Abstract
A man with non-small-cell lung cancer who was negative for anti-nuclear antibodies was admitted for dyspnea after immune checkpoint inhibitor (ICI) administration. Computed tomography (CT) showed complexed radiologic features, including subpleural and basal predominant reticular shadow with cystic structures and peribronchovascular consolidation. Although we treated him with high-dose steroid under a diagnosis of ICI-related pneumonitis, he developed acute exacerbation of pneumonitis with progressive fibrosis and volume loss. A re-evaluation identified anti-aminoacyl-tRNA synthetase antibody in the serum collected before ICI administration. This case highlights the importance of re-evaluating pre-existing autoimmune disorders in patients who develop ICI-related pneumonitis with atypical radiologic features.
Keywords: Non-small-cell lung cancer, Immune checkpoint inhibitor, Pneumonitis, Anti-aminoacyl-tRNA synthetase antibody
Abbreviations: ICIs, immune checkpoint inhibitors; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand-1; NSCLC, non-small-cell lung cancer; PFS, progression-free survival; OS, overall survival; RR, response rate; irAEs, immune-related adverse events; ARS, aminoacyl tRNA synthetases; HRCT, high-resolution computed tomography; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ANAs, anti-nuclear antibodies; GGO, ground-glass opacity; OP, organizing pneumonia; HP, hypersensitivity pneumonitis; NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia; ILD, interstitial lung disease; CTCAE, common terminology criteria for adverse events; ASS, anti-synthetase syndrome; IIM, idiopathic inflammatory myopathy
1. Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1)/programmed cell death-ligand-1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) have dramatically transformed the non-small-cell lung cancer (NSCLC) treatment paradigm. Although ICI monotherapy showed a favorable progression-free survival (PFS) and overall survival (OS) in some cohorts of patients with advanced-stage NSCLC, the response rate (RR) is not necessarily high, and relatively few patients can benefit from it. Based on these backgrounds, several regimens of combined cancer immunotherapy, including combination therapy of an ICI with a cytotoxic agent, angiogenesis inhibitor, or another ICI, have been developed for advanced-stage NSCLC in recent years [[1], [2], [3]].
Among multiple options, the IMpower130 regimen of atezolizumab in combination with carboplatin plus nanoparticle albumin-bound (nab)-paclitaxel showed safety and favorable therapeutic efficacy compared with chemotherapy alone in a phase III trial [1]. Therefore, it is now regarded as one of the standard regimens in the first-line treatment for non-squamous NSCLC.
However, it is well known that increasing activity of the immune system induced by ICIs often causes inflammatory side effects, termed immune-related adverse events (irAEs), in multiple organs, including the gastrointestinal tract, endocrine glands, skin, central nervous system, and lung [4]. ICI-related pneumonitis is an irAE that should carefully be managed, as it is one of the most common causes of ICI-related death [[5], [6], [7]].
We herein report a rare case of ICI-related pneumonitis by atezolizumab with atypical radiologic features. A retrospective evaluation after the development of pneumonitis revealed that pre-existing anti-aminoacyl tRNA synthetases (ARS) antibody may be involved in the pathogenesis of such pneumonitis.
2. Case presentation
A 61-year-old man was referred to our hospital with abnormal thoracic opacity. He had smoked for one pack-year but had no notable medical history. Physical examinations did not show any significant findings. Chest X-ray showed a huge mass shadow in his left upper lung field (Fig. 1A). High-resolution computed tomography (HRCT) revealed a lung mass shadow in his left upper lobe along with mediastinal and supraclavicular lymph node swelling. Based on the transbronchial biopsy and imaging studies, we diagnosed him with advanced-stage lung adenocarcinoma (cT3N3M1b stage IVB) with brain metastasis. The tumor cells were negative for oncogenic driver mutations, such as epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) fusion gene, but strongly positive for PD-L1 determined by PD-L1 22C3 pharmDx (tumor proportion score: 100%). Blood tests revealed that anti-nuclear antibodies (ANAs) were negative (Table 1).
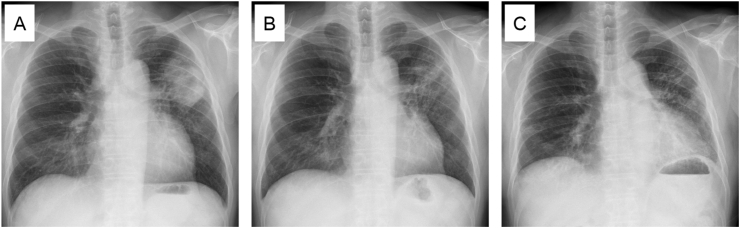
Fig. 1.
Chest X-ray findings. (A) A huge mass shadow was observed in left upper lung field at the initial visit. (B) The tumor had regressed after second cycle of chemotherapy. (C) Bilateral GGOs, predominantly in left lower lung field, appeared after the third cycle of chemotherapy.
