Abstract
Purpose of Review
A number of sequelae after acute coronavirus disease 2019 (COVID-19) significantly affect the quality of life of patients. The chemosensory disorders including olfactory dysfunction (OD) and gustatory dysfunction (GD) are two of the commonest symptoms complained by patients with COVID-19. Although chemosensory function has been reported improved in over 60% of COVID-19 patients in a short time after acute infection, it may last as a major symptom for patients with long COVID-19. This narrative review discussed current literatures on OD and GD in long COVID-19 including the prevalence, risk factors, possible mechanisms, and potential therapies.
Recent Findings
Although the prevalence of OD and GD has declined continuously after acute COVID-19, a considerable number of patients had persistent chemosensory disorders 3 months to 2 years after symptom onset. Female gender, initial severity of dysfunction, nasal congestion, emotional distress and depression, and SARS-CoV-2 variants have been identified as risk factors for persistent OD and GD in long COVID-19. The pathogenesis of OD and GD in long COVID-19 remains unknown, but may be analogous to the persistent OD and GD post common respiratory viral infection. Corticosteroids and olfactory training might be a potential choice regarding the treatment of lasting OD and GD after SARS-CoV-2 infection; however, more studies are needed to prove it.
Summary
OD and GD are common long-term consequences of COVID-19 and influenced by gender, initial severity of dysfunction, emotional distress and depression, and SARS-CoV-2 variants. More studies are needed to illustrate their pathogenesis and to establish therapeutic strategies.
Keywords: Olfactory dysfunction, Gustatory dysfunction, Recovery, Long COVID-19
Introduction
The global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is the worst catastrophic public health event of the twenty-first century so far. In addition to the fatal cases in the first few weeks after infection, up to 69% of COVID-19 survivors may develop long-term sequelae [1, 2]. This is called post-COVID-19 condition or “long COVID-19,” which has been used to describe sustained symptoms beyond 3 months of SARS-CoV-2 infection [3]. In a recent meta-analysis including 72 articles, 167 COVID-19-related long-term sequelae have been identified in 88,769 patients, and the most frequently reported sequelae were fatigue (27.5%), somnipathy (20.1%), anxiety (18.0%), dyspnea (15.5%), post-traumatic stress disorder (14.6%), hypomnesia (13.4%), arthralgia (12.9%), depression (12.7%), and alopecia (11.2%) over 3–13.2 months of follow-up [4]. Although the acute phase treatment for COVID-19 is well developed, there is still a long way to go in the evaluation of the clinical course and management of long-term complications of COVID-19. The chemosensory disorders including olfactory dysfunction (OD) and gustatory dysfunction (GD) are the two frequently reported symptoms of acute COVID-19 [5, 6], affecting over 70% of COVID-19 patients in Western countries [7, 8]. The persistence of OD and GD symptoms after acute COVID-19 is attracting more and more interest and attention from health professionals and publics since it will significantly impact on the quality of life of patients with long COVID-19. The clinical course of chemosensory disorder post-COVID-19 and the influencing factors are important to identify, which may guide the management of long COVID-19.
OD and GD in Acute and Short-Term COVID-19
Studies from different geographic areas have found that the prevalence of OD ranged from 33 to 85.6%, and that of GD varied from 64 to 88% in patients with acute COVID-19 [5, 6, 9, 10]. Although the prevalence of chemosensory disorders is high in acute COVID-19, the recovery starts in a few weeks after infection. A study from the UK used symptom rating scales to determine the OD and GD at 4 weeks after disease onset and demonstrated that 89% of the SARS-CoV-2–positive mildly symptomatic patients with abrupt changes in their sense of smell or taste reported a complete resolution or improvement of chemosensory symptoms [11]. Consistently, a number of following investigations showed that about 64% to 89% of COVID-19 patients with OD, and 70% to 89% of COVID-19 patients with GD reported the improvement of symptoms within 3 months after disease onset [12–14]. Although the recovery rate of OD and GD in a short time post-SARS-CoV-2 infection seems promising, the high infectious rate of SARS-CoV-2 worldwide would still translate to a large number of patients having persistent OD and GD after acute COVID-19. Therefore, it is critical to determine the clinical course of OD and GD in long-term follow-up and the relevant prognostic factors, and to establish effective interventions.
