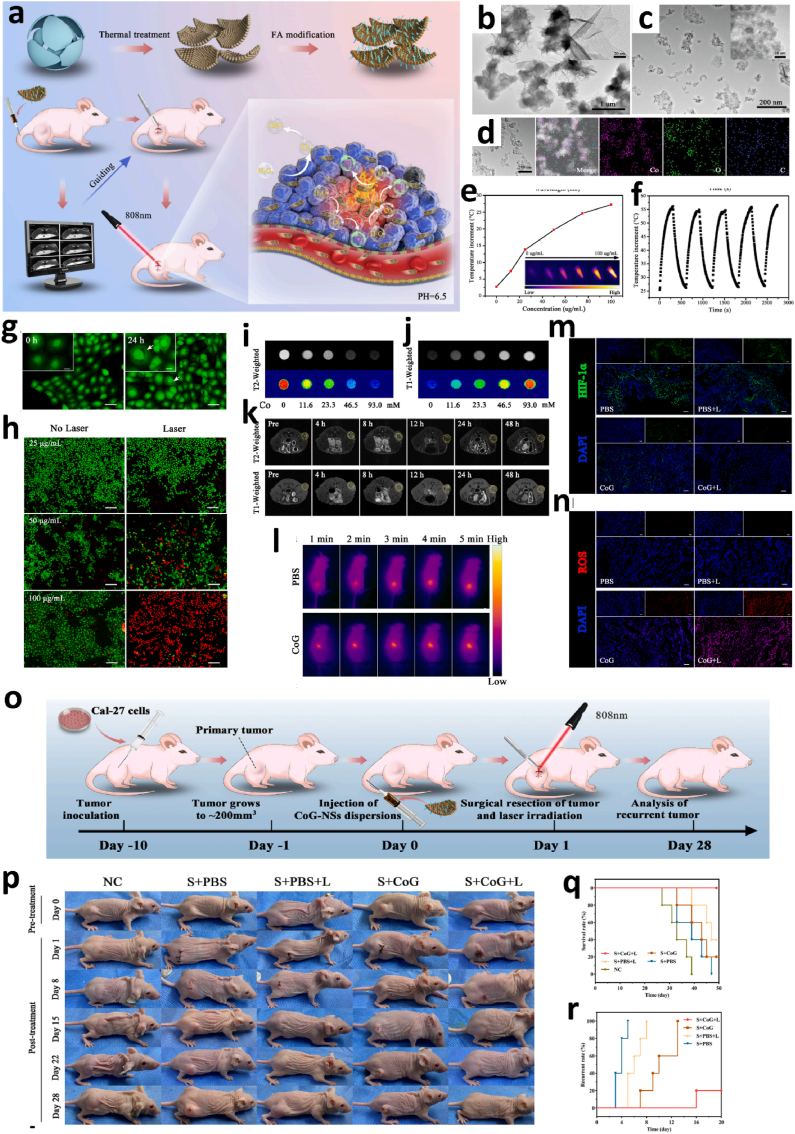
Phototherapy, including photothermal therapy (PTT) and photodynamic therapy (PDT), is a new medical treatment using natural or artificial light. Phototherapy has the advantages of high efficiency and reduced damage to the surrounding tissues, and thus, has been thoroughly investigated via basic research and applied widely in clinical practice, including tumor treatment [[135], [136], [137], [138]]. Recently, some preclinical research tried to apply phototherapy as a neoadjuvant treatment for preoperative application. For example, Huang et al. developed a versatile cobalt-glycerate nanoplatform (CoG-NS) with enhanced MRI for imaging-guided synergistic neoadjuvant PTT and PDT treatment of oral squamous cell carcinoma (OSCC) (Fig. 6) [139]. The constructed CoG-NS accumulated in the OSCC through enhanced permeability and retention (EPR)-based passive targeting. Folic acid grafted on the nanoparticles surface mediated active targeting, which could be used as an MRI contrast agent for tumor imaging guidance following phototherapy. Upon 808-nm laser irradiation, CoG-NS could efficiently transform the light to heat, inducing hyperthermia, and simultaneously activating the intrinsic catalytic potential of CoG-NS, generating oxygen and reactive oxygen species (ROS), realizing the synergetic phototherapy of combined PTT and PDT. After the tumor was surgically resected, the residual CoG-NS in unresected tumor cells could still provide syngeneic phototherapy by PTT and PDT, under laser irradiation, decreasing OSCC local recurrence and increasing the total survival time [139].
Fig. 6.
CoG-NS-mediated MR-guided neoadjuvant phototherapy for oral squamous cell carcinoma. a. Scheme of the synthesis of CoG-NSs and its MR-guided preoperative phototherapy for oral squamous cell carcinoma. b. TEM image of CoG-FSs. c. TEM image of CoG-NSs. d. Elemental mapping of CoG-NSs (EDS). e. Concentration dependent photothermal effect of CoG-NSs. f. Photothermal stability of CoG-NSs over laser on/off cycles. g. Uptake of CoG-NSs by CAL-27 cells (scale bar: 20 μm). h. Live/dead cells staining of CAL-27 cells with Calcein–AM (green: live cells) and PI (red: dead cells) after treatment with various concentration of CoG-NSs with or without laser (scale bar: 100 μm). i. Phantom T2-weighted MR images of different concentrations of CoG-NSs suspensions. j. Phantom T1-weighted MR images of different concentrations of CoG-NSs suspensions. k. In vivo axial T1 and T2 MR imaging of tumor at different time point post injection of CoG-NSs. l. In vivo infrared photothermal imaging of CAL-27 tumor. m. Immunofluorescence of HIF-1a expression in the tumor after different treatments (scale bar: 100 μm). n. DHE staining of ROS in tumor tissue in different groups (scale bar: 100 μm). o. The schedule of CoG-NS-mediated MR-guided neoadjuvant photo-therapy anticancer recurrence. p. Representative photos of CAL-27 tumor-bearing BALB/c nude mice in different treatment groups at different time. q. Survival curve of CAL-27 tumor-bearing BALB/c nude mice in different treatment groups. r. The recurrence of CAL-27 tumor-bearing BALB/c nude mice in different treatment groups [139]. Copyright 2022, Elsevier.
In a different approach from targeting tumor tissue with phototherapy for NACT, Deng et al. constructed a mitochondrially targeted and X-ray-activated PDT nanoconstruct for treatment of rectal cancer [140]. The photosensitizer verteporfin and ultrasmall Au-NPs, which are radiation enhancers, were co-loaded in biodegradable polymer poly(lactic-co-glycolic acid) (PLGA) nanocarriers, and conjugated with triphenylphosphonium, a mitochondria targeting moiety, on the surface of the nanoconstruct, generating X-PDT. It was founded that a single, low fraction of radiotherapy of up to 4 Gy (compared to high doses of 45 Gy used in standard fractionated radiotherapy) could induce significant cytotoxic-ROS generation within the mitochondria. This triggered loss of mitochondrial membrane potential and led to apoptosis of cancer cells. One prominent benefit of X-PDT is that it overcomes limitation of universal PDT, i.e., only acting on surface tumors, by acting on deep tumors using the tissue-penetrating properties of X-rays. Therefore, the direct integration of X-radiotherapy and PDT has significant potential for future clinical translation.
As aforementioned, an intrinsic disadvantage of near-infrared (NIR) light is its insufficient penetration depth, which limits the application of NIR-based phototherapy to surface tumors, and ineffective for deep abdominal tumors. To overcome this limitation, Li et al. developed a multifunctional IR820@PEG-SPIO, which integrated fluorescence, photoacoustic and magnetic resonance imaging capabilities to realize multi-modal–guided ablation of deep orthotopic hepatocellular carcinoma (HCC), with the assistance of minimally invasive laparoscopic-assisted photothermal ablation (L-A PTA) as a preoperative neoadjuvant treatment [141]. The IR820@PEG-SPIO was comprised of biodegradable superparamagnetic iron oxide (SPIO), MRI contrast agent gadolinium, and organic fluorescent contrast agent IR820. This formulation exhibited an ultrasmall size (14.3 nm), good biocompatibility, and excellent EPR-based passive target distribution capability. The integrated trimodal imaging (fluorescence imaging, PAI, and MRI) could cooperatively detect and co-locate HCC, even at the early-stage (≤2 mm), which would pave the way for subsequent accurate invasive laparoscopic-assisted PTT to completely ablate interior HCC. This study provided an excellent paradigm of multimodal-imaging–guided phototherapy for deep abdominal small tumor or micro-metastases.
6. Nanotechnology-mediated preoperative multimodal theranostics
Nanotechnology-mediated nanomedicine is a comprehensive discipline, involving in medicine, pharmaceutical science, materials science, chemistry, and biology. Its generalist feature makes it easy to realize the integration of multiple diagnostic and therapeutic modalities. Therefore, advances in nanotechnology can increase the precision of imaging-guided surgery and surgery-assisted synergistic therapy with adjuvant therapy (e.g., chemotherapy, immunotherapy, and phototherapy) [2]. Seizing this momentum, many attempts are being made to realize the promise of synthetic neoadjuvant therapy for the comprehensive tumor therapy.
