Abstract
Novel anti-HER2 antibody-drug conjugates trastuzumab deruxtecan (DS-8201a) showed its effect in previously-treated HER2-low metastatic breast cancer, suggesting a promising future in HER2-low breast cancer. We retrospectively reviewed the clinicopathological data of 325 patients with stage I–III HER2 negative breast cancer who received neoadjuvant chemotherapy in the First Affiliated Hospital of Sun Yat-sen University from January 2016 to June 2021. In general, 91 patients (28.0%) were HER2-zero, and 234 patients (72.0%) were HER2-low. The pathological complete response (pCR) rate of the entire cohort was 17.3%. The pCR rate was 16.7% in HER2-low group, and 18.9% in HER2-zero group, showing no significant difference. Patients with HER2-low tumors had significantly longer overall survival (OS) than patients with HER2-zero tumors. ER status was the affecting factor of OS in HER2-low patients in both univariate and multivariate analysis. In conclusion, evidence for HER2-low BC as a distinct entity is insufficient, and more efforts are needed to standardize the scoring of HER2-low breast cancer.
Keywords: HER2-low breast cancer, Neoadjuvant chemotherapy, Pathological complete response, Survival
Highlights
-
•
HER2-low breast cancer has a higher proportion of positive hormone receptor status compared with HER-zero breast cancer.
-
•
HER2-low and HER2-zero breast cancer had similar pathological response rate to neoadjuvant chemotherapy.
-
•
HER2-low tumors had a significantly longer overall survival than HER2-zero tumors.
-
•
Estrogen receptor and androgen receptor may play a role in HER2-low breast cancer.
1. Introduction
In the era of precision medicine, human epidermal growth factor receptor 2 (HER2) is an important therapeutic and prognostic biomarker of breast cancer (BC), which is positive in 15%–20% of BC [1]. Anti-HER2 targeted therapy, including monoclonal antibodies, tyrosine kinase inhibitor (TKI), and antibody-drug conjugates (ADC) targeting HER2, has brought dawn to HER2 positive patients [[2], [3], [4], [5], [6]], while HER2 negative breast cancer patients failed to benefit from traditional anti-HER2 treatment in clinical practice [7]. With the emergence of ADC targeting HER2 and the release of the result of DESTINY-Breast 04 (ClinicalTrials.gov Identifier: NCT03734029) study [8], we begin to pay more attention to HER2-low breast cancer.
The expression of HER2 is generally detected by immunohistochemistry (IHC), combined with in situ hybridization (ISH) to detect the amplification of ERBB2 gene. According to American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) Clinical Practice Guideline, HER2 positive is defined as HER2 IHC result of 3+, or 2+ with ISH detected ERBB2 gene amplification [9]. The widely accepted definition of HER2-low was HER2 IHC 1+ or 2+ with negative ISH results [[10], [11], [12], [13], [14], [15], [16]]. Studies have shown that HER2-low breast cancer accounted for 45%–55% of all breast cancer [17]. HER2-low patients can't benefit from conventional HER2-targeted drugs such as trastuzumab and pertuzumab [7,18,19]. However, the results of DESTINY-Breast 04 showed that previously-treated HER2-low metastatic breast cancer patients treated with trastuzumab deruxtecan (DS-8201a), an ADC drug targeting HER2, had longer progress-free survival (PFS) and overall survival (OS) compared with patients treated with chemotherapy of physicians' choice [8]. There are several ongoing clinical trials comparing DS-8201a with the standard regimen in HER2-positive early breast cancer (DESTINY-Breast 05 and DESTINY-Breast 11), and clinical trials concerning early-stage HER2-low breast cancer are still recruiting (ClinicalTrials.gov Identifier: NCT05165225). With more and more ADC drugs targeting HER2 in breast cancer being researched and developed [15], whether HER2-low breast cancer is a real entity worth researching and discussing.
Studies about HER2-low patients treated with neoadjuvant chemotherapy (NACT) were rarely reported. The current retrospective studies [[20], [21], [22]] on HER2-low breast cancer's response to NACT had different reports about pathological complete response (pCR) rate and survival, therefore, we reviewed data from our center. The purpose of this article is to compare the differences in clinicopathological characteristics, pCR rate, and survival of HER2-low and HER2-zero BC.
