Abstract
Phase variation in the colonial opacity of Streptococcus pneumoniae has been implicated as a factor in bacterial adherence, colonization, and invasion in the pathogenesis of pneumococcal disease. Additionally, the synergistic effects of influenza A virus and S. pneumoniae in the development of otitis media (OM) have been reported. This study examined the ability of opaque or transparent S. pneumoniae from the same strain in combination with an antecedent influenza A virus infection to colonize the nasopharynx and invade the middle ear in the chinchilla model. Our data indicated that there was no significant difference in the level of nasopharyngeal colonization and induction of OM between the opaque and transparent variants unless there was a prior challenge with influenza A virus. Subsequent to influenza A virus infection, there was a significant difference between the variants in the ability to colonize and persist in the nasopharynx and middle ear. The concentrations of the opaque variant in nasopharyngeal-lavage samples and middle-ear fluid remained consistently higher than those of the transparent variant for 10 days postinoculation. Data from this study indicate that the effects of influenza A virus on the pathogenesis of experimental S. pneumoniae-induced OM differ depending on the opacity phenotype involved.
Otitis media (OM) is one of the most common childhood diseases. The prevalence, medical care costs, and hearing-related morbidity of OM are significant. Streptococcus pneumoniae, the primary etiological agent, has been isolated from approximately 20 to 50% of middle-ear effusions from children with OM (4). The process whereby S. pneumoniae becomes established in the human nasopharynx and then affects the transition from a colonized to a diseased state in the middle ear is not yet known.
Many children with OM experience an antecedent viral upper-respiratory-tract infection, and viruses are also recognized as important etiological agents of OM, either alone or in combination with bacterial pathogens. Influenza A virus, adenovirus, and respiratory syncytial virus are the primary respiratory-tract viruses associated with this disease (5, 6). A recent study with adult human volunteers indicates that influenza A virus infection promotes significant colonization of the nasopharynx by S. pneumoniae (21).
Comparable data have also been derived from experimental studies. Giebink et al. previously reported that influenza A virus infection promotes the development of OM in chinchillas coinoculated with influenza A virus and S. pneumoniae (11). The mechanism underlying this phenomenon was suggested to involve, in part, viral compromise of eustachian tube mucosal integrity and function, including epithelial damage and accumulation of cellular and mucous debris in the tubal lumen in association with the development of negative middle-ear pressure (MEP) during experimental influenza A virus infection (12). Furthermore, in vitro studies have found that influenza A virus-infected HEp-2 cells have significantly higher binding indices for S. pneumoniae strains than do uninfected cells (2).
S. pneumoniae undergoes spontaneous phase variation between a transparent and an opaque colony phenotype. The relationship between S. pneumoniae colonial opacity and nasopharyngeal (NP) colonization in an infant rat model of carriage was first described by Weiser et al. (22). Transparent variants demonstrate an increased ability to adhere to human lung epithelial cells and are selected for during NP colonization in rodent models but are unable to induce sepsis (8). The opaque phenotype, however, is characteristically more virulent and is associated with invasive infection in the mouse model (13). Recent studies have showed that transparent S. pneumoniae has 2.1 to 3.8 times more cell wall teichoic acid. Choline in the form of phosphorylcholine (ChoP) binds to teichoic acid and has been implicated in direct adherence to host cells via the receptor for platelet-activating factor (PAF) (9). Opaque S. pneumoniae, in contrast, has 1.2- to 5.6-fold more capsular polysaccharide than does related transparent S. pneumoniae (13). The amount of immune human serum required to achieve 50% opsonophagocytic killing is 1.2- to 30-fold greater for the opaque variants, and they bind less C-reactive protein (CRP) than do the related transparent variants. The increased amount of capsular polysaccharide appears to be the major factor in the decreased opsonophagocytic killing of opaque pneumococci (14).
A recent study demonstrates that influenza A virus also promotes a significant increase in NP colonization and an increased incidence and severity of OM in the chinchilla induced by S. pneumoniae type 6A, which is a predominately transparent variant (19). This study was designed to further assess the effects of influenza A virus on adherence, the kinetics of colonization, and invasion of the middle ear by isogenic opaque and transparent variants of S. pneumoniae type 6A in the chinchilla model of OM.
Study design.