6.1. Nanotechnology integrated neoadjuvant chemo-radiotherapy
The combined application of chemotherapy and radiotherapy is a universal therapeutic regimen for adjuvant and neoadjuvant treatment of tumors [[142], [143], [144]]. However, the overlapping toxicity of chemotherapy and radiotherapy is impediment to radiosensitizing chemotherapy [145]. Most recently, some preclinical research has put this combination into practice as adjuvant and neoadjuvant therapy, with the assistance of nanotechnology to provide the advantage of targeted drug delivery [[146], [147], [148]]. In a phase Ib/II trial of nanomedicine-based NACT and radiotherapy for locally-advanced rectal cancers, it was shown that the addition of nano-camptothecin CRLX101 to the standard neoadjuvant CRT was well tolerated in advanced rectal cancer patients at the weekly maximum tolerated dose (15 mg/m2), which represents a feasible clinical potential for nanomedicine use in CRT to improve therapeutic windows [149]. To reduce CRT-induced severe, general toxicity and improve its therapeutic efficacy and benefit, Zhang et al. developed a novel NACT-based on Abraxane/human neutrophils (NEs) cytopharmaceuticals with radiotherapy [148]. By virtue of the natural chemotaxis of NEs to inflammatory tumor, NEs were employed as Trojan Horse-like nanodrug carriers to deliver Abraxane, a nano-formulation of albumin-bound PTX, to cancer with guidance to the tumor through secreted inflammatory factors [127,150]. They further found that local, low dose radiotherapy not only caused tumor disruption, but also increased the release of inflammatory factors to guide more NEs to home in on the tumor. Simultaneously, these activated NEs burst to release Abraxane through neutrophil extracellular traps, realizing increased synthetic anti-tumor efficiency of chemotherapy and radiotherapy [148]. Moreover, by integrating targeting ligands and diagnostic probes into the neoadjuvant chemoradiotherapy nano-systems, it is easy to realize active and accurate tumor targeted neoadjuvant therapy and diagnostic imaging, providing primary data to develop the subsequent optimal treatment strategy. For example, Lee et al. developed an active targeted chemoradiotherapy nanosystem (Cy7–B5–HSA–5-FU NPs) by integrating low-density lipoprotein receptor-related protein-1 (LRP-1) affinity peptide B5 as the targeted ligand, 5-FU for chemo-radiotherapy, and Cy7 fluorophore for diagnostic imaging, into human serum albumin nanoparticles (HSA NPs), improving the effectiveness of neoadjuvant treatment and increasing the ratio of tumor CR [37].
6.2. Nanotechnology-integrated neoadjuvant chemo-phototherapy
For effective preoperative remission and prevention of postoperative recurrence and adhesion, Wei et al. developed a multifunctional injectable supramolecular drug-loaded nanocomposite hydrogel (NCH) by integrating oxaliplatin@cucurbit and AuNPs into chitosan/oxidized-konjac glucomannan matrixes (OxPt@CB-NCH) [151], to launch local and controllable NACT and PTT. Compared to systemic administration, this localized and injectable hydrogel, applied as a preoperative therapy strategy to eradicate or shrink tumors, has the several advantages. Firstly, oxaliplatin could response TME-overexpressed spermine to release from the hydrogel, to exert its selective anti-tumor effect and enhanced anti-tumor activity by consequent consumption of spermine, which would minimize its adverse effect. Secondly, in virtue of the non-invasiveness and high spatiotemporal controllability of PTT, a mild and uninjurious PTT (around 45 °C) was executed to facilitate the release of oxaliplatin, its intratumoral permeation and cellular internalization, and sensitization tumor cells to chemotherapy [152,153], leading to a synergistic enhancement by chemo-phototherapy. Moreover, it was also found that a single application of OxPt@CB-NCH for resection could still execute high anti-tumor efficiency in residual tumors to prevent tumor recurrence and act as an excellent barrier material to prevent postoperative adhesion. To reduce cisplatin-based NACT induced dose-dependent toxic side effects in its implementation for preventing bladder cancer progression and recurrence, Ding et al. developed a photoenhanced cancer chemotherapy (PECC) strategy based on AIEgen (BITT) [154]. They loaded BITT and the cisplatin (IV) prodrug (DSP) into the biocompatible and biodegradable bovine serum albumin (BSA) nanocarrier, yielding BITT@BSA−DSP NPs. It was found that integration of BITT could execute the NIR-imaging–guided photodynamic therapy (PDT) and photothermal therapy (PTT), and increase the sensitivity of bladder cancer to cisplatin chemotherapy, thereby realizing enhanced PECC efficiency with negligible side effects [154].
6.3. Nanotechnology integrated neoadjuvant combination immunotherapy
The unprecedented benefits of immunotherapy in treating advanced solid tumors has led to increased research into the adoption of immunotherapy for early stage solid tumors, including preoperative tumor treatment with neoadjuvant immunotherapy [155]. Similar to other neoadjuvant therapies, the primary objective of neoadjuvant immunotherapy is shrinking the primary tumor to minimize the extent and morbidity of subsequent surgery, evaluating and optimizing the therapeutic treatment regimens, and activating the immune system by making full use of the rich array of tumor antigens to combat micro-metastases and reduce recurrence [155]. These objectives (in other words benefits) are largely verified in recently published preclinical research [117,156,157]. It was found that most neoadjuvant immunotherapy was combined with other therapeutic modalities, including chemotherapy and radiotherapy [90]. The main function of these immune boosters is not to exhaust their capacity to eliminate to tumor cells, but to trigger tumor specific antigen release to elicit tumor specific immune response, thus taking full advantage of the “hot” feature of tumors in the early stage [158]. Recently, some research tentatively integrates neoadjuvant immunotherapy and other therapeutic modalities into the blooming nanomedicine field to maximize their anti-tumor effect, based on the ability of nanomedicine for targeted delivery of drug combinations and multi-modal tumor theranostics. For example, Atukorale et al. developed a neoadjuvant therapeutic strategy by combining the immune checkpoint inhibitor anti-PD1 with a powerful immunostimulatory nanoparticle (immuno-NP) to combat drug-resistant tumors and prevent relapse and metastasis [119]. It was shown that two synergistic immune-stimulators, the STING agonist (cdGMP) and the TLR agonist (MPLA) were readily co-encapsulated in lipid-based nanoparticles in a specific ratio (immune-NP) [159,160]. The immune-NP could trigger a 75-fold increase in production of interferon β in primary splenocytes compared to single-agonist treatment. An in vivo investigation using B16F10 melanoma and breast cancer models, found two key effects. First, the combination of immune-NP with anti-PD1 primed local CD8+ T cells and augmented their tumor-killing activity against the primary tumor by targeting and co-activating APC. Second, the treatment also generated a protective immunological memory response to prevent tumor recurrence [119]. Another powerful strategy is the amalgamation of NACT and neoadjuvant immunotherapy by leveraging the nanoplatform, termed neoadjuvant chemoimmunotherapy, to minimize post-surgical tumor metastasis and recurrence. Nanoplatform-mediated chemotherapy induces immunogenic tumor cell death, producing multiple tumor-specific antigen epitopes to mobilize the host immune system. Simultaneously, immunotherapeutic agents loaded in the nanoparticles, such as immune checkpoint inhibitors and immunoadjuvant, would boost and amplify the ICD-induced anti-tumor immune response [161]. One example is the tactical nanomissile (TALE) for neoadjuvant chemoimmunotherapy, developed by Gong, to mobilize anti-tumor immunity and minimize post-surgical tumor metastasis and recurrence [121]. The TALE was comprised of three functional ingredients, including mitoxantrone (Mit) as the ICD inducer, a PD-L1 immune checkpoint inhibitor and tumor targeting ligand, and AZO as the tumor intracellular azoreductase-responsive nanocarrier and immunoadjuvant, referred to mitoxantrone@anti-PD-L1/azobenzene/lipo (MPAL). MPAL as the neoadjuvant chemoimmunotherapy could generate an in situ tumor vaccine to stimulate the host immune response, and achieved primary tumor regression while minimizing post-surgical tumor metastases and recurrence [121].