2. Materials and methods
2.1. Cohort and study design
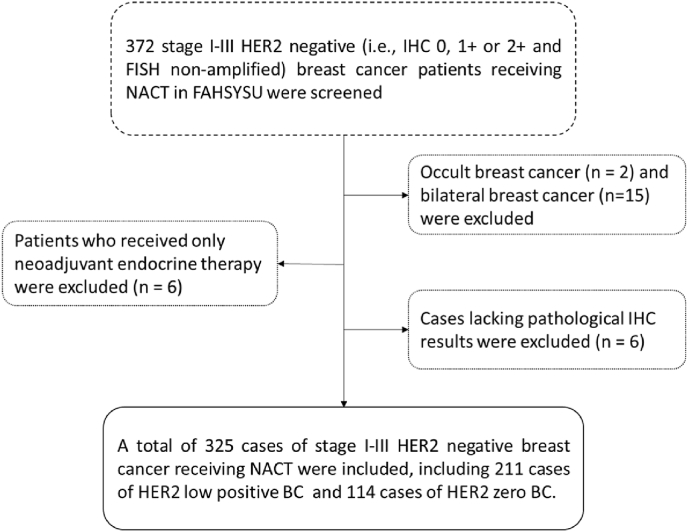
We included a retrospective cohort consisting of patients with early HER2-negative BC who were treated in the First Affiliated Hospital of Sun Yat-sen University (FAHSYSU) from January 2016 to June 2021 (Fig. 1). All patients were treated with anthracycline- and taxane-based NACT before surgery and pCR was used to measure response to NACT. The included patients with HER2-negative BC were divided into HER2-low (defined as HER2 IHC1+ or IHC2+ with negative FISH detection) [[10], [11], [12], [13], [14], [15], [16]] and HER2-zero (defined as HER2 IHC0) groups for further analysis.
Fig. 1.
Flow chart of case inclusion and exclusion. NACT: neoadjuvant chemotherapy; FAHSYSU: the First Affiliated Hospital of Sun Yat-sen University; IHC: immunohistochemistry; BC: breast cancer.
2.2. Patient characteristics
Hormone receptor (HR) positive was defined as estrogen receptor (ER) and/or progesterone receptors (PR) expressing cells percentage >10% by IHC. Androgen receptor (AR) positive was defined as AR expressing cells percentage >80% by IHC [23]. Evaluation of the staging was based on the 8th edition American Joint Committee on Cancer (AJCC) Cancer Staging Manual.
2.3. Treatment protocols
NACT regimens received in this study were based on anthracycline and paclitaxel. The operation mode was determined based on the actual condition and wishes of patients, including but not limited to modified radical mastectomy, breast-conserving surgery, etc.
2.4. Outcomes and follow-up
Pathological complete response was defined as absence of invasive disease in breast and lymph nodes as shown by hematoxylin-eosin staining after NACT (i.e., pT0/pTis pN0) [24]. The adapted survival indicators were overall survival (OS) and disease-free survival (DFS). OS was defined as the time from the date of first admission to the hospital to the date of death (any causes). DFS was defined as the time from the date of first admission to the hospital to the date of event defined as the first documented relapse or metastasis of the disease.
2.5. Statistical analysis
We used R 4.2.1 and SPSS 27.0.0 software for statistical analysis. The continuous data were described as median [IQR], and Mann Whitney's test was used for inter group comparison of quantitative data. Categorical data were described as n (%), and Fisher's exact test or chi square test were used for comparison between groups. Chi square test was used to compare the pCR rates between the HER2-low and HER2-zero groups. Factors influencing pCR rate of NACT were analyzed by logistic regression. The survival probability was described by Kaplan-Meier method. The survival between HER2-low patients and HER2-zero patients was compared by log-rank test. Cox proportional hazard model analysis was performed to analyze prognostic factors in the whole cohort, HER2-low patients, and HER2-zero patients.
3. Result
3.1. Clinicopathological characteristics between HER2-low and HER2-zero BC patients
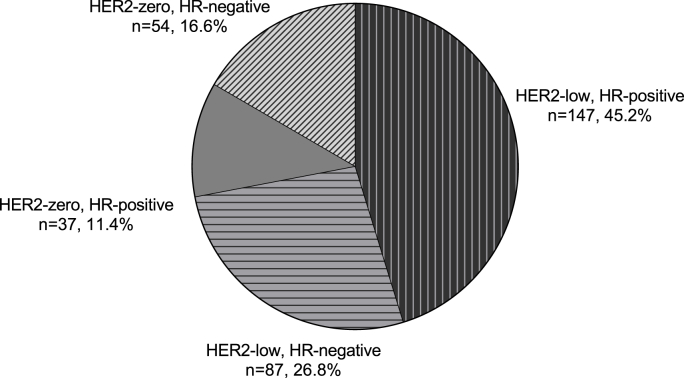
Of the 325 women eligible for the present study (Fig. 1), 91 patients (28.0%) were HER2-zero, and 234 patients (72.0%) were HER2-low, among which there were 118 patients (50.4%) with HER2 IHC1+ and 116 patients (49.6%) with HER2 IHC2+/FISH non-amplified. The clinicopathological characteristics were shown in Table 1. One hundred and forty-seven cases were HR positive among the HER2-low population (n = 234), accounting for 62.8%, while 37 cases were HR-positive among HER2-zero patients (n = 91), accounting for 40.7% (p < 0.001). The distribution of patients based on the HER2 expression and HR status was shown in Fig. 2. The proportion of HER-low increased as ER and AR expression got higher (p < 0.001) (Supplementary Fig. 1).
Table 1.
