Highlights
-
•
We proposed an automated tractography approach for individualized TAI detection.
-
•
We tested the feasibility of automated tractography in patients with acute severe TBI.
-
•
We investigated associations with behavioral measures of consciousness.
-
•
The TRACULA pipeline successfully reconstructed 93% of the tracts across patients.
-
•
The pipeline accurately discriminated between patients and controls (AUC: 0.91)
Keywords: TBI, Tractography, Traumatic Axonal Injury, Diffusion MRI, White Matter
Abstract
New techniques for individualized assessment of white matter integrity are needed to detect traumatic axonal injury (TAI) and predict outcomes in critically ill patients with acute severe traumatic brain injury (TBI). Diffusion MRI tractography has the potential to quantify white matter microstructure in vivo and has been used to characterize tract-specific changes following TBI. However, tractography is not routinely used in the clinical setting to assess the extent of TAI, in part because focal lesions reduce the robustness of automated methods. Here, we propose a pipeline that combines automated tractography reconstructions of 40 white matter tracts with multivariate analysis of along-tract diffusion metrics to assess the presence of TAI in individual patients with acute severe TBI. We used the Mahalanobis distance to identify abnormal white matter tracts in each of 18 patients with acute severe TBI as compared to 33 healthy subjects. In all patients for which a FreeSurfer anatomical segmentation could be obtained (17 of 18 patients), including 13 with focal lesions, the automated pipeline successfully reconstructed a mean of 37.5 ± 2.1 white matter tracts without the need for manual intervention. A mean of 2.5 ± 2.1 tracts resulted in partial or failed reconstructions and needed to be reinitialized upon visual inspection. The pipeline detected at least one abnormal tract in all patients (mean: 9.1 ± 7.9) and accurately discriminated between patients and controls (AUC: 0.91). The number and neuroanatomic location of abnormal tracts varied across patients and levels of consciousness. The premotor, temporal, and parietal sections of the corpus callosum were the most commonly damaged tracts (in 10, 9, and 8 patients, respectively), consistent with prior histopathological studies of TAI. TAI measures were not associated with concurrent behavioral measures of consciousness. In summary, we provide proof-of-principle evidence that an automated tractography pipeline has translational potential to detect and quantify TAI in individual patients with acute severe TBI.
1. Introduction
Traumatic axonal injury (TAI) is the most common pathologic substrate of head trauma, as assessed by post-mortem examination (Brody et al., 2015, McGinn and Povlishock, 2016). TAI is caused by high-velocity translational and rotational forces that stretch and shear axons, leading to either primary axotomy or a secondary cascade that can result in further axonal injury (Hill et al., 2016). The overall extent and location of TAI have been shown to correlate with level of consciousness, coma duration, and with short and long-term cognitive dysfunction (Benson et al., 2007, Gennarelli et al., 1982, Jolly et al., 2021, Moe et al., 2020, Newcombe et al., 2010; Smith et al., 2000). Therefore, the diagnosis of TAI currently relies upon neurological examination of level of consciousness and the presence of visible white matter (WM) damage using routine neuroimaging techniques. However, due to its diffuse and microscopic nature (Adams et al., 1989, Blumbergs et al., 1995, Johnson et al., 2013), TAI is challenging to quantify using CT and conventional MRI (Betz et al., 2012), and its extent is likely underestimated in clinical practice. Furthermore, individual scores obtained from the neurological examination (e.g., the Glasgow Coma Scale) have limited diagnostic utility, given that neurologic deficits may reflect a broad spectrum of pathophysiologic processes.
For clinicians and the families of patients with acute severe traumatic brain injury (TBI), the lack of reliable diagnostic tools that measure the burden of TAI creates prognostic uncertainty and complicates discussions about continuation of life-sustaining therapy in the intensive care unit (ICU) (Izzy et al., 2013, Turgeon et al., 2011). Given the multifocal and heterogenous nature of TAI, neuroimaging studies that compare TBI patients at the group level are insensitive to TAI variability and therefore are suboptimal for gaining insight into TAI mechanisms in individual patients (Betz et al., 2012). New techniques for the individualized assessment of WM injury (Jolly et al., 2021, Kim et al., 2013, Yuh et al., 2014) are therefore needed to enhance detection of TAI and improve the accuracy of outcome prediction in critically ill patients with acute severe TBI.
Diffusion MRI (dMRI) probes WM tissue microstructure non-invasively (Basser et al., 1994), making it possible to identify TAI in vivo (Hashim et al., 2017, Li et al., 2011, Xu et al., 2007). Numerous studies have used dMRI to assess WM integrity in patients with TBI (Arfanakis et al., 2002, Asken et al., 2018, Galanaud et al., 2012, Hulkower et al., 2013, Inglese et al., 2005, Newcombe et al., 2007, Zhang et al., 2017) and have shown its sensitivity to detect WM abnormalities across TBI severities (i.e., mild, moderate, severe) and recovery phases (i.e., acute, subacute, chronic). Typically, summary metrics such as fractional anisotropy (FA) and mean diffusivity (MD) are extracted from WM regions of interest (ROIs) that are manually drawn in subject’s space (Inglese et al., 2005, Donald et al., 2011) or segmented from an atlas (Galanaud et al., 2012, O’Phelan et al., 2018). Whole-brain voxel-based analysis (Van Hecke et al., 2016) and tract-based spatial statistics ( Smith et al., 2006) approaches are also used to compare normalized scalar maps between patient groups (Kinnunen et al., 2011), or between a control group and a patient (Jolly et al., 2021). While these approaches have demonstrated sensitivity to TAI (Jolly et al., 2021, Perlbarg et al., 2009), they do not assess injury of specific WM tracts at the individual level.
Diffusion-based tractography allows for the delineation and quantification of specific WM pathways at the individual level. WM tracts of the human brain subserve specific cognitive domains (Catani and Mesulam, 2008, Mesulam, 1990), and diffusion abnormalities within these tracts correlate with domain-specific cognitive deficits after TBI (Sharp et al., 2014). By providing tract-specific evaluation of WM damage, tractography methods thus have the potential to elucidate the complex relationship between WM damage and cognitive deficits, while also informing prognostication and early decisions about continuation of life-sustaining therapy. However, few studies have utilized tractography in patients with acute TBI (D’Souza et al., 2015, Ordóñez-Rubiano et al., 2017, Snider et al., 2019, Wang et al., 2008, Warner et al., 2010) or acute severe TBI (Ordóñez-Rubiano et al., 2017, Snider et al., 2019, Wang et al., 2008). Furthermore, dMRI tractography techniques are not routinely used in the clinical setting to assess the extent of TAI (Schweitzer et al., 2019) because the presence of focal lesions, the lower quality of the dMRI data compared to research settings, and the time feasibility constraints all limit the application of available tractography pipelines.
To address these barriers to clinical translation, we tested a pipeline for individualized TAI assessment in patients with acute severe TBI that combines automated tractography with a multivariate analysis of along-tract diffusion metrics. We used TRACULA (TRActs Constrained by UnderLying Anatomy), a method for global probabilistic tractography with anatomical neighborhood priors (Yendiki et al., 2011), to automatically reconstruct 40 WM tracts at the individual level (Maffei et al., 2021). TRACULA has been successfully applied to clinical populations before (Kreilkamp et al., 2017, Sarica et al., 2014) and also in patients with mild TBI (Goodrich-Hunsaker et al., 2018). The anatomical neighborhood priors in TRACULA encode information about the relative position of the tracts with respect to their surrounding anatomical structures, rather than their absolute coordinates in an atlas space. Therefore, TRACULA does not necessitate accurate registration to an atlas (Zöllei et al., 2019, Maffei et al., 2021), which can be challenging in the presence of lesions and may not reflect interindividual anatomical differences. Furthermore, in contrast to local tractography methods that consider the local diffusion orientation at each step, TRACULA uses a global approach and would thus not “stop” in the presence of a lesion. We previously showed that TRACULA, when trained on high-quality, high angular resolution, multi-shell data from the Human Connectome Project (Fan et al., 2016), improves the accuracy of tractography reconstructions in routine-quality single-shell data (Maffei et al., 2021) when compared to a commonly used multi-ROI automated local tractography approach (Behrens et al., 2007). These methodologic advantages of TRACULA suggest potential for translation of this technique to critically ill patients with acute severe TBI, who are not medically stable enough to be scanned with high angular and spatial resolution multi-shell dMRI sequences that may require long scan times.
We applied TRACULA to a dMRI dataset prospectively acquired in a cohort of 18 critically ill patients with acute severe TBI who were imaged on a clinical 3 Tesla MRI scanner. We used a multivariate approach based on the Mahalanobis distance to capture the distinct ways in which WM tracts may differ in individual patients compared to 33 healthy subjects. The Mahalanobis distance allows us to integrate multiple along-tract diffusion metrics (e.g., FA, MD) into a single measure, and it has been shown to provide more robust results than traditional univariate analyses (Dean et al., 2017, Owen et al., 2021). The goals of this study were: i) to test the feasibility of obtaining correct automated TRACULA reconstructions of WM tracts in patients with acute severe TBI, including those with focal lesions; ii) to implement an individualized multivariate approach to measure patient-specific TAI severity, using tract reconstructions and along-tract dMRI metrics derived from the automated TRACULA tractography pipeline; and iii) to test for associations between the degree of TAI, as measured by tract-specific multivariate measures, and behavioral measures of consciousness.
