Abstract
Background
Vietnam introduced a 3-dose hepatitis B (HBV) immunization program comprising 1 dose immediately after birth and 2 or 3 in infancy in the past 20 years, but the impact of the vaccine has not been systematically evaluated. Thus, we conducted this survey aiming to estimate the age-specific chronic HBV prevalence in the general population and to evaluate HBV immunization effectiveness.
Methods
Population-based, four-stage cluster sampling was used in the South Central Coast region of Vietnam. The point-of-care Determine rapid test was used to assess hepatitis B surface antigen (HBsAg) positivity.
Results
A total of 2,075 samples were included in the study. HBsAg prevalence was significantly higher among adults aged 20–39 years than in the population aged 1–19 years (8.0% [95% confidence interval 5.0–12.0] vs. 2.0% [95% confidence interval 1.0–6.0], p<0.01). HBsAg prevalence decreased after implementation of the 3-dose vaccination schedule during infancy from 1997 to 2002, whereas the change in prevalence after implementation of the birth dosing was not significant. A slight increase in HBsAg prevalence was observed for the cohort born in 2011, 2012, and 2013, when there was a vaccine shortage and media reports of immunization resistance.
Conclusions
This is the first population-based assessment of the introduction of the HBV vaccine in Vietnam performed by estimating the HBsAg prevalence across a wide range of ages. The results showed that the HBV immunization policy effectively reduces HBsAg prevalence in general, although birth dosing of the vaccine and low immunization coverage should be carefully monitored.
Keywords: Hepatitis B surface Antigen, Seroprevalence survey, Cluster sampling, Immunization, Vietnam
1. Introduction
Hepatitis B (HBV) is a serious infectious disease that may cause liver cirrhosis and hepatocellular carcinoma, mainly due to persistent virus infection, and kills about 1 million people worldwide every year. The World Health Organization (WHO) estimated that, in 2015, 257 million people were living with chronic HBV infection (defined as hepatitis B surface antigen [HBsAg] positive), with highly endemic countries concentrated in Southeast Asia and Africa.1
Vaccination is the core tool for HBV prevention. The WHO recommends that all infants receive the HBV vaccine as soon as possible after birth, preferably within 24 h, followed by 2 or 3 doses of vaccine at least 4 weeks apart to complete the schedule.1
In the WHO Western Pacific region, resolutions were adopted in 2003 and 2005 to reduce the HBV infection rate in children aged 5 years to less than 1% and to promote the prevention of mother-to-child transmission through HBV vaccination in each country.2,3 Vietnam has a very high prevalence of HBV, around 10% in adults,4,5 but routine immunization against HBV in children has achieved a 97% immunization rate due to the efforts of the parties involved.6
Evaluation of the vaccination program is essential for continuing and improving HBV control in the country. Because chronic HBV infection is mostly asymptomatic, HBsAg prevalence represents a surrogate marker for the cumulative incidence of chronic HBV infection.7 However, most cross-sectional epidemiological studies of HBV conducted in developing countries target only specific generations or specific populations. Possible reasons are the high cost, insufficient human resources, and unsatisfactory laboratory systems in those countries. Such resource-limited settings can benefit from multistage, cluster sampling surveys combined with a rapid diagnostic test kit or the collection of dried blood spot samples. In the Lao People’s Democratic Republic, these methods were used to estimate the nationwide HBV prevalence and population immunity against measles and rubella.8, 9, 10
The first objective of this study was to estimate the age-specific chronic HBV infection prevalence in the general population by measuring HBsAg with point-of-care diagnostic kits from a stratified multistage cluster sampling survey with random selection at each stage. The second objective was to evaluate the effectiveness of HBV immunization by comparing HBsAg prevalence and immunization coverage.
2. Methods
A field survey was conducted at the end of May 2019 in four provinces (Khanh Hoa, population 1,247,915; Ninh Thuan, 670,299; Quang Ngai, 1,325,845; Binh Dinh, 1,612,151) in the South Central Coast region of Vietnam. To collect a wide range of data on people both before and after the introduction of the immunization program, from infants to childbearing age, we targeted people aged 1–39 years on May 20, 2019.