OD and GD at 3–12 Months After Symptom Onset
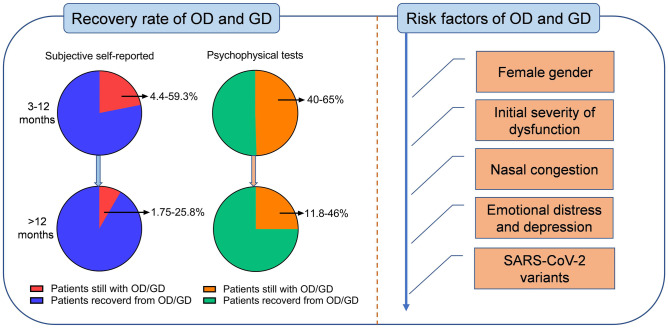
Subjective Self-Reported OD and GD: Based on the self-reported evaluation, OD and GD have been found improved 3–12 months after disease onset by a plenty of studies as illustrated in Table 1 [15••]. For example, Nguyen et al. found that 24% of 125 patients with initial OD and GD reported persistent symptoms 7 months after the onset of COVID-19 in France by using a telephone questionnaire [16]. An Israel study recruited 65 non-hospitalized patients with OD and GD, and by using a web-based questionnaire, showed that a complete recovery of smell and taste function occurred in 52% and 61.5% of COVID-19 patients at 8-month follow-up, respectively [17]. A prospective longitudinal study from the UK evaluated 434 COVID-19 patients with OD and GD through an email-based survey and found that there was a significant improvement in the severity of OD where over 40% of the patients had a normal sense of smell and only 2.8% remained complete loss of smell at 6-month follow-up [18]. In addition, it was found that the ratio of patients with GD decreased from 94.8 to 35.3% at 6-month follow-up [18]. A longitudinal web-based survey of 290 COVID-19 patients with OD in the USA found that 36% of them reported incompletely recovered olfactory function and 58% still complained of taste problems at 6 months after the onset of symptoms [19]. A study from Brazil showed that the reported prevalence of OD and GD was 72.9% and 67.4%, respectively, at the moment of diagnosis, and after a mean follow-up period of 6 months, 45% and 50% of affected patients still had some degree of chemosensory symptoms [20]. A prospective study recruited 1009 COVID-19 patients with OD and 780 with GD and evaluated the chemosensory function by phone call survey or physically to document the change 6 months after the onset of symptoms [21]. It found that 34% of the patients with OD and GD were not completely recovered. Given to difference in the initial severity of chemosensory dysfunction, evaluation method, follow-up time, and the definition of recovery (recovery vs partial recovery vs full recovery), there are considerable variations in the prevalence of persistent DO and GD 3 to 12 months post-COVID-19. In a recent meta-analysis of reconstructed time-to-event individual patient data from 18 studies, it showed that at 30, 60, 90, and 180 days post-COVID-19, respectively, about 74%, 86%, 90%, and 96% of patients self-reported smell recovery and 79%, 88%, 90%, and 98% of patients self-reported taste recovery [15••]. About 5.6% and 4.4% of patients might develop long-lasting self-reported smell and taste dysfunction [15••]. However, sensitivity analyses in this meta-analysis suggest these could be underestimated [15••].
OD and GD Evaluated by Psychophysical Test: The majority of studies determined chemosensory disorders based on self-reported symptoms in COVID-19 patients. However, self-reporting may lead to an underestimation of the prevalence of OD and GD [22, 23]. Therefore, several recent studies have attempted to estimate the chemosensory disorders in long COVID-19 by using psychophysical tests. Hintschich et al. used the NHANES Pocket Smell Test and Taste Strips Test to assess olfaction and gustation function and found that 40% and 32.5% of the patients still suffered from OD and GD 8 months after acute COVID-19, respectively [24]. Another study, based on a self-administered chemosensory test identifying 3 substances, found that 64% and 51.4% of patients had OD and GD 7 months after symptom onset, respectively [25]. Similarly, a prospective study using a psychophysical test found that 42.6% of 190 outpatients experienced persistent OD and 16.3% of patients had persistent GD at 6-month follow-up, respectively [26]. Cristillo et al. studied hospitalized patients with the Sniffin’ Sticks test and found that the prevalence of OD was 50% at 6 months after disease onset [27]. Based on the psychophysical test, the prevalence of chemosensory disorders in long COVID-19 ranges from 40 to 65% at 6–12 months follow-up, which is higher than those self-reported (Fig. 1).
OD and GD Evaluated by Both Subjective Self-Reported Symptoms and Psychophysical Tests: A study from Italy recruited 145 mild to moderate COVID-19 patients who completed a 6-month follow-up [22]. This study used both self-reported symptoms and Smell Identification Test (CA-UPSIT) to evaluate olfactory function, and found that despite 77% of patients reported a normal sense of smell, over 60% of patients were determined to have persistent OD by the psychophysical test at 6 months from the diagnosis of COVID-19. A weak correlation was observed between the self-reported alteration of the sense of smell and olfactory test scores [22]. Previously, self-reported symptoms and psychophysical tests of OD have been compared in several studies, and no significant correlation was revealed [27–29•]. It suggests that psychophysical tests should be incorporated in the evaluation of recovery of OD and GD in long COVID-19 in order to avoid the underestimation of dysfunction based on self-reported evaluation [22, 27, 28] (Fig. 1).
Table 1.