Clinicopathological parameters of included patients divided by HER2 status.
| Variable | HER2-low |
HER2-zero |
Overall |
p |
|---|---|---|---|---|
| (n = 234) | (n = 91) | (n = 325) | ||
| Age (median [IQR]) | 47.0 [40.0, 53.0] | 46.0 [38.0, 54.0] | 46.0 [40.0, 53.0] | 0.6457† |
| HR Status (%). | ||||
| Negative | 87 (37.2) | 54 (59.3) | 141 (43.4) | 0.0005‡ |
| Positive | 147 (62.8) | 37 (40.7) | 184 (56.6) | |
| Grading (%). | ||||
| Grade Ⅰ | 19 (8.1) | 1 (1.1) | 20 (6.2) | 0.0048※ |
| Grade Ⅱ | 105 (44.9) | 29 (31.9) | 134 (41.2) | |
| Grade Ⅲ | 55 (23.5) | 31 (34.1) | 86 (26.5) | |
| Missing | 55 (23.5) | 30 (33.0) | 85 (26.2) | |
| T stage (%). | ||||
| cT1 | 10 (4.3) | 6 (6.6) | 16 (4.9) | 0.7413 |
| cT2 | 130 (55.6) | 54 (59.3) | 184 (56.6) | |
| cT3 | 57 (24.4) | 17 (18.7) | 74 (22.8) | |
| cT4 | 33 (14.1) | 13 (14.3) | 46 (14.2) | |
| Missing | 4 (1.7) | 1 (1.1) | 5 (1.5) | |
| N stage (%). | ||||
| cN0 | 33 (14.1) | 13 (14.3) | 46 (14.2) | 0.8319 |
| cN1-3 | 198 (84.6) | 76 (83.5) | 274 (84.3) | |
| Missing | 3 (1.3) | 2 (2.2) | 5 (1.5) | |
| Clinical Stage (%). | ||||
| 1 | 2 (0.9) | 1 (1.1) | 3 (0.9) | 0.4708 |
| 2 | 88 (37.6) | 37 (40.7) | 125 (38.5) | |
| 3 | 140 (59.8) | 49 (53.8) | 189 (58.2) | |
| Missing | 4 (1.7) | 4 (4.4) | 8 (2.5) | |
| Histological Type (%). | ||||
| No special type | 6 (2.6) | 4 (4.4) | 287 (88.3) | 0.5166 |
| Invasive lobular | 206 (88.0) | 81 (89.0) | 10 (3.1) | |
| Other | 22 (9.4) | 6 (6.6) | 28 (8.6) | |
| AR status (%). | ||||
| Positive | 115 (49.1) | 49 (53.8) | 76 (23.4) | 0.4569 |
| Negative | 59 (25.2) | 17 (18.7) | 164 (50.5) | |
| Missing | 60 (25.6) | 25 (27.5) | 85 (26.2) | |
| Ki67 status (%). | ||||
| ≤20% | 67 (28.6) | 15 (16.5) | 82 (25.2) | 0.0072 |
| 20.1%–30.0% | 40 (17.1) | 8 (8.8) | 48 (14.8) | |
| >30% | 126 (53.8) | 68 (74.7) | 194 (59.7) | |
| Missing | 1 (0.4) | 0 (0.0) | 1 (0.3) | |
Data are described as n (%) or median [IQR]. †Mann-Whitney test for continuous data. ‡Fisher's exact test for data with 2 categories. ※χ2 test for data with more than two categories. HR: hormone receptor; AR: androgen receptor.
Fig. 2.
Distribution of HER2 negative patients based on HR and HER2 status.
Most of the HER2-low tumors were grade I and II, lower than that of the HER2-zero population (p < 0.01). Ki-67 expression level in HER2-low breast cancer was significantly lower than that in the HER2-zero population (p < 0.01). As for age, AR status, clinical stage, and histological type, no significant difference between the HER2-low and the HER2-zero groups were found.
3.2. Efficacy of neoadjuvant chemotherapy between HER2-low and HER2-zero BC patients
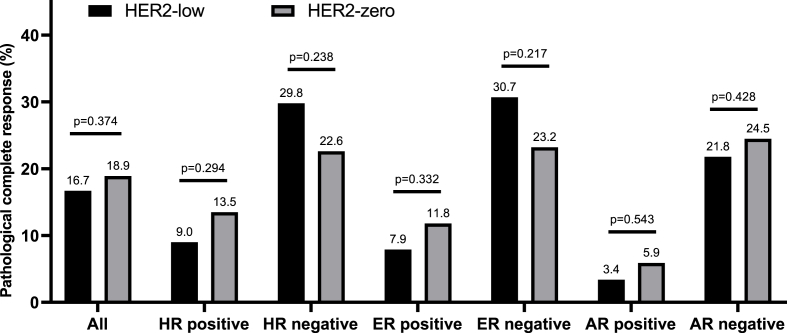
Among the 318 cases eligible to evaluate response to NACT, the pCR rate of the entire cohort, the HER2-low group, and the HER2-zero group was 17.3%, 16.7%, and 18.9%, respectively, showing no significant difference between the two groups (p = 0.374, Fig. 3). Stratified analyses according to different HR, ER, and AR status showed similar pCR rates between HER2-low and HER2-zero patients (p > 0.1, Fig. 3).