A total of 48 healthy chinchillas (Chinchilla lanigera) (220 to 450 g) free of middle-ear disease, as determined by otoscopy and tympanometry, were used in these studies. Two cohorts of 12 chinchillas each were inoculated intranasally (i.n.) with influenza A virus followed 7 days later by i.n. inoculation with the S. pneumoniae type 6A opaque or transparent variant. Another two cohorts of 12 chinchillas each were inoculated i.n. with diluent only (without influenza A virus) followed 7 days later by i.n. inoculation of either opaque or transparent S. pneumoniae; these cohorts served as controls. Three chinchillas (preselected and randomized) from each cohort were evaluated for NP colonization and OM at each of four different time periods after S. pneumoniae type 6A inoculation as described below. The ability of influenza A virus to promote NP colonization by S. pneumoniae and development of OM was compared for the opaque and transparent variants. All experiments were performed in duplicate.
i.n. inoculation with influenza A virus.
Influenza virus A/Alaska/6/77 (H3N2) has previously been used by our laboratories and has been described in detail (7, 16). Briefly, the virus stock was diluted in sterile Eagle's minimal essential medium (MEM) (Whittaker, Walkersville, Md.). Two cohorts of 12 chinchillas each were inoculated i.n. with 0.2 ml of a virus suspension containing approximately 6 × 106 PFU of influenza A virus/ml. The inoculum was divided equally between both nares. Two cohorts of 12 chinchillas each received 0.2 ml of MEM without virus and served as controls. All virus-inoculated animals were housed separately from those receiving MEM alone.
S. pneumoniae inoculation.
S. pneumoniae type 6A (EF3114; kindly provided by B. Andersson, Department of Clinical Immunology, University of Göteborg, Göteborg, Sweden) was used for these experiments and has been described in detail previously (3). The isogenic opaque and transparent variants of S. pneumoniae type 6A were isolated by Jeffrey Weiser, Children's Hospital of Philadelphia, and confirmed prior to inoculation and at various time points according to the method established by Weiser et al. (22). Log-phase cultures were prepared from chocolate agar plate subcultures by inoculating Todd-Hewitt broth supplemented with 5% yeast extract (Difco Laboratories, Detroit, Mich.) with S. pneumoniae type 6A opacity variants grown overnight on chocolate agar and obtained by washing the plates with 5 ml of phosphate-buffered saline (PBS), pH 7.2. After a 3-h incubation, the cultures were centrifuged at 3,500 × g for 20 min, washed twice, and resuspended in PBS. The concentration of S. pneumoniae (expressed as CFU per milliliter) was determined by standard dilution and plate count. Seven days after challenge with influenza A virus, the virus-infected and control chinchillas were inoculated i.n. with 0.5 ml of either the S. pneumoniae type 6A opaque-phenotype suspension or the transparent-phenotype suspension, each containing 5 × 107 CFU/ml. All virus-inoculated animals were housed separately from controls receiving S. pneumoniae type 6A only.
Disease course assessment.
All assessments were made blindly and by the same observers throughout this study. Tympanic membrane (TM) inflammation was assessed daily in both ears of each chinchilla by means of otoscopy. MEP changes were also evaluated daily in both ears of each chinchilla for up to 10 days after inoculation with S. pneumoniae type 6A by means of tympanometry (Ear Scan; Micro Audiometric, South Daytona, Fla.). Normal chinchilla MEP was considered to be between −60 and +40 daPa (17).
Assessment of NP colonization and invasion of the middle ear.
Three chinchillas, preselected and randomized, were evaluated by tympanocentesis and NP lavage on days 1, 3, 7, and 10 after inoculation with S. pneumoniae type 6A as previously described (20). Tympanocentesis was first performed on both ears of each of the three chinchillas by aspiration with a tuberculin syringe fitted with a 25-gauge needle. If no middle-ear fluid (MEF) was present, the bullae were lavaged with 0.5 ml of prewarmed sterile saline. Subsequent to tympanocentesis, NP lavage was performed on each chinchilla as described previously (20). Chinchillas were not subjected to repeated tympanocentesis or lavage. Tympanocentesis and bulla lavage were always performed before NP lavage to prevent contamination of the middle ear. The MEF or lavage samples, and the NP lavage samples, were cultured on chocolate agar plates by overnight incubation in a humidified atmosphere with 5% CO2, and the concentrations of S. pneumoniae in the samples were determined by standard dilution and plate count. The phase variation of colonial morphology was determined by the method of Weiser et al. (22).
