Abstract
Time perception is known as a mental ability to discern time. Although relative nature of time leaves its numerous aspects undefined, several models have been developed to describe temporal information processing in the brain as well as several areas of the brain have shown to be involved. Time perception alteration has been reported in several neurological conditions; however, the effect of multiple sclerosis (MS) on time perception has yet to be explained. In this study, we aimed to investigate the domains of temporal processing involved in patients with MS and the probable factors affecting it, such as the location of brain demyelinating plaques and gender. Two groups of participants (MS: n = 27 (8 men, 19 women), mean age = 33.85; control: n = 30 (10 men, 20 women), mean age = 28.46) were asked to perform quadruplet time perception tasks (prospective time estimation, duration discrimination, temporal reproduction, and paced motor timing) designed with a software program. Patients with MS had significantly higher scores in time estimation (p < 0.01) and duration discrimination (p < 0.001, in 100-ms interval; p < 0.05, in 1000-ms interval), indicating that MS patients overestimate the time. Since a slower internal clock for MS patients was expected as a result of axonal demyelination, these results suggest the time overestimation in patients with MS which is in contrast with the internal clock model. It means that a slow internal clock causes underestimating and perceiving the time slower.
Keywords: Time estimation, Time perception, Multiple sclerosis
Introduction
While time perception is usually described as a mental ability to discern time and has an essential role in daily activities [26, 11], time’s diverse application and its relative nature has made an obstacle to its transparency and a unified definition. Aiming more simplicity, time perception is practically measured by how someone perceives time intervals between two consecutive events, recognizing a stimulus duration, and learning a rhythmic movement [4].
Previous studies have indicated that the suprachiasmatic nucleus is responsible for circadian rhythms and functional MRI (fMRI) studies have shown that several brain regions such as the prefrontal cortex, cerebellum, and basal ganglia are involved in temporal processing on a millisecond time scale. In other words, the network that is involved in the millisecond scale of time perception is widely distributed in the brain [6, 18, 25]. While degeneracy is shaping a more abstract concept of how the same function can be exerted by different groups of neurons, localizing brain areas corresponding to a specific function is trending so far [16].
Although new time perception models such as state-dependent networks have been developed recently [18], the internal-clock model has remained the most popular model that explains the altered time perception in different conditions, stating a neural network oscillator oscillates rhythmically in relation to external time intervals, and these oscillations are accumulated to keep track of the elapsing time [18], leading to an explanation of several conditions which make the internal clock tick faster causing the time perceived to slow down [30].
In addition to certain synthetic substances and herbs, several medical conditions can alter the perception of time and have led to a more accurate definition of the temporal processing mechanism. Neurodegenerative diseases such as Parkinson’s and Alzheimer’s have been shown that could alter time perception [2, 8, 9, 14, 15, 17, 31]. In Parkinson’s disease, time perception impairment is shown to be associated with dopaminergic system disturbance [2, 15]. El Haj et al. have shown that Alzheimer’s patients have impaired prospective and retrospective time estimation—the ability to keep track of upcoming time and gathering information of elapsed time respectively—as other cognitive functions dimmed [9].
However, the effects of multiple sclerosis on time perception ability have yet to be explored [8, 13]. Multiple sclerosis is a neurodegenerative disorder characterized by acquired distributed (in time and location) demyelination in the central nervous system (CNS). These demyelinating plaques afflict the myelin sheath and lower the axonal conduction speed [20]. Patients with multiple sclerosis (PwMS) deal with several cognitive disorders, which impair daily living activity and decrease individuals’ quality of life [5]. The delay in perceiving external stimuli such as auditory and visual inputs has been shown in patients with MS [1, 20]. In addition, ventricular enlargement and corpus callosum atrophy are common findings in multiple sclerosis and are correlated with cognitive deficits and slow information processing [29]. The fronto-striatal circuitry which seems to be a vital component of the internal clock circuit and responsible for temporal information processing is damaged in multiple sclerosis [12, 19]. Since enough information about time perception ability in PwMS is missing and lacks the concept of time as a cognitive entity, and related factors like education and gender differences are not yet cleared [8, 13],a set of tasks and methods are employed for explicating different aspects of this cognitive performance and probable factors affecting it. Our initial hypothesis based on that neuronal conduction slowing down due to a damaged myelin sheath can affect internal clock speed.
