Abstract
Aims
Osteoporosis is common in total hip arthroplasty (THA) patients. It plays a substantial factor in the surgery’s outcome, and previous studies have revealed that pharmacological treatment for osteoporosis influences implant survival rate. The purpose of this study was to examine the prevalence of and treatment rates for osteoporosis prior to THA, and to explore differences in osteoporosis-related biomarkers between patients treated and untreated for osteoporosis.
Methods
This single-centre retrospective study included 398 hip joints of patients who underwent THA. Using medical records, we examined preoperative bone mineral density measures of the hip and lumbar spine using dual energy X-ray absorptiometry (DXA) scans and the medications used to treat osteoporosis at the time of admission. We also assessed the following osteoporosis-related biomarkers: tartrate-resistant acid phosphatase 5b (TRACP-5b); total procollagen type 1 amino-terminal propeptide (total P1NP); intact parathyroid hormone; and homocysteine.
Results
The prevalence of DXA-proven hip osteoporosis (T-score ≤ -2.5) among THA patients was 8.8% (35 of 398). The spinal osteoporosis prevalence rate was 4.5% (18 of 398), and 244 patients (61.3%; 244 of 398) had osteopenia (-2.5 < T-score ≤ -1) or osteoporosis of either the hip or spine. The rate of pharmacological osteoporosis treatment was 22.1% (88 of 398). TRACP-5b was significantly lower in the osteoporosis-treated group than in the untreated group (p < 0.001).
Conclusion
Osteoporosis is common in patients undergoing THA, but the diagnosis and treatment for osteoporosis were insufficient. The lower TRACP-5b levels in the osteoporosis-treated group — that is, osteoclast suppression — may contribute to the reduction of the postoperative revision rate after THA.
Cite this article: Bone Joint Res 2022;11(12):873–880.
Keywords: Bone mineral density, TRACP-5b, Total P1NP, Osteoporosis, Total hip arthroplasty (THA), Hip, biomarkers, T-scores, bone mineral density (BMD), osteopenia, hip joints, dual-energy X-ray absorptiometry, spine
Article focus
The prevalence of osteoporosis and osteopenia in prospective total hip arthroplasty (THA) patients.
The low rate of pharmacological treatment for osteoporosis in THA patients.
The difference in osteoporosis-related biomarkers between treated and untreated groups.
Key messages
Osteoporosis is common in THA patients.
Pharmacological treatment for THA patients is insufficient.
Osteoporosis-related biomarkers are significantly lower in THA patients than in untreated groups (p < 0.001).
Strengths and limitations
This is a unique study in the medical literature.
Few studies have investigated osteoporosis-related biomarkers.
The clinical significance of the results requires further investigation.
Treatment of osteoporosis does not always follow standard operating procedure for consensus of treating osteoporosis based on Fracture Risk Assessment Tool/dual energy X-ray absorptiometry score.
Introduction
Total hip arthroplasty (THA) provides excellent functional recovery for patients with hip disorders, such as hip osteoarthritis (OA) and femoral head necrosis. The number of THAs performed is increasing globally, 1,2 and this trend likely contributes to the increasing incidence of periprosthetic fracture, 3-5 aseptic loosening, and periprosthetic joint infection, 6 an uncommon but potentially disabling side-effect.
Osteoporosis is common before THA, especially in elderly patients, 7,8 and the condition is undertreated and undervalued preoperatively. 9 Previous studies suggest that osteoporosis is a risk factor for aseptic loosening and periprosthetic fractures, which are common causes of revision after THA. 10 Randomized controlled trials (RCTs) indicate that osteoporosis medication prevents periprosthetic bone loss after THA. 11 Despite this awareness, bone mineral density (BMD) and bone quality are not routinely investigated, and preoperative osteoporosis is not treated preoperatively. 12 Given that the preoperative optimization of BMD and bone quality may help to mitigate these complications, they are important perioperative considerations; however, they have been underemphasized to date. 9
Various osteoporosis-related biomarkers have been identified and measured to help assess bone quality. 13,14 The relationship between these biochemical markers and osteoporotic fractures has been previously reported in osteoporosis patients. 15-17 However, few reports exist on the relationships between such biomarkers in preoperative THA patients. 18,19 The purpose of this study was to investigate the prevalence and treatment rates of osteoporosis and compare osteoporosis-related biomarkers in patients treated and untreated for osteoporosis preoperatively.
Methods
Study design
This single-centre retrospective study was performed according to the principles of the Declaration of Helsinki. 20 This study is still ongoing, and all patients whose data were included in this manuscript provided written informed consent through a preoperative informed consent process to publish the case details.