Fig. 3.
The pCR rate in HER2-low and HER2-zero breast cancer. Stratified analyses according to different HR, ER, and AR status showed similar pCR rates between HER2-low and HER2-zero patients (p > 0.1). Caption: Hormone receptor positive was defined as estrogen receptor (ER) and/or progesterone receptor (PR) expressing cells percentage >10% by IHC. ER positive was defined as ER expressing cells percentage >10% by IHC. AR positive was defined as AR expressing cells percentage >80% by IHC. Intra-group differences in pCR rate were analyzed using the chi-square test. No significant difference was found in each group.
The HER2-low status was not significantly associated with pCR (OR = 0.859, 95% CI: 0.456–1.616, p = 0.637, Table 2). The factors that affected pCR rate in HER2 negative patients included ER/PR status, Ki-67, AR status, and T stage. After adjusting confounding factors, PR status (OR = 0.251, 95% CI: 0.069–0.907, p = 0.035), AR status (OR = 0.256, 95% CI: 0.070–0.937, p = 0.040), and lymph node (LN) status (OR = 0.325, 95% CI: 0.112–0.943, p = 0.039) remained significantly associated with pCR in HER2-negative patients.
Table 2.
Logistic analysis of pCR in HER2-low and HER2-zero patients.
| HER2-low (n = 236) |
HER2 zero (n = 89) |
Overall (n = 318) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| univariate analysis |
multivariate analysis |
univariate analysis |
multivariate analysis |
univariate analysis |
multivariate analysis |
|||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
|
HER2 status low vs zero |
– | – | – | – | – | – | – | – | 0.859(0.456–1.616) | 0.637 | 1.038(0.460–2.339) | 0.929 |
|
ER status positive vs negative |
0.193(0.086–0.404) | 0.000 | 0.736(0.185–2.927) | 0.664 | 0.441(0.115–1.386) | 0.186 | 3.494(0.452–27.007) | 0.231 | 0.245(0.126–0.458) | 0.000 | 1.187(0.391–3.599) | 0.762 |
|
PR status positive vs negative |
0.175(0.068–0.396) | 0.000 | 0.289(0.059–1.412) | 0.125 | 0.214(0.032–0.837) | 0.051 | 0.169(0.018–1.577) | 0.119 | 0.186(0.082–0.377) | 0.000 | 0.251(0.069–0.907) | 0.035 |
|
Ki-67 >20% vs ≤ 20% |
9.216(2.695–57.839) | 0.003 | 3.778(0.775–18.429) | 0.100 | 3.797(0.681–71.344) | 0.214 | 0.803(0.045–14.229) | 0.881 | 7.348(2.601–30.806) | 0.001 | 2.935(0.774–11.137) | 0.113 |
|
AR status positive vs negative |
0.128(0.020–0.454) | 0.007 | 0.231(0.048–1.106) | 0.067 | 0.193(0.010–1.108) | 0.128 | 0.150(0.007–3.004) | 0.215 | 0.142(0.034–0.413) | 0.002 | 0.256(0.070–0.937) | 0.040 |
|
LN status N1-3 vs N0 |
0.352(0.152–0.852) | 0.016 | 0.300(0.083–1.083) | 0.066 | 1.230(0.284–8.547) | 0.803 | 0.487(0.236–1.054) | 0.057 | 0.325(0.112–0.943) | 0.039 | ||
|
T stage T4 vs T1-3 |
0 | 0.988 | 0.739(0.107–3.147) | 0.713 | 0.190(0.030–0.643) | 0.024 | 0.584(0.120–2.858) | 0.507 | ||||
| Age | 0.974(0.938–1.010) | 0.151 | 0.973(0.924–1.021) | 0.266 | 0.973(0.944–1.002) | 0.066 | ||||||
|
Histological type NST vs Other |
1.147(0.360–5.103) | 0.834 | 0.462(0.082–3.548) | 0.397 | 0.883(0.340–2.746) | 0.812 | ||||||
|
Histological type Invasive lobular vs Other |
1.133(0.050–11.414) | 0.921 | 0 | 0.993 | 0.467(0.023–3.475) | 0.513 | ||||||
OR: odds ratio; ER: estrogen receptor; PR: progesterone receptor; AR: androgen receptor; NST: no special type.
ER/PR status, AR status, Ki67, and N stage were the significant influencing factors of pCR in the HER2-low group, while in the HER2-zero group, only PR was the significant influencing factor of pCR, but none of the above factors were statistically significant after adjusting confounding factors (Table 2).
