Abstract
Platelet transfusion is a common clinical approach to providing platelets to patients suffering from thrombocytopenia or other ailments that require an additional platelet source. However, a stable supply of platelet products is challenged by aging societies, pandemics, and other factors. Many groups have made extensive efforts toward the in vitro generation of platelets for clinical application. We established immortalized megakaryocyte progenitor cell lines (imMKCLs) from human induced pluripotent stem cells (iPSCs) and achieved clinical-scale manufacturing of iPSC-derived platelets (iPSC-PLTs) from them by identifying turbulent flow as a key physical condition. We later completed the iPLAT1 study, the first-in-human clinical trial using autologous iPSC-PLTs. This review summarizes current findings on the ex vivo generation of iPSC-PLTs that led to the iPLAT1 study and beyond. We also discuss new insights regarding the heterogeneity of megakaryocytes and the implications for the ex vivo generation of iPSC-PLTs.
Keywords: iPSC, Megakaryocytes, Platelets, Clinical trial
Introduction
Platelets are anucleate blood cells derived from megakaryocytes and play crucial roles in the maintenance of blood vessel integrity and also suggested in various immune responses and cancer physiology [1]. Platelet transfusions are the common clinical approach to providing platelets to patients suffering from thrombocytopenia or other ailments that require an additional platelet source [2, 3]. These transfusions are entirely reliant on blood donors as the source of platelets. However, blood supplies are challenged by recent pandemics and aging societies, in which younger donors are rarer and therefore less able to meet the demand. In addition, the resulting platelet products have a short shelf life and, in cases of alloimmune platelet transfusion refractoriness (allo-PTR), must be compatible for human leukocyte antigen class I (HLA-I) or human platelet antigen (HPA) [4]. These limitations have motivated the development of alternative platelet sources from human induced pluripotent stem cells (iPSCs).
Given that human iPSCs can be expanded and differentiated into potentially any cell type, extensive efforts have been made to differentiate iPSCs to platelets [5, 6]. Theoretically, iPSC-derived platelets (iPSC-PLTs) generated ex vivo can also address the issues inherent to donor platelets by reducing the risk of recipient exposure to infectious pathogens and selecting or engineering the iPSCs to reduce immunogenicity.
Based on the establishment of immortalized megakaryocyte progenitor cell lines (imMKCLs) from iPSCs [7] and the development of turbulent flow-based bioreactors [8] and novel drugs [6], we started the iPLAT1 study, the first-in-human clinical trial using autologous iPSC-PLTs in 2019 [9, 10]. This review summarizes current findings on the ex vivo generation of iPSC-PLTs that led to the iPLAT1 trial and also discusses new insights regarding the cellular heterogeneity of megakaryocytes and their implications in the ex vivo generation of iPSC-PLTs.
iPLAT1: The first-in-human clinical trial using autologous iPSC-PLTs
Allo-PTR, which is caused by alloantibodies against HLA-I and HPA, is a serious clinical issue present in about 5–15% of platelet transfusion patients [4]. These patients require HLA-I/HPA-compatible platelet transfusions and thus face serious logistical challenges [4]. To provide a compatible platelet product for an aplastic anemia patient in Japan with allo-PTR due to anti-HPA-1a antibodies and without a compatible platelet donor due to a rare HPA-1b/1b type, we developed an ex vivo production system for autologous iPSC-PLTs [9, 10]. We then performed an extensive non-clinical evaluation of the master cell bank (MCB) and iPSC-PLTs to confirm the safety, quality, and efficacy of the cells [9]. Compared with donor platelets, iPSC-PLTs were larger but showed comparable function [10].
In the iPLAT1 study (Table 1), autologous iPSC-PLTs were administrated sequentially at three escalating doses up to 1 × 1011 (1/2 the dose of a standard transfusion in Japan) [10]. Regarding the primary endpoint of safety, the patient did not present any significant clinical symptoms [10]. Unexpectedly, no increase in the corrected count increment was observed after the transfusions, but the existence of larger platelets was confirmed in peripheral blood, suggesting their circulation. This outcome could be attributed to the difficulty in detecting small increases over the high baseline platelet count, the suboptimal observation times for detection, less circulation due to early coagulation, or some other cause [10]. Nevertheless, we believe the iPLAT1 study marks a significant milestone for the clinical application of iPSC-PLTs and the regenerative medicine field.