Study population
This study included 487 patients who underwent primary THA between July 2017 and December 2020. The mean age was 65.5 years (standard deviation (SD) 11.8). A total of 24 hip joints with femoral neck fractures were excluded from the analysis because the BMD measurement of the displaced femoral neck was not appropriate for accurately measuring BMD. In total, 65 hip joints without both BMD and osteoporosis-related biomarkers were also excluded from the analysis. Overall, 398 patients with a mean age of 65.1 years (SD 11.6) who underwent THA between July 2017 and December 2020 were included (Figure 1). Table I presents the demographic details of the study group.
Fig. 1.
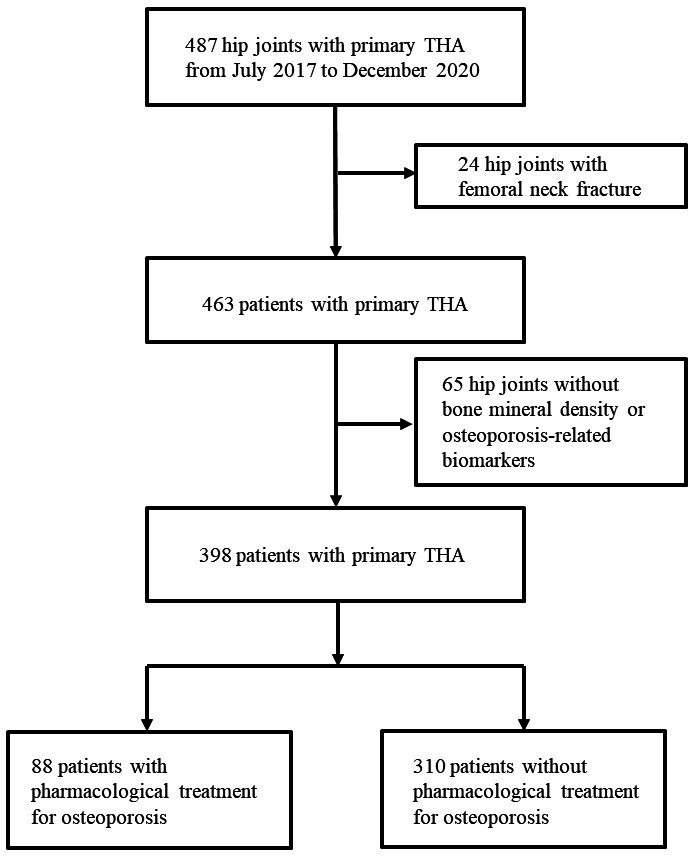
Flowchart of patient selection. THA, total hip arthroplasty.
Table I.
Baseline clinical and demographic characteristics.
| Characteristic | Data |
|---|---|
| Patients, n | 398 |
| Mean age, yrs (SD) | 65.1 (11.6) |
| Sex, n | |
| Male | 68 |
| Female | 330 |
| Preoperative diagnosis, n | |
| DDH-OA | 308 |
| Osteonecrosis of the femoral head | 48 |
| Primary OA | 17 |
| Rapidly destructive coxarthrosis, a rare syndrome that typically involves the rapid and aggressive destruction of the unilateral hip joint | 15 |
| Rheumatoid arthritis | 7 |
| Others | 3 |
DDH, developmental dysplasia of the hip; OA, osteoarthritis; SD, standard deviation.
We reviewed electronic medical records retrospectively to obtain preoperative pharmacological osteoporosis treatment data.
Preoperative measurement
We focused on preoperative BMD and osteoporosis-related biomarkers, as these data have been collected routinely since July 2017 to assess preoperative osteoporosis and bone quality in all patients undergoing primary THA. Among the 487 hip joints, 398 (82%) included data for both preoperative BMD and the following osteoporosis-related biomarkers: serum tartrate-resistant acid phosphatase 5b (TRACP-5b); total procollagen type 1 amino-terminal propeptide (total P1NP); intact parathyroid hormone (intact PTH); and homocysteine. The preoperative BMDs of the operation side of the hip and spine were measured using dual-energy X-ray absorptiometry (DXA), with the results expressed as total hip and spine BMD. The T-score represents the number of SDs from the mean BMD of a young adult, with osteoporosis defined as a BMD T-score ≤ -2.5 and osteopenia as a T-score between -1 and -2.5, according to the criteria of the World Health Organization (WHO). 21
Covariates
Patient background data (age, sex, height, weight, BMI, and preoperative diagnosis) were recorded.