Statistical analysis.
Data are expressed as means ± standard errors from the duplicate experiments. Differences in S. pneumoniae concentrations in nasal and middle-ear lavage samples between the cohorts inoculated with the opaque variant and those inoculated with the transparent variant in combination with an antecedent influenza A virus infection were analyzed by the Mann-Whitney rank sum test. The Student t test was used to compare the differences in MEP. A P value of <0.05 was accepted as the minimal level of significance.
Effect of the opacity phenotype on NP colonization.
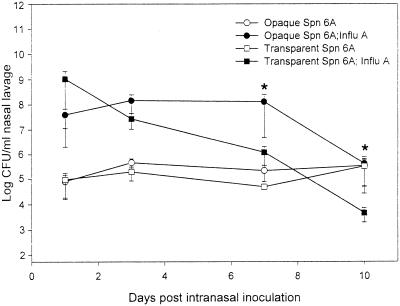
There was no significant difference in the level of NP colonization between the opaque and transparent variants of S. pneumoniae type 6A in the control cohorts which had not been inoculated previously with influenza A virus. Both phenotypes colonized the nasopharynx at approximately 105 CFU/ml of nasal-lavage fluid for as long as 10 days after i.n. challenge with S. pneumoniae type 6A (Fig. 1).
FIG. 1.
NP colonization dynamics in chinchillas inoculated i.n. with either opaque or transparent S. pneumoniae (Spn) type 6A 7 days after i.n. challenge with either diluent without influenza A virus (Influ A) or diluent with Influ A. Each data point represents the geometric mean number of CFU of S. pneumoniae bacteria (± the standard error of the mean) per milliliter of nasal-lavage fluid from a total of six animals combined from two separate experiments. ∗, P < 0.05 compared to the cohort inoculated with the transparent variant and Influ A.
As expected from our previous report (19), the chinchillas receiving an antecedent influenza A virus infection demonstrated a significant 2-log-unit increase in S. pneumoniae type 6A NP colonization, compared to the chinchillas not previously infected with virus. An increase was observed for both the opaque and transparent variants within 24 h after i.n. inoculation of S. pneumoniae type 6A (Fig. 1). Although the concentration of transparent S. pneumoniae in the nasal-lavage fluid was 1.5 log units higher than that of opaque S. pneumoniae on day 1, the data were not statistically significant. However, the clearance kinetics for the transparent and opaque variants were markedly different. The concentration of the transparent phenotype in the nasal-lavage fluid declined steadily, so that by 7 to 10 days postinoculation a statistically significant decrease (P < 0.05) was evident in the log CFU of the transparent phenotype per milliliter of nasal-lavage fluid compared with that of the opaque phenotype (Fig. 1). The concentration of the opaque variant, however, remained constant for 7 days postinoculation and then declined (Fig. 1). Neither phenotype was completely cleared from the nasopharynx during the 10-day experiment. Ninety-five to ninety-nine percent of the nasal-lavage isolates cultured at each sampling time were of the same opacity phenotype as the respective original inoculum.
Effect of opacity phenotype on invasion of the middle ear and induction of OM.
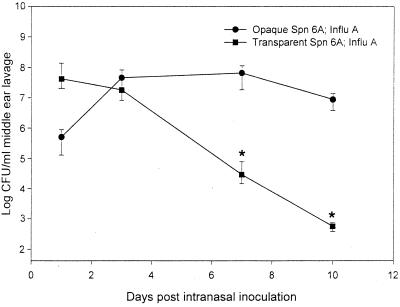
The combination of an antecedent influenza A virus infection of the nasopharynx with i.n. challenge with opaque or transparent S. pneumoniae type 6A resulted in a significant increase in the incidence of culture-positive OM. Nine out of 12 chinchillas with the combined opaque S. pneumoniae type 6A and influenza A virus infection developed OM compared to 3 out of 12 chinchillas inoculated i.n. with opaque S. pneumoniae type 6A only. Similarly, 9 out of 12 chinchillas with influenza A virus and the transparent phenotype developed OM compared to 4 out 12 chinchillas in the control cohort. Although influenza A virus increased the development of OM in both opaque and transparent S. pneumoniae type 6A cohorts, the survival times and clearance kinetics for the two phenotypes in the middle-ear cavity were markedly different, as was observed during NP colonization. Although the concentration of transparent S. pneumoniae in the middle ear was 1.8 log units higher than that of opaque S. pneumoniae on day 1 post-i.n. challenge, the opaque S. pneumoniae type 6A multiplied and persisted at a higher level in the middle ear on days 7 and 10 than the transparent S. pneumoniae type 6A (Fig. 2). The transparent S. pneumoniae was cleared at a steady and continuous rate without any evidence of multiplication.