Table 1.
Laboratory data.
| Before the administration of chemotherapy | After third cycle of chemotherapy | The onset of acute exacerbation | |
|---|---|---|---|
| WBC (/μL) | 11,600 | 6200 | 13,400 |
| HGB (g/dL) | 14.2 | 9.8 | 12.6 |
| PLT (x104 /μL) | 38.4 | 21.4 | 25.9 |
| AST (U/L) | 12 | 18 | 16 |
| ALT (U/L) | 11 | 11 | 18 |
| LDH (U/L) | 241 | 216 | 343 |
| T-bil (mg/dL) | 0.4 | 0.6 | 0.6 |
| CK (U/L) | 56 | 26 | 16 |
| BUN (mg/dL) | 13 | 16 | 16 |
| Cr (mg/dL) | 0.72 | 0.92 | 0.68 |
| Na (mEq/L) | 139 | 139 | 141 |
| K (mEq/L) | 4.1 | 4.1 | 3.9 |
| Cl (mEq/L) | 103 | 100 | 103 |
| CRP (mg/dL) | 0.93 | 6.81 | 7.80 |
| KL-6 (U/mL) | 1161 | 797 | 1559 |
| SP-D (ng/mL) | 84.4 | N.E. | 141 |
| ANA (dils) | 40 | N.E. | <40 |
| Anti-CCP Ab (U/mL) | N.E. | N.E. | <0.6 |
| Anti-ARS Ab (Index) | 35.7a | N.E. | 129 |
| MPO-ANCA (U/mL) | N.E. | N.E. | <1.0 |
| PR3-ANCA (U/mL) | N.E. | N.E. | <1.0 |
N.E.; not examined.
Retrospectively evaluated after the development of ICI-related pneumonitis.
We started to treat him with carboplatin (AUC = 6) and nab-paclitaxel (100 mg/m2) combined with atezolizumab (1200 mg/body) every 3 weeks with local palliative radiotherapy (RT) to brain metastasis (18.8 Gy/1Fr). The chemotherapy induced a partial response (Fig. 1B), but he developed respiratory failure after the third cycle of chemotherapy (Fig. 1C) and was admitted.
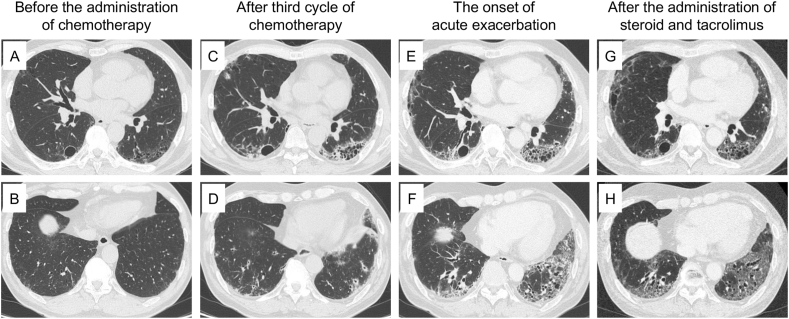
The chest auscultation revealed fine crackles in bilateral lower lung fields, but other physical examination did not reveal any abnormal findings, such as muscle or skin abnormalities. Although HRCT before the administration of chemotherapy showed slight cystic changes, we considered that these findings were mainly due to paraseptal emphysema (Fig. 2A and B). However, HRCT on the day of admission showed subpleural and basal predominant reticular shadow, cystic structures, and peripheral traction bronchiectasis with volume loss in his left lower lobe (Fig. 2C). In the right lower lobe, peribronchovascular consolidation with ground-glass opacity (GGO) was also observed. (Fig. 2D). We discontinued the chemotherapy, including atezolizumab, and administered antibiotics, but his symptoms did not improve.
Fig. 2.
Chest computed tomography findings. The images before the administration of chemotherapy showed paraseptal emphysema but not GGOs or reticular shadows (A, B). After the third cycle of chemotherapy, subpleural and basal predominant reticular shadow, cystic structures, and peripheral traction bronchiectasis with volume loss were observed in the left lower lobe (C), and peribronchovascular consolidation with GGO was observed in the right lower lobe (D). The images at the onset of acute exacerbation showed additional bilateral GGO and consolidation with progressive traction bronchiectasis and volume loss of bilateral lower lobes (E, F). Methylprednisolone pulse followed by high-dose prednisolone and tacrolimus improved the GGO and consolidation (G, H).