Table 1.
Outline of the first-in-human clinical trial of autologous iPSC-derived platelets
| Contents | |
|---|---|
| Name | Clinical study of autologous transfusion of iPSC-derived platelets for thrombocytopenia (iPLAT1) |
| Purpose | To verify the safety of the autologous transfusion of platelets made from the peripheral blood mononuclear cells (PBMCs) reprogrammed into iPSCs of a patient with aplastic anemia complicated by platelet transfusion refractoriness |
| Number of patients | One |
| Experimental design | Single-center, open-label, uncontrolled dose escalation study |
| The patient received three dose cohorts | |
| Cohort 1: 0.5 JPN units (1×1010 platelets, 2019.5.22.) | |
| Cohort 2: 1.5 JPN units (3×1010 platelets, 2019.8.21.) | |
| Cohort 3: 5.0 JPN units (1×1011 platelets, 2020.1.22.) | |
| Cell process | PBMCs were collected from the patient and reprogrammed into iPSCs. The iPSCs were then differentiated into a megakaryocyte cell line that was stored frozen as a master cell line. The master cell line was thawed, proliferated, and differentiated into platelets. The platelets were purified, concentrated, washed, irradiated and finally transfused into the patient |
| Follow-up period | One year from the time of the final transfusion |
| Endpoints | Primary: safety (frequency and extent of adverse events) |
| Secondary: efficacy (platelet count) | |
| Outcomes | Primary: No significant clinical symptoms or signs were observed |
| Secondary: No increase in platelet count, but larger platelets (iPSC-PLTs) were detected by flow cytometry |
Ex vivo generation of iPSC-PLTs for clinical application
Since the first description of an in vitro culture for the generation of human platelets and the cloning of human thrombopoietin (TPO) and its receptor c-MPL [11], significant effort has been spent on developing systems for megakaryocyte differentiation, mainly starting from hematopoietic stem/progenitor cells (HSPCs) [12–14]. This effort has led to research toward the generation of functional platelets from human iPSCs, which are potentially an inexhaustible source for ex vivo manufacturing [6–8, 15, 16].
Establishing expandable megakaryocyte progenitor cell lines from iPSCs
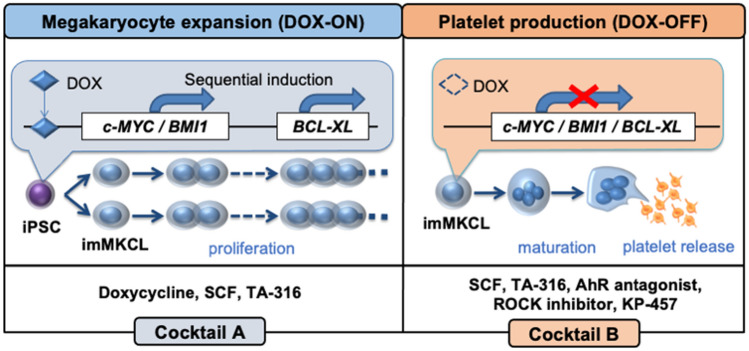
In the direct hematopoietic differentiation of human pluripotent stem cells, we found a unique expression pattern of c-MYC, in which the gene was expressed at the megakaryocyte proliferation stage but then suppressed at the maturation stage [17]. Accordingly, we established immortalized megakaryocyte progenitor cell lines (imMKCLs) by overexpressing c-MYC together with anti-senescent BMI-1 and anti-apoptotic BCL-XL genes in human iPSC-derived HPCs [7]. In this Tet-On inducible expression system, the proliferation and maturation stage can be switched by the addition and removal of doxycycline (DOX) (Fig. 1) [18]. Because imMKCLs are cryopreservable, they are suitable as master cells for ex vivo iPSC-PLT production. However, only less than 5% of iPSC clone-derived imMKCLs continuously proliferate. By profiling the transcriptional differences in several clones, we found that the proliferation arrest is correlated with the expression of specific cyclin-dependent kinase inhibitors and the silencing of CDKN1A and p53 improves the stable establishment of imMKCLs [19]. Developing methods to establish stable imMKCLs should expand the application of iPSC-PLTs extensively.