6.4. Other nanotechnology-integrated neoadjuvant treatments
Apart from the aforementioned nanotechnology-mediated multiple neoadjuvant therapies, some other nanomedicine-integrated multiple neoadjuvant treatment models are being developed and display tremendous therapeutic potential and preventive anti-tumor immune benefits. For example, we developed biomimetic mesoporous silica nanoparticles to mediate the combination of TME reprogramming and NACT, which could significantly reduce the metastasis of breast cancers to other organs, including lungs and liver [20]. Deng et al. developed a mitochondrially-targeted poly(lactic-co-glycolic acid) (PLGA) nanocarrier loaded with the photosensitizer verteporfin and ultra-small gold nanoparticles to provide synergism with neoadjuvant X-ray radiation and photodynamic therapy (PDT), termed X-PDT treatment, which could amplify the efficacy of radiation for rectal cancer and reduce radiation-associated side effects [140]. By leveraging nano-photothermal therapy (NPTT), Yan et al. developed the new idea of “neoadjuvant NPTT” [21]. The key notion of neoadjuvant NPTT was that multimodal imaging guidance nanomedicine-mediated tumor preoperative phototherapy could shrink the tumor in a short time and make the tumor distinguishable from the adjacent normal tissue, which has high clinical potential when preparing for surgical resection [21]. Similar to neoadjuvant NPTT, nanoplatform-mediated MR-guided neoadjuvant photothermal/photodynamic therapy for oral squamous cell carcinoma was also developed and verified [139]. There is no doubt that all these integrated neoadjuvant treatments are based on the distinguishing multifunctional features of the nanoplatform, including the ability to deliver multiple cargos, targeted accumulation at specific destinations, and spatiotemporal coordination of cargo delivery. These properties will lead to a significant increase in the development of additional synergistic neoadjuvant treatments.
7. Outlook
For most solid tumors, surgical resection is still the primary and most effective therapeutic option. Therefore, the preoperative preparation for tumor resection (such as determination of malignant tumor margins), intraoperative imaging-guided surgery and postoperative complete resection verification and care are crucial for positive surgical outcomes [2]. The development and application of multifunctional nanoplatforms, which combine medical and optical imaging technologies, will pave the way for improved cancer theranostics and surgical resection. Conventional preoperative and intraoperative single imaging modalities have several short comings, such as limited spatial resolution of MRI, rapid clearance from body, insufficient surgical window of small molecular imaging, poor specificity, and limited penetration for optical imaging. By comparison, nanoplatform-based multimodal imaging could realize unparalleled advantages in increased sensitivity, specificity, and spatial resolution by integrating the merits of a range of imaging modalities and the superiority of nanotechnology into a single application. For example, triple-modality MRI-photoacoustic-Raman nanoparticles exerts obvious superiority in accurate brain-tumor imaging and resection [125]. The natural cuttlefish melanin nanoprobe exhibits specific advantages in preoperative and intraoperative mapping of lymphatic metastases in sentinel lymph nodes (SLN), by integrating NIR-II imaging and MRI into the cuttlefish melanin nanoparticle [131]. Although many imaging modalities could be formulated into the nanoplatform to construct multimodal imaging nanoprobes, appropriate and accessible imaging equipment must be identified, which is a challenge in the development of new multimodal imaging nanoprobes.
Tumor surgical resection is part of a comprehensive treatment management program which includes preoperative diagnosis, evaluation, neoadjuvant treatment, intraoperative real-time multimodal imaging and evaluation, multiple drug combination management and postoperative adjuvant treatment, recurrence prevention, wound healing care, and anti-infection management. However, few existing therapeutic paradigms are capable of simultaneously considering and covering this all-encompassing cancer management plan before and/or after surgery. Therefore, developing some reliable therapeutic modalities that can handle and cover the overall course of tumor management may be a convenient and universal strategy. Nanotechnology-based nanomedicine may turn these into reality. For example, Chen et al. developed the nanomedicine-based multifunctional injectable hydrogel, which not only displayed preoperative tumor burden remission via mild PTT-enhanced chemotherapy, but also effectively inhibited the postoperative tumor recurrence and prevented postoperative adhesion [151]. It is worth mentioning that nanomedicine which are not just limited to preoperative administration but adapted to the whole process of tumor management will be an exciting outcome.
Although nanomedicine-mediated tumor immunotherapy has achieved great progress, its application in neoadjuvant immunotherapy, especially neoadjuvant combination immunotherapy, is in its infancy. In fact, at early stages of the tumor, many proinflammatory anti-tumor immune cells, including M1 macrophages, DCs and CD8+ T cells exist in the TME in a quiescent manner, which has led to the label “hot” tumor [162]. This characterization of a “hot” tumor holds much therapeutic potential compared to its counterpart, the “cold” tumor. The need to transform the “cold” tumor to a “hot” tumor requires attention [163], and focusing on how to awaken and augment the anti-tumor immune reaction is critical. These dispositions include the targeted delivery of immune-stimulators to early-stage tumor microenvironment to activate innate and adaptive immune cells, and early interventions to instigate the release of tumor specific antigens to induce and enhance tumor specific immune response. Thus, it can be foreseen that nanoparticle-mediated delivery of immunomodulators to the primary tumor immunosuppressive environment, in combination with other therapy modalities, such as chemotherapy or phototherapy, to trigger the release of tumor specific antigens which activate the immune response, would hold great anti-tumor potential as neoadjuvant immunotherapy. In these situations, apart from the selection of immune modulatory agents, more attention should be paid to the optimization of the nano-delivery system, including their composition, surface modification, size and cargo release.
In addition to the primary tumor, another preoperative imaging and surgical target is the lymph nodes (LNs). Accurate preoperative imaging of LNs and their acquisition during and after surgery is of great significance to grading, treatment, and prognosis evaluation for tumors, especially in patients with gastric cancer [164,165]. Some studies have indicated that obtaining an adequate number of dissected lymph nodes is positively associated with improved survival rate [166,167]. This puts a higher demand on the LN imaging. Traditional optical dyes (such as Evans blue, indocyanine green, and IR820) and radiolabeled-tracer-based LN imaging have clinical limitations due to their insufficient accumulation, rapid clearance, and short retention time [165,168]. The advanced development of nanotechnology brings many new opportunities to preoperative LN imaging. For example, a dual-modal magnetic iron oxide nanoparticle, which combines MRI and NIR imaging, was successfully constructed and applied in intraoperative sentinel lymph node mapping, and exhibited high tissue penetration and sensitive detection [168]. A similar nanoprobe integrated near-infrared-II imaging, with naked eye identification and MRI, was also developed for preoperative and intraoperative evaluation of lymphatic metastasis with high accumulation, high sensitivity, and high signal-to-noise ratio [131]. More recently, it was found that preoperative gastroscopic submucosal injection of carbon nanoparticles in patients undergoing laparoscopic radical gastric cancer surgery could significantly shorten operation time and increase the number of total LNs resected to improve their accurate pathological staging [164].
Credit author statement
Xiaogang Qu: Writing-original draft. Dong Zhou: Writing-original draft. Jianpu Lu: Writing-original draft. Duotian Qin: Writing-original draft. Jun Zhou: Conceptualization, Writing-review & editing, Validation, Supervision. Hai-Jun Liu: Conceptualization, Writing-review & editing, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jun Zhou, Email: jzhou30@bwh.harvard.edu.
Hai-Jun Liu, Email: hliu40@bwh.harvard.edu.