Bronchoscopy revealed that the fraction of lymphocytes was elevated in the bronchoalveolar lavage fluid (29.3%), consistent with the drug-induced pneumonitis. As the frequency of pneumonitis by atezolizumab was relatively higher than that by carboplatin plus nab-paclitaxel in the previous studies [1], we diagnosed him with ICI-related pneumonitis induced by atezolizumab (Grade 3 according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0), though the imaging findings were not necessarily typical, and we treated him with high-dose prednisolone (60 mg/day). This temporary improved his symptoms, but at two months after starting steroid therapy, he again complained of progressive dyspnea with a fever when prednisolone was tapered to 20 mg/day. Laboratory data showed elevated levels of C-reactive protein (CRP) and Krebs von den Lungen-6 (KL-6) (Table 1). HRCT showed additional bilateral GGO and consolidation with progressive traction bronchiectasis and volume loss of bilateral lower lobes (Fig. 2E and F). These imaging findings were consistent with acute exacerbation of pneumonitis but atypical for ICI-related pneumonitis, which typically shows the imaging pattern of organizing pneumonia (OP) or GGO [[6], [7], [8]] but generally not progressive fibrosis with obvious cystic changes and volume loss. Therefore, we re-evaluated the pre-existing comorbidities and finally identified anti-PL7 antibody, a representative anti-ARS antibody. A retrospective evaluation revealed that this antibody had also been present in the serum collected before the administration of ICI, and the titer was elevated after ICI administration (Table 1). Based on these findings, we finally considered the possible presence of pre-clinical anti-synthetase syndrome (ASS), with atezolizumab likely triggering the development of ASS with pneumonitis.
Accordingly, we treated the patient with methylprednisolone pulse followed by high-dose prednisolone and tacrolimus, an immunosuppressive agent. The treatments improved the GGO and consolidation and suppressed the progression of fibrosis (Fig. 2G and H). We have observed neither re-exacerbation of pneumonitis after administration of tacrolimus nor recurrence of the tumor since discontinuation of chemotherapy.
3. Discussion
Information on the radiologic features of ICI-related pneumonitis is still limited [9]. The largest study to date included 64 cases, with the most frequent pattern being OP (23%), followed by hypersensitivity pneumonitis (HP) (16%) and nonspecific interstitial pneumonia (NSIP) (8%) [6]. Other reports found that GGO was the most dominant pattern [7,8]. To our knowledge, only one study has previously reported patients who developed ICI-related pneumonitis with a honeycomb [10], although detailed information, including whether or not the honeycomb had been present before ICI administration, was not documented in these reports.
HRCT in our patient demonstrated complex radiologic findings. The findings of subpleural and basal predominant reticular shadow, cystic structures, and peripheral traction bronchiectasis in his left lower lobe were consistent with usual interstitial pneumonia (UIP) (Fig. 2C). In contrast, however, the peribronchovascular consolidation with GGO in his right lobe was consistent with NSIP (Fig. 2D). Furthermore, the rapidly progressive fibrosis with obvious volume loss seemed to be a distinctive feature and atypical for ICI-related pneumonitis.
ICI-related pneumonitis is more frequently observed among patients with lung cancer than among those with other types of cancer, mainly due to smoking and underlying lung conditions [11,12]. The incidence of ICI-related pneumonitis was around 4.5% among patients with lung cancer [13], and a younger age (≤60 years old), a poor performance status (PS) (≥2), pre-existing autoimmune diseases or lung diseases (especially interstitial lung disease (ILD)), and a history of thoracic radiotherapy should be regarded as risk factors [14]. In clinical practice, examinations to evaluate auto-antibodies, such as ANAs, or the existence of ILD are often performed before ICI administration. In the present case, although the patient had been negative for these risk factors at the pre-treatment evaluation, he developed pneumonitis. Atypical radiologic findings motivated us to re-evaluate the possibility of autoimmune disorders, and we finally identified anti-ARS antibody even in the serum collected before administration of the ICI. We diagnosed the patient with ICI-related pneumonitis based on the clinical course and elevated lymphocytes fraction in the bronchoalveolar lavage fluid, although the radiologic findings were not necessarily typical. However, in retrospect, we should have re-evaluated the pre-existing auto-immune diseases when the steroid therapy was initiated. Although it would not be appropriate to measure the several auto-antibodies for all patients who develop pneumonitis after ICI administration, the current findings highlight the importance of re-evaluating the presence of pre-existing comorbidities especially in a patient with ICI-related pneumonitis presenting with atypical radiologic features.
Anti-ARS antibodies are detected in 35%–40% of idiopathic inflammatory myopathy (IIM) as well as in patients who are negative for ANAs, as their antigens are located in the cytoplasm and not the nucleus [15]. ASS, characterized by the presence of anti-ARS antibodies, clinically manifests with ILD, myositis, and arthritis [16]. The frequency of ILD among patients with ASS was reported to range from 70% to 100%, and ILD often manifests in advance of myositis [17,18]. While NSIP is dominant as the radiologic pattern, UIP is also observed in some patients with ASS [19]. Furthermore, ICIs are known to induce the exacerbation of pre-existing autoimmune diseases, and Shikano et al. previously reported that nivolumab, an anti-PD-1 antibody, induced anti-ARS antibody-positive polymyositis [20]. Based on these reports, we finally considered that ASS triggered by atezolizumab might have been involved in the development of pneumonitis in this case, resulted in the appearance of complex radiologic features.