FIG. 2.
Survival of the opaque or transparent phenotype in the middle ears of chinchillas subsequent to i.n. inoculation with S. pneumoniae (Spn) type 6A 7 days after i.n. challenge with influenza A virus (Influ A). Each data point represents the geometric mean number of CFU of S. pneumoniae bacteria (± the standard error of the mean) per milliliter of MEF or middle-ear-lavage fluid from a total of two to four animals combined from two separate experiments. ∗, P < 0.05 compared to the cohort inoculated with the opaque variant and Influ A.
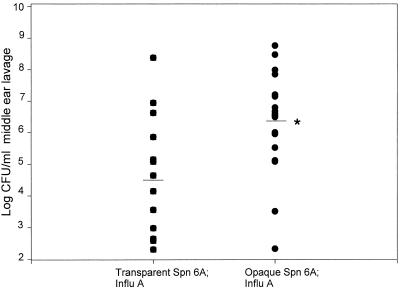
The 18 ears infected with opaque S. pneumoniae type 6A during the course of the experiment had a median concentration of 3.7 × 106 CFU/ml of MEF, whereas the 13 ears infected with transparent S. pneumoniae type 6A had a median concentration of 4.4 × 104 CFU/ml (P = 0.02) (Fig. 3). Ninety-nine percent of the isolates from each MEF sample were of the same colony opacity phenotype as the inoculum.
FIG. 3.
Comparison of S. pneumoniae (Spn) concentrations in the middle ears for cohorts inoculated i.n. with the opaque or transparent variant of S. pneumoniae type 6A after a prior i.n. inoculation with influenza A virus (Influ A). Horizontal bars, median values; ∗, P = 0.02.
Effect of opacity phenotype on MEP.
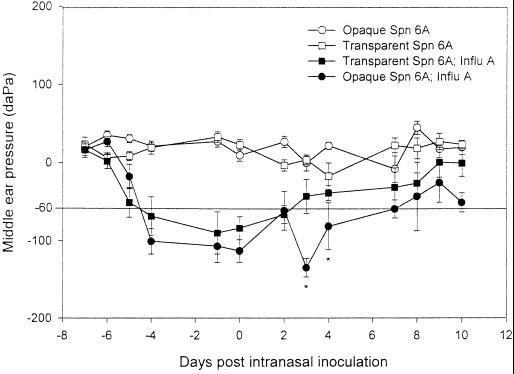
Neither the transparent nor the opaque variant of S. pneumoniae type 6A alone induced negative MEP (Fig. 4). Influenza A virus induced a significant negative MEP as early as 3 days after i.n. inoculation of virus. Subsequent to inoculation with S. pneumoniae type 6A, the opaque phenotype induced a statistically significant decline in MEP on days 3 and 4 post-i.n. challenge compared to that for the cohort receiving the transparent phenotype together with virus.
FIG. 4.
Comparison of mean MEPs (± standard errors of the means) for cohorts inoculated with the S. pneumoniae (Spn) type 6A opaque or transparent phenotype either with or without prior i.n. inoculation with influenza A virus (Influ A). MEPs were determined by tympanometry over a 10-day observation period. Values below −60 daPa are considered abnormal for the chinchilla (17). ∗, P < 0.05 for the comparison of cohorts inoculated with influenza A virus and the opaque variant versus influenza A virus and the transparent variant.
The data presented here demonstrate the effects of influenza A virus on NP colonization and induction of OM by S. pneumoniae opaque and transparent variants in the chinchilla model.