Materials and methods
Subjects
A case–control study was designed to assess time perception characteristics in patients with multiple sclerosis using a set of tasks. This study was conducted in the rehabilitation school of Ahvaz Jundishapur University of medical sciences (AJUMS), Ahvaz, Iran. The study protocol has been reviewed and approved by the ethics committees of the center and AJUMS. All the patients with MS were eligible to include; outpatient individuals who were referred by Khuzestan Multiple Sclerosis Association. We excluded those patients who had comorbid diseases of the CNS like stroke, alcohol or drug dependence, and a history of head trauma. We also excluded those patients aged less than 18 or over 50. The control group was healthy participants from the rehabilitation school’s staff and students. All the participants were asked about their years of education including years of school and university (healthy group: mean = 15.9, MS group: mean = 14.19). In patients with MS, the time since their initial diagnosis was registered (mean = 4.7 years). Finally, we included 8 men and 19 women (mean age = 33.85 in the MS group, and 10 men and 20 women (mean age = 28.46) in the healthy group. The groups’ average ages were not considerably different. Two groups did not differ in age. Written informed consent was taken from all the participants.
Procedures
All the participants were instructed to complete time perception tasks designed with a software program. Testing was conducted at the Musculoskeletal Rehabilitation Research Center on a personal computer (PC) of examiners. Each participant performs all of the tests in a single session that lasted 20 min. The tests consist of (1) prospective time estimation, (2) duration discrimination, (3) temporal reproduction, and (4) paced motor timing.
Time perception tasks
Prospective time estimation
The participants were given instructions to determine how much time had elapsed from the start point; which start with a green ball displayed on the screen until it disappeared and replaced with a red ball and an alert tone, indicating the end of the task. The true duration that must be estimated was 53 s. Participants were also informed not to count during the trial; instead, they were instructed to mark their estimations on a time arrow with a length of 3 min and an accuracy of 1 s. This is for visualizing the task; to reduce the numerical bias that occurs in verbal counting when estimating time [30]. In this test, the ability to estimate prospective time, i.e., how much time has passed from a set point, would be assessed. Ɵ ratio was used to calculate subjective to objective (53 s) time.
Duration discrimination
Two auditory stimuli including white noises displayed via a headset at 100 decibels (dB) SPL [30] were presented to the participants to decide which play longer in each set. In this test, a time duration estimation comparison between two stimuli was assessed. The whole comparison was conducted in two conditions which played randomly: short and long conditions, using 100 ms (ms) as a base tone and 101 to 400 ms as the comparison tone for short mode and 1000 ms as a base tone, and 1005 to 2000 ms as the comparison tone for long condition. There was a pause interval of 500 ms between each base and comparison tone. Weber fraction which showed ∆t/t was used to assess participant performance in which ∆t indicates the difference of mean comparison interval in which estimation threshold was reached difference with the base duration (100 or 1000 ms) and t is the base duration. The stimulation presentation stops while reaching the threshold of ± 10 ms of estimated time.
Temporal reproduction
The participants were instructed to reconstruct the duration of a standard stimulus. Two tones were played via a headset at a 100 dB sound pressure level in each trial; first, a 300-Hz reference tone was sounded, then, after a fixed 1000-ms gap, the 600-Hz tone was played, which should be terminated anytime the participant felt it attained the first tone length. Five different standard tones (1000, 2000, 3000, 4000, and 5000 ms) were repeated randomly (each tone repetition was six times). The mean reproduced duration was calculated over 6 trials for each standard tone. The participants were informed not to count and to decide when the second tone reached the first duration based on their feelings. Another distractive task was assigned to further dissuade them from counting. Two symbols were displayed on the screen before the first tone was played, and the participants were asked to memorize them. After reconstructing the duration, the participants were shown a symbol, and they had to remember whether the symbol shown at the end of the trial was one of the symbols shown at the beginning or not [30]. The ability to reconstruct the duration between two stimuli is the primary outcome measure in this test.