2.1. Sample size
The sample size was calculated based on an expected HBsAg seroprevalence rate of 10%. A sample size of 208 for each 5-year age group was calculated at a 5% level of significance with ±5% precision in consideration of an assumed design effect of 1.5. To cover 40 years (ages 1–39 years), we needed eight 5-year age groups, giving a total of 1664 participants. We also assumed that about 30% of households would be absent or have no household members of the target age at the time of our visit and that the response rate of the target household members in the visited households would be 70%. The required number of households to be approached was thus calculated as 1887 in consideration of the population pyramid information in Vietnam in 2014, in which the number of household members aged 1–39 years per household was estimated to be 2.16, with an additional 20% to make up shortfalls due to variabilities among age groups.
2.2. Sampling
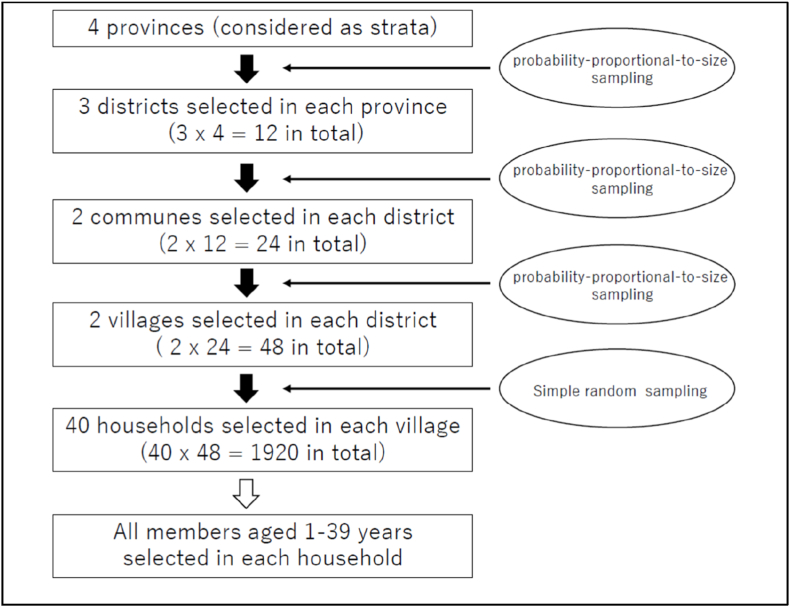
The target provinces were considered as four strata. In the first stage of sampling, 3 districts were randomly selected from each stratum using probability proportional to size (PPS) sampling based on the population data from the 2015 census conducted in Vietnam. In the second stage, 2 communes were randomly selected from each selected district by PPS sampling. In the third stage, 2 villages were randomly selected from each selected commune by PPS sampling, and a total of 48 villages, as clusters, were selected.
In each selected village, 40 households were randomly selected and all household members aged 1–39 years were targeted in this survey (Fig. 1).
Fig. 1.
Sampling flowchart.
2.3. Questionnaire
A brief face-to-face questionnaire was administered to the sampled household members. Information was collected on the demographic status and history of children (i.e., age, sex, place of birth, and immunization history) from all samples. In addition, the sample aged 18 years or older was asked about socioeconomic status and exposure to potential risk factors for HBV infection (e.g., surgery, blood transfusion, and history of past hepatitis infection among other family members).
2.4. HBsAg testing
A simple rapid test (Alere Determine HBsAg; Alere Medical Co. Ltd., Chiba, Japan) with a fingerstick whole-blood sensitivity and specificity of 97.2% and 98.5%, respectively,11 was used instead of the traditional ELISA test because it is better suited for field use. Testing for HBsAg was performed according to the manufacturer's instructions. Blood obtained from a finger prick using a safety lancet and glass capillary tube was applied to the sample pad of the rapid test kit. After the addition of chase buffer, surveyors evaluated the results after at least 15 min. If no control bar was present after 15 min, the results were deemed invalid and the test was repeated. In addition, blood spots were applied to filter paper for additional testing.
2.5. Ethical considerations
The surveyors explained the study’s objectives and procedures in detail to the selected household members verbally and in writing. Written informed consent was obtained from the participants or from their parents if they were less than 18 years old. Informed consent, questionnaires, and the blood collection process were overseen by supervisors from the Pasteur Institute of Nha Trang. The survey proposal was reviewed and approved by the Ethics Committees of the Pasteur Institute of Nha Trang and the National Center Global Health and Medicine, Japan (NCGM-3091).