3.3. Survival analysis
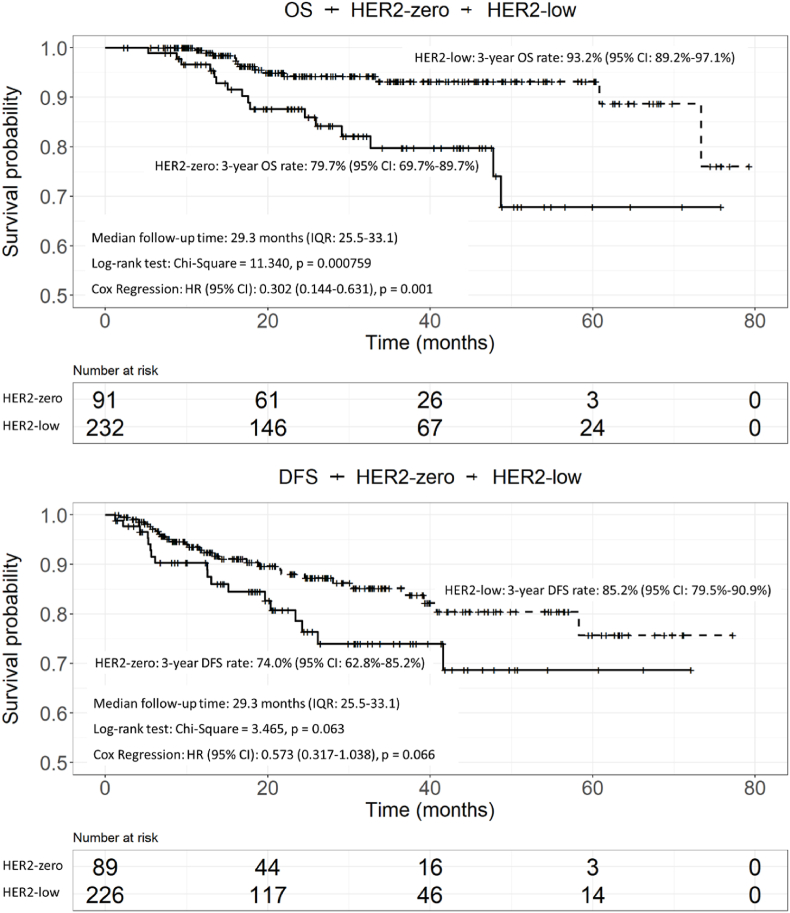
The median follow-up time was 29.3 months (IQR: 25.5–33.1). In general, we detected 49 locoregional and distant recurrences (28 cases in HER2-low group and 18 cases in HER2-zero group) and 29 death events (13 cases in HER2-low group and 16 cases in HER2-zero group). The 3-year overall survival (OS) and disease-free survival (DFS) rate of the included patients was 89.1% (95% CI: 85.0%–93.2%) and 82.0% (95% CI: 76.7%–87.3%), respectively.
Patients with HER2-low BC had a significantly longer OS than patients with HER2-zero BC, and the effect of HER2 status was quantified in the univariate and multivariate COX regression analysis (HR = 0.302, 95% CI: 0.144–0.631, p = 0.001, Fig. 4, Table 3). HER2-low patients had a longer DFS than HER2-zero patients but with no statistical difference (HR = 0.573, 95% CI: 0.317–1.038, p = 0.066). Stratified analyses according to ER and AR status is shown in Supplementary Figs. 2–4. The OS advantage of HER2-low over HER2-zero remained significant in ER-negative patients (p = 0.046) and AR-negative patients (p = 0.005). HER2-low status has no significant impact on DFS in patients with different ER and AR status.
Fig. 4.
Kaplan-Meier's survival curve of HER2-low and HER2-zero patients. P values are from the stratified log-rank test and Cox regression.
Table 3.
Univariate and multivariate COX-regression of overall survival (OS), and disease-free survival (DFS) in HER2-low and HER2-zero patients.