A relationship between S. pneumoniae opacity phenotype and adherence was originally suggested by Weiser et al. These authors reported that colonies of encapsulated S. pneumoniae could be classified as “transparent” or “opaque” when viewed with transmitted light and that spontaneous phase variation occurs between these two phenotypes at a rate of 10−3 to 10−6 per generation (22). Of great interest are the relationships between S. pneumoniae opacity phenotypes and S. pneumoniae adherence, colonization, and virulence (13, 14). A previous study demonstrated that influenza A virus promoted a significant increase in NP colonization by S. pneumoniae type 6A, an increased incidence and severity of OM, and a sustained presence of S. pneumoniae in the MEF (19). Data from the present study expand previous findings and provide for the first time an assessment of the specific effects of influenza A virus infection on the virulence of isogenic transparent and opaque variants of S. pneumoniae type 6A in the chinchilla OM model.
S. pneumoniae with the transparent phenotype has been shown to be more efficient than S. pneumoniae with the opaque phenotype at colonization of the nasopharynx in an infant rat model of carriage (22). Opaque variants of the same strain, however, are significantly more virulent and are associated with invasive diseases (13). Opaque and transparent pneumococcal variants adhere to a similar degree to nonactivated epithelial and endothelial cells; however, only transparent variants demonstrate enhanced adherence to cytokine-stimulated cells or PAF receptor-transfected human TSA cells (8). In contrast, our data indicated that there was no difference in the level of NP colonization and induction of OM in the chinchilla model between the opaque and transparent phenotypes unless there was a prior challenge with influenza A virus. Species as well as age differences between infant rats and adult chinchillas may account for these different results.
Data from this study indicate that while influenza A virus induced an increase in NP colonization levels for both the transparent and the opaque variant, different clearance kinetics for the two phenotypes were evident. These data are contrary to what one would have expected based on a two-step model proposed for pneumococcal adherence by Cundell et al. (10). According to this model, the virus infection would presumably “activate” the epithelium and upregulate cytokine production and PAF receptors, resulting, theoretically, in enhanced adherence and an increase in the concentration of the transparent phenotype in nasal-lavage fluid. A higher concentration of transparent S. pneumoniae was observed in the nasal-lavage fluid and MEF only on day 1 post-i.n. challenge. After 3 days postchallenge, different effects were observed. Persistence of the opaque phenotype was evident in the MEF and in bulla and nasal-lavage fluids. The transparent phenotype, however, was cleared at a steady rate. This may be due, in part, to transudation of serum components and a concomitant increase in CRP in the nasopharynx induced by the antecedent influenza A virus infection. Transparent S. pneumoniae has more teichoic acid and therefore a greater propensity to bind ChoP. ChoP is a target for CRP, an acute-phase reactant in human serum which facilitates opsonophagocytosis in the absence of specific antibody (13). An increased concentration of CRP may, therefore, facilitate enhanced elimination of the transparent variants by the host.
Prior influenza A virus infection may also create a favorable microenvironment which provides for the selection of the opaque phenotype in the nasopharynx and middle-ear cavity for as long as 10 days post-i.n. challenge. Previous studies have demonstrated that polymorphonuclear leukocyte (PMN) dysfunction was induced by influenza A virus during experimental pneumococcal OM in the chinchilla model (1), as well as in humans following influenza A virus infection (15, 18).
Moreover, the persistence of the opaque variant in the nasopharynx and middle ear may also be due, in part, to the greater amount of capsular polysaccharide contained in the opaque variants (14). The primary mechanism of clearance of the pneumococcus is opsonophagocytosis, and thus the greater amount of antiphagocytic capsular polysaccharide present on the opaque variants, together with the possible suppression of PMN function after i.n. challenge with influenza A virus, may account for the persistence and altered rate of clearance from the middle ear observed in the present study. No assessment of contributions of the host's specific immune response to opacity phase variation has yet been undertaken, but presumably there would be significant impacts, and such studies are planned.
In conclusion, the results from the present study indicate that the effects of influenza A virus on the pathogenesis of experimental S. pneumoniae-induced OM vary depending on the opacity phenotype of the S. pneumoniae inoculum.
Acknowledgments
This study was supported, in part, by a grant from the NIDCD/NIH (R01 DC03105-03).
We thank Lisa Routt and Kathy Holloway for manuscript preparation.