Loss of smell and taste in the long COVID-19 (3–12 months)*
| Author/area | Study type | Time period after onset of disease | Severity of COVID-19 at baseline | The number of patients with OD/GD at baseline | OD/GD evaluation method | OD outcome | GD outcome |
|---|---|---|---|---|---|---|---|
| Tan et al./NM [15••] | Systematic review and meta-analysis | 6 months | Hospitalized and non-hospitalized | 3699 | Self-reported and brief smell identification test | 5.6% patients reported long lasting OD by self-reported test | 4.4% patients reported long lasting GD by self-reported test |
| Nguyen et al./ France [16] | Cross-sectional | 7 months | Non-severe | 125 | Telephone questionnaire | 24.0% patients reported persistent OD | 24.0% patients reported persistent GD |
| Biadsee et al./Israel [17] | Cross-sectional | 8 months | Non-hospitalized | 65 | Telephone questionnaire | 48% patients experienced OD | 38.5% patients experienced GD |
| Hopkins et al./UK [18] | Prospective longitudinal | 6 months | Mild to moderate | 434 | Online survey | 59.3% patients were not recovered | 35.3% patients were not recovered |
| Coelho et al./USA [19] | Prospective longitudinal | 6 months | NM | 290 | Online survey | 36% patients were not completely recovered | 58% of patients were not completely recovered |
| Reis et al./Brazil [20] | Cross-sectional | 6 months | Non-hospitalized | 222/205 | Online self-report questionnaire | 45% still had some degree of OD | 50% still had some degree of GD |
| Teaima et al./Egypt [21] | Prospective longitudinal | 6 months | Mild to moderate | 866 | Phone call or physically to document the change | 40.4% patients with OD or GD were not completely recovered | 40.4% patients with OD or GD were not completely recovered |
| Boscolo-Rizzo et al./Italy [22] | Prospective longitudinal | 6 months | Mild to moderate | 145 | SNOT-22 and CA-UPSIT | over 60% of patients had OD by objective test | NM |
| Hintschich et al./Germany [24] | Cross-sectional | 8 months | Hospitalized | 212/224 | NHANES pocket smell test/taste strips test | 40% patients were not completely recovered | 32.5% patients were not completely recovered |
| Bussiere et al./Canada [25] | Cross-sectional | 7 months | NM | 572/574 | Online questionnaire and chemosensory perception test by smell 3 substances | 64% patients were not completely recovered | 51% patients were not completely recovered |
| Petrocelli et al./Italy [26] | Prospective longitudinal | 6 months | NM | 190/184 | Self-administered psychophysical test | 42.6% patients experienced OD | 16.3% of patients experienced GD |
| Cristillo et al./ Italy [27] | cross-sectional | 6 months | Mild to moderate | 50 | Sniffin’ sticks test/taste strips test | 16% patients with OD | 16% patients with GD |
| Prem et al./ Austria [28] | Prospective longitudinal | 7 months | Non hospitalized | 102 | PROMs and questionnaires; sniffin’ sticks/taste strips test | 76.5% patients experienced OD based on objective test | 18.6% patients experienced GD based on objective test |
| Bordin et al./Italy [29•] | Prospective longitudinal | 6 months | NM | 101 | SNOT-22 and sniffin’ sticks | 81.8% of normosmics self-reported their olfaction as recovered, and 72.7% of hyposmics reported smell as recovered | NM |
| Klein et al./Israel [36] | Prospective longitudinal | 6 months | Mild | 103 | Telephone questionnaire | 15% patients with OD | 8% patients with GD |
| Riestra-Ayora et al./Spain [38] | Prospective case–control | 6 months | NM | 125/118 | VAS scale | 11% patients with OD | 12% of the patients with GD |
| Lechien et al./Europe [41] | Cross-sectional | 6 months | Hospitalized | 1363 | NHANES and sniffin’ sticks tests | 4.7% patients with OD based on objective evaluation | NM |
CA-UPSIT culturally adapted University of Pennsylvania Smell Identification Test, GD gustatory dysfunction, NM not mentioned, NHANES national health and nutrition examination surveys, OD olfactory dysfunction, PROMs patient reported outcome measurement survey, SNOT-22 sinonasal outcome test-22, VAS visual analog scale
*The publications were searched in Pubmed till Aug 1, 2022
Fig. 1.
The recovery rates of OD and GD in long COVID-19 and associated risk factors. OD, olfactory dysfunction; GD, gustatory dysfunction
OG and GD at Follow-Up Longer than 12 Months After Symptom Onset
Fewer studies investigated the recovery of olfactory and gustatory function longer than 12 months after COVID-19. As illustrated in Table 2, a study of 178 patients in Italy found that OD and GD were self-reported in 25.8% and 21.3% of patients at one year after the onset of COVID-19, respectively [30]. Tan et al. followed up previously hospitalized COVID-19 patients in London and revealed that 12.8% of patients reported persistent problems with smell or taste 1 year after infection [31]. Fernandez-de-Las-Penas et al. enrolled 1593 hospitalized patients and found that 3.1% of patients still had OD and 1.75% of patients had GD at 13-month follow-up by telephone review of self-reported symptoms [32]. Ferreli et al. enrolled 97 patients with COVID-19-related OD and found that 13.1% of patients reported OD at 18-month follow-up by telephone review of self-reported symptoms [33]. A matched case–control study from Italy compared the chemosensory disorders in long COVID-19 patients and normal controls without SARS-CoV-2 infection using psychophysical tests [34]. This study found that OD was identified in 46% of cases and 10% of controls, while GD was in 27% of cases versus 10% of controls at 13 months post-COVID-19 [34]. By setting the control group, this study confirmed the long-lasting chemosensory disorders due to COVID-19. The longest follow-up time of COVID-19-related OD and GD so far is about 2 years. Paolo Boscolo-Rizzo et al. reported that 11.8% of 119 COVID-19 patients still complained of OD or GD 2 years after the onset of symptoms by telephone evaluation with SNOT-22 [35•].