References
- 1.King T.A., Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 2015;12:335–343. doi: 10.1038/nrclinonc.2015.63. [DOI] [PubMed] [Google Scholar]
- 2.Wang C., Fan W., Zhang Z., Wen Y., Xiong L., Chen X. Advanced nanotechnology leading the way to multimodal imaging‐guided precision surgical therapy. Adv. Mater. 2019;31 doi: 10.1002/adma.201904329. [DOI] [PubMed] [Google Scholar]
- 3.Berber E., Akyildiz H.Y., Aucejo F., Gunasekaran G., Chalikonda S., Fung J. Robotic versus laparoscopic resection of liver tumours. HPB. 2010;12:583–586. doi: 10.1111/j.1477-2574.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoo C.K., Vickery C.J., Forsyth N., Vinall N.S., Eyre-Brook I.A. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann. Surg. 2007;245:867. doi: 10.1097/01.sla.0000259219.08209.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd D.R., Brierley J.D., Baker T.P., Sullivan D.C., Gress D.M. Current and future cancer staging after neoadjuvant treatment for solid tumors. CA A Cancer J. Clin. 2021;71:140–148. doi: 10.3322/caac.21640. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang J., Bu Q., Tao N., Chen M., Liu H., Zhou J., Liu J., Deng B., Kong N., Zhang X. A facile and general method for synthesis of antibiotic-free protein-based hydrogel: wound dressing for the eradication of drug-resistant bacteria and biofilms. Bioact. Mater. 2022;18:446–458. doi: 10.1016/j.bioactmat.2022.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillen S., Schuster T., Meyer zum Büschenfelde C., Friess H., Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liedtke C., Mazouni C., Hess K.R., André F., Tordai A., Mejia J.A., Symmans W.F., Gonzalez-Angulo A.M., Hennessy B., Green M. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 9.Mauri D., Pavlidis N., Ioannidis J.P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J. Natl. Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 10.Zaborowski A., Stakelum A., Winter D. Systematic review of outcomes after total neoadjuvant therapy for locally advanced rectal cancer. Journal of British Surgery. 2019;106:979–987. doi: 10.1002/bjs.11171. [DOI] [PubMed] [Google Scholar]
- 11.Bear H.D., Tang G., Rastogi P., Geyer C.E., Jr., Robidoux A., Atkins J.N., Baez-Diaz L., Brufsky A.M., Mehta R.S., Fehrenbacher L. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N. Engl. J. Med. 2012;366:310–320. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Meel R., Sulheim E., Shi Y., Kiessling F., Mulder W.J., Lammers T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao W., Ji X., Zhu X., Li L., Wang J., Zhang Y., Saw P.E., Li W., Kong N., Islam M.A. Two‐dimensional antimonene‐based photonic nanomedicine for cancer theranostics. Adv. Mater. 2018;30 doi: 10.1002/adma.201802061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie F., Chen R., Zhang L., Yin Z., Zhu Q., You S., Jiang C., Li Y., Li S., Zha X. Efficacy of two-weekly nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy for breast cancer. Nanomedicine. 2019;14:1595–1603. doi: 10.2217/nnm-2018-0485. [DOI] [PubMed] [Google Scholar]
- 16.Futamura M., Nagao Y., Ishihara K., Takeuchi M., Nakada T., Kawaguchi Y., Asano M., Kumazawa I., Shiroko T., Morimitsu K. Preoperative neoadjuvant chemotherapy using nanoparticle albumin-bound paclitaxel followed by epirubicin and cyclophosphamide for operable breast cancer: a multicenter phase II trial. Breast Cancer. 2017;24:615–623. doi: 10.1007/s12282-016-0748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Untch M., Jackisch C., Schneeweiss A., Conrad B., Aktas B., Denkert C., Eidtmann H., Wiebringhaus H., Kümmel S., Hilfrich J. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto—GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17:345–356. doi: 10.1016/S1470-2045(15)00542-2. [DOI] [PubMed] [Google Scholar]
- 18.Saracchini S., Foltran L., Tuccia F., Bassini A., Sulfaro S., Micheli E., Del Conte A., Bertola M., Gion M., Lorenzon M. Phase II study of liposome-encapsulated doxorubicin plus cyclophosphamide, followed by sequential trastuzumab plus docetaxel as primary systemic therapy for breast cancer patients with HER2 overexpression or amplification. Breast. 2013;22:1101–1107. doi: 10.1016/j.breast.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Campos S. Liposomal anthracyclines: adjuvant and neoadjuvant therapy for breast cancer. Oncol. 2003;8:10–16. doi: 10.1634/theoncologist.8-suppl_2-10. [DOI] [PubMed] [Google Scholar]
- 20.Liu H.j., Wang J., Wang M., Wang Y., Shi S., Hu X., Zhang Q., Fan D., Xu P. Biomimetic nanomedicine coupled with neoadjuvant chemotherapy to suppress breast cancer metastasis via tumor microenvironment remodeling. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 21.Yan H., Shang W., Sun X., Zhao L., Wang X., Zhang S., Xu N., Xu W., Tian J., Kang F. Neoadjuvant nano-photothermal therapy used before operation effectively assists in surgery for breast cancer. Nanoscale. 2019;11:706–716. doi: 10.1039/c8nr08109c. [DOI] [PubMed] [Google Scholar]
- 22.Fraguas-Sánchez A., Martín-Sabroso C., Fernández-Carballido A., Torres-Suárez A. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother. Pharmacol. 2019;84:689–706. doi: 10.1007/s00280-019-03910-6. [DOI] [PubMed] [Google Scholar]
- 23.Oda N., Shimazu K., Naoi Y., Morimoto K., Shimomura A., Shimoda M., Kagara N., Maruyama N., Kim S.J., Noguchi S. Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Res. Treat. 2012;136:107–116. doi: 10.1007/s10549-012-2245-8. [DOI] [PubMed] [Google Scholar]
- 24.Bear H.D., Anderson S., Brown A., Smith R., Mamounas E.P., Fisher B., Margolese R., Theoret H., Soran A., Wickerham D.L. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Stover D.G., Bell C.F., Tolaney S.M. Neoadjuvant and adjuvant chemotherapy considerations for triple-negative breast cancer. AJHO. 2016;12:6–12. [Google Scholar]
- 26.DeMichele A., Yee D., Esserman L. Mechanisms of resistance to neoadjuvant chemotherapy in breast cancer. N. Engl. J. Med. 2017;377:2287–2289. doi: 10.1056/NEJMcibr1711545. [DOI] [PubMed] [Google Scholar]
- 27.Fisher B., Bryant J., Wolmark N., Mamounas E., Brown A., Fisher E.R., Wickerham D.L., Begovic M., DeCillis A., Robidoux A. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 28.Kim D.-W., Kim S.-Y., Kim H.-K., Kim S.-W., Shin S., Kim J., Park K., Lee M., Heo D.S. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2007;18:2009–2014. doi: 10.1093/annonc/mdm374. [DOI] [PubMed] [Google Scholar]
- 29.Cabral H., Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J. Contr. Release. 2014;190:465–476. doi: 10.1016/j.jconrel.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z., Xiong X., Wan J., Xiao L., Gan L., Feng Y., Xu H., Yang X. Cellular uptake and intracellular trafficking of PEG-b-PLA polymeric micelles. Biomaterials. 2012;33:7233–7240. doi: 10.1016/j.biomaterials.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 31.Shin H.-C., Alani A.W., Cho H., Bae Y., Kolesar J.M., Kwon G.S. A 3-in-1 polymeric micelle nanocontainer for poorly water-soluble drugs. Mol. Pharm. 2011;8:1257–1265. doi: 10.1021/mp2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho H., Kwon G.S. Polymeric micelles for neoadjuvant cancer therapy and tumor-primed optical imaging. ACS Nano. 2011;5:8721–8729. doi: 10.1021/nn202676u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gogas H., Papadimitriou C., Kalofonos H., Bafaloukos D., Fountzilas G., Tsavdaridis D., Anagnostopoulos A., Onyenadum A., Papakostas P., Economopoulos T. Neoadjuvant chemotherapy with a combination of pegylated liposomal doxorubicin (Caelyx®) and paclitaxel in locally advanced breast cancer: a phase II study by the Hellenic Cooperative Oncology Group. Ann. Oncol. 2002;13:1737–1742. doi: 10.1093/annonc/mdf284. [DOI] [PubMed] [Google Scholar]
- 34.Dong M., Luo L., Ying X., Lu X., Shen J., Jiang Z., Wang L. Comparable efficacy and less toxicity of pegylated liposomal doxorubicin versus epirubicin for neoadjuvant chemotherapy of breast cancer: a case-control study. OncoTargets Ther. 2018;11:4247. doi: 10.2147/OTT.S162003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R., Tian F., Qi Y., Ma L., Zhou T., Li Y., Hui T., Zhang L., Wang S., Song Z. Pegylated liposomal doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant chemotherapy in locally advanced breast cancer (registration number: ChiCTR1900023052) Sci. Rep. 2019;9:1–8. doi: 10.1038/s41598-019-54387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu E., Zhou Y., Liu Z., Li J., Zhang D., Chen J., Cai Z. Cisplatin loaded hyaluronic acid modified TiO2 nanoparticles for neoadjuvant chemotherapy of ovarian cancer. J. Nanomater. 2015:2015. [Google Scholar]