Paced motor timing
There were two components to this test. The instruction for the first section was to tap fingers synchronized on the space button of a keyboard when the “bib” sound was heard. Throughout each trial, a regular sequence of tones (500 Hz, duration 50 ms) was played 20 times in a row (sensory-motor synchronization). The participants were asked to synchronize their finger tapping with each single bib sound and continue it with the same tempo, even when the tone halted after ten tones during the second part (continuation of tapping). Finger tapping must be continued for another 10, 20, or 40 times in each trial in the second part, depending on their inter-tone interval (inter-tone intervals were 4000, 2000, or 1000 ms for both conditions). Only the taps that followed the tone within 120 ms were considered synchronized. The taps that exceed 120 ms are more likely to be a motor response or reaction rather than synchronization [30]. This activity assessed the capacity to synchronize one’s hand movements to various beats as well as the memory’s ability to maintain track of the rhythm. Outcomes of this test are exerted via three formulas,for mean asynchronization, we used the difference of mean tap onset synchronization to the value 120 ms, while the mean tap onset asynchrony is assessed by a software program. For missed synchronization, the total participant taps percentage, and for intertap interval, Ɵ ratio was used by which mean participant intertap intervals relative to the actual interval were calculated.
Magnetic resonance imaging
All the patients with MS had recent brain MRIs. Lesions in different parts of the CNS including peri ventricular, infratentorial, subcortical, septum semi-oval, pons, parietal lobe, pericallosal, corpus callosum, cerebral hemisphere, callososeptal, white matter, craniocervical junction, parietal white matter, corona radiata, and juxta cortical were frequently reported in MRI findings. Two categories were made based on patients’ magnetic resonance imaging (MRIs), such as the presence of a periventricular lesion and the number of lesions, in order to show a probable relation between the severity of time perception disturbance and the region and number of plaques.
Statistical analysis
We used subjective/objective duration (θ) as an index for temporal performance. Subjective is defined as the produced, reproduced, or estimated interval depending on a task. The objective is defined as the actual time interval. When θ=1, it hints the performance is accurate. When it is greater or less than 1, it indicates longer or shorter producing, reproducing, or estimating of time. We utilized GraphPad Prism version 6.1 for performing statistical analysis. Two-way ANOVA and Tukey’s and post hoc tests were used to compare four groups of healthy men, healthy women, MS men, and MS women scores. The independent sample t-test was applied to compare the two groups. Pearson’s correlation was used to determine the correlation between education level and prospective time estimation task score.
Results
The scores of MS and healthy groups in temporal processing tasks including prospective time estimation, duration discrimination, and temporal reproduction are shown in Table 1. It also includes the data based on an ANOVA analysis that shows their differences.
Table 1.
Temporal processing tasks results
| Temporal processing task | p value | ||
|---|---|---|---|
| MS | Health | ||
| Time estimation θ | 2.052 | 1.102 | 0.0071 (< 0.01) * |
| Duration discrimination ∆t/t | |||
| Interval 100 ms | 1.818 | 0.5888 | < 0.001* |
| Interval 1000 ms | 0.6547 | 0.2560 | 0.0142 (< 0.05) * |
| Temporal reproduction θ | |||
| Interval 1000 ms | 1.306 | 1.148 | 0.2072 |
| Interval 2000 ms | 1.217 | 1.019 | 0.4038 |
| Interval 3000 ms | 1.011 | 0.9459 | 0.689 |
| Interval 4000 ms | 0.7908 | 0.8614 | 0.334 |
| Interval 5000 ms | 0.7501 | 0.8859 | 0.268 |
Temporal processing tasks
Prospective time estimation
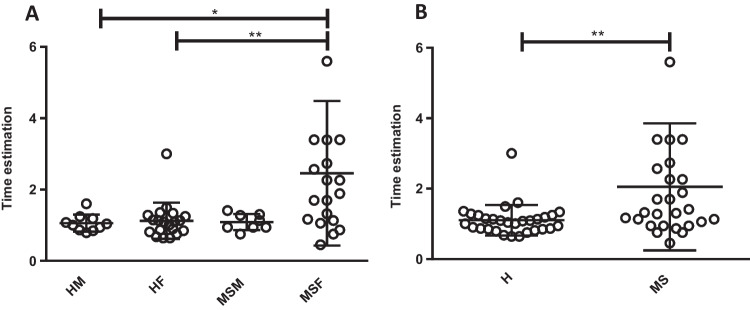
Two-way ANOVA and Tukey’s post hoc tests were used for comparison between the MS group and healthy group regarding both sexes, and a significantly higher score in women of MS groups than both genders in healthy groups was shown (healthy men: p < 0.05 and healthy women: p < 0.01) (Fig. 1A). Furthermore, the result of the independent sample t-test revealed that individuals in MS group get a higher score than others in the healthy group (p < 0.01) (Fig. 1B).