Table 2.
Loss of smell and taste in the long COVID-19 (over 12 months)*
| Author/area | Study type | Time period after onset of disease | Severity of COVID-19 at baseline | The number of patients with OD/GD at baseline | OD/GD evaluation method | OD outcomes | GD outcomes |
|---|---|---|---|---|---|---|---|
| Fortunato et al./Italy [30] | prospective longitudinal | 12 months | Mild | 178 | Telephone interview | 25.8% patients experienced OD | 21.3% patients experienced GD |
| Tan et al./UK [31] | cross-sectional | 12 months | Hospitalized | 149 | Telephone interview | 12.8% of patients reported OD or GD | 12.8% of patients reported OD or GD |
| Fernandez-de-Las-Penas et al./Spain [32] | prospective longitudinal | 13 months | Hospitalized | 1593 | Telephone interview | 3.1% of patients with OD | 1.75% of patients with GD |
| Ferreli et al./Italy [33] | prospective longitudinal | 18 months | NM | 97 | Telephone interview | 13.1% of patients reported OD | NM |
| Boscolo-Rizzo et al./Italy [34] | matched case–control | 13 months | Mild | 100 | ARTIQ, SNOT-22, and sniffin’ sticks test/taste strips test | OD was identified in 46% of cases and 10% of healthy controls | GD was identified in 27% of cases versus 10% of healthy controls |
| Boscolo-Rizzo et al./Italy [35•] | longitudinal | 24 months | Mild | 119 | ARTIQ, SNOT-22 | 11.8% still reported OD or GD | 11.8% still reported OD or GD |
ARTIQ Acute Respiratory Tract Infection Questionnaire, GD gustatory dysfunction, NM not mentioned, OD olfactory dysfunction, SNOT-22 sinonasal outcome test-22
*The publications were searched in Pubmed till Aug 1, 2022
Risk Factors of Persistent OD and GD After COVID-19
Given the lasting of OD and GD in a considerable number of patients even 2 years after disease onset, it is crucial to determine the demographic and clinical factors associated with chemosensory disorders in long COVID-19. There is a controversy regarding the age and persistent OD and GD in long COVID-19. Some studies indicated a positive correlation between age and the prevalence of OD in long COVID-19 [1, 2]. It might be explained by more comorbidities and consequently more medications used in elderly patients, which might influence the clinical course of OD and GD [20, 27]. In contrast, some studies showed that younger age was a risk factor for OD and GD [24–27], and some found no correlation between age and OD [16, 36]. Since the prevalence of OD in the general population increases with aging [37], the association between age and chemosensory disorders in long COVID-19 should be determined with a comparison of age and gender-matched subjects without COVID-19. Female patients were found more likely to report persistent symptoms of OD and GD than male patients [15••, 24–26, 36] (Fig. 1). Patients with greater initial severity of chemosensory dysfunction were less likely to recover [15••, 24], and phantosmia was associated with a lower probability of complete recovery of olfactory function [21] (Fig. 1). In addition, dyspnea and nasal obstruction were found as predictors of poor olfactory and gustatory function recovery [38] (Fig. 1). Allergies and prior radiotherapy of the head and neck region had a significant influence on GD [24]. Emotional distress and depression have been reported associated with OD and GD in long COVID-19 [34] (Fig. 1). Consistently, in the recent elegant meta-analysis by Tan et al., female gender, greater initial severity of dysfunction, and nasal congestion have been identified as risk factors for poor recovery of chemosensory dysfunction [15••] (Fig. 1). Importantly, SARS-CoV-2 variants have been found with different ability to cause chemosensory disorders. Individuals with mild COVID-19 infection during the Gamma and Omicron waves had a lower risk of reporting OD than individuals infected during the period of the original lineages [39]. Thus, different SARS-CoV-2 variants can lead to different clinical courses of OD and GD (Fig. 1).