- 37.Lee K.J., Ko E.J., Park Y.-Y., Park S.S., Ju E.J., Park J., Shin S.H., Suh Y.-A., Hong S.-M., Park I.J. A novel nanoparticle-based theranostic agent targeting LRP-1 enhances the efficacy of neoadjuvant radiotherapy in colorectal cancer. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120151. [DOI] [PubMed] [Google Scholar]
- 38.Dong X., Liu H.-J., Feng H.-Y., Yang S.-C., Liu X.-L., Lai X., Lu Q., Lovell J.F., Chen H.-Z., Fang C. Enhanced drug delivery by nanoscale integration of a nitric oxide donor to induce tumor collagen depletion. Nano Lett. 2019;19:997–1008. doi: 10.1021/acs.nanolett.8b04236. [DOI] [PubMed] [Google Scholar]
- 39.Netti P.A., Berk D.A., Swartz M.A., Grodzinsky A.J., Jain R.K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 40.Lu D., Wientjes M.G., Lu Z., Au J.L.-S. Tumor priming enhances delivery and efficacy of nanomedicines. J. Pharmacol. Exp. Therapeut. 2007;322:80–88. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- 41.Wong H.L., Shen Z., Lu Z., Wientjes M.G., Au J.L.-S. Paclitaxel tumor-priming enhances siRNA delivery and transfection in 3-dimensional tumor cultures. Mol. Pharm. 2011;8:833–840. doi: 10.1021/mp1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geretti E., Leonard S.C., Dumont N., Lee H., Zheng J., De Souza R., Gaddy D.F., Espelin C.W., Jaffray D.A., Moyo V. Cyclophosphamide-mediated tumor priming for enhanced delivery and antitumor activity of HER2-targeted liposomal doxorubicin (MM-302) predose with cyclophosphamide enhances MM-302 tumor delivery. Mol. Cancer Therapeut. 2015;14:2060–2071. doi: 10.1158/1535-7163.MCT-15-0314. [DOI] [PubMed] [Google Scholar]
- 43.Khawar I.A., Kim J.H., Kuh H.-J. Improving drug delivery to solid tumors: priming the tumor microenvironment. J. Contr. Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Luan X., Guan Y.-Y., Lovell J.F., Zhao M., Lu Q., Liu Y.-R., Liu H.-J., Gao Y.-G., Dong X., Yang S.-C. Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel. Biomaterials. 2016;95:60–73. doi: 10.1016/j.biomaterials.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Guan Y.-Y., Luan X., Xu J.-R., Liu Y.-R., Lu Q., Wang C., Liu H.-J., Gao Y.-G., Chen H.-Z., Fang C. Selective eradication of tumor vascular pericytes by peptide-conjugated nanoparticles for antiangiogenic therapy of melanoma lung metastasis. Biomaterials. 2014;35:3060–3070. doi: 10.1016/j.biomaterials.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y.-R., Guan Y.-Y., Luan X., Lu Q., Wang C., Liu H.-J., Gao Y.-G., Yang S.-C., Dong X., Chen H.-Z. Delta-like ligand 4-targeted nanomedicine for antiangiogenic cancer therapy. Biomaterials. 2015;42:161–171. doi: 10.1016/j.biomaterials.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 47.Luan X., Guan Y.Y., Liu H.J., Lu Q., Zhao M., Sun D., Lovell J.F., Sun P., Chen H.Z., Fang C. A tumor vascular‐targeted interlocking trimodal nanosystem that induces and exploits hypoxia. Adv. Sci. 2018;5 doi: 10.1002/advs.201800034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu D.-H., Liu Y.-R., Luan X., Liu H.-J., Gao Y.-G., Wu H., Fang C., Chen H.-Z. IF7-conjugated nanoparticles target Annexin 1 of tumor vasculature against P-gp mediated multidrug resistance. Bioconjugate Chem. 2015;26:1702–1712. doi: 10.1021/acs.bioconjchem.5b00283. [DOI] [PubMed] [Google Scholar]
- 49.Dong X., Mu L.L., Liu X.L., Zhu H., Yang S.C., Lai X., Liu H.J., Feng H.Y., Lu Q., Zhou B.B.S. Biomimetic, hypoxia‐responsive nanoparticles overcome residual chemoresistant leukemic cells with co‐Targeting of therapy‐induced bone marrow niches. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 50.Whiteside T. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinshaw D.C., Shevde L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., Fearon D., Greten F.R., Hingorani S.R., Hunter T. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X., Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 54.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen M., Miao Y., Qian K., Zhou X., Guo L., Qiu Y., Wang R., Gan Y., Zhang X. Detachable liposomes combined immunochemotherapy for enhanced triple-negative breast cancer treatment through reprogramming of tumor-associated macrophages. Nano Lett. 2021;21:6031–6041. doi: 10.1021/acs.nanolett.1c01210. [DOI] [PubMed] [Google Scholar]
- 56.Li Z., Ding Y., Liu J., Wang J., Mo F., Wang Y., Chen-Mayfield T.-J., Sondel P.M., Hong S., Hu Q. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat. Commun. 2022;13:1–15. doi: 10.1038/s41467-022-29388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georgoudaki A.-M., Prokopec K.E., Boura V.F., Hellqvist E., Sohn S., Östling J., Dahan R., Harris R.A., Rantalainen M., Klevebring D. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000–2011. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 58.Mitrofanova I., Zavyalova M., Telegina N., Buldakov M., Riabov V., Cherdyntseva N., Kzhyshkowska J. Tumor-associated macrophages in human breast cancer parenchyma negatively correlate with lymphatic metastasis after neoadjuvant chemotherapy. Immunobiology. 2017;222:101–109. doi: 10.1016/j.imbio.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Yildirim E., Bektas S., Pelen Z., Yanik I., Er A.M., Cengel F., Gumuskaya P.Ö. Histopathological, radiological, and demographic factors predicting the response to neoadjuvant therapy for rectal cancer. J. Gastrointest. Cancer. 2022;53:22–30. doi: 10.1007/s12029-021-00697-9. [DOI] [PubMed] [Google Scholar]
- 60.Glynne-Jones R., Wyrwicz L., Tiret E., Brown G., Rödel C.d., Cervantes A., Arnold D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 61.Feeney G., Sehgal R., Sheehan M., Hogan A., Regan M., Joyce M., Kerin M. Neoadjuvant radiotherapy for rectal cancer management. World J. Gastroenterol. 2019;25:4850. doi: 10.3748/wjg.v25.i33.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valls C., Andía E., Sánchez A., Gumà A., Figueras J., Torras J., Serrano T. Hepatic metastases from colorectal cancer: preoperative detection and assessment of resectability with helical CT. Radiology. 2001;218:55–60. doi: 10.1148/radiology.218.1.r01dc1155. [DOI] [PubMed] [Google Scholar]
- 63.Sauer R., Becker H., Hohenberger W., Rödel C., Wittekind C., Fietkau R., Martus P., Tschmelitsch J., Hager E., Hess C.F. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 64.Ansari N., Solomon M.J., Fisher R.J., Mackay J., Burmeister B., Ackland S., Heriot A., Joseph D., McLachlan S.-A., McClure B. Acute adverse events and postoperative complications in a randomized trial of preoperative short-course radiotherapy versus long-course chemoradiotherapy for T3 adenocarcinoma of the rectum. Ann. Surg. 2017;265:882–888. doi: 10.1097/SLA.0000000000001987. [DOI] [PubMed] [Google Scholar]
- 65.Chatterjee D.K., Wolfe T., Lee J., Brown A.P., Singh P.K., Bhattarai S.R., Diagaradjane P., Krishnan S. Convergence of nanotechnology with radiation therapy—insights and implications for clinical translation. Transl. Cancer Res. 2013;2:256. doi: 10.3978/j.issn.2218-676X.2013.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Overchuk M., Zheng G. Overcoming obstacles in the tumor microenvironment: recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials. 2018;156:217–237. doi: 10.1016/j.biomaterials.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 67.Dipetrillo T., Milas L., Evans D., Akerman P., Ng T., Miner T., Cruff D., Chauhan B., Iannitti D., Harrington D. Paclitaxel poliglumex (PPX-Xyotax) and concurrent radiation for esophageal and gastric cancer: a phase I study. Am. J. Clin. Oncol. 2006;29:376–379. doi: 10.1097/01.coc.0000224494.07907.4e. [DOI] [PubMed] [Google Scholar]
- 68.Koukourakis M.I., Patlakas G., Froudarakis M.E., Kyrgias G., Skarlatos J., Abatzoglou I., Bougioukas G., Bouros D. Hypofractionated accelerated radiochemotherapy with cytoprotection (Chemo-HypoARC) for inoperable non-small cell lung carcinoma. Anticancer Res. 2007;27:3625–3631. [PubMed] [Google Scholar]
- 69.Koukourakis M., Romanidis K., Froudarakis M., Kyrgias G., Koukourakis G., Retalis G., Bahlitzanakis N. Concurrent administration of Docetaxel and Stealth® liposomal doxorubicin with radiotherapy in non-small cell lung cancer: excellent tolerance using subcutaneous amifostine for cytoprotection. Br. J. Cancer. 2002;87:385–392. doi: 10.1038/sj.bjc.6600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varveris H., Kachris S., Mazonakis M., Lyraraki E., Markouizou A., Karabekios S., Tzedakis A., Perisinakis K., Damilakis J., Vlachaki M. Pegulated liposomal doxorubicin and cisplatin given concurrently with conventional radiotherapy: A phase I dose-escalation trial for patients with squamous cell carcinoma of head and neck and lung. Oncol. Rep. 2004;12:473–481. doi: 10.3892/or.12.2.473. [DOI] [PubMed] [Google Scholar]