Fig. 1.
Diagram showing prospective time estimation analysis. A gender-based analysis showed women in the MS group obtained higher scores in the time estimation task than both genders in the healthy group, healthy men p < 0.05 and healthy women p < 0.01. (HM: healthy male, HF: healthy female, MSM: MS male, MSF: MS female). B MS group showed a higher score in the time estimation task than the healthy group, p < 0.01. (H: healthy, MS: multiple sclerosis)
Duration discrimination
Duration discrimination for 100-ms interval
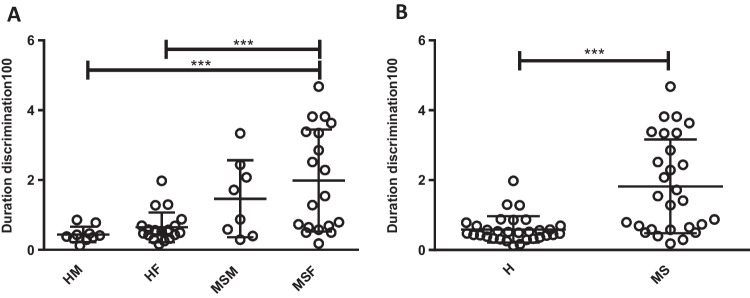
Our results showed that the Weber fraction score of the female MS group was significantly higher than that of healthy males (p < 0.001) and healthy females (p < 0.001) (Fig. 2A). Moreover, the Weber fraction in PwMS was significantly higher than in the healthy group, indicating higher estimation threshold (p < 0.001) (Fig. 2B).
Fig. 2.
Duration discrimination for 100-ms interval analysis. A Women in the MS group showed higher score in duration discrimination (interval 100) task than healthy men and women, p < 0.001. (HM: healthy male, HF: healthy female, MSM: MS male, MSF: MS female). B Duration discrimination for 100-ms interval, general analysis between MS and healthy group MS group showed higher score duration discrimination task (interval 100) than health group, p < 0.001. (H: healthy, MS: multiple sclerosis)
Duration discrimination in 1000-ms interval
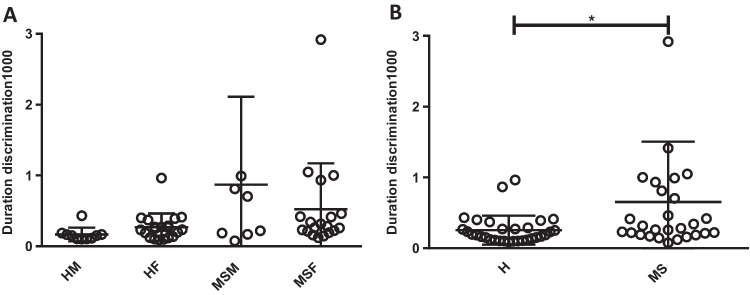
Although no comparable difference between genders was observed (Fig. 3A), there was a significant increase in the Weber fraction in the MS group compared to that in the healthy group (p < 0.05) (Fig. 3B).
Fig. 3.
Duration discrimination for 1000-ms interval, gender analysis. A No significant difference is detected between genders of four groups in the duration discriminate task (interval 1000). (HM: healthy male, HF: healthy female, MSM: MS male, MSF: MS female). B Duration discrimination for 100-ms interval, general analysis between MS and healthy group MS group showed a higher score in duration discrimination task (interval 1000) than the healthy group, p < 0.05. (H: healthy, MS: multiple sclerosis)
Temporal reproduction
In the MS group, men had higher scores at 2000-, 3000-, and 4000-ms intervals than women (p < 0.05). Furthermore, there were no significant differences in this task between the two healthy and MS groups; however, smaller scores for the MS group indicate mild under reproduction by them in this task.