2. Methods
2.1. Participants
We prospectively enrolled 18 patients (mean ± standard deviation age: 28.6 ± 8.7 years, age range: 18–51; 13 male) with acute severe TBI, sixteen of whom were enrolled as part of a previously described pilot study (Edlow et al., 2017), and two of whom were subsequently enrolled as pilot cases for an ongoing observational study (ClinicalTrials.gov NCT03504709; see Table 1 for additional information). Inclusion criteria were: 1) age 18 to 65 years; and 2) traumatic coma, defined by Glasgow Coma Scale (GCS) (Teasdale & Jennett, 1974) total score ≤ 6 without eye opening on at least one neurologic examination before ICU admission; and 3) no eye opening for at least 24 h after injury. Exclusion criteria were: 1) prior history of severe brain injury or neurodegenerative disease; 2) life expectancy less than six months per physician judgment; 3) presence of metal contraindicating MRI; and/or 4) no pre-injury English fluency.
Table 1.
Demographics and Clinical Characteristics of the TBI patients. CRS-R: Coma Recovery Scale-Revised; CRS-R-T: Total score of the CRS-R; CRS-R-M: Motor CRS-R sub-scale; CRS-R-A: Auditory CRS-R sub-scale; CRS-R-O/V: Oromotor-verbal CRS-R sub-scale; DRS: Disability Rating Scale; GCS: Glasgow Coma Scale; GCS-T: Total score of the GSC; GOS-E: Glasgow Outcome Scale-Extended; LOC: level of consciousness; MCS+ = minimally conscious state plus, evidence of language function (e.g., reproducible movement to command, object recognition, intelligible verbalization); MCS- = minimally conscious state minus, no evidence of language function, but shows at least one behavior consistent with a minimally conscious state (e.g., automatic motor response); MVA: motor vehicle accident; Ped vs car: pedestrian versus car; PTCS: post-traumatic confusional state; SD: standard deviation; VS: vegetative state. *Unable to determine exact date based on intensive care unit clinician assessments. We used 1 day for further analysis. **Patients who died in the ICU after withdrawal of life sustaining therapy and before command-following was observed.
| ID | Age | Sex | Mechanism of injury | Days in Coma | Days Until Command-Following | Day of MRI | LOC at MRI | GCS-T at MRI | CRS-R-T at MRI | CRS-R-M at MRI | CRS-R-A at MRI | CRS-R-O/V at MRI | DRS 6 month |
GOS-E 6 month |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 27 | M | MVA | 1 | 1 | 16 | PTCS | 15 | 23 | 6 | 4 | 3 | 7 | 3 |
| P2 | 21 | M | Ped vs car | 1 | 6 | 1 | MCS- | 7 | 4 | 3 | 0 | 1 | 0 | 7 |
| P3 | 19 | F | MVA | 5 | 10 | 3 | Coma | 5 | 1 | 1 | 0 | 0 | 0 | 7 |
| P4 | 19 | M | Fall | 1 | 2 | 17 | PTCS | 14 | 23 | 6 | 4 | 3 | 0 | 7 |
| P5 | 34 | M | Fall | 6 | 26 | 15 | VS | 7 | 3 | 0 | 0 | 2 | 8 | 3 |
| P6 | 28 | F | MVA | 2 | 9 | 7 | VS | 9 | 6 | 2 | 0 | 1 | 5 | 3 |
| P7 | 45 | M | MVA | 1 | 6 | 13 | MCS+ | 13 | 18 | 5 | 3 | 3 | 4 | 5 |
| P8 | 33 | M | Fall | 1 | 7 | 8 | PTCS | 11 | 20 | 5 | 4 | 2 | 5 | 3 |
| P9 | 32 | M | Ped vs car | 1 | 8 | 11 | MCS+ | 10 | 9 | 2 | 3 | 1 | 5 | 4 |
| P10 | 24 | M | Assault | 1 | 7 | 12 | MCS- | 10 | 10 | 5 | 1 | 1 | 3 | 5 |
| P11 | 22 | F | Ped vs car | 1 | 9 | 14 | PTCS | 14 | 22 | 6 | 4 | 3 | 0 | 7 |
| P12 | 27 | F | Fall | 13 | ** | 8 | Coma | 5 | 1 | 1 | 0 | 0 | 30 | 1 |
| P13 | 18 | M | Fall | 1 | 2 | 4 | MCS+ | 10 | 12 | 5 | 3 | 1 | 0 | 7 |
| P14 | 51 | M | Ped vs car | 8 | ** | 8 | VS | 6 | 3 | 1 | 0 | 0 | 30 | 1 |
| P15 | 29 | M | Ped vs car | 3 | 8 | 7 | MCS- | 7 | 3 | 3 | 0 | 0 | 3 | 5 |
| P16 | 33 | M | Fall | 3 | 3 | 3 | MCS+ | 10 | 12 | 5 | 4 | 0 | 2 | 5 |
| P17 | 26 | F | Ped vs car | 4 | ** | 12 | VS | 8 | 3 | 1 | 0 | 0 | 30 | 1 |
| P18 | 26 | M | Fall | 1 | 0–2* | 28 | PTCS | 11 | 18 | 6 | 3 | 2 | 18 | 3 |
| Mean (SD) | 28.6 (8.6) | 3.0 (3.3) | 7.0 (6.1) | 10.4 (6.5) | 9.6 (3.1) | 10.6 (8.1) | 3.5 (2.2) | 1.8 (1.8) | 1.3 (1.2) | |||||
| Range | 18–51 | 1–13 | 1–26 | 1–28 | 5–15 | 1–23 | 0–6 | 0–4 | 0–3 | 0–30 | 1–7 |
We also enrolled 33 healthy control subjects (mean ± standard deviation age 30.9 ± 8.8 years, age range: 21–59; 21 male) with no history of neurological (including concussions), psychiatric, cardiovascular, pulmonary, renal or endocrinological disease (Edlow et al., 2017). We tested for significant differences in age and sex between the two groups using respectively a nonparametric Wilcoxon sum rank test and a chi-square test in Python 3 (SciPy Stats 1.3.1) (Jones et al., 2001). Written informed consent was obtained from healthy subjects and from patients’ surrogate decision-makers in accordance with a study protocol approved by the Mass General Brigham Institutional Review Board.
2.2. Behavioral assessment of consciousness
The level of consciousness (LOC) was prospectively assessed by a neurologist on the investigator team (B.L.E.) immediately prior to the MRI. For each patient, the LOC was assessed via behavioral evaluation with the Coma Recovery Scale-Revised (CRS-R) (Giacino et al., 2004), and the Confusion Assessment Protocol (CAP) (Sherer et al., 2005). Per routine ICU care at our institution, neurological examinations and GCS assessments were performed off of sedation every-two-to-four hours (depending on clinical stability and safety considerations) by treating clinicians. ICU clinician GCS assessments were used to determine time from injury to coma emergence (i.e., eye opening or localization to noxious stimulation) and time to command-following. If a patient’s neurological examination fluctuated, the first behavioral evidence of coma emergence and command-following were reported. The GCS Total was included as a primary dependent variable in this study given its widespread use clinically; however, its limitations (i.e., only moderate psychometric properties, administration process is not standardized) must be acknowledged (Bodien et al., 2021). The CRS-R, which has strong psychometric properties and a standardized administration process (Giacino et al., 2004, Teasdale et al., 2014), along with the CAP, were used in addition to the GCS to more precisely classify each patient’s LOC into the following categories: coma, vegetative state, minimally conscious state minus (MCS-; MCS without language function), MCS plus (MCS+; MCS with language function), and post-traumatic confusional state (PTCS). Functional outcome at 6 months was measured with the Glasgow Coma Scale-Extended (GOS-E) (Wilson et al., 1998) and with the Disability Rating Scale (DRS) (Rappaport et al., 1982). Patients and their surrogates were assessed either in-person at the study site or, if this was not feasible, through validated phone questionnaires. All outcome assessments were conducted by a single investigator (B.L.E).
2.3. MRI data acquisition
MRI data were acquired as soon as the treating clinicians deemed patients stable for transport. Diffusion weighted imaging (DWI) data were acquired in the ICU on a 3 T Skyra scanner (Siemens Medical Solutions, Malvern, PA) with a 32-channel head coil using an echo-planar imaging (EPI) sequence with the following parameters: , and volumes, ms, and . Out of 33 healthy controls and 18 patients, 4 healthy controls and 9 patients were scanned using simultaneous multi-slice (SMS) acceleration (acceleration factor = 2) (Setsompop et al., 2012) which resulted in slightly different and (, ). All other parameters remained the same. A previous publication from our group that included data from the same patients showed that diffusion-derived connectivity metrics did not differ significantly between these two acquisitions (Snider et al., 2019). In addition, we conducted a linear mixed effects model with a random intercept for subject to test whether our dependent variables (i.e., average of affected tracts and number of affected tracts) differed for patients with SMS () and without SMS (). The length of the diffusion sequence was 8 min and 46 s. Multi-echo MPRAGE structural images (van der Kouwe et al., 2008) were also acquired with the following parameters: , acquisition matrix = , , , °. All patients and healthy subjects were imaged using the same scanner and head coil.