Fig. 1.
Expansion and platelet production of imMKCLs. The imMKCLs are established by the sequential introduction of c-MYC and BMI1 genes followed by the BCL-XL gene into human iPSC-derived hematopoietic progenitor. These transgenes were under the control of a Tet-On system, which allowed doxycycline to switch imMKCLs from a self-renewing proliferation phase (DOX-ON) to a platelet-producing maturation phase (DOX-OFF). In DOX-ON stage, human stem cell factor (SCF), TPO receptor agonist (TA-316) and doxycycline are applied, while in subsequent DOX-OFF stage, SCF, TA-316 along with AhR antagonist, ROCK inhibitor and KP-457, a selective ADAM17 inhibitor, are applied for platelet production in feeder-free conditions
Identification of turbulent flow as a key condition for platelet generation
An in vivo imaging study identified shear stress in bone marrow blood flow as crucial for platelet release from megakaryocytes [20]. Based on this concept, various bioreactors were developed to recapitulate flow-dependent shear stress in in vitro platelet generation systems [13, 21–23], but the production efficiency and platelet quality fell far short of realizing clinically applicable levels.
In our later in vivo live imaging analysis of mouse bone marrow megakaryocytes with flow dynamic visualization, we found that megakaryocytes actively generating platelets were exposed to turbulent flow, leading to the development of a novel turbulence-controllable bioreactor [8]. We further identified turbulent energy in addition to shear stress as determinant hydrodynamic parameters of platelet production [8]. By adopting a novel turbulent flow-based system with optimal levels of shear stress and turbulent energy produced by a vertical reciprocal-motion stirrer, we achieved the clinically required hundred billion-order scale of “healthy” platelet manufacturing [8].
Developing drugs to promote ex vivo platelet generation
The utilization of recombinant TPO in clinical trials was stopped due to the immunogenicity [24]. Accordingly, TPO mimetics have been developed for clinical use, and we have developed TA-316, a chemically synthesized c-MPL agonist that favors megakaryocyte-biased differentiation from bone marrow CD34+ hematopoietic stem cells (HSCs) in vitro and promotes the proliferation and maturation of imMKCLs comparable to recombinant TPO [25].
Compared with in vivo thrombopoiesis, megakaryocytes differentiated in vitro generate far fewer platelets. Thus, many groups have searched for chemicals that improve megakaryocyte maturity and subsequent platelet release [6]. We identified that an aryl hydrocarbon receptor (AhR) antagonist combined with a ROCK inhibitor markedly promotes platelet production from imMKCLs under feeder-free conditions [8]. AhR antagonists are known to promote HSC expansion [26] and increase the number of proplatelet-generating megakaryocytes [27]. In addition, we developed KP-457, which prevents the cleavage activity of a disintegrin and metalloproteinase 17 (ADAM17), blocking CD42b (GPIbα) shedding [28]. These achievements enabled the development of a feeder-free culture condition with additive cocktails (Fig. 1) to realize clinical-scale iPSC-PLT manufacturing. Meanwhile, we established a fluorescence microscopy platform to evaluate megakaryocyte maturity by monitoring the expression of β1-tublin, a main cytoskeletal component of the cytoplasm in mature megakaryocytes [29]. Using this platform, we further identified a series of compounds that may further facilitate megakaryocyte maturation and subsequent platelet production from imMKCLs [29].