| OS |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2-low |
HER2-zero |
Overall |
||||||||||
| univariate analysis |
multivariate analysis |
univariate analysis |
multivariate analysis |
univariate analysis |
multivariate analysis |
|||||||
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
|
HER2 status low vs zero |
– | – | – | – | – | – | – | – | 0.302(0.144–0.631) | 0.001 | 0.428(0.202–0.908) | 0.027 |
|
ER status positive vs negative |
0.220(0.068–0.716) | 0.012 | 0.082(0.013–0.533) | 0.009 | 0.197(0.045–0.866) | 0.032 | 0.329(0.031–3.529) | 0.358 | 0.173(0.07–0.425) | 0.000 | 0.160(0.041–0.628) | 0.009 |
|
PR status positive vs negative |
0.503(0.163–1.548) | 0.231 | 2.576(0.470–14.123) | 0.276 | 0.221(0.050–0.975) | 0.046 | 0.465(0.042–5.111) | 0.531 | 0.301(0.128–0.706) | 0.006 | 1.425(0.394–5.161) | 0.589 |
|
T stage T4 vs T1-3 |
1.741(0.479–6.330) | 0.400 | 3.526(0.842–14.771) | 0.085 | 1.182(0.336–4.153) | 0.794 | 1.108(0.311–3.948) | 0.874 | 1.464(0.596–3.597) | 0.406 | ||
|
LN status N1-3 vs N0 |
0.483(0.131–1.784) | 0.275 | 0.505(0.130–1.960) | 0.323 | 1.005(0.284–3.561) | 0.994 | 0.961(0.256–3.610) | 0.952 | 0.639(0.259–1.576) | 0.331 | ||
|
Ki-67 >20% vs ≤ 20% |
1.493(0.410–5.445) | 0.543 | 1.335(0.302–5.903) | 0.703 | 1.689(0.643–4.435) | 0.287 | ||||||
|
AR status positive vs negative |
0.997(0.183–5.448) | 0.998 | 0 | 0.998 | 0.357(0.080–1.597) | 0.178 | ||||||
| Age | 1.009(0.949–1.073) | 0.781 | 0.999(0.956–1.044) | 0.960 | 1.005(0.967–1.044) | 0.814 | ||||||
| DFS | ||||||||||||
| HER2-low | HER2-zero | Overall | ||||||||||
| univariate analysis | multivariate analysis | univariate analysis | multivariate analysis | univariate analysis | multivariate analysis | |||||||
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
|
HER2 status low vs zero |
– | – | – | – | – | – | – | – | 0.573(0.317–1.038) | 0.066 | 0.683(0.326–1.433) | 0.314 |
|
ER status positive vs negative |
0.432(0.205–0.911) | 0.027 | 0.541(0.094–3.113) | 0.492 | 0.672(0.252–1.797) | 0.429 | 1.068(0.232–4.91) | 0.932 | 0.471(0.261–0.847) | 0.012 | 0.890(0.258–3.071) | 0.854 |
|
PR status positive vs negative |
0.600(0.283–1.273) | 0.183 | 0.960(0.155–5.948) | 0.965 | 0.576(0.205–1.621) | 0.296 | 0.549(0.102–2.941) | 0.483 | 0.558(0.306–1.017) | 0.057 | 0.907(0.258–3.186) | 0.879 |
|
Ki-67 >20% vs ≤ 20% |
2.460(0.853–7.100) | 0.096 | 1.504(0.472–4.791) | 0.490 | 1.620(0.372–7.050) | 0.520 | 0.977(0.188–5.070) | 0.978 | 2.313(0.980–5.459) | 0.056 | 2.081(0.685–6.327) | 0.196 |
|
AR status positive vs negative |
0.224(0.052–0.967) | 0.045 | 0.311(0.069–1.412) | 0.130 | 0.244(0.031–1.899) | 0.178 | 0.226(0.069–0.744) | 0.014 | 0.297(0.085–1.033) | 0.056 | ||
|
T stage T4 vs T1-3 |
2.339(1.030–5.312) | 0.042 | 4.126(1.375–12.378) | 0.011 | 1.250(0.360–4.339) | 0.725 | 1.888(0.959–3.719) | 0.066 | 2.431(1.024–5.772) | 0.044 | ||
|
LN status N1-3 vs N0 |
0.699(0.266–1.840) | 0.468 | 1.753(0.401–7.672) | 0.456 | 0.949(0.424–2.123) | 0.898 | ||||||
| Age | 1.016(0.975–1.058) | 0.458 | 0.958(0.920–0.999) | 0.043 | 0.959(0.920–1.000) | 0.050 | 0.986(0.957–1.016) | 0.361 | ||||
OS: overall survival; DFS: disease-free survival; ER: estrogen receptor; PR: progesterone receptor; AR: androgen receptor.
We further investigated the prognostic factors in HER2-low and HER2-zero patients (Table 3). For OS, ER-positive status was the only factor affecting survival in HER2-low patients (p = 0.012), and the significance remained after adjusting confounding factors (HR = 0.082, 95% CI: 0.013–0.533, p = 0.009). Both ER-positive status (HR = 0.197, 95% CI: 0.045–0.866, p = 0.032) and PR-positive status (HR = 0.221, 95% CI: 0.050–0.975, p = 0.046) associated with better OS in HER2-zero patients, but the significance lost in multivariate analysis. For DFS, ER status (HR = 0.432, 95% CI: 0.205–0.911, p = 0.027), T stage (p = 0.042), and AR status (HR = 0.224, 95% CI: 0.052–0.967, p = 0.045) were prognostic factors in HER2-low patients, and T stage (HR = 4.126, 95% CI: 1.375–12.378, p = 0.011) remained a prognostic factor in multivariate analysis, while no factors were identified associated with DFS in HER2-zero patients.