Statistical analysis
Statistical analyses were performed using SPSS v27 (IBM, USA). Chi-squared tests were used to compare categorical values, and continuous variables were evaluated with independent-samples t-tests assuming unequal variance. A Pearson’s rank correlation test was used to assess the linear correlation between osteoporosis-related biomarkers (TRACP-5b, total P1NP, intact PTH, and homocysteine) and hip T-scores. A p-value less than 0.05 were considered statistically significant.
Results
Prevalence and treatment rate of osteoporosis before THA
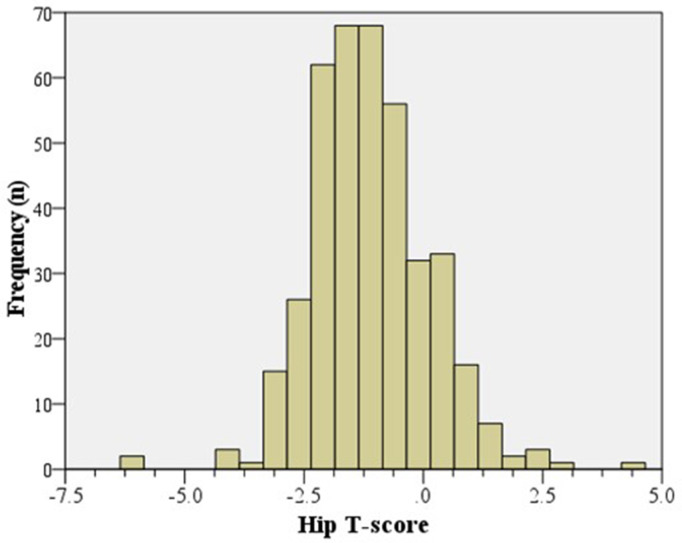
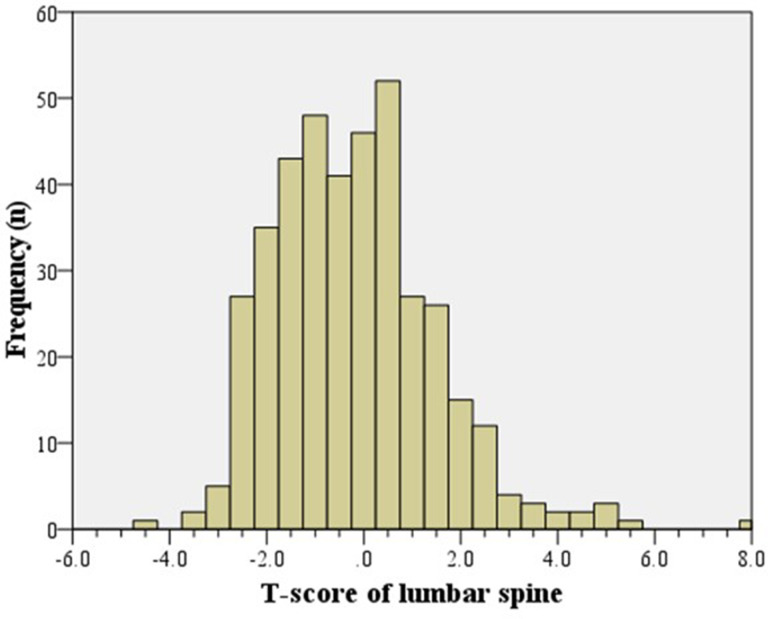
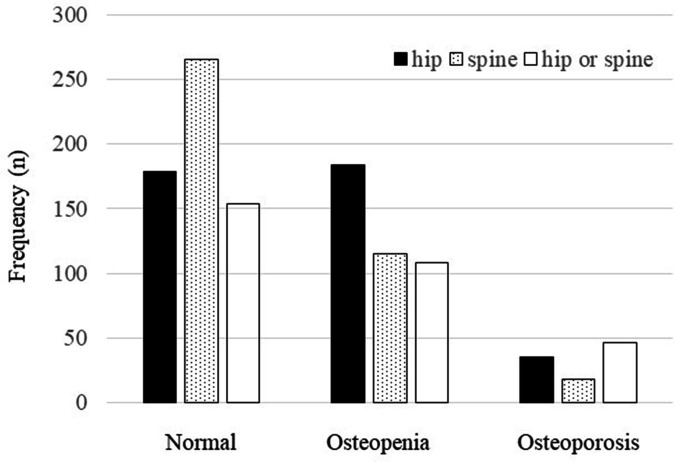
The mean T-score was -1.1 (SD 1.2) for the hip (Figure 2) and -0.19 (SD 1.6) for the lumbar spine (Figure 3). The cohort’s mean hip Z-score and mean spine Z-score were positive, at 0.26 (SD 1.2) and 1.2 (SD 1.7), respectively. The prevalence of DXA-proven hip osteoporosis (T-score ≤ -2.5) and spinal osteoporosis was 8.7% (35 of 398) and 4.5% (18 of 398), respectively, and 244 patients (61.3%; 244 of 398) had osteopenia or osteoporosis of either the hip or spine (Figure 4, Table II).