Paced motor timing
The mean rate of asynchronization or tempo matching differs significantly between the healthy and MS groups in sensory-motor synchronization at 2000 ms (p < 0.0001) and 4000 ms (p = 0.0002) with higher scores or greater error in the MS group. However, no significant differences were seen at the 1000-ms interval (p = 0.0640). The MS group had a higher rate of missing synchronization percentage at the 1000-ms interval (p = 0.0032) but not at 2000 ms (p = 0.680) or 4000 ms (p = 0.232). In the continuation tap phase, though no significant difference between the two groups, the MS group had a smaller Ɵ ratio indicating faster tapping (1000 ms: p = 0.156, 2000 ms: p = 0.09 and 4000 ms: p = 0.094) (Table 2).
Table 2.
Paced motor timing test results
| Paced motor timing | Mean score | p value | |
|---|---|---|---|
| Health | MS | ||
| Mean async (ms) (sensorimotor synchronization phase) | |||
| Interval 1000 ms | − 53.84 | − 113.5 | 0.064 |
| Interval 2000 ms | − 123.6 | − 258.9 | < 0.0001* |
| Interval 4000 ms | − 187.7 | − 354.1 | 0.0002* |
| Missed sync % (sensorimotor synchronization phase) | |||
| Interval 1000 ms | 1.8% | 6.2% | 0.0032* |
| Interval 2000 ms | 1% | 1.2% | 0.680 |
| Interval 4000 ms | 1.5% | 2.7% | 0.232 |
| Mean intertap interval θ (continuation tapping phase) | |||
| Interval 1000 ms | 1.02 | 1.09 | 0.156 |
| Interval 2000 ms | 1.04 | 0.94 | 0.09 |
| Interval 4000 ms | 0.94 | 0.86 | 0.094 |
Educational differences
Interestingly, the mean years of education in the healthy group (y = 15.9) was significantly higher than in the multiple sclerosis group (y = 14.19) (p value < 0.03). We made a correlation between years of education and prospective time estimation task score due to the higher levels of education in healthy individuals than in patients (p < 0.03). There was no significant correlation between these two parameters (p < 0.05, r = − 0.2747), according to the Pearson R correlation.
Imaging analysis
The most common region of lesions in this study was the periventricular area (plaques seen in 12 patients). Patients were separated into two groups: those with periventricular plaques and those without. Patients with periventricular plaques scored 1.62 on the duration discrimination task (interval 100), while those without scored 1.91, p = 0.27. Hence, no remarkable relation was found. Another analysis was based on the number of plaques. Patients were divided into four groups, each with one to four plaques. One-way ANOVA revealed no significant difference in duration discrimination scores (interval 100) between the four groups (p = 0.412).
Years of morbidity
The correlation between the number of years since the patient was diagnosed with multiple sclerosis and the prospective time estimation task score was not statistically significant (p = 0.032, r = 0.414). In addition, there was no significant difference in mean years of morbidity in PwMS (4.74 ± 3.6) between men (3.79) and women (5.06) (p = 0.428).
Discussion
Time perception tasks
The results of this study indicate that patients with MS have impaired temporal processing. Though fundamental inter-task differences, patients with MS showed higher scores than healthy individuals in most of the tasks, particularly in prospective time estimation, duration discrimination, and several aspects of paced motor timing. The prospective time estimation task is a visual-based task based on judging a vanishing circle on the screen, as opposed to the duration discrimination task, which is more auditory and is based on comparing two noise durations. Disturbance in both tasks indicates impairment of both visual and auditory time perception in patients with multiple sclerosis. Prospective time estimation is a more subjective task and is more related to one’s previous experience of how much time has elapsed; therefore, it could have been more accurate in educated people who use clocks more frequently; however, we excluded such a correlation (r = − 0.27).
The most significant difference was in the 0.1-s scale as seen in the duration discrimination with 100-ms intervals and then 53-s intervals in the prospective time estimation task, but there was no strong evidence to support impairment in the 1–2-s scale as in the current study’s third and fourth tasks. Troche et al. support impairment in sub-second but not a second interval timing in pediatrics with Multiple sclerosis which is more likely to be caused by subcortical function alteration [28]. This is in contrast with circumstances that affect the dopaminergic system and result in a deficit in 1–2 s [2, 15]. Although impairment in 1–2 s is more likely to involve cognition and is based on memory reconstruction, hundreds of millisecond time scale which is more affected in multiple sclerosis less engage with these functions [30]. Impairment in the 53 s which is categorized as a long time period estimation and is highly related to attention and working memory, can also be affected by participant sense of boredom which is seen more in MS patients.