4. Discussion
In the current clinical practice, HER2-low BC is classified either as HR-positive BC or TNBC, and the presence of low HER2 expression is not considered a factor in treatment decision-making. However, with the emergence of novel anti-HER2 ADC, the treatment paradigm for HER2 negative BC would be shifted. Previous studies have revealed that HER2-low BC could be a new disease entity with distinct clinical and biological characteristics. More data concerning HER2-low BC were warranted to discover its unique biology. In this study, we included a total of 325 patients with stage I-III HER2 negative BC in our center. All the patients were treated with anthracycline- and taxane-based NACT followed by breast surgery. Of them, 234 patients (72%) were HER2-low, 91 patients (28%) were HER2-zero, and the proportion of HER2-low and HER2-zero BC was similar to some of the previous reports [[25], [26], [27]]. The proportion of HER2-low BC was different from other studies [28,29]. We found that HER2-low BC was associated with higher proportion of positive HR status, lower tumor grade, and lower Ki-67 expression levels compared with HER2-zero BC. Though not predictive of pCR rates after NACT, HER2-low status was an independent prognostic factor for OS and associated with better survival in HR-negative patients. Distinct prognostic factors were identified in HER2-low patients. Overall, our study provided new evidence about HER2-low BC, which may be less likely a unique disease entity.
Our data showed that about two-thirds of HER2-low patients were HR-positive, while only 40.7% of the HER2-zero tumors were HR-positive, which was concordant with previous reports [30,31]. Patients with HER2-low BC tend to have lower histological grades and lower Ki-67 expression, according to our analysis. Denkert et al. [21] reported similar characteristics and supplemented that HER2-low BC carried a reduced number of TP53 mutations compared with HER2-zero tumors. More evidence is needed to support HER2-low as a distinct biological entity.
The pCR rate from our center showed no difference between HER2-low and HER2-zero patients, concordant with logistic regression analysis indicating that HER2-low status was not predictive for pCR. Similarly, Domergue et al. [22] and Leite et al. [20] showed that the pCR rate of NACT was not significantly different between HER2-low and HER2-zero groups. However, a pooled analysis of four prospective, neoadjuvant clinical trials (GeparSepto, NCT01583426; GeparOcto, NCT02125344; GeparX, NCT02682693; Gain-2 neoadjuvant, NCT01690702) [21] demonstrated that HER2-low tumors had a significantly lower pCR rate than HER2-zero tumors, pCR rate being 29.2% (321/1098) and 39.0% (473/1212), respectively. The discrepancy in pCR rate may be partly due to the different regimens used in those studies. Whether novel anti-HER2 ADC could be used in HER2-low tumors in the context of NACT to improve pCR rate is worth further research.
According to our data, HER2-low tumors had a significantly longer OS than HER2-zero tumors. Survival differences between HER2-low and HER2-zero BC varied across studies. Single-center studies such as Xu HC et al. [32] and Zhang GC et al. [30] detected no significant difference in DFS and OS between the two groups, and Rosso et al. [33] reported longer OS in HER2-low tumors compared with HER2-zero. Multicenter studies [28,29] about early HER2-negative breast cancer from Asia demonstrated better outcomes in HER2-low tumors than HER2-zero tumors. Patients with HER2-low tumors were associated with a higher frequency of luminal disease, more grade I/II tumors, and a lower expression of Ki-67, which suggested a less aggressive characteristic than HER2-zero tumors that may explain the survival benefit in HER2-low patients. Further basic research was warranted to investigate the biology of HER2-low BC.
The proportion of HER-low BC increased as the ER or AR expression got higher in our research, suggesting that HER2-low BC may have interaction with the signal pathway of ER and AR. Expression of HER2 and its downstream pathway such as MAPK may have crosstalk with ER-mediated tumor cell regulation [34]. Furthermore, the androgen receptor pathway may be a potential target in triple-negative breast cancer, and there are literatures providing biological insight into the luminal androgen receptor (LAR) subtype of TNBC. Jiang YZ et al. [35] represented 4 transcriptome-based subtypes in TNBC and pointed out that the LAR subtype was highly prevalent in Asian women and was enriched with ERBB2 mutation. We found that pCR rate of AR negative tumor (22.6%) was significantly higher than AR positive tumor (4.0%) (p < 0.001), regardless of HER2 status or ER status, and AR status was found to be associated with pCR and DFS in the entire cohort and HER2-low tumors, but not in HER2-zero tumors (Table 2, Table 3). When classifying our patients based on ER status and AR status, patients with ER-/AR + tumors had longer DFS than other groups (Supplementary Fig. 4), which is concordant with previous report [23]. The clinical role of AR in the HER2-low BC needs further study.
5. Conclusion
In conclusion, our study suggests that there was no significant difference in the pCR rate between HER2-low group and HER2-zero group treated with anthracycline- and taxane-based NACT. Patients with HER2-low tumors had significantly longer OS than patients with HER2-zero tumors. ER status was the affecting factor of OS in HER2-low patients in both univariate and multivariate analysis. Although HER2-low BC could benefit from anti-HER2 ADC, evidence for HER2-low BC as a distinct entity is insufficient and more efforts are needed to standardize the scoring of HER2-low BC.
Data availability
This study involved data from the internal studies of the First Affiliated Hospital of Sun Yat-sen University, which are not publicly available to protect patient privacy. Further inquiries can be directed to the corresponding author.