Fig. 2.
Histogram showing the distribution of hip T-scores (n = 398).
Fig. 3.
Histogram showing the distribution of lumbar spine T-scores (n = 398).
Fig. 4.
Prevalence of osteoporosis and osteopenia.
Table II.
Prevalence of osteopenia and osteoporosis.
| Location | Osteoporosis, n (%) | Osteopenia, n (%) | Osteopenia or osteoporosis, n (%) |
|---|---|---|---|
| Hip (n = 398) |
35 (8.7) | 184 (46.2) | 219 (55.0) |
| Spine (n = 398) |
18 (4.5) | 115 (28.9) | 133 (33.4) |
| Hip or spine (n = 398) |
46 (11.6) | 198 (49.7) | 244 (61.3) |
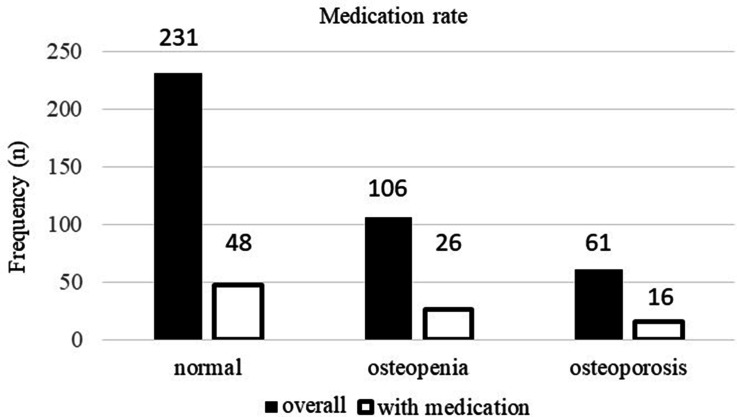
The osteoporosis treatment rate was 22.1% (88 of 398; Table III). The treatment rate of osteoporosis in normal, osteopenia, and osteoporosis patients was 20.8% (48 of 231), 24.5% (26 of 106), and 26.2% (16 of 61), respectively (Figure 5). The breakdown of the therapeutic agents used for osteoporosis included 40 patients on vitamin D analogues (ten for alfacalcidol and 30 for eldecalcitol), 24 on bisphosphonates, eight on a combination of bisphosphonates and vitamin D analogues, six on selective oestrogen receptor modulators, seven on teriparatides, and three on denosumab.
Table III.
Breakdown of preoperative osteoporosis drugs used for patients.
| Medication | n |
|---|---|
| Vitamin D | |
| Alfacalcidol | 10 |
| Eldecalcitol | 30 |
| BP | 24 |
| BP + vitamin D | |
| BP + alfacalcidol | 4 |
| BP + eldecalcitol | 4 |
| SERM | 6 |
| Teriparatide | 7 |
| Denosumab | 3 |
| Total | 88 |
BP, bisphosphonate; SERM, selective oestrogen receptor modulator.
Fig. 5.
The rate of pharmacological osteoporosis treatment in patients from the normal, osteopenia, and osteoporosis groups.
Correlations between the continuous variables, hip T-score, and the osteoporosis-related biomarkers of serum TRACP-5b, total P1NP, intact PTH, and homocysteine were assessed using Pearson’s rank correlation. A Pearson’s test revealed a weak negative correlation between hip T-score and TRACP-5b (r = -0.197, p < 0.001) and total P1NP (r = -0.121, p = 0.016). The negative correlation was more pronounced when observed in the untreated group. No significant correlation was shown between hip T-score and the other biomarkers (Figure 6, Table IV).
Fig. 6.