The Pathogenesis of OD and GD in Long COVID-19
SARS-CoV-2 uses the receptors angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) for entering host cells [40]. Enrichment of ACE2 and TMPRSS2 is found in the olfactory epithelium, a phenomenon that is particularly pronounced in sustentacular non-neural and neural stem cells [41, 42]. Although the pathogenesis of OD in the acute phase has been described, the etiology of lasting OD in long COVID-19 is barely studied. By drawing on the findings from other viral infection diseases which may cause long-term OD, we may infer the potential mechanisms of OD in long COVID-19. In a 3-month follow-up study based on MRI, significantly higher bilateral gray-matter volumes in olfactory cortices as well as a prevailing reduction of diffusivity in white matter have been reported [43], which implied the persistent alteration of olfactory center in long COVID-19. In addition, previous studies suggested that the basic process of olfactory coding is modified following the initial viral insult, inducing permanent impairment of olfaction [44]. This theory is supported by human fMRI studies showing abnormalities in olfactory functional connectivity in patients with post-viral olfactory dysfunction (PVOD) as compared to controls [45]. It has been shown that influenza A and respiratory syncytial virus infection lead to prolonged changes in synaptic plasticity even after virus clearance [46, 47]. Neuroinvasive propensity has been documented in almost all the β-coronaviruses (CoVs), including SARS-CoV [48], MERS-CoV [49], and HCoV-229E [50]. ACE2 expression is delineated in olfactory neurons [51], indicating that SARS-CoV-2 might enter the brain via the olfactory nerves and impact the formation of olfactory sensation [52]. However, the direct damage caused by SARS-CoV-2 to the olfactory pathway in the central nervous system is not supported by the study by Khan et al. [53]. Based on postmortem studies, they found that the sustentacular cells were the main target cell type in the olfactory mucosa, and no evidence supported the infection of olfactory sensory neurons or olfactory bulb parenchyma [53]. Therefore, the alteration of the olfactory pathway in the central nervous system in COVID-19 patients might not be caused by the direct attack of SARS-CoV-2, but by some indirect ways. One possible mechanism of long-term OD may be related to chronic unresolved inflammation in long COVID-19. A recent study revealed that the plasma levels of cytokines such as IL-4 and IL-6 remained upregulated in individuals recovered from COVID-19 [54]. The persistent peripheral and neuroinflammation may contribute to neurological sequelae in long COVID-19. Obviously, more studies are needed to clarify the pathogenesis of long-term OD and GD after SASR-CoV-2 infection.
The Treatment of OD and GD in Long COVID-19
Although high percentages of patients reported improvement of OD and GD after 3 months of disease onset, a large number of patients would have lasting chemosensory disorders given over 600 million people globally confirmed as having COVID-19 as of September 2022. It is important to establish efficient therapies for OD and GD in long COVID-19 [55]. Oral and topical corticosteroids are frequently used in the treatment of PVOD [56–58]. Topical corticosteroids may be beneficial for acute OD after SARS-CoV-2 infection. A prospective interventional study reported statistically significant improvement in recognizing odors after treating with fluticasone nasal sprays for 5 days immediately after proven SARS-CoV-2 infection [59]. Nevertheless, there is contradictory evidence concerning the effectiveness of intranasal corticosteroids. Another prospective, randomized, controlled trial was performed in patients with anosmia after COVID-19. The results showed that the addition of mometasone furoate nasal spray to olfactory training did not provide superior benefits over olfactory training alone in a 3-week treatment period [60]. For oral corticosteroids, a study was conducted on 72 COVID-19 patients 5 weeks after the loss of their smell [61]. The participants were under olfactory training and were given methylprednisolone for 10 days or not [61]. It was found that only those having methylprednisolone treatment simultaneously had significantly improved olfactory scores [61].
Although the corticosteroid treatments showed benefit in acute COVID-19 with OD, do these treatments have a beneficial effect on long-term OD? There is no direct evidence demonstrating the effect of corticosteroids on long-term OD after COVID-19. Nevertheless, in theory, the use of corticosteroids in the acute phase of COVID-19 with OD may prevent the development of chronic inflammation and subsequent persistent damage of the mucosa of the olfactory region, and therefore may reduce the risk of long-term OD. Apparently, more clinical experiments are warranted to evaluate the efficacy of topical and oral corticosteroids for the prevention and treatment of long-term OD.
Olfactory training is an effective intervention for PVOD [62–64]. A recent meta-analysis demonstrated that 12-week olfactory training was able to significantly improve the olfactory function of patients with PVOD [65]. Denis et al. described a trial wherein 548 participants with persistent OD for one to over 12 months after COVID-19 underwent olfactory training with concurrent visual depictions of the scents. After 4 weeks, 64% of patients reported improved symptoms [66]. Despite the major limitation of the lack of a control cohort, the results demonstrated that a large proportion of patients experienced clinically significant benefit from olfactory training. Nevertheless, high-level evidence is needed to confirm the effectiveness of olfactory training for OD in long COVID-19.