In summary, we experienced a rare case of lung adenocarcinoma in a patient who developed ICI-related pneumonitis with atypical radiologic features after the administration of atezolizumab. A retrospective re-evaluation allowed us to identify the pre-existing anti-ARS antibody, suggesting that pre-existing ASS might have been involved in the exacerbation of ICI-induced pneumonitis. This case highlights the importance of the re-evaluation of pre-existing autoimmune diseases in patients who develop ICI-related pneumonitis with atypical radiologic features.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that all authors have no conflict of interests.
References
- 1.West H., McCleod M., Hussein M., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. Jul 2019;20(7):924–937. doi: 10.1016/s1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. May 31 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. Nov 22 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 4.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. Jan 11 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 5.Nishino M., Ramaiya N.H., Awad M.M., et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin. Cancer Res. Dec 15 2016;22(24):6051–6060. doi: 10.1158/1078-0432.Ccr-16-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaunay M., Cadranel J., Lusque A., et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. Aug 2017;50(2) doi: 10.1183/13993003.00050-2017. [DOI] [PubMed] [Google Scholar]
- 7.Naidoo J., Wang X., Woo K.M., et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J. Clin. Oncol. Mar 2017;35(7):709–717. doi: 10.1200/jco.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atchley W.T., Alvarez C., Saxena-Beem S., et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in North Carolina. Chest. Aug 2021;160(2):731–742. doi: 10.1016/j.chest.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalisz K.R., Ramaiya N.H., Laukamp K.R., Gupta A. Immune checkpoint inhibitor therapy-related pneumonitis: patterns and management. Radiographics. Nov-Dec 2019;39(7):1923–1937. doi: 10.1148/rg.2019190036. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z., Wang S., Du H., Shen H., Zhu J., Li Y. Immunotherapy-induced pneumonitis in non-small cell lung cancer patients: current concern in treatment with immune-check-point inhibitors. Invest. N. Drugs. Jun 2021;39(3):891–898. doi: 10.1007/s10637-020-01051-9. [DOI] [PubMed] [Google Scholar]
- 11.Nobashi T.W., Nishimoto Y., Kawata Y., et al. Clinical and radiological features of immune checkpoint inhibitor-related pneumonitis in lung cancer and non-lung cancers. Br. J. Radiol. Nov 1 2020;93(1115) doi: 10.1259/bjr.20200409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino M., Giobbie-Hurder A., Hatabu H., Ramaiya N.H., Hodi F.S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. Dec 1 2016;2(12):1607–1616. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 13.Shao J., Wang C., Ren P., Jiang Y., Tian P., Li W. Treatment- and immune-related adverse events of immune checkpoint inhibitors in advanced lung cancer. Biosci. Rep. May 29 2020;40(5) doi: 10.1042/bsr20192347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chennamadhavuni A., Abushahin L., Jin N., Presley C.J., Manne A. Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.779691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirakata M. Autoantibodies to aminoacyl-tRNA synthetases. Intern. Med. Jun 2005;44(6):527–528. doi: 10.2169/internalmedicine.44.527. [DOI] [PubMed] [Google Scholar]
- 16.Marco J.L., Collins B.F. Clinical manifestations and treatment of antisynthetase syndrome. Best Pract. Res. Clin. Rheumatol. Aug 2020;34(4) doi: 10.1016/j.berh.2020.101503. [DOI] [PubMed] [Google Scholar]
- 17.Dugar M., Cox S., Limaye V., Blumbergs P., Roberts-Thomson P.J. Clinical heterogeneity and prognostic features of South Australian patients with anti-synthetase autoantibodies. Intern. Med. J. Sep 2011;41(9):674–679. doi: 10.1111/j.1445-5994.2010.02164.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamaguchi Y., Fujimoto M., Matsushita T., et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waseda Y., Johkoh T., Egashira R., et al. Antisynthetase syndrome: pulmonary computed tomography findings of adult patients with antibodies to aminoacyl-tRNA synthetases. Eur. J. Radiol. Aug 2016;85(8):1421–1426. doi: 10.1016/j.ejrad.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Shikano K., Kaneko K., Kaburaki K., et al. Nivolumab-induced anti-aminoacyl-tRNA synthetase antibody-positive polymyositis complicated by interstitial pneumonia in a patient with lung adenocarcinoma. Scand. J. Rheumatol. Jan 2020;49(1):82–83. doi: 10.1080/03009742.2019.1596309. [DOI] [PubMed] [Google Scholar]