Due to motor involvement in MS [10], a larger impairment in two motor-based tasks (temporal reproduction and paced motor timing) was expected; however, we found no compelling evidence to support this hypothesis, and in contrast, a faster tapping (smaller Ɵ) in continuation tapping phase in paced motor timing task for MS group was observed (Table2) though was not significant. In the sensory-motor synchronization task, there was also significantly higher asynchrony at 2000 ms and 4000 ms, indicating that PwMS press the bottom with a greater error. However, tapping in rhythm is an automatic movement which is hardly under control; it is possibly caused by their inability to synchronize their finger movements with a rhythm. This situation is specific to PwMS and would not occur in cocaine or amphetamine abusers [30].
Higher scores of MS patients in tasks suggest poorer time resolution and that they are overestimating time; that is, PwMS dedicate larger numbers to the same time interval than the healthy group; i.e., time passes slower to them. While overestimation of time is caused by a fast-ticking internal clock according to the internal clock hypothesis [18], This is contrary to our initial hypothesis in which we expected the internal clock of PwMS would slow down due to demyelinating axons in the CNS and delayed electrical impulse transmission. In other words, our findings mismatch the internal clock hypothesis. Our results indicate that this model cannot make an accurate prediction of this disease unless there is a compensatory mechanism that led them to overestimate the time with a slow clock or the disease is accelerating their clock. Dogru-Huzmeli et al. have shown overestimating time in multiple sclerosis patients, and while they found higher scores in estimation and lower scores in production for PwMS, we found higher scores in both estimation and production of time in PwMS than healthy individuals [8]. Compared to other medical conditions, there is increasing evidence that Parkinson’s disease patients underestimate time. As M.A. Pastor et al. advocated, these patients tend to underproduce time, and their internal clock ticks slower [21].
Cocaine and amphetamine addicts tend to overestimate time [30]. The dopaminergic system is manipulated by both these drugs and Parkinson’s disease. However, they have the opposite effect on time estimation, which is due to an increase in dopamine activity in drug abusers and a decrease in dopamine activity in Parkinson’s disease patients [21].
Gender differences
A noticeable task-related gender difference in task performance was found in this study, in which women in the MS group showed higher scores, i.e., poorer time resolution in the first two tasks (prospective time estimation and duration discrimination), while men in the MS group showed a higher score in the temporal reproduction task. It is supported by a meta-analysis on 9482 subjects where females showed larger duration estimation errors than males [3] and better scores in discrimination of duration for men in another study [22]. On the opposite, a study has shown there is no significant difference between males and females in time estimation task but females performed better and more accurate [23].
Overall, in the present study, multiple sclerosis males and females could account for this diversity in conclusions as in previous studies only healthy males and females participated.
Cognitive and anatomic correlations
Different cognitive scores correlate with different stages of multiple sclerosis [27]. However, in this study, years of morbidity showed no effect on time estimation performance and indicates that temporal processing impairment is independent of the chronicity of the disease. This is in contrast to the findings of Parkinson’s disease studies, which have shown that the severity of the disease leads to increased time estimation errors [21]. However, our findings could be attributed to the significant variation in years of morbidity among patients.
Several imaging studies have shown a correlation between severe ventricular dilatation and corpus callosum atrophy and low scores in cognitive tests in PwMS [7]. Conventional MRI studies have shown a weak correlation between total lesion load and cognitive deficits [24]. We found no relation between either plaques’ location or their numbers and temporal processing impairments in this study. Though the damaged brain areas in multiple sclerosis are not necessarily involved in time perception, this finding might be biased by the low sample size. Proposing the fronto-striatal cortex as the main character of time perception impairment in stimulant-dependent individuals with almost normal cognitive scores [30], it can be inferred that even in patients without visible brain lesions nor plaques in conventional MRI, some anatomical features can be involved functionally.
One of the main limitations of this study was that we did not classify PwMS based on their MS type and the stage of the disease. However, we utilized years of morbidity as an indicator for inter-patient differences and disease chronicity.
We should emphasize that test durations and no rest between the four tests exhausted the participants, and four patients left the trials before completion. Also, due to the COVID-19 pandemic, data gathering faced some problems, and individuals did not prefer to participate in in-person examinations. The next projects would be based on online tests with a shorter time frame for each session.