2.4. Diffusion MRI data processing
Diffusion weighted images were skull stripped and corrected for eddy-current distortions and movement in FSL 6.0.1 (Andersson and Sotiropoulos, 2016). The tensor and the ball-and-stick model were fit to the data using DTIFIT and BEDPOSTX (Behrens et al., 2003) in FSL 6.0.1, respectively. Automated reconstruction of 40 major WM pathways was performed using the global probabilistic tractography algorithm TRACULA in FreeSurfer 7.2.0 (Maffei et al., 2021, Yendiki et al., 2011) using default parameters (200 initial iterations [nburnin]; 7500 sample paths [nsample]). Although TRACULA can reconstruct a total of 42 WM tracts, we decided to exclude the right and left fornix, because optimal identification of these WM bundles requires segmentation of thalamic subnuclei that are not provided by the standard “recon-all” FreeSurfer processing pipeline. The mathematical formulation of TRACULA has been described elsewhere (Yendiki et al., 2011). Briefly, the algorithm models a pathway as a cubic spline, which is initialized with the median streamline of the training set. A random sampling algorithm is used to draw samples from the posterior probability distribution of the pathway, which is decomposed into the likelihood term and the prior term. The likelihood term fits the shape of the spline to the diffusion orientations obtained from the ball-and-stick model in the voxels that it goes through. The prior term fits the shape of the spline to its anatomical neighborhood, given the manually labeled examples of this pathway from the training set and the anatomical segmentation volumes of both test and training subjects. The number of control points of the cubic spline were chosen as previously described (Maffei et al., 2021).
Along-tract tensor-derived metrics obtained from DTIFIT were extracted for each of the reconstructed tracts to perform a pointwise assessment of streamline tractography attributes (Jones et al., 2005). For each of the 40 tracts, 1D along-tract profiles of FA and MD were generated by projecting the value of each measure from every point on every automatically reconstructed streamline to its nearest point on a reference streamline, as previously described (Maffei et al., 2021). The reference streamline is the mean of the training streamlines for each tract, ensuring that all subject data were sampled at the same number of cross-sections along a given bundle. Before extracting the diffusion measures, the posterior probability distribution estimated by TRACULA was thresholded by masking out all values below 20 % of the maximum, which is the default threshold in TRACULA (Maffei et al., 2021, Yendiki et al., 2011, Zöllei et al., 2019). For each subject, the quality of the DWI data was assessed by extracting volume-by-volume translation and rotation estimates obtained from the results of the eddy-current distortion correction step, as well as slice-by-slice signal drop-out measures specific to DW-MRI (Benner et al., 2011, Yendiki et al., 2014). The quality of the WM tract reconstruction was visually assessed as described in Section 2.6. All the pre-processing steps detailed above are performed automatically by the TRACULA pipeline.
2.5. Structural MRI data processing
The anatomical segmentation that was needed to compute the anatomical neighborhood priors in TRACULA was obtained by analyzing the structural T1-weighted image in FreeSurfer 7.1.1 (Dale et al., 1999, Fischl et al., 1999, Fischl et al., 2004). The automated “recon-all” pipeline was run with default parameters, except for the “bigventricle” option, which was added to optimize segmentation in patients that may have enlarged ventricles post-injury. The obtained segmentation volumes included a combination of the Desikan-Killiany cortical parcellation labels (Desikan et al., 2006) and the standard FreeSurfer subcortical segmentation (Fischl et al., 2002). We did not visually inspect the accuracy of the obtained segmentations and did not manually edit the recon-all outputs, as we aimed at assessing the feasibility of obtaining automated TRACULA reconstructions independent of the accuracy of the segmentation volumes. To compute the prior probabilities on the anatomical neighbors of the tracts using TRACULA, the anatomical segmentations were transformed to the subject’s individual dMRI space. This within-subject, dMRI-to-T1 alignment was performed using a boundary-based, affine registration method (Greve & Fischl, 2009). To ensure that the relative position of the anatomical structures was the same for all subjects, and to map the median streamline from the training data to the subject during initialization, all subjects’ images were mapped onto a template brain. We used the non-linear symmetric normalization (SyN) in ANTs (Avants et al., 2008) to map each subject’s images onto an FA template constructed from the training dataset (Maffei et al., 2021). It is important to highlight that this step is only used to initialize the reconstruction of the tract, which is then refined by fitting it to the anatomy of the individual subject. For each patient, the results of the within-subject and subject-to-template registration were visually checked to ensure no major mis-alignment errors were present due to lesions.
2.6. Quality assessment of tract reconstructions
To assess the feasibility of implementing TRACULA in a clinical setting, we performed a qualitative assessment to evaluate the accuracy of the tract reconstructions in patients with acute severe TBI. We considered the application of TRACULA in this population to be feasible if more than 50 % of the automated reconstructions were successful. For this qualitative assessment, a study investigator (C.M.) visually inspected all 40 tracts for all patients. Reconstructions were considered successful if the tracts traversed the WM regions and reached the cortical areas used to define these tracts in the manual labeling protocols. These protocols were carefully defined by the known anatomy of each tract and are detailed in (Maffei et al., 2021).
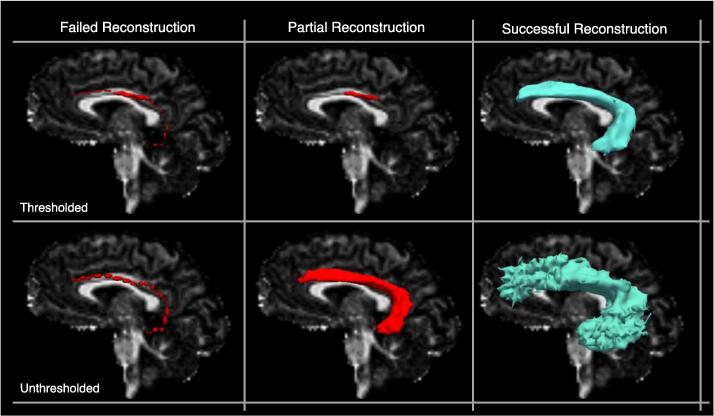
When a focal lesion was present, the reconstruction was considered successful if the tract reached the cortical termination regions delineated in (Maffei et al., 2021), even if the tract’s course was altered by the presence of the lesion. As TRACULA uses a global probabilistic approach to estimate the tracts, post-processing steps to remove false positive reconstructions were not necessary. However, when a suitable solution for the initial reconstruction of the pathway is not found, the tract appears as a single curve. This can be due to errors in the subject-to-template registration step, or to a misplacement of the FreeSurfer segmentation labels in the presence of lesions. We refer to these reconstructions as “failed reconstructions” (See Fig. 1 for a simulated example). Moreover, as a single threshold is used across all tracts and groups to threshold the posterior probability of the tract (i.e., 20 % of the maximum), some reconstructions can result in incomplete tracts or in unusually small tracts for which many voxels did not survive the threshold. We refer to these reconstructions as “partial reconstructions” (See Fig. 1 for an example).
Fig. 1.
Simulated examples of failed, partial, and successful TRACULA reconstructions for a representative tract (the left arcuate fasciculus) in a control subject. Reconstructions are shown thresholded (20% of the maximum posterior probability) and unthresholded. Reconstructions are shown on sagittal fractional anisotropy scalar maps.
We reran TRACULA on the tracts that resulted in either failed or partial reconstructions reinitializing the control points of the initial spline by setting the reinit parameter to 1 (Yendiki et al., 2011). This would rerun the estimation of the specified tract using a different set of initial control points, thereby changing the initial guess of the tract. We retained the re-initialized reconstructions if they resulted in correct tracts as defined above. The same criteria were applied to patients and healthy subjects. This was the only manual intervention performed in the TRACULA pipeline. Tracts that had partial or failed reconstructions after reinitialization were excluded from subsequent analyses.