Conclusion and Take-Home Messages
In conclusion, although the prevalence of OD and GD has declined continuously after acute COVID-19, a considerable number of patients reported chemosensory disorders as a long consequence of COVID-19. Further investigations are required to clarify the long clinical course of OD and GD in COVID-19 patients. The risk factors of persistent OD and GD after COVID-19 have been revealed to be female gender, initial severity of dysfunction, nasal congestion, emotional distress and depression, and SARS-CoV-2 variants. The use of corticosteroids in the acute phase of COVID-19 may be able to prevent chronic inflammation and the development of persistent OD and GD theoretically; nevertheless, clinical evidence is needed to support the use of corticosteroids for chemosensory disorders in long COVID-19. Olfactory training is an effective therapy for PVOD and can be considered for the treatment of long COVID-19-related OD, which is also pending the confirmation with high-level clinical evidence.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) grants 82130030 (Z.L.), 81920108011(Z.L.), 82071025 (M.Z.), 82071026 (B.L.), 82201260 (Y.K.D.), and the Key Research and Development Program of Hubei Province 2021BCA119 (Z.L.).
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bo Liao and Yi-Ke Deng contributed equally to the completion of this article
Contributor Information
Ming Zeng, Email: zmsx77@163.com.
Zheng Liu, Email: zhengliuent@hotmail.com.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healey Q, Sheikh A, Daines L, Vasileiou E. Symptoms and signs of long COVID: a rapid review and meta-analysis. J Glob Health. 2022;12:05014. doi: 10.7189/jogh.12.05014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang T, Yan MZ, Li X, Lau EHY. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: a systematic review and meta-analysis. Infection. 2022. [DOI] [PMC free article] [PubMed]
- 5.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323(20):2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rojas-Lechuga MJ, Izquierdo-Dominguez A, Chiesa-Estomba C, et al. Chemosensory dysfunction in COVID-19 out-patients. Eur Arch Otorhinolaryngol. 2021;278(3):695–702. doi: 10.1007/s00405-020-06266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng M, Wang DY, Mullol J, Liu Z. Chemosensory dysfunction in patients with COVID-19: what do we learn from the global outbreak? Curr Allergy Asthma Rep. 2021;21(2):6. doi: 10.1007/s11882-020-00987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nouchi A, Chastang J, Miyara M, et al. Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur J Clin Microbiol Infect Dis. 2021;40(4):691–697. doi: 10.1007/s10096-020-04056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan C, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806–813. [DOI] [PMC free article] [PubMed]
- 11.Boscolo-Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(8):729–732. doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy K, Saniasiaya J, Abdul GN. Olfactory and gustatory dysfunctions as a clinical manifestation of Coronavirus disease 2019 in a Malaysian Tertiary Center. Annals of Otology, Rhinology & Laryngology. 2020;130(5):513–519. doi: 10.1177/0003489420963165. [DOI] [PubMed] [Google Scholar]
- 13.Song J, Deng YK, Wang H, et al. Self-reported taste and smell disorders in patients with COVID-19: distinct features in China. Curr Med Sci. 2021;41(1):14–23. doi: 10.1007/s11596-021-2312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paderno A, Mattavelli D, Rampinelli V, et al. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg. 2020;163(6):1144–1149. doi: 10.1177/0194599820939538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.•• Tan BKJ, Han R, Zhao JJ, et al. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ. 2022;378:e069503. This meta-analysis reported that 5.6% and 4.4% of patients might develop long lasting self-reported OD and GD at 6 months post-covid-19. Women, greater initial severity of dysfunction and nasal congestion were risk factors for poor recovery of chemosensory dysfunctions.
- 16.Nguyen NN, Hoang VT, Lagier JC, Raoult D, Gautret P. Long-term persistence of olfactory and gustatory disorders in COVID-19 patients. Clin Microbiol Infect. 2021;27(6):931–932. doi: 10.1016/j.cmi.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biadsee A, Dagan O, Ormianer Z, Kassem F, Masarwa S, Biadsee A. Eight-month follow-up of olfactory and gustatory dysfunctions in recovered COVID-19 patients. Am J Otolaryngol. 2021;42(4):103065. doi: 10.1016/j.amjoto.2021.103065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins C, Surda P, Vaira LA, et al. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinology. 2021;59(1):26–31. doi: 10.4193/Rhin20.544. [DOI] [PubMed] [Google Scholar]
- 19.Coelho DH, Reiter ER, Budd SG, Shin Y, Kons ZA, Costanzo RM. Quality of life and safety impact of COVID-19 associated smell and taste disturbances. Am J Otolaryngol. 2021;42(4):103001. doi: 10.1016/j.amjoto.2021.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reis D, Sartoretto SC, Calasans-Maia MD, Louro RS, Moraschini V. Long-term prevalence of taste and olfactory dysfunction in COVID-19 patients: a cross-sectional study. Oral Dis. 2022. [DOI] [PMC free article] [PubMed]