2.6. Data entry and analysis
All completed questionnaires were brought to the Pasteur Institute of Nha Trang. To reduce any data entry-related errors, double data entry was performed in Microsoft Excel (Microsoft Office 2019) along with validation and correction. STATA16 (Stata Corp., College Station, TX) was used for the data analysis. To compare and evaluate differences among generations, HBsAg prevalence was calculated for each 5-year age group, considering the individual weight of each sample and the multistage clustered sampling design to ensure representative and unbiased results. The Clopper–Pearson exact method was used to calculate the 95% confidence intervals (CIs) of HBsAg prevalence for each group. Binary logistic regression models were used to investigate the predictors of different individual characteristics for HBsAg positivity. A p value less than 0.05 was considered statistically significant.
3. Results
Information was collected on 2,093 participants in 1,093 households. After the exclusion of 1 participant who refused a blood test, 5 participants with unknown blood test results, and 12 participants whose age was out of the target range, 2,075 participants were finally enrolled into our survey. Participants were almost equally distributed among the four provinces: 533 from Khanh Hoa, 523 from Ninh Thuan, 525 from Quang Ngai, and 494 from Binh Dinh. In total, 61.1% of the participants were female, and the overall mean age ±standard deviation was 18.0 ± 11.7 years. Adults aged 18–39 years (n = 954) accounted for 46.0% of participants, and children aged 1–17 years (n = 1,121) for 54.0%. The numbers of participants in each age group exceeded the required sample size of 208, except in the group aged 20–24 years.
The background characteristics of the adult participants revealed that history of blood transfusion and family history of hepatitis were associated with HBsAg positivity (p = 0.016 and p<0.001, respectively), whereas it had no association with sex, province of residence, level of education, annual income, and history of surgery (Table 1a). The background characteristics of the participants less than 18 years old showed that children born in private clinics had higher HBsAg prevalence (5.81%) than those born in hospitals (1.20%) and in health centers (0.82%) (Table 1b); this association was statistically significant (p = 0.004). Immunization certificates were available for 246 children, and history of HBV vaccination at birth and history of HBV vaccination during infancy were not associated with HBsAg prevalence.
Table 1a.
Prevalence of HBsAg among participants aged ≥18 years in four provinces in the South Central Coast region of Vietnam by selected background characteristics.
| n | HBsAg(+) | 95% CI | Odds ratio | 95%CI | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Province | ||||||||||
| Khanh Hoa | 277 | 8.66% | 5.63 | ー | 12.62 | (reference) | ||||
| Nhin Thuan | 256 | 12.11% | 8.38 | ー | 16.75 | 1.45 | 0.83 | ー | 2.55 | 0.193 |
| Quang Ngai | 225 | 7.11% | 4.11 | ー | 11.29 | 0.81 | 0.42 | ー | 1.56 | 0.523 |
| Binh Dinh | 196 | 8.16% | 4.73 | ー | 12.92 | 0.94 | 0.48 | ー | 1.81 | 0.847 |
| Sex | ||||||||||
| Male | 256 | 11.72% | 8.05 | ー | 16.31 | 1.49 | 0.94 | ー | 2.38 | 0.093 |
| Female | 698 | 8.17% | 6.24 | ー | 10.45 | (reference) | ||||
| Education | ||||||||||
| None or some primary | 59 | 8.47% | 2.81 | ー | 18.67 | (reference) | ||||
| Completed 'primary | 159 | 8.18% | 4.43 | ー | 13.58 | 1.23 | 0.42 | ー | 3.58 | 0.706 |
| Completed junior high | 309 | 9.39% | 6.38 | ー | 13.20 | 1.43 | 0.53 | ー | 3.83 | 0.477 |
| Completed high school | 230 | 10.00% | 6.45 | ー | 14.63 | 1.53 | 0.56 | ー | 4.19 | 0.404 |
| Higher than high school | 182 | 9.34% | 5.53 | ー | 14.53 | 1.42 | 0.50 | ー | 4.01 | 0.506 |
| Annual income | ||||||||||
| < 25,000,000 VND | 202 | 8.42% | 4.98 | ー | 13.13 | 0.95 | 0.53 | ー | 1.70 | 0.865 |
| 25,000,000 ~ 50,000,000 | 191 | 8.38% | 4.86 | ー | 13.25 | (reference) | ||||
| > 50,000,000 VND | 230 | 10.43% | 6.80 | ー | 15.13 | 1.21 | 0.72 | ー | 2.03 | 0.481 |
| History of blood transfusion | ||||||||||
| Yes | 46 | 19.57% | 9.36 | ー | 33.91 | 2.53 | 1.17 | ー | 5.44 | 0.017 |
| No | 879 | 8.76% | 7.0 | ー | 10.80 | (reference) | ||||
| History of surgery | ||||||||||
| Yes | 248 | 9.68% | 6.30 | ー | 14.06 | 1.09 | 0.67 | ー | 1.79 | 0.727 |
| No | 683 | 0.89% | 6.90 | ー | 11.32 | (reference) | ||||
| Family history of hepatitis | ||||||||||
| Yes | 91 | 30.77% | 21.5 | ー | 41.32 | 7.73 | 4.41 | ー | 13.55 | <0.001 |
| No | 644 | 5.43% | 3.81 | ー | 7.48 | (reference) | ||||
Table 1b.