2.7. Multivariate analysis
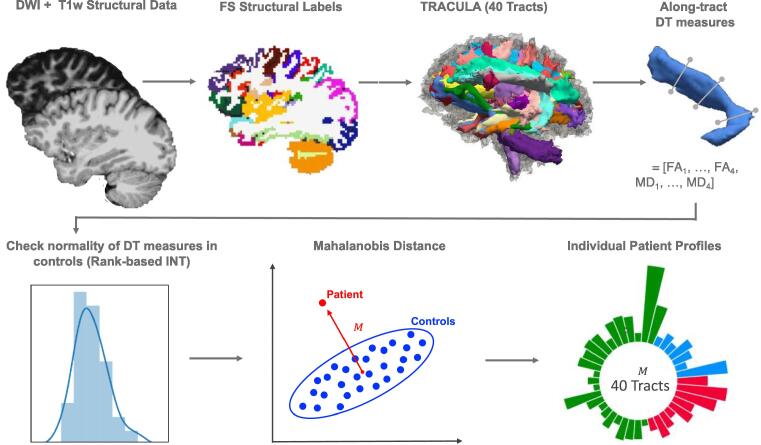
Fig. 2 shows the steps of the multivariate analysis. To measure the extent of TAI in each patient, we employed a multivariate analysis that included along-tract measures of both FA and MD. We focused on along-tract measures of FA and MD, as these microstructural measures are the most commonly studied in the field of TBI (Hulkower et al., 2013, Mac Donald et al., 2007). For this analysis we used both the tracts that had been successfully reconstructed without need of reinitialization and the tracts that needed to be reinitialized (Section 2.6). For each subject, each tract was divided uniformly in segments and the along-tract measures of FA and MD, extracted at each point along the tract in TRACULA (Section 2.4), were averaged within each segment to obtain a total of eight features for each tract (, , , , ). We collapsed the along-tract measurements in four equidistant segments to ensure that, for each tract, the number of features (i.e., the measures for each tract; = 8) was smaller than the number of observations (i.e., the number of healthy control subjects; = 33). The number of segments was kept constant across all 40 tracts to allow inter-tract comparison of multivariate measures. To meet normality assumptions, we conducted Shapiro-Wilk tests in Python 3 using SciPy Stats 1.3.1 (Jones et al., 2001) and tested for the normality of and for each of the 40 tracts in the control group. For the tracts for which the distribution of or departed significantly from normality () we applied a rank-based inverse normal transformation to the data for both healthy controls and patients in Python 3 (Blom constant = , (Blom, 1958)), as in (Jolly et al., 2021).
Fig. 2.
Schematic of the automated individualized pipeline. After pre-processing of diffusion weighted imaging (DWI) and structural T1-weighted imaging data, which included extraction of the FreeSurfer (FS) anatomical labels, the 40 white matter tracts were reconstructed in TRACULA. Along-tract measures derived from the diffusion tensor (DT) were then extracted for four equidistant segments for each of the 40 tracts. We checked the normality of the distribution for these measures in the controls and applied a Rank-based inverse normal transformation (INT) to the tracts for which assumptions of normality were not met (). We then computed the Mahalanobis distance ( for each tract using along-tract fractional anisotropy (FA) (, …, ) and mean diffusivity (MD) (, …, ) as features () (here shown as a simplified 2D example). Finally, we built individual patient profiles based on the distance of each patient from the controls for each of the 40 white matter tracts.
For each of the 40 WM tracts, we compared the 8-D feature vector of dMRI measures ( and ) between each patient and the healthy population based on the Mahalanobis distance , which is defined as (Mahalanobis, 1936):
where is the feature vector of a specific WM tract in a patient, is the mean feature vector of the same tract over controls, and is the covariance matrix of the feature vector in controls. The values of reflect how far a patient, represented as a point in the 8-D feature space, is from the distribution estimated from the healthy controls. The Mahalanobis distance has been used in neuroimaging studies, e.g., to classify between patients with neurological diseases and controls (Lindemer et al., 2015) and to detect individual neurodevelopmental differences (Dean et al., 2017). We computed the between each individual patient and the healthy controls for each of the 40 tracts to build individual profiles and identify the location and severity of WM abnormalities in patients. For multivariate Gaussian data, the distribution of values is known to be Chi-squared with degrees of freedom equal to the number of features (Gnanadesikan & Kettenring, 1972). We computed the p-values corresponding to the Chi-square statistic in Python 3 using SciPy Stats 1.3.1 (Jones et al., 2001) and considered a WM tract to be abnormal if its was (, Bonferroni adjusted for multiple comparisons across 40 tracts). We extracted the total number of abnormal tracts for each patient.
To measure the accuracy of the pipeline in the task of detecting TAI, we computed its performance in discriminating between patients and controls. First, we computed the between each control and the remaining control population in a leave-one-out fashion. For each of the 33 health controls, we used the multivariate distribution of along-tract dMRI measures ( and ) from the remaining 32 heathy controls to compute the of each WM tract and extract the total number of abnormal tracts at . We used a Wilcoxon rank sum test in Python 3 (SciPy Stats 1.3.1) to assess differences in the number of abnormal tracts between patients and controls. We then performed a receiver operating characteristic (ROC) analysis by varying simultaneously the alpha threshold used to obtain the number of abnormal tracts () for each subject, and the number of abnormal tracts used to label a subject as control or patient (). For each combination of alpha and number of abnormal tracts, we computed the false positives (FP; number of controls classified as patients), the false negative (FN; number of patients classified as controls), the true positives (TP; number of patients classified as patients), and the true negatives (TN; number of controls classified as controls) to compute the true-positive rate () and false-positive rate (). We obtained the ROC curve by plotting the TPR as a function of FPR and computed the area under the ROC curve (AUC) to quantify accuracy. To visualize the difference in distribution of values between the two populations we plotted the probability density functions of values for patients and controls for each of the 40 tracts.
Finally, we compared the results obtained using all tracts – those that were successfully reconstructed and those that were reinitialized – to the results obtained using only the tracts that were successfully reconstructed without reinitialization (Section 2.6). We used a Wilcoxon signed-rank test in Python 3 (SciPy Stats 1.3.1) to assess differences in the number of abnormal tracts and their mean per patient.
2.8. Testing for the effect of head motion
For each subject, we computed a total motion index (TMI) as previously described in (Yendiki et al., 2014). The TMI is a composite score of the four motion measures automatically extracted in TRACULA: i) average volume-by-volume translation (degrees), ii) average volume-by-volume rotation (mm), iii) percent of slices with excessive intensity drop-out, and iv) average drop-out score for slices with excessive intensity drop-out (Section 2.4) (Yendiki et al., 2014). For each subject, the TMI was computed in the following manner:
where are the four motion measures listed above, is the value of the j-th motion measure for the m-th subject and , , and are respectively the median, upper quartile, and lower quartile of the j-th measure over all the subjects (Yendiki et al., 2014). Rather than excluding subjects based on their TMI, we have included this measure in our analyses. Specifically, to ensure differences in values between patients and controls were not influenced by head motion in the scanner, we first used a nonparametric Wilcoxon rank sum test in Python 3 (SciPy Stats 1.3.1) to assess if there were statistically significant differences in head motion between the patients and controls. Then, we conducted a linear mixed effects regression model in R (RStudio Team, 2020) using the ‘lmer’ function in the “lme4” package to test if TMI significantly predicted , while controlling for group (i.e., control, patient) and tract (i.e., 40 reconstructed tracts), and including participant as a random intercept.
2.9. Testing the relationship with behavioral measures
We conducted a series of analyses to test for associations between the degree of TAI, as measured by tract-specific multivariate measures, and the following behavioral measures of consciousness: GCS Total, CRS-R Total, days in coma as defined by the GCS, days to command-following as defined by the GCS.
First, we performed non-parametric Spearman correlations to support variable selection and model building for the primary regression analyses. A correlation matrix was constructed in R (RStudio Team, 2020) using the ‘cor’ function in the “stats” package. The matrix consisted of the dependent variables (i.e., GCS Total, CRS-R Total, days in coma as defined by the GCS, days to command-following as defined by the GCS), the independent variables (i.e., average of affected tracts and number of affected tracts), and potential confounding variables (i.e., age, TMI, days to MRI) (Edlow et al., 2016). P-values were adjusted for multiple comparisons using the Bonferroni correction method () (Jafari & Ansari-Pour, 2019).
Next, linear regressions were planned for completion in R using the ‘lm’ function (RStudio Team, 2020). Separate linear regression models would be conducted for each of the dependent variables (i.e., GGS Total, CRS-R Total, days in coma, days to command-following) with the average of affected tracts and the number of affected tracts as independent variables, and any identified confounding variables as covariates. Predictor variables that were significantly correlated with one another would be included in separate models to avoid multicollinearity.
Then, an ordinal logistic regression analysis was applied to predict LOC, given the categorical nature of this variable as defined by the CRS-R (i.e., coma, vS MCS-, MCS+, PTCS). The ordinal logistic regression was performed in R (RStudio Team, 2020) using the ‘plor’ function from the “MASS” package (Venables & Ripley, 2002) with LOC as the dependent variable; the average of affected tracts and the number of affected tracts as independent variables, and any identified confounding variables as covariates (i.e., determined via univariate ordinal logistic regression analyses between LOC and age, days to MRI, and TMI).
Finally, to assess the association between dMRI-derived measures of TAI and level of function after TBI, non-parametric Spearman correlations were conducted to test the relationship between average of affected tracts and number of affected tracts with DRS at 6-month follow-up. To examine the relationship between dMRI-derived measure of TAI and global outcome after TBI, an ordinal logistic regression was applied to assess the relationship between average DM of affected tracts and number of affected tracts and the GOS-E at 6-month follow-up with the following levels: 1 = Dead, 2 = Vegetative State, 3 = Low Severe Disability, 4 = Upper Severe Disability, 5 = Low Moderate Disability, 6 = Upper Moderate Disability, 7 = Low Good Recovery, 8 = Upper Good Recovery.