- 21.Teaima AA, Salem OM, Teama M, et al. Patterns and clinical outcomes of olfactory and gustatory disorders in six months: prospective study of 1031 COVID-19 patients. Am J Otolaryngol. 2022;43(1):103259. [DOI] [PMC free article] [PubMed]
- 22.Boscolo-Rizzo P, Menegaldo A, Fabbris C, et al. Six-Month Psychophysical evaluation of olfactory dysfunction in patients with COVID-19. Chem Senses. 2021;46. [DOI] [PMC free article] [PubMed]
- 23.Mazzatenta A, Neri G, D'Ardes D, et al. Smell and taste in severe CoViD-19: self-reported vs. testing. Front Med (Lausanne). 2020;7:589409. [DOI] [PMC free article] [PubMed]
- 24.Hintschich CA, Fischer R, Hummel T, Wenzel JJ, Bohr C, Vielsmeier V. Persisting olfactory dysfunction in post-COVID-19 is associated with gustatory impairment: results from chemosensitive testing eight months after the acute infection. PLoS ONE. 2022;17(3):e0265686. doi: 10.1371/journal.pone.0265686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bussiere N, Mei J, Levesque-Boissonneault C, et al. Chemosensory dysfunctions induced by COVID-19 can persist up to 7 months: a study of over 700 healthcare workers. Chem Senses. 2021;46. [DOI] [PMC free article] [PubMed]
- 26.Petrocelli M, Cutrupi S, Salzano G, et al. Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study. J Laryngol Otol. 2021;135(5):436–441. doi: 10.1017/S002221512100116X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristillo V, Pilotto A, Cotti Piccinelli S, et al. Age and subtle cognitive impairment are associated with long-term olfactory dysfunction after COVID-19 infection. J Am Geriatr Soc. 2021;69(10):2778–2780. doi: 10.1111/jgs.17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prem B, Liu DT, Besser G, et al. Long-lasting olfactory dysfunction in COVID-19 patients. Eur Arch Otorhinolaryngol. 2022;279(7):3485–3492. doi: 10.1007/s00405-021-07153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.• Bordin A, Mucignat-Caretta C, Gaudioso P, et al. Comparison of self-reported symptoms and psychophysical tests in coronavirus disease 2019 (COVID-19) subjects experiencing long-term olfactory dysfunction: a 6-month follow-up study. Int Forum Allergy Rhinol. 2021;11(11):1592–1595. This study compared the results of self-reported symptoms and psychophysical tests in COVID-19 patients with long-term OD at 6-month follow-up. The results confirmed that psychophysical smell tests were more sensitive than patient-reported outcome measures, and that the latter could be unreliable when used to assess smell recovery in the long-term. [DOI] [PMC free article] [PubMed]
- 30.Fortunato F, Martinelli D, Iannelli G, et al. Self-reported olfactory and gustatory dysfunctions in COVID-19 patients: a 1-year follow-up study in Foggia district, Italy. BMC Infect Dis. 2022;22(1):77. doi: 10.1186/s12879-022-07052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan HQM, Pendolino AL, Andrews PJ, Choi D. Prevalence of olfactory dysfunction and quality of life in hospitalised patients 1 year after SARS-CoV-2 infection: a cohort study. BMJ Open. 2022;12(1):e054598. doi: 10.1136/bmjopen-2021-054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-de-Las-Penas C, Martin-Guerrero JD, Navarro-Pardo E, Cancela-Cilleruelo I, Moro-Lopez-Menchero P, Pellicer-Valero OJ. Exploring trajectory curves from loss of smell and taste in previously hospitalized COVID-19 survivors: the LONG-COVID-EXP-CM Multicenter Study. J Gen Intern Med. 2022;37(7):1821–1823. doi: 10.1007/s11606-022-07459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreli F, Gaino F, Russo E, et al. Long-term olfactory dysfunction in COVID-19 patients: 18-month follow-up study. Int Forum Allergy Rhinol. 2022;12(8):1078–1080. doi: 10.1002/alr.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boscolo-Rizzo P, Hummel T, Hopkins C, et al. High prevalence of long-term olfactory, gustatory, and chemesthesis dysfunction in post-COVID-19 patients: a matched case-control study with one-year follow-up using a comprehensive psychophysical evaluation. Rhinology. 2021;59(6):517–527. doi: 10.4193/Rhin21.249. [DOI] [PubMed] [Google Scholar]
- 35.• Boscolo-Rizzo P, Fabbris C, Polesel J, et al. Two-Year Prevalence and recovery rate of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2022;148(9):889–891. This 2 years follow-up study reported that 88.2% of patients reporting a COVID-19–related OD or GD completely recovered within 2 years. A late recovery was observed in 10.9% of patients. [DOI] [PMC free article] [PubMed]
- 36.Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect. 2021. [DOI] [PMC free article] [PubMed]
- 37.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54(26):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 38.Riestra-Ayora J, Yanes-Diaz J, Esteban-Sanchez J, et al. Long-term follow-up of olfactory and gustatory dysfunction in COVID-19: 6 months case-control study of health workers. Eur Arch Otorhinolaryngol. 2021;278(12):4831–4837. doi: 10.1007/s00405-021-06764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardoso CC, Rossi AD, Galliez RM, Faffe DS, Tanuri A, Castineiras T. Olfactory dysfunction in patients with mild COVID-19 during Gamma, Delta, and Omicron Waves in Rio de Janeiro. Brazil JAMA. 2022;328(6):582–583. doi: 10.1001/jama.2022.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e278. [DOI] [PMC free article] [PubMed]