GMP-based production and preclinical assessment of autologous iPSC-PLTs
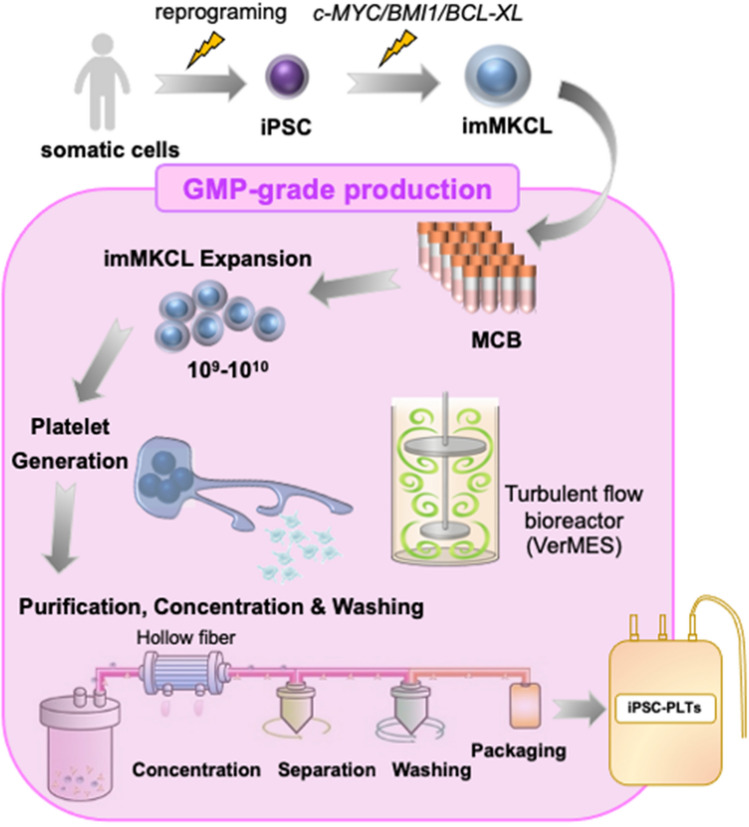
One of the major risks associated with cell therapies is the potential emergence of tumors after administration [30]. Platelets are considered safe in terms of tumorigenic risk since they do not contain a nucleus. However, this assumption is only valid when all nucleated precursor cells have been removed from the transfusion product. Therefore, in iPLAT1, after iPSC-PLTs were produced based on good manufacturing practice (GMP) using certified pathogen-free imMKCLs as the MCB, they were gamma irradiated (25 Gy) to eliminate any tumorigenicity potential [9, 10]. The MCB was established from patient iPSCs and, to produce autologous iPSC-PLTs from the MCB, we set up a production system comprised of a 23-day DOX-ON expansion phase, 6-day DOX-OFF platelet production phase, and final 1-day purification-washing-packaging phase (Fig. 2) [9]. Before the clinical trial took place, the safety and quality of the iPSC-PLTs were assessed through multiple preclinical tests in vitro and animal models following consultation with the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) [9, 10]. The toxicity and mutagenicity of any additives used in the production process were also tested. Thus, our study could serve as a comprehensive reference for the development of cell products which use iPSC-derived progenitor cells as an MCB.
Fig. 2.
Overview of the GMP-grade production of iPSC-PLTs. iPSCs are reprogramed from patient-derived somatic cells, then imMKCLs are established and stocked frozen as master cell bank (MCB). Upon need, a vial of MCB is thawed, and after sufficient expansion in DOX-ON culture, transferred to the bioreactor (VerMES) to yield platelets in DOX-OFF conditions. The platelets including media are concentrated, separated, washed, and packaged as transfusion products, followed by radiation for eliminating tumorigenicity of remaining imMKCLs
Heterogeneity of megakaryocytes and implications for ex vivo platelet generation
MegakaryocyteS exist as a heterogenous population that differ in size, ploidy, and location. Classically, megakaryocytes per se were considered as just the source of platelets, but megakaryocytes have several other functions [31, 32]. Specifically, they regulate HSC function by secreting cytokines such as TGF-b, FGF-1, and CXCL4 (PF4) [33]. Further, emerging evidence has supported the notion that megakaryocytes participate directly in immunity, as they express multiple receptors that sense pathogens and present antigens, release cytokines, and engage with other immune cells [34]. Megakaryocytes also possess antiviral immunity by producing interferon (IFN)-induced transmembrane protein 3 (IFITM3) to limit viral infections [35]. In the COVID-19 pandemic, a megakaryocyte subset was associated with the presence of cytokine storms [36] and an increased response in IFN-activated megakaryocytes was identified as a hallmark of severe cases [37]. Interestingly, the immune function of megakaryocytes likely depends on where they reside, as megakaryocytes in the lungs exhibit an immune-skewed gene expression signature compared with those in the bone marrow, and the immune phenotype of mouse lung MKs is similar to that of antigen-presenting dendritic cells [38–40].