Prevalence of HBsAg among participants aged <18 years in four provinces in the South Central Coast region of Vietnam by selected background characteristics.
| n | HBsAg(+) | 95% CI | Odds ratio | 95%CI | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Province | ||||||||||
| Khain Hoa | 256 | 2.34% | 0.86 | ー | 5.03 | (reference) | ||||
| Nhin Thuan | 267 | 1.50% | 0.41 | ー | 3.80 | 0.63 | 0.18 | ー | 2.27 | 0.484 |
| Quang Ngai | 300 | 2.33% | 0.94 | ー | 4.75 | 1.00 | 0.33 | ー | 3.00 | 0.994 |
| Binh Dinh | 298 | 2.68% | 1.17 | ー | 5.22 | 1.15 | 0.39 | ー | 3.36 | 0.799 |
| Sex | ||||||||||
| Male | 550 | 1.64% | 0.75 | ー | 3.08 | 0.57 | 0.25 | ー | 1.31 | 0.189 |
| Female | 569 | 2.81% | 1.62 | ー | 4.53 | (reference) | ||||
| Time to nearest health facility | ||||||||||
| < 15 min | 870 | 2.30% | 1.41 | ー | 3.53 | 1.69 | 0.50 | ー | 5.75 | 0.398 |
| 15-30 min | 219 | 1.37% | 0.28 | ー | 3.95 | (reference) | ||||
| > 30 min | 32 | 6.25% | 0.77 | ー | 20.8 | 4.80 | 0.77 | ー | 29.91 | 0.093 |
| Birth place | ||||||||||
| Hospital | 753 | 1.20% | 0.58 | ー | 2.26 | (reference) | ||||
| Health center | 122 | 0.82% | 0.02 | ー | 4.48 | 0.68 | 0.09 | ー | 5.44 | 0.719 |
| Private clinic | 86 | 5.81% | 1.91 | ー | 13.1 | 5.10 | 1.67 | ー | 15.59 | 0.004 |
| History of hepatitis B vaccination at birth | ||||||||||
| Yes | 142 | 2.11% | 0.44 | ー | 6.05 | 0.82 | 0.28 | ー | 3.18 | 0.919 |
| No or unknown | 979 | 2.25% | 1.41 | ー | 3.38 | (reference) | ||||
| History of regular hepatitis B vaccination | ||||||||||
| Received 3 times | 218 | 2.29% | 0.74 | ー | 5.27 | 1.04 | 0.38 | ー | 2.79 | 0.944 |
| Less than twice or unknown | 903 | 2.21% | 1.36 | ー | 3.40 | (reference) | ||||
To compare HBsAg prevalence by age group, we calculated HBsAg prevalence considering the sampling design and sampling weight of each participant (Table 2). Overall, younger age groups showed lower HBsAg prevalence, with a marked change observed between individuals aged 20–24 years and those aged 15–19 years (8.8% [95% CI 5.3–14.2] vs. 3.8% [95% CI 0.9–14.4], p<0.04). The HBsAg prevalence was relatively stable among individuals aged 1–19 years (0.8%–3.8%) and those aged 20–39 years (7.3%–8.8%), whereas the group aged 5–9 years showed a small increase compared with the groups aged 1–4 years and 10–14 years. The design effect was assumed to be 1.5 before the survey, and post-survey calculations revealed that it was distributed around 1.0 except for the groups aged 15–19 years and 35–39 years.