- 71.Vares G., Jallet V., Matsumoto Y., Rentier C., Takayama K., Sasaki T., Hayashi Y., Kumada H., Sugawara H. Functionalized mesoporous silica nanoparticles for innovative boron-neutron capture therapy of resistant cancers. Nanomed. Nanotechnol. Biol. Med. 2020;27 doi: 10.1016/j.nano.2020.102195. [DOI] [PubMed] [Google Scholar]
- 72.Miladi I., Aloy M.-T., Armandy E., Mowat P., Kryza D., Magné N., Tillement O., Lux F., Billotey C., Janier M. Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomed. Nanotechnol. Biol. Med. 2015;11:247–257. doi: 10.1016/j.nano.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 73.Xiao J., Zeng L., Ding S., Chen Y., Zhang X., Bian X.w., Tian G. Tumor‐tropic adipose‐derived mesenchymal stromal cell mediated Bi2Se3 nano‐radiosensitizers delivery for targeted radiotherapy of non‐small cell lung cancer. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202200143. [DOI] [PubMed] [Google Scholar]
- 74.Zhu L., Zhao Y., Liu T., Chen M., Qian W.P., Jiang B., Barwick B.G., Zhang L., Styblo T.M., Li X. Inhibition of NADPH oxidase-ROS signal using hyaluronic acid nanoparticles for overcoming radioresistance in cancer therapy. ACS Nano. 2022;16:18708–18728. doi: 10.1021/acsnano.2c07440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheung A., Bax H.J., Josephs D.H., Ilieva K.M., Pellizzari G., Opzoomer J., Bloomfield J., Fittall M., Grigoriadis A., Figini M. Targeting folate receptor alpha for cancer treatment. Oncotarget. 2016;7 doi: 10.18632/oncotarget.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui F.-b., Liu Q., Li R.-T., Shen J., Wu P.-y., Yu L.-X., Hu W.-j., Wu F.-l., Jiang C.-P., Yue G.-f. Enhancement of radiotherapy efficacy by miR-200c-loaded gelatinase-stimuli PEG-Pep-PCL nanoparticles in gastric cancer cells. Int. J. Nanomed. 2014;9:2345. doi: 10.2147/IJN.S60874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Werner M.E., Cummings N.D., Sethi M., Wang E.C., Sukumar R., Moore D.T., Wang A.Z. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:463–468. doi: 10.1016/j.ijrobp.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang X., Du X., Yu Y., Qin M., Qian L., Zhang M., Yang Y., Yu Q., Gan Z. Deep‐penetrating triple‐responsive prodrug nanosensitizer Actuates efficient chemoradiotherapy in pancreatic ductal adenocarcinoma models. Small. 2022;18 doi: 10.1002/smll.202202834. [DOI] [PubMed] [Google Scholar]
- 79.Yook S., Cai Z., Lu Y., Winnik M.A., Pignol J.-P., Reilly R.M. Intratumorally injected 177Lu-labeled gold nanoparticles: gold nanoseed brachytherapy with application for neoadjuvant treatment of locally advanced breast cancer. J. Nucl. Med. 2016;57:936–942. doi: 10.2967/jnumed.115.168906. [DOI] [PubMed] [Google Scholar]
- 80.Bonvalot S., Le Pechoux C., De Baere T., Kantor G., Buy X., Stoeckle E., Terrier P., Sargos P., Coindre J.M., Lassau N. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin. Cancer Res. 2017;23:908–917. doi: 10.1158/1078-0432.CCR-16-1297. [DOI] [PubMed] [Google Scholar]
- 81.Bonvalot S., Rutkowski P.L., Thariat J., Carrère S., Ducassou A., Sunyach M.-P., Agoston P., Hong A., Mervoyer A., Rastrelli M. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In. Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20:1148–1159. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- 82.Heeke A.L., Tan A.R. Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Rev. 2021;40:537–547. doi: 10.1007/s10555-021-09972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou F., Qiao M., Zhou C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell. Mol. Immunol. 2021;18:279–293. doi: 10.1038/s41423-020-00577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee A.Y., Brady M.S. Neoadjuvant immunotherapy for melanoma. J. Surg. Oncol. 2021;123:782–788. doi: 10.1002/jso.26229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roller J.F., Veeramachaneni N.K., Zhang J. Exploring the evolving scope of neoadjuvant immunotherapy in NSCLC. Cancers. 2022;14:741. doi: 10.3390/cancers14030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bilusic M., Gulley J.L. Oxford University Press; 2021. Neoadjuvant Immunotherapy: an Evolving Paradigm Shift? pp. 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao S., Li N., Gao S., Xue Q., Ying J., Wang S., Tao X., Zhao J., Mao Y., Wang B. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J. Thorac. Oncol. 2020;15:816–826. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 88.Song Y., Straker R.J., Xu X., Elder D.E., Gimotty P.A., Huang A.C., Mitchell T.C., Amaravadi R.K., Schuchter L.M., Karakousis G.C. Neoadjuvant versus adjuvant immune checkpoint blockade in the treatment of clinical stage III melanoma. Ann. Surg Oncol. 2020;27:2915–2926. doi: 10.1245/s10434-019-08174-1. [DOI] [PubMed] [Google Scholar]
- 89.Pinato D.J., Fessas P., Sapisochin G., Marron T.U. Perspectives on the neoadjuvant use of immunotherapy in hepatocellular carcinoma. Hepatology. 2021;74:483–490. doi: 10.1002/hep.31697. [DOI] [PubMed] [Google Scholar]
- 90.Yang C., Xia B.-R., Zhang Z.-C., Zhang Y.-J., Lou G., Jin W.-L. Immunotherapy for ovarian cancer: adjuvant, combination, and neoadjuvant. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.577869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanani A., Veen T., Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br. J. Surg. 2021;108:1417–1425. doi: 10.1093/bjs/znab342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Topalian S.L., Taube J.M., Pardoll D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367 doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gajewski T.F., Schreiber H., Fu Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y., Xiao Y., Sun G., Jin X., Guo L., Li T., Yin H. Elsevier; 2021. Harnessing the Therapeutic Potential of Extracellular Vesicles for Cancer Treatment, Seminars in Cancer Biology; pp. 92–104. [DOI] [PubMed] [Google Scholar]
- 95.Yu H.-j., De Geest B.G. Nature Publishing Group; 2020. Nanomedicine and Cancer Immunotherapy; pp. 879–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dhar R., Seethy A., Singh S., Pethusamy K., Srivastava T., Talukdar J., Rath G.K., Karmakar S. Cancer immunotherapy: recent advances and challenges. J. Cancer Res. Therapeut. 2021;17:834. doi: 10.4103/jcrt.JCRT_1241_20. [DOI] [PubMed] [Google Scholar]
- 97.Gao S., Yang X., Xu J., Qiu N., Zhai G. Nanotechnology for boosting cancer immunotherapy and remodeling tumor microenvironment: the horizons in cancer treatment. ACS Nano. 2021;15:12567–12603. doi: 10.1021/acsnano.1c02103. [DOI] [PubMed] [Google Scholar]
- 98.Tang L., Zhang M., Liu C. Advances in nanotechnology-based immunotherapy for glioblastoma. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.882257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lim S., Park J., Shim M.K., Um W., Yoon H.Y., Ryu J.H., Lim D.-K., Kim K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics. 2019;9:7906. doi: 10.7150/thno.38425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lakshmanan V.-K., Jindal S., Packirisamy G., Ojha S., Lian S., Kaushik A., Alzarooni A.I.M.A., Metwally Y.A.F., Thyagarajan S.P., Do Jung Y. Nanomedicine-based cancer immunotherapy: recent trends and future perspectives. Cancer Gene Ther. 2021;28:911–923. doi: 10.1038/s41417-021-00299-4. [DOI] [PubMed] [Google Scholar]
- 101.Zhao Y., Bilal M., Qindeel M., Khan M.I., Dhama K., Iqbal H. Nanotechnology-based immunotherapies to combat cancer metastasis. Mol. Biol. Rep. 2021;48:6563–6580. doi: 10.1007/s11033-021-06660-y. [DOI] [PubMed] [Google Scholar]
- 102.Van Herck S., De Geest B.G. Nanomedicine-mediated alteration of the pharmacokinetic profile of small molecule cancer immunotherapeutics. Acta Pharmacol. Sin. 2020;41:881–894. doi: 10.1038/s41401-020-0425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Herck S., Van Hoecke L., Louage B., Lybaert L., De Coen R., Kasmi S., Esser-Kahn A.P., David S.A., Nuhn L., Schepens B. Transiently thermoresponsive acetal polymers for safe and effective administration of amphotericin B as a vaccine adjuvant. Bioconjugate Chem. 2017;29:748–760. doi: 10.1021/acs.bioconjchem.7b00641. [DOI] [PubMed] [Google Scholar]