3. Results
3.1. Patient demographics and clinical characteristics
Patient demographics and clinical characteristics are provided in Table 1, including clinical indicators of coma duration (i.e., days in coma, days until command-following, level of consciousness at MRI), behavioral measures of consciousness (i.e., GCS and CRS-R total scores), including subscales measuring language and motor function, and clinical indicators of functional outcomes at 6 months post-injury. A nonparametric Wilcoxon sum rank showed no significant differences in age between the two groups (, ). A chi-square test of independence showed no significant difference in sex between groups ().
3.2. Quality assessment and feasibility of tractography reconstructions
Fig. 3 shows the reconstruction of the 40 tracts for two representative patients, one without focal lesions and one with a large right frontal hemorrhagic contusion. We observed that the TRACULA pipeline successfully reconstructed 93 % of the tracts (mean: 37.52; SD: 2.18) across all patients without manual intervention, exceeding our feasibility threshold of 50 % by 43 % (Section 2.6).
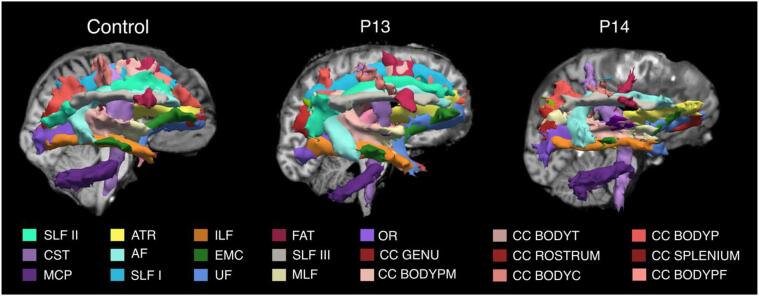
Fig. 3.
TRACULA reconstructions. The 40 tracts automatically reconstructed in TRACULA are shown in 3D sagittal view (right hemisphere) for one control and two patients, one with no focal lesions (P13), and one with a focal lesion in the right frontal lobe (P14). For each subject, tracts are overlaid on the T1-weighted structural volume. Color labels are reported only for tracts visible in the figure. Complete list of WM tracts abbreviations: ACOMM: anterior commissure; AF: arcuate fasciculus; AR: acoustic radiation; ATR: anterior thalamic radiation; CC BODYC: central section of the body of the CC; CC BODYP: parietal section of the body of the CC; CC BODYPF: prefrontal section of the body of the CC; CC BODYPM: premotor section of the body of the CC; CC BODYT: temporal section of the body of the CC; CC GENU: genu of the CC; CC ROSTRUM: rostrum of the CC; CC SPLENIUM: splenium of the CC; CBD: dorsal portion of cingulum bundle; CBV: ventral portion of the cingulum bundle; CC: corpus callosum; CST: cortico-spinal tract; EMC: extreme capsule; FAT: frontal Aslant tract; ILF: inferior longitudinal fasciculus; LH: left hemisphere; ILF: middle longitudinal fasciculus; MVA: motor vehicle accident; OR: optic radiation; Ped vs car: pedestrian versus car; RH: right hemisphere; SLF I,II,III: first, second, and third branch of the superior longitudinal fasciculus; UF: uncinate fasciculus.
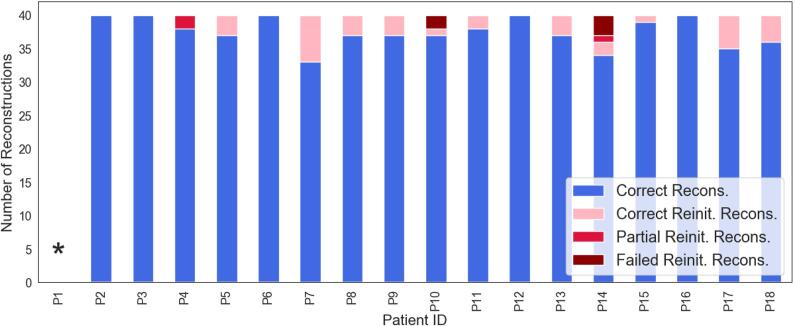
Fig. 4 reports the number of reconstructions for each patient that i) were reconstructed without the need for reinitialization, ii) resulted in either partial or failed reconstruction and were reinitialized successfully, and iii) resulted in partial or failed reconstructions even after reinitialization (See Section 2.6). Overall, an average of 37.5 tracts () resulted in complete reconstructions, 2.5 resulted in partial or failed reconstructions and were reinitialized, 0.2 tracts resulted in partial reconstruction after reinitialization, and 0.3 in failed reconstructions after reinitialization across all patients. For a complete list of tracts that were reinitialized for each patient see Supplementary Table S1. In one patient (P1) the FreeSurfer “recon-all” stream failed due to the presence of a massive lesion that injured a substantial portion of the cerebral cortex in the right hemisphere. This patient was thus excluded from the TRACULA pipeline and is not reported in further results. For five of the remaining 17 patients (P2, P3, P6, P12, P16), reconstructions were complete and did not require reinitialization, even in the presence of focal lesions (P2, P6, P12) (Supplementary Fig. S1). For the remaining twelve patients, a small number of tracts () resulted in failed or partial reconstructions and required reinitialization. For nine patients out of twelve, reinitialization led to successful reconstructions (as defined in Section 2.6). For one of these twelve patients (P4) two tracts were only partially reconstructed after reinitialization (MCP, LH CST), and for two patients (P10, P14) two and three tracts respectively (P10: ACOMM, RH UF; P14: ACOMM, RH SLFII, CC BODYPF) did not improve after reinitialization and resulted in failed reconstructions (Fig. 3, P14). Upon visual inspection, failed or partial reconstructions were attributable to hemorrhagic contusions in the temporal and frontal lobes, respectively, which caused disruption of three tracts each (Supplementary Fig. S2).
Fig. 4.
The number of correct reconstructions that did not need to be reinitialized (blue), the number of correct reconstructions after reinitialization (pink), and number of tracts that resulted in partial (red) or failed (maroon) reconstructions even after reinitialization are shown for each patient. * For P1, the FreeSurfer automated segmentation pipeline failed due to the presence of a massive lesion, which made it infeasible to run the TRACULA pipeline. Recons: reconstructions; Reinit: reinitialized. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Multivariate individualized assessment of white matter damage
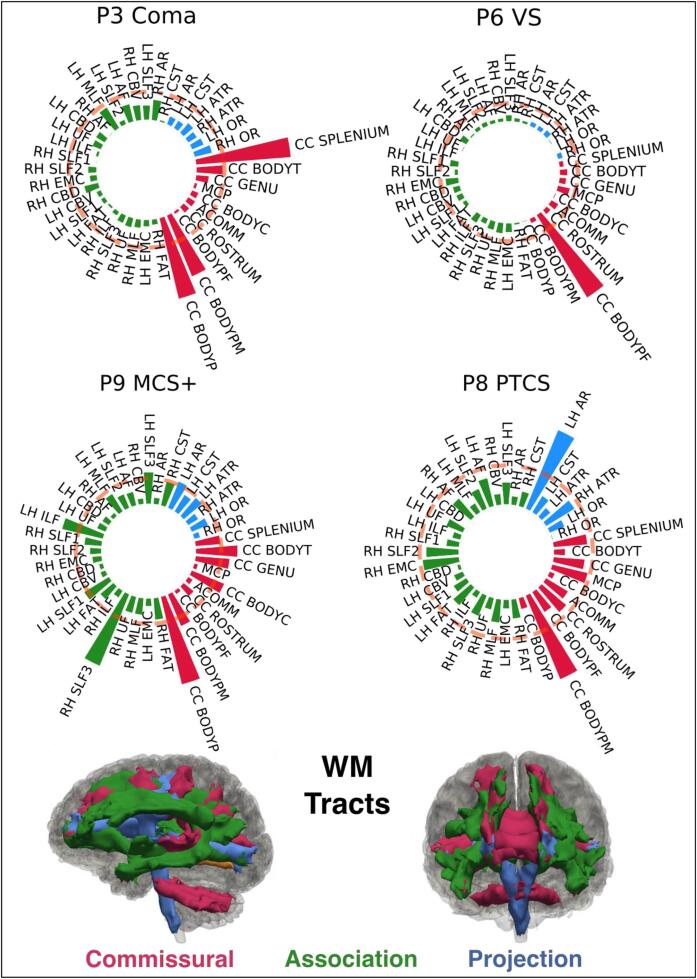
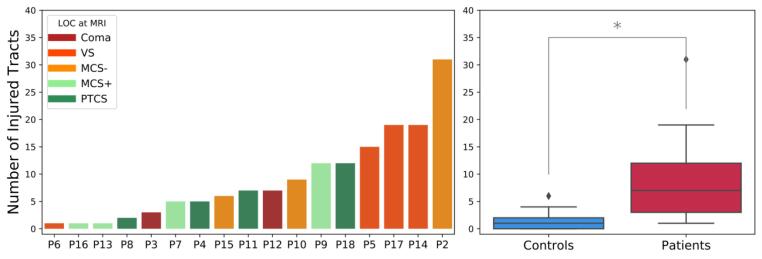
The Shapiro-Wilk test showed that the distribution of and departed significantly from normality () in 8.6 tracts per measure on average in the controls. Supplementary Table S2 reports the tracts along with the and . We applied a rank-based inverse normal transformation to these tracts for both healthy controls and patients before proceeding with the multivariate analysis (Section 2.7). Fig. 5 shows the -based individual profiles obtained from the multivariate analysis for four representative patients at different levels of consciousness (Coma, vS MCS, PTCS). Individual profiles for all 17 patients are shown in Supplementary Fig. S3. The individual profiles show the Mahalanobis distance of each of the 40 WM tracts from the multivariate distribution of diffusion measures (along-tract FA and MD values) for that tract in the control population. We considered a WM tract to be abnormal if its distance exceeded the critical value of the Chi-squared statistic at (pink dashed line in Fig. 5) (Section 2.7). The number and type of abnormal tracts varied across LOC (Fig. 5, Supplementary Fig. S3). To investigate whether a common pattern of TAI localization was visible across individuals, we grouped the reconstructed WM tracts as follows: i) commissural tracts: ACOMM, CC-BODYC, CC-BODYT, CC-BODYPF, CC-BODYP, CC-BODYPM, CC-GENU, CC-ROSTRUM, CC-SPLENIUM; ii) projection tracts: CST, ATR, AR, OR; iii) association tracts: all the other WM tracts. Commissural tracts were shown to have overall higher values (Supplementary Fig. S4) that resulted in a higher number of significantly extreme values () across patients compared to projection and association tracts (Supplementary Fig. S5). Specifically, the CC-BODYPM, the CC-BODYP, and CC-BODYT resulted abnormal in 10, 9, and 8 patients respectively. To ensure these results were not driven by the presence of significant midline shift in the patients, the amount of midline shift at the septum pellucidum and pineal gland in each patient was measured by an neurocritical care physician (author B.L.E.) on the T1-weighted structural images (Ropper, 1986). Minimal mass effect and midline shift in the patient cohort was observed at the time of the MRI (Supplementary Table S1), indicating that midline shift does not account for the higher values observed in the CC-BODYPM, the CC-BODYP, and CC-BODYT. For each patient we extracted the total number of abnormal tracts. On average, 9.1 ± 7.9 SD WM tracts were abnormal in patients with TBI (Fig. 6).