Table 2.
Prevalence of HBsAg calculated considering the sampling design for each age group in four provinces in the South Central Coast region of Vietnam.
| Age group | n | HBsAg(+) | 95% CI | Design effect | ||
|---|---|---|---|---|---|---|
| 1–4 | 292 | 1.39% | 0.42 | ー | 4.46 | 1.12 |
| 5–9 | 358 | 3.11% | 1.67 | ー | 5.70 | 0.84 |
| 10–14 | 310 | 0.80% | 0.18 | ー | 3.43 | 1.10 |
| 15–19 | 230 | 4.03% | 0.96 | ー | 15.42 | 3.55 |
| 20–24 | 169 | 8.72% | 5.36 | ー | 13.90 | 0.61 |
| 25–29 | 236 | 8.01% | 5.02 | ー | 12.55 | 0.77 |
| 30–34 | 238 | 8.57% | 5.7 | ー | 12.68 | 0.62 |
| 35–39 | 242 | 7.49% | 3.45 | ー | 15.51 | 2.23 |
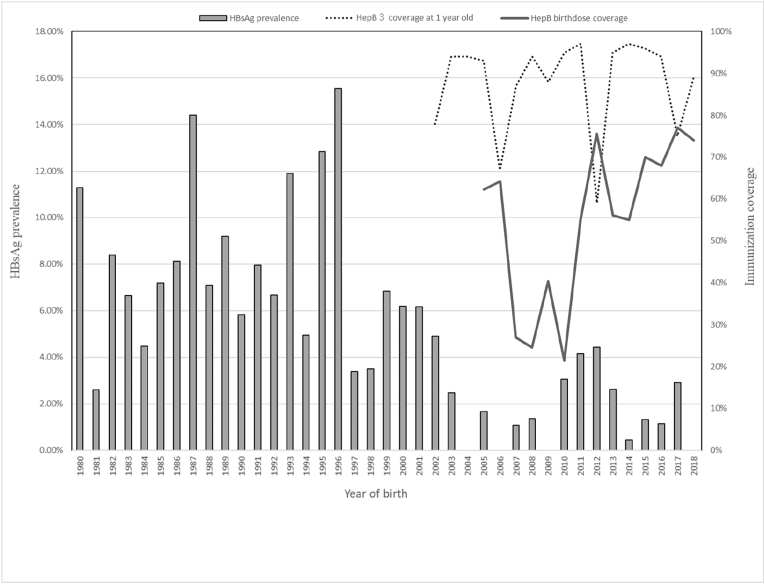
To evaluate the effectiveness of the universal HBV vaccine implementation, we plotted the HBsAg prevalence by year of birth, by coverage of the third HBV dose at 1 year old, and by HBV vaccine birth dosing coverage. The HBsAg prevalence for each age cohort fluctuated (2.40%–20.9%) before the introduction of the HBV vaccine in 2001, when individuals aged 17–18 years at the time of the survey were born, but generally decreased after that year (0%–4.5%). Coverage of the third HBV dose rapidly decreased to 59% in 2013 (among children born in 2012) and HBsAg prevalence among children aged 6–7 years temporarily increased, but the same phenomenon was not observed in 2007 when this coverage fell to 67%.
4. Discussion
This study has three main findings. First, the age-specific HBsAg prevalence was successfully estimated using a population-based survey from representative data of the South Central Coast region. Second, the HBsAg prevalence decreased after HBV vaccine introduction. Third, the HBsAg prevalence fluctuated by year of birth.
The characteristics of participants in this study are similar to those of the sampled population of the Viet Nam Multiple Indicator Cluster Survey by UNICEF (MICS2014). For example, 93.7% of the participants older than 18 years had completed elementary school, whereas 90.4% of the children of a lower secondary school age were attending lower secondary school or higher in the MICS 2014. Moreover, 76.8% of participants had completed lower secondary school, whereas 70.7% of children of an upper secondary school age were attending upper secondary school or higher in the MICS 2014.12 Therefore, the results of the present study could be regarded as representative of a population with a demographic composition similar to that of Vietnam as a whole (Table 1a, Table 1ba and 1b).