- 104.Yang F., Shi K., Jia Y.-p., Hao Y., Peng J.-r., Qian Z.-y. Advanced biomaterials for cancer immunotherapy. Acta Pharmacol. Sin. 2020;41:911–927. doi: 10.1038/s41401-020-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lai C., Duan S., Ye F., Hou X., Li X., Zhao J., Yu X., Hu Z., Tang Z., Mo F. The enhanced antitumor-specific immune response with mannose-and CpG-ODN-coated liposomes delivering TRP2 peptide. Theranostics. 2018;8:1723. doi: 10.7150/thno.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu J., Wang S., Qi J., Yu Z., Xian Y., Liu W., Wang X., Liu C., Wei M. Mannose-modified liposome designed for epitope peptide drug delivery in cancer immunotherapy. Int. Immunopharm. 2021;101 doi: 10.1016/j.intimp.2021.108148. [DOI] [PubMed] [Google Scholar]
- 107.Le Moignic A., Malard V., Benvegnu T., Lemiègre L., Berchel M., Jaffrès P.-A., Baillou C., Delost M., Macedo R., Rochefort J. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J. Contr. Release. 2018;278:110–121. doi: 10.1016/j.jconrel.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 108.Mai Y., Guo J., Zhao Y., Ma S., Hou Y., Yang J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 2020;354 doi: 10.1016/j.cellimm.2020.104143. [DOI] [PubMed] [Google Scholar]
- 109.Miura N., Akita H., Tateshita N., Nakamura T., Harashima H. Modifying antigen-encapsulating liposomes with KALA facilitates MHC class I antigen presentation and enhances anti-tumor effects. Mol. Ther. 2017;25:1003–1013. doi: 10.1016/j.ymthe.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei X., Liu L., Li X., Wang Y., Guo X., Zhao J., Zhou S. Selectively targeting tumor-associated macrophages and tumor cells with polymeric micelles for enhanced cancer chemo-immunotherapy. J. Contr. Release. 2019;313:42–53. doi: 10.1016/j.jconrel.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 111.Wan Z., Zheng R., Moharil P., Liu Y., Chen J., Sun R., Song X., Ao Q. Polymeric micelles in cancer immunotherapy. Molecules. 2021;26:1220. doi: 10.3390/molecules26051220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren C., Gao Y., Liu J., Zhang Y., Pu G., Yang L., Huang F., Yang C., Yang Z., Liu J. Anticancer supramolecular hydrogel of D/L-peptide with enhanced stability and bioactivity. J. Biomed. Nanotechnol. 2018;14:1125–1134. doi: 10.1166/jbn.2018.2564. [DOI] [PubMed] [Google Scholar]
- 113.Nguyen T.L., Choi Y., Kim J. Mesoporous silica as a versatile platform for cancer immunotherapy. Adv. Mater. 2019;31 doi: 10.1002/adma.201803953. [DOI] [PubMed] [Google Scholar]
- 114.Mu Q., Lin G., Jeon M., Wang H., Chang F.-C., Revia R.A., Yu J., Zhang M. Iron oxide nanoparticle targeted chemo-immunotherapy for triple negative breast cancer. Mater. Today. 2021;50:149–169. doi: 10.1016/j.mattod.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen X.-Y., Yung L.-Y.L., Tan P.H., Bay B.H. Harnessing the immunogenic potential of gold nanoparticle-based platforms as a therapeutic strategy in breast cancer immunotherapy: a mini review. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.865554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kwon M., Jung H., Nam G.-H., Kim I.-S. The right Timing, right combination, right sequence, and right delivery for Cancer immunotherapy. J. Contr. Release. 2021;331:321–334. doi: 10.1016/j.jconrel.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 117.O'Donnell J.S., Hoefsmit E.P., Smyth M.J., Blank C.U., Teng M.W. The promise of neoadjuvant immunotherapy and surgery for cancer TreatmentNeoadjuvant immunotherapy for the treatment of cancers. Clin. Cancer Res. 2019;25:5743–5751. doi: 10.1158/1078-0432.CCR-18-2641. [DOI] [PubMed] [Google Scholar]
- 118.Zhang M., Kim J.A., Huang A.Y.-C. Optimizing tumor microenvironment for cancer immunotherapy: β-glucan-based nanoparticles. Front. Immunol. 2018;9:341. doi: 10.3389/fimmu.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atukorale P.U., Moon T.J., Bokatch A.R., Lusi C.F., Routhier J.T., Deng V.J., Karathanasis E. Dual agonist immunostimulatory nanoparticles combine with PD1 blockade for curative neoadjuvant immunotherapy of aggressive cancers. Nanoscale. 2022;14:1144–1159. doi: 10.1039/d1nr06577g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park C.G., Hartl C.A., Schmid D., Carmona E.M., Kim H.-J., Goldberg M.S. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aar1916. [DOI] [PubMed] [Google Scholar]
- 121.T. He, M. Hu, S. Zhu, M. Shen, X. Kou, X. Liang, L. Li, X. Li, M. Zhang, Q. Wu, A tactical nanomissile mobilizing antitumor immunity enables neoadjuvant chemo-immunotherapy to minimize postsurgical tumor metastasis and recurrence, Acta Pharmaceutica Sinica B,doi:10.1016/j.apsb.2022.09.017. [DOI] [PMC free article] [PubMed]
- 122.Chung C.K., Da Silva C., Kralisch D., Chan A., Ossendorp F., Cruz L.J. Combinatory therapy adopting nanoparticle-based cancer vaccination with immune checkpoint blockade for treatment of post-surgical tumor recurrences. J. Contr. Release. 2018;285:56–66. doi: 10.1016/j.jconrel.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 123.Kircher M.F., Mahmood U., King R.S., Weissleder R., Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63:8122–8125. [PubMed] [Google Scholar]
- 124.Cheng Y., Morshed R.A., Auffinger B., Tobias A.L., Lesniak M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014;66:42–57. doi: 10.1016/j.addr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kircher M.F., De La Zerda A., Jokerst J.V., Zavaleta C.L., Kempen P.J., Mittra E., Pitter K., Huang R., Campos C., Habte F. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toms S.A., Lin W.-C., Weil R.J., Johnson M.D., Jansen E.D., Mahadevan-Jansen A. Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity. Operative Neurosurgery. 2005;57 doi: 10.1227/01.neu.000176855.39826.2d. ONS-382-ONS-391. [DOI] [PubMed] [Google Scholar]
- 127.Liu H.-j., Wang M., Shi S., Hu X., Xu P. A therapeutic sheep in metastatic wolf's clothing: trojan Horse approach for cancer brain metastases treatment. Nano-Micro Lett. 2022;14:1–17. doi: 10.1007/s40820-022-00861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanai N., Berger M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–766. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 129.Gao X., Yue Q., Liu Z., Ke M., Zhou X., Li S., Zhang J., Zhang R., Chen L., Mao Y. Guiding brain‐tumor surgery via blood–brain‐barrier‐permeable gold nanoprobes with acid‐triggered MRI/SERRS signals. Adv. Mater. 2017;29 doi: 10.1002/adma.201603917. [DOI] [PubMed] [Google Scholar]
- 130.Maletínská L., Blakely E.A., Bjornstad K.A., Deen D.F., Knoff L.J., Forte T.M. Human glioblastoma cell lines: levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000;60:2300–2303. [PubMed] [Google Scholar]
- 131.Dong J., Sun J., Cai W., Guo C., Wang Q., Zhao X., Zhang R. A natural cuttlefish melanin nanoprobe for preoperative and intraoperative mapping of lymph nodes. Nanomed. Nanotechnol. Biol. Med. 2022;41 doi: 10.1016/j.nano.2021.102510. [DOI] [PubMed] [Google Scholar]
- 132.Huang K.-W., Chieh J.-J., Horng H.-E., Hong C.-Y., Yang H.-C. Characteristics of magnetic labeling on liver tumors with anti-alpha-fetoprotein-mediated Fe3O4 magnetic nanoparticles. Int. J. Nanomed. 2012;7:2987. doi: 10.2147/IJN.S30061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi B., Zhang B., Zhang Y., Gu Y., Zheng C., Yan J., Chen W., Yan F., Ye J., Zhang H. Multifunctional gap-enhanced Raman tags for preoperative and intraoperative cancer imaging. Acta Biomater. 2020;104:210–220. doi: 10.1016/j.actbio.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 134.Ray A., Wang X., Lee Y.-E.K., Hah H.J., Kim G., Chen T., Orringer D.A., Sagher O., Liu X., Kopelman R. Targeted blue nanoparticles as photoacoustic contrast agent for brain tumor delineation. Nano Res. 2011;4:1163–1173. doi: 10.1007/s12274-011-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie Z., Fan T., An J., Choi W., Duo Y., Ge Y., Zhang B., Nie G., Xie N., Zheng T. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020;49:8065–8087. doi: 10.1039/d0cs00215a. [DOI] [PubMed] [Google Scholar]
- 136.Ouyang J., Xie A., Zhou J., Liu R., Wang L., Liu H., Kong N., Tao W. Chemical Society Reviews; 2022. Minimally Invasive Nanomedicine: Nanotechnology in Photo-/ultrasound-/radiation-/magnetism-Mediated Therapy and Imaging. [DOI] [PubMed] [Google Scholar]