Fig. 5.
Individual Tract Profiles. The figure shows the individual profiles for four representative subjects, each with a different level of consciousness (Coma, vS MCS+, PTCS). The Mahalanobis distance for the reconstructed tracts is plotted using polar bar plots. The pink dashed line corresponds to the critical value of the Chi-squared statistic at . Bars that extend beyond the dashed line represent tracts for which the multivariate diffusion measures (along-tract FA and MD values) were significantly different from the multivariate distribution of those measures in controls and were considered damaged. White matter tracts are grouped in commissural (red), associative (green), and projection pathways (blue). The 3D reconstructions of the 40 WM tracts are shown for a representative control subject in sagittal and coronal view, color coded based on the group they belong to. MCS+: minimally conscious state plus; PTCS: post-traumatic confusional state; VS: vegetative state; WM: white matter. For a complete list of WM tract abbreviations see Fig. 3. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Number of affected tracts per subject. Left panel: The number of abnormal WM tracts is shown for each patient with TBI. A tract was considered abnormal if its , corresponding to a Chi-square statistic, was ( corrected for multiple comparisons across 40 tracts). Patients are ordered based on the number of abnormal tracts. The color of the bars reflects the level of consciousness (LOC) at the day of MRI. MCS+: minimally conscious state plus; MCS-: minimally conscious state minus; PTCS: post-traumatic confusional state; VS: vegetative state. Patient IDs corresponding to those used in Table 1 () are indicated for each patient. Right panel: Boxplots showing the minimum, first quartile, median, third quartile and maximum number of significantly abnormal tracts for controls and patients. Outliers are defined as points greater than: third quartile + 1.5 * interquartile range. significant difference (Wilcoxon rank sum test: , ).
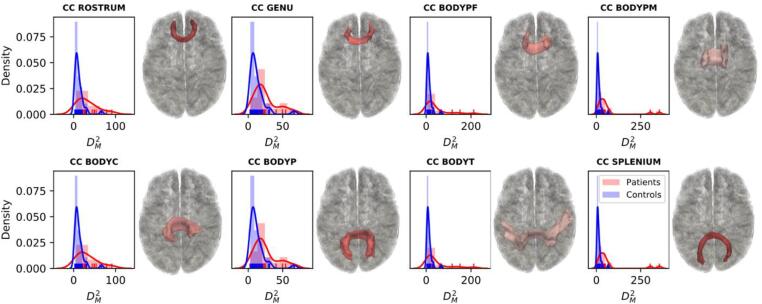
To investigate the accuracy of the multivariate pipeline at detecting TAI, we quantified its performance in classifying patients versus controls. We first computed the for each control in a leave-one-out fashion and extracted the number of tracts that exceeded the critical value of the Chi-squared statistic at for each control. On average 1.36 ± 1.38 tracts appeared to have significantly abnormal values in controls (Fig. 6, right panel). However, the mean of these tracts was lower than the mean of the abnormal tracts in controls (35 and 74 respectively). A Wilcoxon sum rank test showed a significant difference between the number of abnormal tracts (, ) and between the of these tracts () in patients versus controls. We then performed a ROC analysis to measure the performance of the pipeline in discriminating between patients and controls and found an accuracy of 0.91 (Supplementary Fig. S6). To visualize the difference in values between the two populations we plotted the probability density functions of for patients and controls. Fig. 7 shows the probability density functions of values for the different sections of the corpus callosum for patients and controls. For subjects with severe TBI, values are shifted to the right suggesting a larger mean and greater degree of microstructural deviation from the control reference group. Probability density functions of values for patients and controls for all 40 tracts are shown in Supplementary fig. S7.
Fig. 7.
Density plots. Probability density functions of Mahalanobis distance () values are shown for patients (shown in red) and controls (shown in blue) for each of the different sections of the corpus callosum (CC). Representative 3D reconstructions of the correspondent WM tracts are shown to the right of the density plots. CC BODYC: central section of the body of the CC; CC BODYP: parietal section of the body of the CC; CC BODYPF: prefrontal section of the body of the CC; CC BODYPM: premotor section of the body of the CC; CC BODYT: temporal section of the body of the CC; CC GENU: genu of the CC; CC ROSTRUM: rostrum of the CC; CC SPLENIUM: splenium of the CC. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Finally, a Wilcoxon signed-rank test showed no significant difference in the number of abnormal tracts () and in the mean per patient when analyses were conducted including only the tracts that had been successfully reconstructed without the need for reinitialization (i.e., discarding tracts that were reinitialized) (Supplementary Fig. S8). The accuracy in discriminating between patients and controls also did not change (). This suggests that the low number of tracts that needed to be reinitialized across subjects did not significantly impact the overall individualized assessment of TAI.
3.4. Effects of head motion and other confounding variables
The TMI and the four motion measures used to compute it are reported for each subject in Supplementary Table S1. A nonparametric Wilcoxon sum rank test revealed a non-statistically significant difference in TMI between patients and controls (, ). Results of the linear mixed effects model revealed that TMI did not significantly predict (). However, group was a significant predictor of (), with patients demonstrating significantly higher values than controls, as expected given how is calculated. See Supplementary Table S1 for full results of the linear mixed effects model. Taken together, these results emphasize that the greater number of affected tracts detected for patients versus controls was not confounded by head motion in the scanner.
Results of the linear mixed effects model testing for the effect of differences in acquisition sequences (with or without SMS) showed that SMS, dichotomous predictor variable with two levels (i.e., with SMS, without SMS), was not significantly associated with average of affected tracts ( ) or number of affected tracts (. This null effect held whether head motion was accounted for in the model or not, emphasizing the robustness of the result. Finally, this null effect was the same when average of affected tracts or number of affected tracts was derived using tracts that completed reconstruction only (Supplementary Note).
3.5. Relationship with behavioral measures of consciousness
Average DM of affected tracts was not significantly correlated with GCS-Total, (), CRS-R Total (, ), days to command-following (, ), or days in coma (). Similarly, number of affected tracts was not significantly correlated with GCS-Total (), CRS-R Total (), days to command-following (), or days in coma (). Additionally, none of the potentially confounding variables (i.e., age, TMI, days to MRI) showed significant correlations with the average DM of affected tracts or the number of affected tracts. Complete results from the non-parametric Spearman correlations are reported in Supplementary Table S1. The proposed linear regression models using average of affected tracts and number of affected tracts to predict GCS-Total, CRS-R Total, days to command-following, and days in coma were not pursued, given that the correlation analyses did not reveal a significant association between these variables.
No significant relationships were found between LOC and average of affected tracts () or number of affected tracts (). The univariate ordinal logistic regressions testing the association between LOC and age (), TMI (), and days to MRI () did not identify any confounding relationships, and thus, it was not necessary to include these variables as covariates in the model.
Neither average DM of affected tracts (), nor number of affected tracts () were significantly correlated with DRS at 6 months follow-up. The ordinal logistic regression testing for associations between GOS-E at 6 months follow-up and average DM of affected tracts () and number of affected tracts () did not identify any significant relationships between these dMRI-derived measures of TAI and global outcome after TBI. The univariate ordinal logistic regressions testing the association between GOS-E and age (), TMI (), and days to MRI () did not identify any confounding relationships, and thus, it was not necessary to include these variables as covariates in the model.