Recent research has revealed that these diverse functions depend on specialized megakaryocyte subsets. The subsets have been characterized by single-cell transcriptome profiling of embryonic megakaryocytes isolated from human yolk sacs/fetal livers [41] and adult bone marrow [42, 43]. Although distinct morphology and molecular signatures of human megakaryocytes from different development sites have been observed [44], both embryonic and adult megakaryocytes exhibit similar cellular heterogeneity with transcriptionally distinct subpopulations, which can be simplified as platelet-generating, HSC niche-supporting, and immune subpopulations [41, 43]. Importantly, the embryonic megakaryocyte subpopulation with immune properties is likely generated through a distinct trajectory [41], suggesting the emergence of megakaryocytes with distinct functions following a genetically programmed lineage ontogeny. Notably, the cellular heterogeneity of megakaryocytes may result in platelet heterogeneity with consequences on platelet function [1].
Given that imMKCLs may partially recapitulate the heterogeneity of primary megakaryocytes and possess immune properties, imMKCLs, the competent source for iPSC-PLTs, should be further investigated with regards to functional and cellular heterogeneity if they are to be considered master cells. Although significant progress has been made in understanding the heterogeneity of human megakaryocytes, little is known regarding the key molecular events/pathways that regulate the heterogenous ontology. Our established iPSC-derived megakaryocyte progenitor models, including imMKCLs, are useful tools for these studies and may inspire the studies on the heterogenous ontology of megakaryocytes. The ability to explore the immune properties of iPSC-derived megakaryocyte models will expand our understanding of megakaryocyte cell biology and facilitate the development of therapeutic and diagnostic strategies using patient-derived iPSCs.
Conclusions
In this review, we provide an update on the ex vivo generation of platelets from iPSCs that led to the iPLAT1 study. Following this research, in 2022, the first-in-human clinical trial using allogenic human iPSC-derived HLA homozygous platelets was initiated. Using the same imMKCL system, we have also succeeded in generating HLA class I-depleted iPSC-PLTs, which can serve as a single off-the-shelf product [45]. These advances should greatly contribute to the worldwide supply of transfusable iPSC-PLTs at low cost. Despite these significant advances, however, many challenges remain such as the heterogeneity of the imMKCL master cells.
Acknowledgements
This work was supported in part by the Regenerative Innovation grant (22bm0704051h0003) and Core Center for iPS Cell Research, Research Center Network for Realization of Regenerative Medicine from Japan Agency for Medical Research and Development (AMED) (N.S. and K.E.), the CiRA Foundation Fund (K.E.), the iPS Cell Research Fund (S.J.C.), a grant-in-aid for scientific research (S) (21H05047, K.E.), and a grant-in-aid for early-career scientists (22K13124, S.J.C.) from the Japan Society for the Promotion of Science (JSPS).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Declarations
Conflict of interest
K.E. is a founder and scientific advisor for Megakaryon Co. Ltd. without salary. The interests of K.E. and N.S. were reviewed and are managed by Kyoto University in accordance with its conflict-of-interest policies. S.J.C. declares no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Si Jing Chen, Email: sjchen@cira.kyoto-u.ac.jp.
Koji Eto, Email: kojieto@cira.kyoto-u.ac.jp.