Conclusion
Time perception is impaired in patients with MS in an overestimating manner and for these patients; the time passes more slowly for them. This impairment is not correlated to education level or years of morbidity but is relevant to gender. We could not detect any relation between the location or number of brain plaques with poorer scores in the tasks. However, our results clear a road to design studies with larger sample sizes and dedicated brain MRIs. As time perception impairment has been suggested by our results, and the necessity of this cognitive ability in humans’ daily living life has largely been established, more investigation into multiple sclerosis patients’ rehabilitation seems to be vital to maintain their quality of life.
Acknowledgements
This article was financially supported by the Research Affairs Deputy of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1399.931).
Author contribution
• Mina Echreshavi: conceptualization, methodology, formal analysis, investigation, writing—original draft
• Narges Shakerian: conceptualization, software, methodology, writing—review and editing
• Hassan Kiani Shahvandi: conceptualization, methodology, review and editing
• Mohammad Momeni: data curation, review and editing, supervision
• Asieh Mehramiri: data curation, review and editing, supervision
• Samireh Ghafouri: data curation, data analysis, writing—review and editing, supervision
Data availability statement
Data will be made available upon request.
Declarations
Conflict of interest
The authors declare no competing interests.
References
- 1.Alusi SH, et al. Tremor and Dysmetria in Multiple Sclerosis: A Neurophysiological Study. Tremor and other hyperkinetic movements (New York, N.Y.) 2021;11:30. doi: 10.5334/tohm.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard, M. et al. (2019) ‘Differential Temporal Perception Abilities in Parkinson ’ s Disease Patients Based on Timing Magnitude’, Scientific Reports, pp. 1–16. 10.1038/s41598-019-55827-y. [DOI] [PMC free article] [PubMed]
- 3.Block RA, Hancock PA, Zakay D. Sex differences in duration judgments: A meta-analytic review. Memory and Cognition. 2000;28(8):1333–1346. doi: 10.3758/BF03211834. [DOI] [PubMed] [Google Scholar]
- 4.Buonomano, D. V (2018) Your brain is a time machine;the neuroscience and physics of time, W. W. Norton & Company.
- 5.Calandri E, et al. Depression, Positive and Negative Affect, Optimism and Health-Related Quality of Life in Recently Diagnosed Multiple Sclerosis Patients: The Role of Identity, Sense of Coherence, and Self-efficacy. Journal of Happiness Studies. 2018;19(1):277–295. doi: 10.1007/s10902-016-9818-x. [DOI] [Google Scholar]
- 6.Casini L, Ivry RB. Effects of divided attention on temporal processing in patients with lesions of the cerebellum or frontal lobe. Neuropsychology. 1999;13(1):10. doi: 10.1037/0894-4105.13.1.10. [DOI] [PubMed] [Google Scholar]
- 7.Desousa EA, Albert RH. Cognitive impairments in multiple sclerosis : A review. American Journal of Alzheimer’s Disease and Other Dementias. 2002;17(1):23–29. doi: 10.1177/153331750201700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dogru-Huzmeli E, Duman T. There is an impairment in time perception of patients with multiple sclerosis. Somatosensory & Motor Research. 2021;38(2):140–145. doi: 10.1080/08990220.2021.1879777. [DOI] [PubMed] [Google Scholar]
- 9.El Haj M, et al. Prospective and retrospective time perception are related to mental time travel: Evidence from Alzheimer’s disease. Brain and Cognition. 2013;83(1):45–51. doi: 10.1016/j.bandc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Giannì, C. et al. (2021) ‘Altered sensorimotor integration in multiple sclerosis: A combined neurophysiological and functional MRI study’, Clinical Neurophysiology, 132(9), pp. 2191–2198. 10.1016/j.clinph.2021.05.028. [DOI] [PubMed]
- 11.Grondin, S. (2010) ‘Timing and time perception: a review of recent behavioral and neuroscience findings and theoretical directions’, Attention, Perception, & Psychophysics, 72(3), pp. 561–582. [DOI] [PubMed]