All statistical analyses were repeated using only the tracts that were successfully reconstructed without the need for reinitialization and results did not differ (Supplementary note, Supplementary Table S1).
4. Discussion
In this prospective observational study, we propose a pipeline that combines automated tractography reconstructions with multivariate analysis of along-tract diffusion metrics to assess the presence of TAI in individual patients with acute severe TBI. We show that i) TRACULA (Maffei et al., 2021, Yendiki et al., 2011) provides automated reconstruction of WM tracts from dMRI data in critically ill patients with acute severe TBI, even in the presence of large focal lesions; and ii) the multivariate analysis detected at least one abnormal WM tract per patient and could accurately differentiate patients from healthy controls (AUC = 0.91). These proof-of-principle results suggest that TRACULA has the potential to be used by clinicians, in conjunction with conventional MRI results, for the acute detection of TAI burden in critically ill patients. We discuss the clinical implications and technical limitations of the current results and provide further directions for optimization and translation of the proposed pipeline.
With respect to TAI detection, the automated pipeline found that, on average, 9.1 ± 7.9 tracts were abnormal in patients with TBI. The individualized profiles of WM damage highlighted the degree of heterogeneity in magnitude, number, and location of WM changes in patients (Fig. 5, Fig. 6 and Supplementary Fig. S3). This finding builds on recent studies showing that diffusion measures can be used to identify the neuroanatomic distribution and severity of TAI at the individual level (Jolly et al., 2021), an important goal given the well-established heterogeneity of this condition (Douglas et al., 2019). Despite the heterogeneity of the specific WM tracts that identified as abnormal across patients, and the fact that no clear pattern of WM damage was visible within LOC groups (Fig. 5), the multivariate individual analysis revealed commissural connections to be more significantly and more frequently affected compared to projection and association fibers across all LOC (Fig. 5, Supplementary Figs. S4 and S5). Specifically, the premotor, parietal, and temporal sections of the CC were the most affected, consistent with previous histological and neuroimaging studies in humans with severe TBI (Moen et al., 2014, Nolan et al., 2020, Ubukata et al., 2016) and with studies in animal models of TAI (Baker et al., 2004, Gennarelli et al., 1982). Changes in diffusion measures in these WM tracts reflect a series of pathological events caused by shearing and straining forces exerted on the axons during TBI. Previous studies investigating the association between dMRI and histological measures reported correlations between decreased FA and axonal degeneration, between increased MD and axonal demyelination, but also between increased FA and neuroinflammation (Hutchinson et al., 2018). In this work we combined along-tract measures of both FA and MD, as multivariate approaches have shown higher power in distinguishing different clinical populations from controls (Dean et al., 2017). Therefore, changes in WM tracts in TBI patients might include both or either FA/MD increase or decrease and reflect a combination of degeneration, demyelination, or inflammatory mechanisms. Future studies are needed to investigate the prognostic value of different combinations of dMRI metrics.
Interestingly, individual multivariate measures of TAI did not show a significant relationship with behavioral measures of consciousness (i.e., GCS-Total, CRS-R Total, days to command-following, days in coma). There are several potential explanations for this observation. First, many clinical factors beyond TAI influence a patient’s LOC in the ICU, including other traumatic lesions (e.g., contusions and subdural hemorrhages) and subclinical seizures, as well as toxic, metabolic, endocrinologic or infectious causes of encephalopathy. Second, widespread tract disruption in the brainstem may have confounded any associations between the global burden of TAI and behavioral measures of consciousness. TRACULA does not yet provide automated reconstructions of brainstem tracts – a key direction for future work in this field, especially as the presence of TAI in brainstem pathways can cause altered consciousness (Edlow et al., 2013, Snider et al., 2019). Third, the small sample size and the variability in days to MRI across patients, along with the substantial variation in the patients’ states of consciousness and the high number of tracts being tested, could have underpowered our analyses. For instance, a post-hoc power analysis, conducted via G*Power (Faul et al., 2007), revealed that our sample size of 17 participants was only sufficient to detect correlation of 0.62 or higher with 80 % power and an unadjusted Type-1 error control rate of 0.05. Nevertheless, given that prior studies have identified correlations between acute tractography data and long-term cognitive function (Wang et al., 2008), these data provide the basis for future studies with larger samples to test whether multivariate tract-specific measurements in the ICU predict recovery.
Across the 17 patients included in this study, TRACULA provided, on average, complete automated reconstructions of 93 % of the WM tracts () without the need of manual intervention. These proof-of-principle results suggest its potential for dissemination to a broad range of hospital settings without the need for local tractography expertise. However, the clinical translation of this pipeline for TAI detection in the ICU will require optimization and validation of the proposed methods in future studies. While on average TRACULA could successfully reconstruct 37.5 of 40 tracts without the need of manual intervention, an average of 2.4 tracts resulted in failed or partial reconstructions requiring reinitialization of at least one tract in 12 patients (Supplementary Table S1). Even if our results did not change when using only the WM tracts that did not require reinitialization, this step relied upon a visual quality assessment from a tractography expert that would limit TRACULA’s applicability to clinical settings. Furthermore, some reinitialized tracts could not be successfully reconstructed due to the presence of extensive focal lesions in two patients. While it has been previously shown that TRACULA is robust to errors in the anatomical segmentation (Zöllei et al., 2019), large lesions can lead to missing labels in the FreeSurfer parcellation that can affect tract reconstructions, as prior probabilities are based on the relative positions of the bundles with respect to these labels. Further optimization of the TRACULA pipeline will include i) improved automated assessment of tract reconstructions, ii) correct handling of missing FreeSurfer anatomical labels, including in cases where the FreeSurfer “recon-all” pipeline fails due to the presence of lesions (e.g., Patient 1 in this study), and iii) assessment of TRACULA parameter setting in clinical populations. While in this feasibility study we used all default parameters, including the threshold applied to the posterior probability of the reconstructed tracts (20 % of the maximum), future studies should address how different TRACULA parameter settings affect the accuracy of the pipeline. Additionally, the dMRI data used in this study were acquired with high angular resolution (, 60 diffusion-encoding directions) and relatively high spatial resolution (2 mm isotropic) compared to more standard clinical diffusion sequences (Chilla et al., 2015), leading to a longer acquisition time (∼9 min). Thus, while obtained on a clinical scanner, the dMRI data collected in this study were likely of higher quality than dMRI data acquired on clinical scanners in most hospitals, and it will be important to determine whether automated reconstruction of WM tracts with TRACULA is robust in the setting of lower-resolution, lower-quality dMRI data in the future. Similarly, future studies should address whether multi-shell dMRI data and additional pre-processing steps (e.g., correction of susceptibility-induced distortions through the acquisition of data with reversed phase-encoding) would improve the accuracy of these results.
It is also important to consider that the individualized assessment proposed here can only be performed by comparing patients to a control cohort scanned with the same dMRI sequence. For this pipeline to be applied in a clinical setting that does not have data from healthy controls, future optimizations of this pipeline should explore its robustness to dMRI data acquired on a different scanner and with a different dMRI sequence. Furthermore, although we found no statistically significant differences in age between the patient and control cohorts, and age did not correlate with any of the results of the multivariate analysis (Supplementary Table S1), the controls used in this study were not enrolled with an even distribution of age. As normal ageing is associated with changes in tensor-derived diffusion metrics (Pfefferbaum et al., 2000, Salat et al., 2005), differences in age between the patient and control cohorts (patient mean age: 28.6 ± 8.7, control mean age: 30.9 ± 8.8) may have impacted the designation of normal versus abnormal tracts in the present study. Future studies should evaluate the relevance of using age-specific normative data to account for these differences. Identification of optimal characteristics and sample sizes for control cohorts is an area of active inquiry (Jolly et al., 2021), and future studies may leverage large normative databases such as the Human Connectome Project in this effort (Bookheimer et al., 2019, Harms et al., 2018). Finally, it is important to consider that some patients with acute severe TBI are not stable enough to travel to an MRI scanner, and thus this pipeline may not be feasible to use until these patients reach the subacute stage of care.
In summary, this study demonstrates the feasibility and utility of an automated tractography pipeline for detecting TAI in the acute stage of severe TBI. The overall burden and location of TAI, as measured by dMRI in the subacute stage, have been shown to predict functional and cognitive outcome (Hellyer et al., 2013, Jolly et al., 2021, Kumar et al., 2009). However, whether these results are extendable to measures obtained in the acute stage, and whether a correlation between TAI and outcome is due to the total lesion burden or to its location, remains to be determined (Kampfl et al., 1998). Thus, while the present results indicate that dMRI has diagnostic utility in assessing acute TAI, its potential prognostic utility and role in guiding therapeutic decision-making will need to be evaluated in future studies.
5. Funding:
This study was supported by the U.S. Department of Defense (USSOCOM Contract No. H9240520D0001), NIH National Institute of Neurological Disorders and Stroke (R01NS119911, R21NS109627, RF1NS115268, U01NS1365885, U01NS086090), NIH Director’s Office (DP2 HD101400), NIH National Institute of Biomedical Imaging and Bioengineering (R01EB021265, U01EB026996), NIH National Institute of Mental Health (U01MH108168, R56MH121426), James S. McDonnell Foundation, American Academy of Neurology, Tiny Blue Dot Foundation, and National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR), Administration for Community Living (90DPCP0008-01-00, 90DP0039).