The design effects of the HBsAg prevalence by age calculated after the survey ranged from 0.61 to 2.13, except in the group aged 15–19 years, which was close to the value of 1.5 estimated in advance and suggests that the study’s sampling design was satisfactory. The higher design effects among individuals aged 15–19 years may be partially explained by spotty implementation of the HBV vaccine. The HBV vaccine was introduced in 1997 as a pilot program when individuals aged 21–22 years at the time of this survey were infants and then gradually expanded nationwide until 2002 when individuals aged 16–17 years in the survey were infants. Therefore, the higher design effect of 3.26 might be explained by this uneven expansion.
The age-specific HBsAg prevalence identified here was similar to that of previous studies. Nguyen et al. reported the results of a nationwide survey of children born between 2000 and 2008 in Vietnam, with HBsAg prevalence of about 3%–5% among children born between 2000 and 2004 and about 2% among those born between 2005 and 2008.13 Several studies in the general population or specific populations were conducted in the pre-vaccination generation, with HBsAg prevalence results ranging from about 10% to 20%.13, 14, 15, 16, 17, 18, 19 These results are close to the HBsAg prevalence by year of birth shown in Fig. 2.
Fig. 2.
Prevalence of HBsAg and coverage of hepatitis B immunization by year of birth in four provinces in the South Central Coast region of Vietnam.
If HBsAg prevalence is regarded as stable after 5 years of age,1 marked reductions in HBsAg between the age groups of 20–24 years (8.8%) and 15–19 years (3.8%) can be reasonably attributed to HBV vaccine introductions in 1997 (pilot and partial introduction) and 2002 (nationwide introduction). In Vietnam, a nationwide HBsAg survey conducted in 2011 concluded that 3 doses of the HBV vaccine successfully reduced prevalence.13 The present study strongly supports this conclusion, although we did not find differences in prevalence between birth dose (+) and (−) and between 3 vaccine doses (+) and (−) (Table 1a, Table 1bb). The reason why there was no significant difference may be that there were many cases with an unknown vaccination history.
The HBsAg prevalence was higher in children aged 5–8 years, namely, children born between 2011 and 2014. The HBV vaccine shortages occurred in 2007, 2008, and 2010, with the reported coverage of these years as low as 67% to 88%. In addition, negative public responses after unexpected deaths immediately after immunization might have led to the widespread vaccine hesitancy in 201320 and consequently a low immunization coverage and higher HBsAg prevalence.
The reports from some the WHO regions and member states target single-year cohorts to evaluate the effectiveness of HBV control, including immunization,21, 22, 23, 24 but we believe that this range is too narrow and may miss yearly fluctuations in HBsAg prevalence due to changes in vaccination coverage caused by vaccine hesitancy, stockouts, or logistic issues. Because immunization coverage dramatically changes by year, a single-year cohort study may overestimate or underestimate the effectiveness of vaccination policies. We recommend that each country targets multiple-year cohorts for evaluation to prevent this situation. In addition, the COVID-19 pandemic may substantially have weakened the routine conventional immunization due to its effects on vaccine distribution, stock capacity, human resources, and public fear in 2020 and in the following years.25, 26, 27
4.1. Strengths
This is the first population-based survey to estimate chronic HBV prevalence among a general population including both children and adults before and after the implementation of immunization policies, which enabled us to evaluate the effectiveness of a hepatitis B vaccination program involving 3 doses of the HBV vaccine at birth and during infancy. This survey targeted a wide range of ages and allowed us to assess changes in prevalence due to increased vaccine hesitancy following widespread media reports of unexpected deaths after vaccination.
4.2. Limitations
Several limitations should be kept in mind. Because this study was cross-sectional, we were unable to analyze causes and effects. The current results strongly suggest that implementation of a universal HBV vaccination program lowers HBsAg prevalence and that decreased vaccination coverage increases HBsAg prevalence, but their direct relationship is unclear. Further study, including measuring HBV antibody among vaccinated and non-vaccinated population, is needed. Because hepatitis B surface antibody (HBsAb) was more difficult to measure in resource-limited settings and was affected not only by vaccination but also by exposure to pathogens, collecting dried blood samples and measuring both HBsAb and hepatitis B core antibodies (HBcAb) might be one potential solution.28
The first (districts), second (communes/wards), and thid (villages) stages of the sampling appeared to be successful because we strictly applied PPS sampling, but the fourth stage (individuals) skewed female (73.2%), particularly in the adult population. This sex ratio did not strongly affect our results but should be kept in mind when interpreting them.