References
- 1.van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16(3):166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 2.Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176(3):365–394. doi: 10.1111/bjh.14423. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205–213. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 4.Stanworth SJ, Navarrete C, Estcourt L, Marsh J. Platelet refractoriness–practical approaches and ongoing dilemmas in patient management. Br J Haematol. 2015;171(3):297–305. doi: 10.1111/bjh.13597. [DOI] [PubMed] [Google Scholar]
- 5.Blau HM, Daley GQ. Stem cells in the treatment of disease. N Engl J Med. 2019;380(18):1748–1760. doi: 10.1056/NEJMra1716145. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto N, Eto K. Generation and manipulation of human iPSC-derived platelets. Cell Mol Life Sci. 2021;78(7):3385–3401. doi: 10.1007/s00018-020-03749-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, et al. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014;14(4):535–548. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Nakamura S, Sugimoto N, Shigemori T, Kato Y, Ohno M, et al. Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell. 2018;174(3):636–48.e18. doi: 10.1016/j.cell.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto N, Nakamura S, Shimizu S, Shigemasa A, Kanda J, Matsuyama N et al. Production and nonclinical evaluation of an autologous iPSC-derived platelet product for the iPLAT1 clinical trial. Blood Adv. 2022;6(23):6056–6069. [DOI] [PMC free article] [PubMed]
- 10.Sugimoto N, Kanda J, Nakamura S, Kitano T, Hishizawa M, Kondo T et al. iPLAT1: The first-in-human clinical trial of iPSC-derived platelets as a phase 1 autologous transfusion study. Blood. 2022;140(22):2398–2402. [DOI] [PubMed]
- 11.Kaushansky K. Thrombopoietin. N Engl J Med. 1998;339(11):746–54. doi: 10.1056/NEJM199809103391107. [DOI] [PubMed] [Google Scholar]
- 12.Karagiannis P, Eto K. Manipulating megakaryocytes to manufacture platelets ex vivo. J Thromb Haemost. 2015;13(Suppl 1):S47–53. doi: 10.1111/jth.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, et al. Platelet bioreactor-on-a-chip. Blood. 2014;124(12):1857–1867. doi: 10.1182/blood-2014-05-574913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Liu C, Lei X, Wang H, Su P, Ru Y, et al. Integrated biophysical and biochemical signals augment megakaryopoiesis and thrombopoiesis in a three-dimensional rotary culture system. Stem Cells Transl Med. 2016;5(2):175–185. doi: 10.5966/sctm.2015-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Q, Shabrani N, Thon JN, Huo H, Thiel A, Machlus KR, et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Reports. 2014;3(5):817–831. doi: 10.1016/j.stemcr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreau T, Evans AL, Vasquez L, Tijssen MR, Yan Y, Trotter MW, et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun. 2016;7:11208. doi: 10.1038/ncomms11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takayama N, Nishimura S, Nakamura S, Shimizu T, Ohnishi R, Endo H, et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207(13):2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberichter SL. von Willebrand factor propeptide: biology and clinical utility. Blood. 2015;126(15):1753–1761. doi: 10.1182/blood-2015-04-512731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sone M, Nakamura S, Umeda S, Ginya H, Oshima M, Kanashiro MA, et al. Silencing of p53 and CDKN1A establishes sustainable immortalized megakaryocyte progenitor cells from human iPSCs. Stem Cell Reports. 2021;16(12):2861–2870. doi: 10.1016/j.stemcr.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 21.Sullenbarger B, Bahng JH, Gruner R, Kotov N, Lasky LC. Prolonged continuous in vitro human platelet production using three-dimensional scaffolds. Exp Hematol. 2009;37(1):101–110. doi: 10.1016/j.exphem.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa Y, Nakamura S, Nakajima M, Endo H, Dohda T, Takayama N, et al. Two differential flows in a bioreactor promoted platelet generation from human pluripotent stem cell-derived megakaryocytes. Exp Hematol. 2013;41(8):742–748. doi: 10.1016/j.exphem.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Blin A, Le Goff A, Magniez A, Poirault-Chassac S, Teste B, Sicot G, et al. Microfluidic model of the platelet-generating organ: beyond bone marrow biomimetics. Sci Rep. 2016;6:21700. doi: 10.1038/srep21700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98(12):3241–3248. doi: 10.1182/blood.V98.12.3241. [DOI] [PubMed] [Google Scholar]