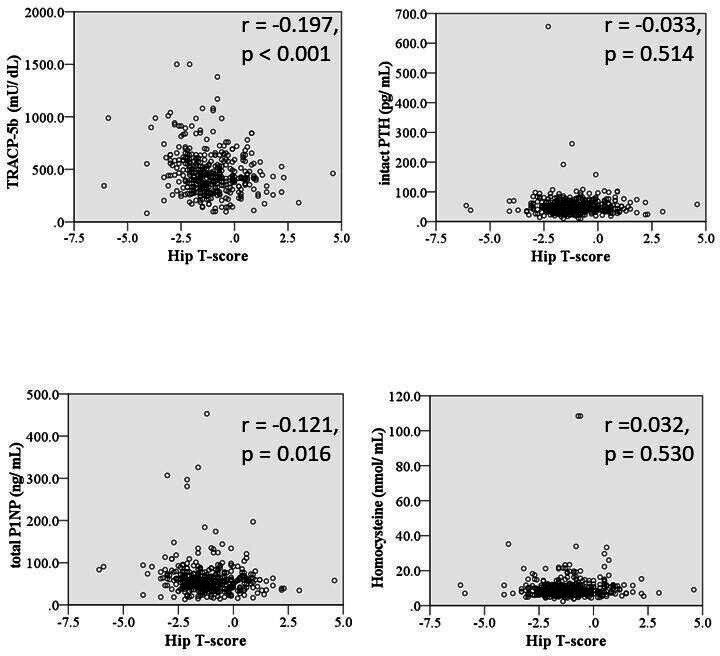
Scatter diagrams showing the relationships between hip T-scores and osteoporosis-related biomarkers (tartrate-resistant acid phosphatase 5b (TRACP-5b), total procollagen type 1 amino-terminal propeptide (total P1NP), intact parathyroid hormone (PTH), and homocysteine). The total number of patients was 398. All p-values were calculated using Pearson’s rank correlation. r = correlation coefficient.
Table IV.
Correlation coefficients between osteoporosis pharmacological treatment and osteoporosis-related biomarkers (r = correlation coefficient, n = 398).
| Biomarker | Medication | Overall (n = 398) | ||||
|---|---|---|---|---|---|---|
| Yes (n = 88) | No (n = 310) | |||||
| r | p-value | r | p-value | r | p-value | |
| TRACP-5b | -0.029 | 0.789 | -0.261 | < 0.001 | -0.197 | < 0.001 |
| Total P1NP | -0.033 | 0.763 | -0.189 | < 0.001 | -0.121 | 0.016 |
| Intact PTH | 0.047 | 0.662 | -0.061 | 0.284 | 0.284 | 0.514 |
| Homocysteine | 0.205 | 0.056 | 0.017 | 0.761 | 0.032 | 0.530 |
Medication: whether patients are using osteoporosis medication.
P1NP, procollagen type 1 amino-terminal propeptide; PTH, parathyroid hormone; TRACP-5b, tartrate-resistant acid phosphatase 5b.
Difference in osteoporosis-related biomarkers with and without treatment for osteoporosis
Of 398 patients, 88 were pharmacologically treated for osteoporosis and 310 were untreated. TRACP-5b was significantly lower in the osteoporosis-treated group than in the untreated group, despite no significant difference in hip T-scores between the two groups. The levels of other osteoporosis-related biomarkers were not significantly different between the groups (Table V).
Table V.
Changes in bone mineral density and osteoporosis-related biomarkers with and without pharmacological treatment for osteoporosis.
| Variable | Medication | p-value* | |
|---|---|---|---|
| Yes (n = 88) | No (n = 310) | ||
| Mean T-score of femoral neck (SD) | -1.3 (1.2) | -1.0 (1.2) | 0.079 |
| Mean T-score of lumbar spine (SD) | -0.5 (1.6) | -0.1 (1.7) | 0.029 |
| Mean TRACP-5b, mU/dl (SD) | 393.6 (207.3) | 490.0 (207.2) | < 0.001 |
| Mean total P1NP, ng/ml (SD) | 58.7 (64.3) | 62.8 (30.8) | 0.402 |
| Mean homocysteine, nmol/ml (SD) | 10.8 (5.3) | 10.1 (8.9) | 0.471 |
| Mean intact PTH, pg/ml (SD) | 50.9 (35.5) | 53.1 (39.2) | 0.625 |
Medication: whether patients are using osteoporosis medication.
Independent-samples t-test.
P1NP, procollagen type 1 amino-terminal propeptide; PTH, parathyroid hormone; SD, standard deviation; TRACP-5b, tartrate-resistant acid phosphatase 5b.
Discussion
In our study, 244 patients (61.3%; 244 of 398) had osteopenia or osteoporosis of either the hip or spine according to WHO criteria. However, the treatment rate for osteoporosis was only 22.1% (88 of 398). Hip T-score had a weak negative correlation with TRACP-5b and total P1NP. TRACP-5b was significantly lower in the osteoporosis-treated group than in the untreated group, although no significant difference in hip T-score existed between the two groups.