- 12.Johnen A, et al. Resolving the cognitive clinico-radiological paradox – Microstructural degeneration of fronto-striatal-thalamic loops in early active multiple sclerosis. Cortex. 2019;121:239–252. doi: 10.1016/j.cortex.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Król J, et al. Time perception and illness acceptance among remitting-relapsing multiple sclerosis patients under treatment. Psychiatria polska. 2015;49(5):911–920. doi: 10.12740/pp/38740. [DOI] [PubMed] [Google Scholar]
- 14.Lake JI, LaBar KS, Meck WH. Emotional modulation of interval timing and time perception. Neuroscience and biobehavioral reviews. 2016;64:403–420. doi: 10.1016/j.neubiorev.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange KW, et al. Subjective time estimation in Parkinson’s disease. Journal of neural transmission. Supplementum. 1995;46:433–438. [PubMed] [Google Scholar]
- 16.Lewis PA, Meek WH. Time and the sleeping brain. The Psychologist. 2012;25(8):594–597. [Google Scholar]
- 17.Lucas M, et al. Time perception impairs sensory-motor integration in Parkinson’s disease. International archives of medicine. 2013;6(1):39. doi: 10.1186/1755-7682-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauk MD, Buonomano DV. The neural basis of temporal processing. Annual Review of Neuroscience. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 19.Meck WH, Benson AM. Dissecting the Brain’s Internal Clock: How Frontal-Striatal Circuitry Keeps Time and Shifts Attention. Brain and Cognition. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- 20.Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: clinical aspects. Current opinion in neurology. 2018;31(6):752–759. doi: 10.1097/WCO.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 21.PASTOR, M. A. et al. (1992) ‘TIME ESTIMATION AND REPRODUCTION IS ABNORMAL IN PARKINSON’S DISEASE’, Brain, 115(1), pp. 211–225. 10.1093/brain/115.1.211. [DOI] [PubMed]
- 22.Rammsayer T, Lustnauer S. Sex Differences in Time Perception. Perceptual and Motor Skills. 1989;68(1):195–198. doi: 10.2466/pms.1989.68.1.195. [DOI] [PubMed] [Google Scholar]
- 23.Roeckelein JE. Sex Differences in Time Estimation. Perceptual and Motor Skills. 1972;35(3):859–862. doi: 10.2466/pms.1972.35.3.859. [DOI] [PubMed] [Google Scholar]
- 24.Rovaris, Marco et al “Cortical/Subcortical Disease Burden and Cognitive Impairment in Patients with Multiple Sclerosis.” American journal of Neuroradiology 21.2 (2000):402 408. Web. 14 Dec. 2022 [PMC free article] [PubMed]
- 25.Rubia K, et al. Synchronization, anticipation, and consistency in motor timing of children with dimensionally defined attention deficit hyperactivity behaviour. Perceptual and motor skills. 1999;89(3_suppl):1237–1258. doi: 10.2466/pms.1999.89.3f.1237. [DOI] [PubMed] [Google Scholar]
- 26.Rubia, K. (2006) ‘The Neural Correlates of Timing Functions’, Timing the Future, pp. 213–238.
- 27.Stepleman, L. and Barwick, F. (2007) ‘The Association of Illness Severity, Self-Reported Cognitive Impairment, and Perceived Illness Management With D ... in a Multiple Sclerosis Clinic Population’, Journal of behavioral medicine, 30(2), pp. 177–186. 10.1007/s10865-007-9095-6. [DOI] [PubMed]
- 28.Troche SJ, et al. Interval Timing in Pediatric Multiple Sclerosis: Impaired in the Subsecond Range but Unimpaired in the One-Second Range. Frontiers in Neurology. 2020;11(October):1–7. doi: 10.3389/fneur.2020.575780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsolaki M, et al. Correlation of Dementia, Neuropsychological and MRI Findings in Multiple Sclerosis. Dementia and Geriatric Cognitive Disorders. 1994;5(1):48–52. doi: 10.1159/000106694. [DOI] [PubMed] [Google Scholar]
- 30.Wittmann M, et al. Impaired time perception and motor timing in stimulant-dependent subjects. Drug and Alcohol Dependence. 2007;90(2):183–192. doi: 10.1016/j.drugalcdep.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittmann M, Lehnhoff S. Age effects in perception of time. Psychological reports. 2005;97(3):921–935. doi: 10.2466/pr0.97.3.921-935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.