- 41.Lechien JR, Radulesco T, Calvo-Henriquez C, et al. ACE2 & TMPRSS2 Expressions in head & neck tissues: a systematic review. Head Neck Pathol. 2020;15(1):225–235. doi: 10.1007/s12105-020-01212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sungnak W, Huang N, Becavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu Y, Li X, Geng D, et al. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JC, Nallani R, Cass L, Bhalla V, Chiu AG, Villwock JA. A systematic review of the neuropathologic findings of post-viral olfactory dysfunction: implications and novel insight for the COVID-19 pandemic. Am J Rhinol Allergy. 2021;35(3):323–333. doi: 10.1177/1945892420957853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kollndorfer K, Jakab A, Mueller CA, Trattnig S, Schopf V. Effects of chronic peripheral olfactory loss on functional brain networks. Neuroscience. 2015;310:589–599. doi: 10.1016/j.neuroscience.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 46.Hosseini S, Wilk E, Michaelsen-Preusse K, et al. Long-term neuroinflammation induced by influenza a virus infection and the impact on hippocampal neuron morphology and function. J Neurosci. 2018;38(12):3060–3080. doi: 10.1523/JNEUROSCI.1740-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Espinoza JA, Bohmwald K, Cespedes PF, et al. Impaired learning resulting from respiratory syncytial virus infection. Proc Natl Acad Sci U S A. 2013;110(22):9112–9117. doi: 10.1073/pnas.1217508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glass WG, Subbarao K, Murphy B, Murphy PM. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol. 2004;15(173(6)). [DOI] [PubMed]
- 49.Li K, Wohlford-Lenane C, Perlman S, et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talbot PJ, Ekandé S, Cashman NR, Mounir S, Stewart JN. Neurotropism of human coronavirus 229E. Adv Exp Med Biol. 1993;342. [DOI] [PubMed]
- 51.Cecchini MP, Brozzetti L, Cardobi N, et al. Persistent chemosensory dysfunction in a young patient with mild COVID-19 with partial recovery 15 months after the onset. Neurol Sci. 2022;43(1):99–104. doi: 10.1007/s10072-021-05635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan M, Yoo S-J, Clijsters M, et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184(24):5932–5949. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun B, Tang N, Peluso MJ, et al. Characterization and biomarker analyses of post-COVID-19 complications and neurological manifestations. Cells. 2021;10(2). [DOI] [PMC free article] [PubMed]
- 55.Whitcroft KL, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 2019;145(9):846–853. doi: 10.1001/jamaoto.2019.1728. [DOI] [PubMed] [Google Scholar]
- 56.Seo BS, Lee HJ, Mo J-H, Lee CH, Rhee CS, Kim J-W. Treatment of postviral olfactory loss with glucocorticoids, Ginkgo biloba, and mometasone nasal spray. Arch Otolaryngol Head Neck Surg. 2009;135(10):1000–1004. [DOI] [PubMed]
- 57.Schriever VA, Merkonidis C, Gupta N, Hummel C, Hummel T. Treatment of smell loss with systemic methylprednisolone. Rhinology. 2012;50(3):284–289. doi: 10.4193/Rhino.11.207. [DOI] [PubMed] [Google Scholar]
- 58.Kim DH, Kim SW, Hwang SH, et al. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngol Head Neck Surg. 2017;156(2):371–377. doi: 10.1177/0194599816679952. [DOI] [PubMed] [Google Scholar]
- 59.Singh CV, Jain S, Parveen S. The outcome of fluticasone nasal spray on anosmia and triamcinolone oral paste in dysgeusia in COVID-19 patients. Am J Otolaryngol. 2021;42(3):102892. doi: 10.1016/j.amjoto.2020.102892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdelalim AA, Mohamady AA, Elsayed RA, Elawady MA, Ghallab AF. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am J Otolaryngol. 2021;42(2):102884. doi: 10.1016/j.amjoto.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le Bon SD, Konopnicki D, Pisarski N, Prunier L, Lechien JR, Horoi M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur Arch Otorhinolaryngol. 2021;278(8):3113–3117. doi: 10.1007/s00405-020-06520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neta FI, Fernandes ACL, Vale AJM, et al. Pathophysiology and possible treatments for olfactory-gustatory disorders in patients affected by COVID-19. Curr Res Pharmacol Drug Discov. 2021;2:100035. doi: 10.1016/j.crphar.2021.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinology journal. 2017;54(26):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 64.Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2016;6(3):299–307. doi: 10.1002/alr.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hura N, Xie DX, Choby GW, et al. Treatment of post-viral olfactory dysfunction: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2020;10(9):1065–1086. doi: 10.1002/alr.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denis F, Septans AL, Periers L, et al. Olfactory training and visual stimulation assisted by a web application for patients with persistent olfactory dysfunction after SARS-CoV-2 infection: observational study. J Med Internet Res. 2021;23(5):e29583. doi: 10.2196/29583. [DOI] [PMC free article] [PubMed] [Google Scholar]