We found low bone mass, defined as osteopenia or osteoporosis according to WHO criteria, in a large number (61.3%) of the patients before THA in our study. Lingard et al 8 found that 33% of patients in the UK awaiting arthroplasty for the lumbar spine and 37% for the proximal femur had low bone mass. Our results indicated a much higher prevalence of preoperative osteoporosis before THA than previously reported; 8 however, research has suggested that Japanese patients have an even higher prevalence of osteoporosis than other populations, 22 and our results are consistent with these previous reports. 7,8
In our study group, pharmacological osteoporosis treatment was applied to only around one in five patients preoperatively, regardless of their BMD. Previous studies have also found osteoporosis to be common, underdiagnosed, and undertreated prior to joint arthroplasty. 9,23 Considering the treatment rates for hypertension (70% to 80%) in Japan, 24 the treatment rate for osteoporosis in our country is still low, 25 which suggests that physicians and orthopaedic surgeons may have been paying less attention to osteoporosis than to other chronic diseases. Preoperative information about bone status is particularly important in arthroplasty to avoid intraoperative fractures and other postoperative complications, including stem subsidence, 26 implant loosening, 27 and late periprosthetic fractures. 28
The relationships between BMD and osteoporosis-related biomarkers have been studied previously, 29-32 and these factors have recently been related to gut microbiome. 33-35 However, patients undergoing THA have not been adequately studied regarding the relationships between BMD and osteoporosis-related biomarkers. Our results of correlations between hip T-score and osteoporosis-related biomarkers are consistent with previous reports. 29-32 Osteoporosis treatment cancels the correlation between osteoporosis-related biomarkers and BMD, and the same effect is thought to be caused in vivo. Previous reports include large-scale clinical studies showing that the use of bisphosphonates resulted in a statistically significant reduction in the postoperative revision rate after artificial joint arthroplasty, 36 and RCTs in which postoperative osteoporosis treatment suppressed the reduction in peri-implant BMD. 11 These results may be associated with low TRACP-5b levels — that is, osteoclast suppression — and further studies are warranted.
Our study has three limitations. First, the information about pharmacological osteoporosis treatment before THA was obtained retrospectively from electronic medical records. We did not prospectively investigate the treatment period, so the effect of medication on osteoporosis-related biomarkers could differ according to the treatment period. Regarding bisphosphonates, a previous report indicated that starting bisphosphonates six months before surgery and continuing three years after surgery is desirable. 37 Second, in most of our cases, pharmacological osteoporosis treatment was started by general practitioners or doctors in other departments, and the reasons for starting osteoporosis drugs are not clear. Third, changes in biomarker values may be different depending on the drug in the treatment group. However, we believe that our results regarding the severe osteoarthritic population provide valuable information. As the number of subjects increases, investigation into changes in biomarker values with each drug will be possible, and future research in this area is needed.
In conclusion, osteoporosis is common in patients undergoing THA, but the diagnosis and treatment for osteoporosis were insufficient. The lower TRACP-5b levels in the osteoporosis-treated group — that is, osteoclast suppression — may contribute to the reduction of the postoperative revision rate after THA.
Author contributions
N. Watanabe: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft.
K. Miyatake: Supervision, Writing – review & editing.
R. Takada: Supervision, Writing – review & editing.
T. Ogawa: Formal analysis, Supervision, Writing – review & editing.
Y. Amano: Writing – review & editing.
T. Jinno: Supervision, Writing – review & editing.
H. Koga: Supervision, Writing – review & editing.
T. Yoshii: Supervision, Writing – review & editing.
A. Okawa: Supervision, Writing – review & editing.
Funding statement
The authors received no financial or material support for the research, authorship, and/or publication of this article.
ICMJE COI statement
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank Harry Wilson for English editing.
Ethical review statement
Approval was obtained from the institutional research ethics committee of Tokyo Medical and Dental University Hospital (No. M2000-1099, Postoperative Clinical Survey of Total Hip Arthroplasty).
Open access funding
The authors report that the open access funding for their manuscript was self-funded.
Contributor Information
Naoto Watanabe, Email: n-watanabe.arm@tmd.ac.jp.
Kazumasa Miyatake, Email: miyatake.orj@tmd.ac.jp.
Ryohei Takada, Email: takada.orth@tmd.ac.jp.
Takahisa Ogawa, Email: takahisa.o@gmail.com.
Yusuke Amano, Email: amanorj@tmd.ac.jp.
Tetsuya Jinno, Email: jinnot@dokkyomed.ac.jp.
Hideyuki Koga, Email: koga.orj@tmd.ac.jp.
Toshitaka Yoshii, Email: yoshii.orth@tmd.ac.jp.
Atsushi Okawa, Email: okawa.orth@tmd.ac.jp.