- 25.Aihara A, Koike T, Abe N, Nakamura S, Sawaguchi A, Nakamura T, et al. Novel TPO receptor agonist TA-316 contributes to platelet biogenesis from human iPS cells. Blood Adv. 2017;1(7):468–476. doi: 10.1182/bloodadvances.2016000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strassel C, Brouard N, Mallo L, Receveur N, Mangin P, Eckly A, et al. Aryl hydrocarbon receptor-dependent enrichment of a megakaryocytic precursor with a high potential to produce proplatelets. Blood. 2016;127(18):2231–2240. doi: 10.1182/blood-2015-09-670208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirata S, Murata T, Suzuki D, Nakamura S, Jono-Ohnishi R, Hirose H, et al. Selective inhibition of ADAM17 efficiently mediates glycoprotein Ibα retention during ex vivo generation of human induced pluripotent stem cell-derived platelets. Stem Cells Transl Med. 2017;6(3):720–730. doi: 10.5966/sctm.2016-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo H, Chen SJ, Hashimoto K, Endo H, Nishi Y, Ohta A, et al. A β1-tubulin-based megakaryocyte maturation reporter system identifies novel drugs that promote platelet production. Blood Adv. 2018;2(17):2262–2272. doi: 10.1182/bloodadvances.2018019547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatib-Massalha E, Méndez-Ferrer S. Megakaryocyte diversity in ontogeny, functions and cell-cell interactions. Front Oncol. 2022;12:840044. doi: 10.3389/fonc.2022.840044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilburg J, Becker IC, Italiano JE. Don't you forget about me(gakaryocytes) Blood. 2022;139(22):3245–3254. doi: 10.1182/blood.2020009302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhan H, Kaushansky K. Megakaryocytes as the regulator of the hematopoietic vascular niche. Front Oncol. 2022;12:912060. doi: 10.3389/fonc.2022.912060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunin P, Nigrovic PA. Megakaryocytes as immune cells. J Leukoc Biol. 2019;105(6):1111–1121. doi: 10.1002/JLB.MR0718-261RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell RA, Schwertz H, Hottz ED, Rowley JW, Manne BK, Washington AV, et al. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood. 2019;133(19):2013–2026. doi: 10.1182/blood-2018-09-873984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren X, Wen W, Fan X, Hou W, Su B, Cai P, et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184(23):5838. doi: 10.1016/j.cell.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernardes JP, Mishra N, Tran F, Bahmer T, Best L, Blase JI, et al. Longitudinal multi-omics analyses identify responses of megakaryocytes, erythroid cells, and plasmablasts as hallmarks of severe COVID-19. Immunity. 2020;53(6):1296–314.e9. doi: 10.1016/j.immuni.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeung AK, Villacorta-Martin C, Hon S, Rock JR, Murphy GJ. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020;4(24):6204–6217. doi: 10.1182/bloodadvances.2020002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pariser DN, Hilt ZT, Ture SK, Blick-Nitko SK, Looney MR, Cleary SJ, et al. Lung megakaryocytes are immune modulatory cells. J Clin Invest. 2021 doi: 10.1172/JCI137377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, He J, Xu C, Chen X, Yang H, Shi S, et al. Decoding human megakaryocyte development. Cell Stem Cell. 2021;28(3):535–49.e8. doi: 10.1016/j.stem.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Sun S, Jin C, Si J, Lei Y, Chen K, Cui Y, et al. Single-cell analysis of ploidy and the transcriptome reveals functional and spatial divergency in murine megakaryopoiesis. Blood. 2021;138(14):1211–1224. doi: 10.1182/blood.2021010697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Wu D, Xia M, Li M, Sun Z, Shen B, et al. Characterization of cellular heterogeneity and an immune subpopulation of human megakaryocytes. Adv Sci (Weinh) 2021;8(15):e2100921. doi: 10.1002/advs.202100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Huang B, Wang H, Zhou J. The heterogeneity of megakaryocytes and platelets and implications for ex vivo platelet generation. Stem Cells Transl Med. 2021;10(12):1614–1620. doi: 10.1002/sctm.21-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki D, Flahou C, Yoshikawa N, Stirblyte I, Hayashi Y, Sawaguchi A, et al. iPSC-derived platelets depleted of HLA Class I are inert to Anti-HLA Class I and natural killer cell immunity. Stem Cell Reports. 2020;14(1):49–59. doi: 10.1016/j.stemcr.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.