5. Conclusion
In conclusion, the introduction of the 3-dose hepatitis B vaccination schedule during infancy effectively reduced HBsAg prevalence in Vietnam, although the effectiveness of birth dosing is not clear based on the results of our cross-sectional seroprevalence survey of children and adults. Vaccine shortages and vaccine hesitancy may hinder the effectiveness of the HBV vaccine and close monitoring of age-specific HBsAg prevalence is therefore essential.
Author contributions
Conceptualization, H.T.D., M.H.; Methodology, K.K.; Software, K.K., Validation, M.H.; Formal Analysis, K.K; Investigation, Y.I., M.S., M.F., M.K.H, H.T.H.H., N.A.T.T., T.H.L., Q.T.N.; Resources, M.H.; Data Curation, M.F., M.K.H., N.A.T.T.; Writing – Original Draft Preparation, K.K; Writing – Review & Editing, K.K., Y.I., S.M., M.S., T.M., M.H.; Visualization, K.K.; Supervision, H.T.D., H.Y.L, T.T.H., T.B.N., M.H.; Project Administration, K.K.; Funding Acquisition, M.H. All authors contributed to the article and approved the submitted version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our sincere thanks to the sampled persons for their voluntary participation in the survey. We greatly acknowledge all of the surveyors and supervisors from the Pasteur Institutes of Nha Trang and the provincial and district staff from the Department of Health.
This work was supported by Grants for the National Center for Global Health and Medicine (19A01 and 30–2009), JSPS KAKENHI grant number JP19K10656, and Health, Labour and Welfare Sciences Research Grant (JPMH18BA1003). The funding source was not involved in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Data availability
Data will be made available on reasonable request.
References
- 1.World Health Organization Hepatitis B vaccines: WHO position paper - July 2017. Wkly Epidemiol Rec. 2017;92:369–392. https://apps.who.int/iris/handle/10665/255873 [Google Scholar]
- 2.World Health Organization . 2003. Regional Committee for the Western Pacific. Expanded Programme on Immunization : Measles and Hepatitis B (Resolution)http://iris.wpro.who.int/handle/10665.1/9645 [Google Scholar]
- 3.World Health Organization . 2005. Regional Committee for the Western Pacific. Measles Elimination, Hepatitis B Control and Poliomyelitis Eradication (Resolution)https://apps.who.int/iris/handle/10665/249496 [Google Scholar]
- 4.Nguyen V.T. Hepatitis B infection in Vietnam: current issues and future challenges. Asia Pac J Publ Health. 2012;24:361–373. doi: 10.1177/1010539510385220. [DOI] [PubMed] [Google Scholar]
- 5.Huy Do S. Epidemiology of hepatitis B and C virus infections and liver cancer in Vietnam. Euroasian J Hepato-Gastroenterol. 2015;5:49–51. doi: 10.5005/jp-journals-10018-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . 2022. Global Health Observatory Data Repository.https://www.who.int/data/gho [Google Scholar]
- 7.World Health Organization . Geveva; 2020. Prevention of Mother-To-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy.https://www.who.int/publications/i/item/978-92-4-000270-8 [PubMed] [Google Scholar]
- 8.Komada K., Sugiyama M., Vongphrachanh P., et al. Seroprevalence of chronic hepatitis B, as determined from dried blood spots, among children and their mothers in central Lao People's Democratic Republic: a multistage, stratified cluster sampling survey. Int J Infect Dis. 2015;36:21–26. doi: 10.1016/j.ijid.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Xeuatvongsa A., Komada K., Kitamura T., et al. Chronic hepatitis B prevalence among children and mothers: results from a nationwide, population-based survey in Lao People's Democratic Republic. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hachiya M., Miyano S., Mori Y., et al. Evaluation of nationwide supplementary immunization in Lao People's Democratic Republic: population-based seroprevalence survey of anti-measles and anti-rubella IgG in children and adults, mathematical modelling and a stability testing of the vaccine. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avellon A., Ala A., Diaz A., et al. Clinical performance of Determine HBsAg 2 rapid test for Hepatitis B detection. J Med Virol. 2020 doi: 10.1002/jmv.25862. [DOI] [PubMed] [Google Scholar]