References
- 1. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606–2612. 10.1007/s11999-009-0834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinstein AM, Rome BN, Reichmann WM, et al. . Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95-A(5):385–392. 10.2106/JBJS.L.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Della Rocca GJ, Leung KS, Pape HC. Periprosthetic fractures: epidemiology and future projections. J Orthop Trauma. 2011;25 Suppl 2:S66–70. 10.1097/BOT.0b013e31821b8c28 [DOI] [PubMed] [Google Scholar]
- 4. Frenzel S, Vécsei V, Negrin L. Periprosthetic femoral fractures--incidence, classification problems and the proposal of a modified classification scheme. Int Orthop. 2015;39(10):1909–1920. 10.1007/s00264-015-2967-4 [DOI] [PubMed] [Google Scholar]
- 5. Montgomery S, Bourget-Murray J, You DZ, et al. . Cost-effectiveness of dual-mobility components in patients with displaced femoral neck fractures. Bone Joint J. 2021;103-B(12):1783–1790. 10.1302/0301-620X.103B12.BJJ-2021-0495.R2 [DOI] [PubMed] [Google Scholar]
- 6. Slullitel PA, Oñativia JI, Zanotti G, Comba F, Piccaluga F, Buttaro MA. One-stage exchange should be avoided in periprosthetic joint infection cases with massive femoral bone loss or with history of any failed revision to treat periprosthetic joint infection. Bone Joint J. 2021;103-B(7):1247–1253. 10.1302/0301-620X.103B7.BJJ-2020-2155.R1 [DOI] [PubMed] [Google Scholar]
- 7. James SJ, Mirza SB, Culliford DJ, Taylor PA, Carr AJ, Arden NK. Baseline bone mineral density and boneturnover in pre-operative hip and knee arthroplasty patients. Bone Joint Res. 2014;3(1):14–19. 10.1302/2046-3758.31.2000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lingard EA, Mitchell SY, Francis RM, et al. . The prevalence of osteoporosis in patients with severe hip and knee osteoarthritis awaiting joint arthroplasty. Age Ageing. 2010;39(2):234–239. 10.1093/ageing/afp222 [DOI] [PubMed] [Google Scholar]
- 9. Bernatz JT, Brooks AE, Squire MW, Illgen RI, Binkley NC, Anderson PA. Osteoporosis is common and undertreated prior to total joint arthroplasty. J Arthroplasty. 2019;34(7):1347–1353. 10.1016/j.arth.2019.03.044 [DOI] [PubMed] [Google Scholar]
- 10. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91-A(1):128–133. 10.2106/JBJS.H.00155 [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi N, Inaba Y, Uchiyama M, Ike H, Kubota S, Saito T. Teriparatide versus alendronate for the preservation of bone mineral density after total hip arthroplasty - A randomized controlled trial. J Arthroplasty. 2016;31(1):333–338. 10.1016/j.arth.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 12. Maier GS, Kolbow K, Lazovic D, Maus U. The importance of bone mineral density in hip arthroplasty: Results of a survey asking orthopaedic surgeons about their opinions and attitudes concerning osteoporosis and hip arthroplasty. Adv Orthop. 2016;2016:8079354. 10.1155/2016/8079354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waqas K, Chen J, Koromani F, et al. . Skin autofluorescence, a noninvasive biomarker for advanced glycation end-products, is associated with prevalent vertebral and major osteoporotic fractures: The Rotterdam Study. J Bone Miner Res. 2020;35(10):1904–1913. 10.1002/jbmr.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Behera J, Bala J, Nuru M, Tyagi SC, Tyagi N. Homocysteine as a pathological biomarker for bone disease. J Cell Physiol. 2017;232(10):2704–2709. 10.1002/jcp.25693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerdhem P, Ivaska KK, Alatalo SL, et al. . Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19(3):386–393. 10.1359/JBMR.0301244 [DOI] [PubMed] [Google Scholar]
- 16. van Meurs JBJ, Dhonukshe-Rutten RAM, Pluijm SMF, et al. . Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350(20):2033–2041. 10.1056/NEJMoa032546 [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Hu X, Zhang Q, Cao H, Wang J, Liu B. Homocysteine level and risk of fracture: A meta-analysis and systematic review. Bone. 2012;51(3):376–382. 10.1016/j.bone.2012.05.024 [DOI] [PubMed] [Google Scholar]