REFERENCES
- 1.Abramson J S, Giebink G S, Quie P G. Influenza A virus-induced polymorphonuclear leukocyte dysfunction in the pathogenesis of experimental pneumococcal otitis media. Infect Immun. 1982;36:289–296. doi: 10.1128/iai.36.1.289-296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmer O R, Raza M W, Ogilvie M M, Weir D M, Blackwell C C. Binding of bacteria to HEp-2 cells infected with influenza A virus. FEMS Immunol Med Microbiol. 1999;23:331–341. doi: 10.1111/j.1574-695X.1999.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersson B, Dahmen J, Frejd T, Leffler H, Magnusson G, Noori G, Eden C S. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J Exp Med. 1983;158:559–570. doi: 10.1084/jem.158.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluestone C D, Stephenson J S, Martin L M. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11:S7–S11. doi: 10.1097/00006454-199208001-00002. [DOI] [PubMed] [Google Scholar]
- 5.Chonmaitree T, Heikkinen T. Role of viruses in middle-ear disease. Ann N Y Acad Sci. 1997;830:143–157. doi: 10.1111/j.1749-6632.1997.tb51886.x. [DOI] [PubMed] [Google Scholar]
- 6.Chonmaitree T, Howie V M, Truant A L. Presence of respiratory viruses in middle ear fluids and nasal wash specimens from children with acute otitis media. Pediatrics. 1986;77:698–702. [PubMed] [Google Scholar]
- 7.Chung M H, Griffith S R, Park K H, Lim D J, DeMaria T F. Cytological and histological changes in the middle ear after inoculation of influenza A virus. Acta Otolaryngol (Stockholm) 1993;113:81–87. doi: 10.3109/00016489309135771. [DOI] [PubMed] [Google Scholar]
- 8.Cundell D R, Weiser J N, Shen J, Young A, Tuomanen E I. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun. 1995;63:757–761. doi: 10.1128/iai.63.3.757-761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 10.Cundell D, Masure H R, Tuomanen E I. The molecular basis of pneumococcal infection: a hypothesis. Clin Infect Dis. 1995;21(Suppl. 3):S204–S211. doi: 10.1093/clind/21.supplement_3.s204. [DOI] [PubMed] [Google Scholar]
- 11.Giebink G S, Berzins I K, Marker S C, Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect Immun. 1980;30:445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giebink G S, Ripley M L, Wright P F. Eustachian tube histopathology during experimental influenza A virus infection in the chinchilla. Ann Otol Rhinol Laryngol. 1987;96:199–206. doi: 10.1177/000348948709600212. [DOI] [PubMed] [Google Scholar]
- 13.Kim J O, Weiser J N. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 14.Kim J O, Romero-Steiner S, Sorensen U B, Blom J, Carvalho M, Barnard S, Carlone G, Weiser J N. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson H E, Blades R. Impairment of human polymorphonuclear leukocyte function by influenza virus. Lancet. 1976;i:283. doi: 10.1016/s0140-6736(76)91407-0. [DOI] [PubMed] [Google Scholar]
- 16.Park K, Bakaletz L O, Coticchia J M, Lim D J. Effect of influenza A virus on ciliary activity and dye transport function in the chinchilla eustachian tube. Ann Otol Rhinol Laryngol. 1993;102:551–558. doi: 10.1177/000348949310200711. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld R M, Doyle W J, Swarts J D, Seroky J, Pinero B P. Third-generation cephalosporins in the treatment of acute pneumococcal otitis media. An animal study. Arch Otolaryngol Head Neck Surg. 1992;118:49–52. doi: 10.1001/archotol.1992.01880010053015. [DOI] [PubMed] [Google Scholar]
- 18.Sawyer W D. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969;119:541–556. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- 19.Tong, H. H., L. M. Fisher, G. M. Kosunick, and T. F. DeMaria. The effect of adenovirus type 1 and influenza A virus on Streptococcus pneumoniae nasopharyngeal colonization and otitis media in the chinchilla. Ann. Otol. Rhinol. Laryngol., in press. [DOI] [PubMed]
- 20.Tong, H. H., L. E. Blue, M. A. James, and T. F. DeMaria. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:921–924. [DOI] [PMC free article] [PubMed]
- 21.Wadowsky R M, Mietzner S M, Skoner D P, Doyle W J, Fireman P. Effect of experimental influenza A virus infection on isolation of Streptococcus pneumoniae and other aerobic bacteria from the oropharynges of allergic and nonallergic adult subjects. Infect Immun. 1995;63:1153–1157. doi: 10.1128/iai.63.4.1153-1157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]