- 137.Liu H.J., Wang M., Hu X., Shi S., Xu P. Enhanced photothermal therapy through the in situ activation of a temperature and redox dual‐sensitive nanoreservoir of triptolide. Small. 2020;16 doi: 10.1002/smll.202003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ji X., Tang Z., Liu H., Kang Y., Chen L., Dong J., Chen W., Kong N., Tao W., Xie T. Thermoelectric effect mediated cancer Thermoelectric therapy and surgical adjuvant treatment. Adv. Mater. 2022 doi: 10.1002/adma.202207391. [DOI] [PubMed] [Google Scholar]
- 139.Zheng C., Zhang X., Wang L., Zhou X., Yang X., Zhang Z., Huang X. Versatile cobalt-glycerate nanoplatform for MR-guided neoadjuvant photo-therapy of oral squamous cell carcinoma. Chem. Eng. J. 2022;437 [Google Scholar]
- 140.Deng W., McKelvey K.J., Guller A., Fayzullin A., Campbell J.M., Clement S., Habibalahi A., Wargocka Z., Liang L., Shen C. Application of mitochondrially targeted nanoconstructs to neoadjuvant x-ray-induced photodynamic therapy for rectal cancer. ACS Cent. Sci. 2020;6:715–726. doi: 10.1021/acscentsci.9b01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li Q., Chen K., Huang W., Ma H., Zhao X., Zhang J., Zhang Y., Fang C., Nie L. Minimally invasive photothermal ablation assisted by laparoscopy as an effective preoperative neoadjuvant treatment for orthotopic hepatocellular carcinoma. Cancer Lett. 2021;496:169–178. doi: 10.1016/j.canlet.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 142.Steel G.G., Peckham M.J. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int. J. Radiat. Oncol. Biol. Phys. 1979;5:85–91. doi: 10.1016/0360-3016(79)90044-0. [DOI] [PubMed] [Google Scholar]
- 143.Bartelink H., Roelofsen F., Eschwege F., Rougier P., Bosset J., Gonzalez D.G., Peiffert D., Van Glabbeke M., Pierart M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J. Clin. Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 144.Gupta S., Maheshwari A., Parab P., Mahantshetty U., Hawaldar R., Sastri S., Kerkar R., Engineer R., Tongaonkar H., Ghosh J. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J. Clin. Oncol. 2018;36:1548–1555. doi: 10.1200/JCO.2017.75.9985. [DOI] [PubMed] [Google Scholar]
- 145.Greenhalgh T.A., Symonds R.P. Principles of chemotherapy and radiotherapy, Obstetrics. Gynaecology Reproductive Med. 2014;24:259–265. [Google Scholar]
- 146.Cao W., Gu Y., Meineck M., Xu H. The combination of chemotherapy and radiotherapy towards more efficient drug delivery. Chem.--Asian J. 2014;9:48–57. doi: 10.1002/asia.201301294. [DOI] [PubMed] [Google Scholar]
- 147.Gao S., Li T., Guo Y., Sun C., Xianyu B., Xu H. Selenium‐containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv. Mater. 2020;32 doi: 10.1002/adma.201907568. [DOI] [PubMed] [Google Scholar]
- 148.Ju C., Wen Y., Zhang L., Wang Q., Xue L., Shen J., Zhang C. Neoadjuvant chemotherapy based on abraxane/human neutrophils cytopharmaceuticals with radiotherapy for gastric cancer. Small. 2019;15 doi: 10.1002/smll.201905688. [DOI] [PubMed] [Google Scholar]
- 149.Sanoff H.K., Moon D.H., Moore D.T., Boles J., Bui C., Blackstock W., O'Neil B.H., Subramaniam S., McRee A.J., Carlson C. Phase I/II trial of nano-camptothecin CRLX101 with capecitabine and radiotherapy as neoadjuvant treatment for locally advanced rectal cancer. Nanomed. Nanotechnol. Biol. Med. 2019;18:189–195. doi: 10.1016/j.nano.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xue J., Zhao Z., Zhang L., Xue L., Shen S., Wen Y., Wei Z., Wang L., Kong L., Sun H. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 151.Chen J., Gu H., Fu S., Lu J., Tan H., Wei Q., Ai H. Multifunctional injectable hydrogels for three-in-one cancer therapy: preoperative remission via mild photothermal-enhanced supramolecular chemotherapy and prevention of postoperative recurrence and adhesion. Chem. Eng. J. 2021;425 [Google Scholar]
- 152.Song C., Park H., Lee C.K., Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperther. 2005;21:761–767. doi: 10.1080/02656730500204487. [DOI] [PubMed] [Google Scholar]
- 153.Wang L., Lin X., Wang J., Hu Z., Ji Y., Hou S., Zhao Y., Wu X., Chen C. Novel insights into combating cancer chemotherapy resistance using a plasmonic nanocarrier: enhancing drug sensitiveness and accumulation simultaneously with localized mild photothermal stimulus of femtosecond pulsed laser. Adv. Funct. Mater. 2014;24:4229–4239. [Google Scholar]
- 154.Ding K., Wang L., Zhu J., He D., Huang Y., Zhang W., Wang Z., Qin A., Hou J., Tang B.Z. Photo-enhanced chemotherapy performance in bladder cancer treatment via albumin coated AIE aggregates. ACS Nano. 2022;16:7535–7546. doi: 10.1021/acsnano.1c10770. [DOI] [PubMed] [Google Scholar]
- 155.Hong W.X., Haebe S., Lee A.S., Westphalen C.B., Norton J.A., Jiang W., Levy R. Intratumoral immunotherapy for early-stage solid tumors. Clin. Cancer Res. 2020;26:3091–3099. doi: 10.1158/1078-0432.CCR-19-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu J., Blake S.J., Yong M.C., Harjunpää H., Ngiow S.F., Takeda K., Young A., O'Donnell J.S., Allen S., Smyth M.J. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic DiseaseNeoadjuvant immunotherapy is effective against metastases. Cancer Discov. 2016;6:1382–1399. doi: 10.1158/2159-8290.CD-16-0577. [DOI] [PubMed] [Google Scholar]
- 157.Bourgeois-Daigneault M.-C., Roy D.G., Aitken A.S., El Sayes N., Martin N.T., Varette O., Falls T., St-Germain L.E., Pelin A., Lichty B.D. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao1641. [DOI] [PubMed] [Google Scholar]
- 158.Hegde P.S., Karanikas V., Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 2016;22:1865–1874. doi: 10.1158/1078-0432.CCR-15-1507. [DOI] [PubMed] [Google Scholar]
- 159.Wolf N.K., Blaj C., Picton L.K., Snyder G., Zhang L., Nicolai C.J., Ndubaku C.O., McWhirter S.M., Garcia K.C., Raulet D.H. Synergy of a STING agonist and an IL-2 superkine in cancer immunotherapy against MHC I–deficient and MHC I+ tumors. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2200568119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Atukorale P.U., Raghunathan S.P., Raguveer V., Moon T.J., Zheng C., Bielecki P.A., Wiese M.L., Goldberg A.L., Covarrubias G., Hoimes C.J. Nanoparticle encapsulation of synergistic immune agonists enables systemic codelivery to tumor sites and IFNβ-driven antitumor immunity. Cancer Res. 2019;79:5394–5406. doi: 10.1158/0008-5472.CAN-19-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pol J., Vacchelli E., Aranda F., Castoldi F., Eggermont A., Cremer I., Sautes-Fridman C., Fucikova J., Galon J., Spisek R. Trial Watch: immunogenic cell death inducers for anticancer chemotherapy. OncoImmunology. 2015;4 doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Haanen J.B. Converting cold into hot tumors by combining immunotherapies. Cell. 2017;170:1055–1056. doi: 10.1016/j.cell.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 163.Yi Y., Yu M., Feng C., Hao H., Zeng W., Lin C., Chen H., Lv F., Zhu D., Ji X. Transforming “cold” tumors into “hot” ones via tumor-microenvironment-responsive siRNA micelleplexes for enhanced immunotherapy. Matter. 2022;5:2285–2305. [Google Scholar]
- 164.Feng Y., Yang K., Sun H.-h., Liu Y.-p., Zhang D., Zhao Y., Shi W., Lu G.-f., Zhang Z.-y., Jia A. Value of preoperative gastroscopic carbon nanoparticles labeling in patients undergoing laparoscopic radical gastric cancer surgery. Surgical Oncol. 2021;38 doi: 10.1016/j.suronc.2021.101628. [DOI] [PubMed] [Google Scholar]
- 165.Feng H.-Y., Yuan Y., Zhang Y., Liu H.-J., Dong X., Yang S.-C., Liu X.-L., Lai X., Zhu M.-H., Wang J. Targeted micellar phthalocyanine for lymph node metastasis homing and photothermal therapy in an orthotopic colorectal tumor model. Nano-Micro Lett. 2021;13:1–17. doi: 10.1007/s40820-021-00666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim C.H., Huh J.W., Kim H.R., Kim Y.J. Prognostic comparison between number and distribution of lymph node metastases in patients with right-sided colon cancer. Ann. Surg Oncol. 2014;21:1361–1368. doi: 10.1245/s10434-013-3426-3. [DOI] [PubMed] [Google Scholar]
- 167.Lykke J., Roikjaer O., Jess P., Group D.C.C. The relation between lymph node status and survival in S tage I–III colon cancer: results from a prospective nationwide cohort study. Colorectal Dis. 2013;15:559–565. doi: 10.1111/codi.12059. [DOI] [PubMed] [Google Scholar]
- 168.Zhou Z., Chen H., Lipowska M., Wang L., Yu Q., Yang X., Tiwari D., Yang L., Mao H. A dual-modal magnetic nanoparticle probe for preoperative and intraoperative mapping of sentinel lymph nodes by magnetic resonance and near infrared fluorescence imaging. J. Biomater. Appl. 2013;28:100–111. doi: 10.1177/0885328212437883. [DOI] [PMC free article] [PubMed] [Google Scholar]