- 12.General Statistics Office and UNICEF . 2015. Viet Nam Multiple Indicator Cluster Survey 2014, Final Report.https://www.unicef.org/vietnam/reports/monitoring-situation-children-and-women Hanoi, Viet Nam. [Google Scholar]
- 13.Nguyen T.H., Vu M.H., Nguyen V.C., et al. A reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine. 2014;32:217–222. doi: 10.1016/j.vaccine.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duong T.H., Nguyen P.H., Henley K., Peters M. Risk factors for hepatitis B infection in rural Vietnam. Asian Pac J Cancer Prev APJCP. 2009;10:97–102. [PMC free article] [PubMed] [Google Scholar]
- 15.Hipgrave D.B., Nguyen T.V., Vu M.H., et al. Hepatitis B infection in rural Vietnam and the implications for a national program of infant immunization. Am J Trop Med Hyg. 2003;69:288–294. doi: 10.4269/ajtmh.2003.69.288. [DOI] [PubMed] [Google Scholar]
- 16.Kakumu S., Sato K., Morishita T., et al. Prevalence of hepatitis B, hepatitis C, and GB virus C/hepatitis G virus infections in liver disease patients and inhabitants in Ho Chi Minh, Vietnam. J Med Virol. 1998;54:243–248. doi: 10.1002/(SICI)1096-9071(199804)54:4<243::AID-JMV2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Katelaris P.H., Robertson G., Bradbury R., Tippett G., Hoa D.Q., Ngu M.C. Seroprevalence of hepatitis viruses in children in rural Viet Nam. Trans R Soc Trop Med Hyg. 1995;89:487. doi: 10.1016/0035-9203(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen V.T., McLaws M.L., Dore G.J. Highly endemic hepatitis B infection in rural Vietnam. J Gastroenterol Hepatol. 2007;22:2093–2100. doi: 10.1111/j.1440-1746.2007.05010.x. [DOI] [PubMed] [Google Scholar]
- 19.Tran H.T., Ushijima H., Quang V.X., et al. Prevalence of hepatitis virus types B through E and genotypic distribution of HBV and HCV in Ho Chi Minh city, Vietnam. Hepatol Res. 2003;26:275–280. doi: 10.1016/S1386-6346(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Wiesen E., Diorditsa S., et al. Impact of Adverse events following immunization in Viet Nam in 2013 on chronic hepatitis B infection. Vaccine. 2016;34:869–873. doi: 10.1016/j.vaccine.2015.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . World Health Organization; Geneva: 2016. Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief.https://apps.who.int/iris/handle/10665/206453 [Google Scholar]
- 22.World Health Organization . WHO South East Asia Regional Immunization Technical Advisory Group (SEAR-ITAG) WHO Regional Office for South-East Asia; New Delhi: 2016. Regional Office for South-east Asia WHO.https://apps.who.int/iris/handle/10665/254351 [Google Scholar]
- 23.World Health Organization . WHO Regional Office for South-East Asia; New Delhi: 2002. Regional Office for South-East Asia. Prevention of Hepatitis B in India: An Overview.https://apps.who.int/iris/handle/10665/205655 [Google Scholar]
- 24.Soeung S.C., Rani M., Huong V., Sarath S., Kimly C., Kohei T. Results from nationwide hepatitis B serosurvey in Cambodia using simple and rapid laboratory test: implications for national immunization program. Am J Trop Med Hyg. 2009;81:252–257. doi: 10.4269/ajtmh.2009.81.252. [DOI] [PubMed] [Google Scholar]
- 25.Dinleyici E.C., Borrow R., Safadi M.A.P., van Damme P., Munoz F.M. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum Vaccines Immunother. 2021;17:400–407. doi: 10.1080/21645515.2020.1804776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan A., Bibi A., Sheraz Khan K., et al. Routine pediatric vaccination in Pakistan during COVID-19: how can healthcare professionals help? Front Pediatr. 2020;8 doi: 10.3389/fped.2020.613433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsuya H. Fight against COVID-19 but avoid disruption of services for other communicable diseases (CDs) and noncommunicable diseases (NCDs) Glob Health Med. 2020;2:343–345. doi: 10.35772/ghm.2020.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norizuki M., Kitamura T., Komada K., et al. Serologic testing of randomly selected children after hepatitis B vaccination: a cross-sectional population-based study in Lao People's Democratic Republic. BMC Infect Dis. 2019;19:507. doi: 10.1186/s12879-019-4086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request.