- 18. Mäkinen TJ, Alm JJ, Laine H, Svedström E, Aro HT. The incidence of osteopenia and osteoporosis in women with hip osteoarthritis scheduled for cementless total joint replacement. Bone. 2007;40(4):1041–1047. 10.1016/j.bone.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 19. Nyström A, Kiritopoulos D, Ullmark G, et al. . Denosumab prevents early periprosthetic bone loss after uncemented total hip arthroplasty: Results from a randomized placebo-controlled clinical trial. J Bone Miner Res. 2020;35(2):239–247. 10.1002/jbmr.3883 [DOI] [PubMed] [Google Scholar]
- 20. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21. Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–1141. 10.1002/jbmr.5650090802 [DOI] [PubMed] [Google Scholar]
- 22. Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9:182. 10.1007/s11657-014-0182-3 [DOI] [PubMed] [Google Scholar]
- 23. Ha CW, Park YB. Underestimation and undertreatment of osteoporosis in patients awaiting primary total knee arthroplasty. Arch Orthop Trauma Surg. 2020;140(8):1109–1114. 10.1007/s00402-020-03462-y [DOI] [PubMed] [Google Scholar]
- 24. Takase H, Tanaka T, Takayama S, et al. . Recent changes in blood pressure levels, hypertension prevalence and treatment rates, and the rate of reaching target blood pressure in the elderly. Medicine (Baltimore). 2017;96(50):50. 10.1097/MD.0000000000009116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iki M. [Epidemiology of bone and joint disease - the present and future -. epidemiology of osteoporosis and osteoporotic fracture in japan]. Clin Calcium. 2014;24(5):657–664. (Article in Japanese). CliCa1405657664 [PubMed] [Google Scholar]
- 26. Aro HT, Alm JJ, Moritz N, Mäkinen TJ, Lankinen P. Low BMD affects initial stability and delays stem osseointegration in cementless total hip arthroplasty in women: a 2-year RSA study of 39 patients. Acta Orthop. 2012;83(2):107–114. 10.3109/17453674.2012.678798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nixon M, Taylor G, Sheldon P, Iqbal SJ, Harper W. Does bone quality predict loosening of cemented total hip replacements? J Bone Joint Surg Br. 2007;89-B(10):1303–1308. 10.1302/0301-620X.89B10.19038 [DOI] [PubMed] [Google Scholar]
- 28. Franklin J, Malchau H. Risk factors for periprosthetic femoral fracture. Injury. 2007;38(6):655–660. 10.1016/j.injury.2007.02.049 [DOI] [PubMed] [Google Scholar]
- 29. Halleen JM, Ylipahkala H, Alatalo SL, et al. . Serum tartrate-resistant acid phosphatase 5b, but not 5a, correlates with other markers of bone turnover and bone mineral density. Calcif Tissue Int. 2002;71(1):20–25. 10.1007/s00223-001-2122-7 [DOI] [PubMed] [Google Scholar]
- 30. Wei X, Zhang Y, Xiang X, et al. . Exploring the relationship of bone turnover markers and bone mineral density in community-dwelling postmenopausal women. Dis Markers. 2021;2021:6690095. 10.1155/2021/6690095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki K, Sugimoto C, Takizawa M, et al. . Correlations between bone mineral density and circulating bone metabolic markers in diabetic patients. Diabetes Res Clin Pract. 2000;48(3):185–191. 10.1016/s0168-8227(00)00119-4 [DOI] [PubMed] [Google Scholar]
- 32. Bucciarelli P, Martini G, Martinelli I, et al. . The relationship between plasma homocysteine levels and bone mineral density in post-menopausal women. Eur J Intern Med. 2010;21(4):301–305. 10.1016/j.ejim.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 33. Li S, Mao Y, Zhou F, Yang H, Shi Q, Meng B. Gut microbiome and osteoporosis: a review. Bone Joint Res. 2020;9(8):524–530. 10.1302/2046-3758.98.BJR-2020-0089.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Ho WTP, Liu C, et al. . The role of gut microbiota in bone homeostasis. Bone Joint Res. 2021;10(1):51–59. 10.1302/2046-3758.101.BJR-2020-0273.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng B, Wen Y, Yang X, et al. . Gut microbiota is associated with bone mineral density : an observational and genome-wide environmental interaction analysis in the UK Biobank cohort. Bone Joint Res. 2021;10(11):734–741. 10.1302/2046-3758.1011.BJR-2021-0181.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prieto-Alhambra D, Javaid MK, Judge A, et al. . Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ. 2011;343:d7222. 10.1136/bmj.d7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prieto-Alhambra D, Lalmohamed A, Abrahamsen B, et al. . Oral bisphosphonate use and total knee/hip implant survival: validation of results in an external population-based cohort. Arthritis Rheumatol. 2014;66(11):3233–3240. 10.1002/art.38789 [DOI] [PubMed] [Google Scholar]