Declaration of competing interest
There is no conflict of interest.
Acknowledgements
The work was funded by The Science and Technology Development Fund, Macau SAR (FDCT-0011/2019/AKP, and 0034/2019/AGJ). We would like to thank all patients, clinicians and pathologists participating in the clinical studies and the biomaterial collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.12.006.
Contributor Information
Shuling Zhou, Email: zhoushling@mail2.sysu.edu.cn.
Ting Liu, Email: liut65@mail2.sysu.edu.cn.
Xiaying Kuang, Email: kuangxy5@mail.sysu.edu.cn.
Tiantian Zhen, Email: zhentt3@mail.sysu.edu.cn.
Huijuan Shi, Email: shihj@mail.sysu.edu.cn.
Ying Lin, Email: linying3@mail.sysu.edu.cn.
Nan Shao, Email: shaon@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Slamon D.J., et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Perez E.A., et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianni L., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 4.von Minckwitz G., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain S.M., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma S., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehrenbacher L., et al. NSABP B-47/NRG Oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020;38:444–453. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modi S., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff A.C., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 10.van der Lee M.M., et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol Cancer Therapeut. 2015;14:692–703. doi: 10.1158/1535-7163.MCT-14-0881-T. [DOI] [PubMed] [Google Scholar]
- 11.Schneeweiss A., et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest N Drugs. 2018;36:848–859. doi: 10.1007/s10637-018-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerji U., et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–1135. doi: 10.1016/S1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 13.Clifton G.T., et al. Initial safety analysis of a randomized phase II trial of nelipepimut-S+GM-CSF and trastuzumab compared to trastuzumab alone to prevent recurrence in breast cancer patients with HER2 low-expressing tumors. Clin Immunol. 2019;201:48–54. doi: 10.1016/j.clim.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Mittendorf E.A., et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019;25:4248–4254. doi: 10.1158/1078-0432.CCR-18-2867. [DOI] [PubMed] [Google Scholar]
- 15.Ponde N., Aftimos P., Piccart M. Antibody-drug conjugates in breast cancer: a comprehensive review. Curr Treat Options Oncol. 2019;20:37. doi: 10.1007/s11864-019-0633-6. [DOI] [PubMed] [Google Scholar]
- 16.Rinnerthaler G., Gampenrieder S.P., Greil R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarantino P., et al. HER2-Low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38:1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 18.Gianni L., et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131–1137. doi: 10.1200/JCO.2009.24.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mass R.D., et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 20.de Moura Leite, L. et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat 190, 155-163, doi:10.1007/s10549-021-06365-7(2021). [DOI] [PubMed]
- 21.Denkert C., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22:1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 22.Domergue C., et al. Impact of HER2 status on pathological response after neoadjuvant chemotherapy in early triple-negative breast cancer. Cancers. 2022;14 doi: 10.3390/cancers14102509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loibl S., et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;130:477–487. doi: 10.1007/s10549-011-1715-8. [DOI] [PubMed] [Google Scholar]
- 24.Korde L.A., et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39:1485–1505. doi: 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schalper K.A., Kumar S., Hui P., Rimm D.L., Gershkovich P. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria. Arch Pathol Lab Med. 2014;138:213–219. doi: 10.5858/arpa.2012-0617-OA. [DOI] [PubMed] [Google Scholar]
- 26.Rossi V., et al. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncol. 2012;17:1418–1425. doi: 10.1634/theoncologist.2012-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schettini, F. et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7, 1, doi:10.1038/s41523-020-00208-2(2021). [DOI] [PMC free article] [PubMed]
- 28.Won H.S., et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022;24:22. doi: 10.1186/s13058-022-01519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan R., et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022;20:105. doi: 10.1186/s12916-022-02284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, G. et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med 20, 142, doi:10.1186/s12916-022-02346-9(2022). [DOI] [PMC free article] [PubMed]
- 31.Li Y., et al. In real life, low-level HER2 expression may Be associated with better outcome in HER2-negative breast cancer: a study of the national cancer center, China. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.774577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H., et al. Clinicopathological characteristics and prognosis of HER2-low early-stage breast cancer: a single-institution experience. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.906011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosso C., Voutsadakis I.A. Characteristics, clinical differences and outcomes of breast cancer patients with negative or low HER2 expression. Clin Breast Cancer. 2022;22:391–397. doi: 10.1016/j.clbc.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Rasha F., Sharma M., Pruitt K. Mechanisms of endocrine therapy resistance in breast cancer. Mol Cell Endocrinol. 2021;532 doi: 10.1016/j.mce.2021.111322. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y.Z., et al. Genomic and transcriptomic landscape of triple-negative breast cancers: subtypes and treatment strategies. Cancer Cell. 2019;35:428–440 e425. doi: 10.1016/j.ccell.2019.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study involved data from the internal studies of the First Affiliated Hospital of Sun Yat-sen University, which are not publicly available to protect patient privacy. Further inquiries can be directed to the corresponding author.