CRediT authorship contribution statement
Chiara Maffei: Conceptualization, Investigation, Methodology, Software, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Natalie Gilmore: Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Samuel B. Snider: Investigation, Writing – review & editing. Andrea S. Foulkes: Investigation, Methodology, Supervision. Yelena G. Bodien: Investigation, Writing – review & editing. Anastasia Yendiki: Software, Methodology, Writing – review & editing, Supervision. Brian L. Edlow: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank the nursing staffs of the Massachusetts General Hospital Neurosciences ICU, Multidisciplinary ICU, and Surgical ICU. We also thank the Massachusetts General Hospital MRI technologists for assistance with data acquisition. We are grateful to the patients and families in this study for their participation and support.
Disclaimer
The views expressed in this manuscript are entirely those of the authors and do not necessarily reflect the views, policy, or position of the United States Government, Department of Defense, or United States Special Operations Command.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103294.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
We have inserted a data availability statement in the text
References
- Adams J.H., Doyle D., Ford I., Gennarelli T.A., Graham D.I., Mclellan D.R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15(1):49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K., Haughton V.M., Carew J.D., Rogers B.P., Dempsey R.J., Meyerand M.E. Diffusion tensor MR imaging in diffuse axonal injury. Am. J. Neuroradiol. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- Asken B.M., DeKosky S.T., Clugston J.R., Jaffee M.S., Bauer R.M. Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): a systematic critical review. Brain Imaging Behav. 2018;12(2):585–612. doi: 10.1007/s11682-017-9708-9. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, A. J., Phan, N., Moulton, R. J., Fehlings, M. G., Yucel, Y., Zhao, M., Liu, E., & Tian, G. F. (2004). Attenuation of the Electrophysiological Function of the Corpus Callosum after Fluid Percussion Injury in the Rat. Https://Home.Liebertpub.Com/Neu, 19(5), 587–599. https://doi.org/10.1089/089771502753754064. [DOI] [PubMed]
- Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E.J., Johansen-Berg H., Woolrich M.W., Smith S.M., Wheeler-Kingshott C.A.M., Boulby P.A., Barker G.J., Sillery E.L., Sheehan K., Ciccarelli O., Thompson A.J., Brady J.M., Matthews P.M. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6(7):750–757. doi: 10.1227/01.NEU.0000309595.77090.89. [DOI] [PubMed] [Google Scholar]
- Behrens T.E.J., Berg H.J., Jbabdi S., Rushworth M.F.S., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner T., Van Der Kouwe A.J.W., Sorensen A.G. Diffusion imaging with prospective motion correction and reacquisition. Magn. Reson. Med. 2011;66(1):154–167. doi: 10.1002/MRM.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, R. R., Meda, S. A., Vasudevan, S., Kou, Z., Govindarajan, K. A., Hanks, R. A., Millis, S. R., Makki, M., Latif, Z., Coplin, W., Meythaler, J., & Haacke, E. M. (2007). Global White Matter Analysis of Diffusion Tensor Images Is Predictive of Injury Severity in Traumatic Brain Injury. Https://Home.Liebertpub.Com/Neu, 24(3), 446–459. https://doi.org/10.1089/NEU.2006.0153. [DOI] [PubMed]
- Betz J., Zhuo J., Roy A., Shanmuganathan K., Gullapalli R.P. Prognostic value of diffusion tensor imaging parameters in severe traumatic brain injury. J. Neurotrauma. 2012;29(7):1292–1305. doi: 10.1089/neu.2011.2215. [DOI] [PubMed] [Google Scholar]
- Blom G. Wiley; 1958. Statistical estimates and transformed beta-variables. [Google Scholar]
- Blumbergs P.C., Scott G., Vis J.M., Wainwright H., Simpson D.A., Mclean A.J. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J. Neurotrauma. 1995;12(4):565–572. doi: 10.1089/NEU.1995.12.565. [DOI] [PubMed] [Google Scholar]
- Bodien Y.G., Barra A., Temkin N.R., Barber J., Foreman B., Vassar M., Robertson C., Taylor S.R., Markowitz A.J., Manley G.T., Giacino J.T., Edlow B.L., Badjatia N., Duhaime A.-C., Ferguson A.R., Gaudette PhD E., Gopinath S., Keene C.D., McCrea M., Merchant R., Mukherjee P., Ngwenya L.B., Okonkwo D., Puccio A., Sugar G., Schnyer D., Yue J.K., Zafonte R. Diagnosing level of consciousness: the limits of the glasgow coma scale total score. J. Neurotrauma. 2021;38(23):3295–3305. doi: 10.1089/neu.2021.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S.Y., Salat D.H., Terpstra M., Ances B.M., Barch D.M., Buckner R.L., Burgess G.C., Curtiss S.W., Diaz-Santos M., Elam J.S., Fischl B., Greve D.N., Hagy H.A., Harms M.P., Hatch O.M., Hedden T., Hodge C., Japardi K.C., Kuhn T.P., Ly T.K., Smith S.M., Somerville L.H., Uğurbil K., van der Kouwe A., Van Essen D., Woods R.P., Yacoub E. The lifespan human connectome project in aging: an overview. Neuroimage. 2019;185:335–348. doi: 10.1016/j.neuroimage.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody D.L., Benetatos J., Bennett R.E., Klemenhagen K.C., Mac Donald C.L. The pathophysiology of repetitive concussive traumatic brain injury in experimental models; new developments and open questions. Mol. Cell. Neurosci. 2015;66(PB):91–98. doi: 10.1016/J.MCN.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilla G.S., Tan C.H., Xu C., Poh C.L. Diffusion weighted magnetic resonance imaging and its recent trend—a survey. Quant. Imaging Med. Surg. 2015;5(3):407. doi: 10.3978/J.ISSN.2223-4292.2015.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza M.M., Trivedi R., Singh K., Grover H., Choudhury A., Kaur P., Kumar P., Tripathi R.P. Traumatic brain injury and the post-concussion syndrome: a diffusion tensor tractography study. Indian J. Radiol. Imag. 2015;25(4):404. doi: 10.4103/0971-3026.169445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dean D.C., Lange N., Travers B.G., Prigge M.B., Matsunami N., Kellett K.A., Freeman A., Kane K.L., Adluru N., Tromp D.P.M., Destiche D.J., Samsin D., Zielinski B.A., Fletcher P.T., Anderson J.S., Froehlich A.L., Leppert M.F., Bigler E.D., Lainhart J.E., Alexander A.L. Multivariate characterization of white matter heterogeneity in autism spectrum disorder. NeuroImage: Clinical. 2017;14:54–66. doi: 10.1016/j.nicl.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Donald M., Christine L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Snyder A.Z., Raichle M.E., Witherow J.R., Fang R., Flaherty S.F., Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMOA1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas D.B., Ro T., Toffoli T., Krawchuk B., Muldermans J., Gullo J., Dulberger A., Anderson A.E., Douglas P.K., Wintermark M. Neuroimaging of traumatic brain injury. Med. Sci. 2019;7(1):2. doi: 10.3390/MEDSCI7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow B.L., Haynes R.L., Takahashi E., Klein J.P., Cummings P., Benner T., Greer D.M., Greenberg S.M., Wu O., Kinney H.C., Folkerth R.D. Disconnection of the ascending arousal system in traumatic coma. J. Neuropathol. Exp. Neurol. 2013;72(6):505–523. doi: 10.1097/NEN.0b013e3182945bf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow B.L., Copen W.A., Izzy S., Bakhadirov K., van der Kouwe A., Glenn M.B., Greenberg S.M., Greer D.M., Wu O. Diffusion tensor imaging in acute-to-subacute traumatic brain injury: a longitudinal analysis. BMC Neurol. 2016;16(1) doi: 10.1186/S12883-015-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow B.L., Chatelle C., Spencer C.A., Chu C.J., Bodien Y.G., O’Connor K.L., Hirschberg R.E., Hochberg L.R., Giacino J.T., Rosenthal E.S., Wu O. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140(9):2399–2414. doi: 10.1093/brain/awx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q., Witzel T., Nummenmaa A., Van Dijk K.R.A., Van Horn J.D., Drews M.K., Somerville L.H., Sheridan M.A., Santillana R.M., Snyder J., Hedden T., Shaw E.E., Hollinshead M.O., Renvall V., Zanzonico R., Keil B., Cauley S., Polimeni J.R., Tisdall D., Buckner R.L., Wedeen V.J., Wald L.L., Toga A.W., Rosen B.R. MGH-USC Human Connectome Project datasets with ultra-high b-value diffusion MRI. Neuroimage. 2016 Jan;1(124(Pt B)):1108–1114. doi: 10.1016/j.neuroimage.2015.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., Van Der Kouwe A., Destrieux C., Halgren E., Ségonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Galanaud D., Perlbarg V., Gupta R., Stevens R., Sanchez P., Tollard E., de Champfleur N., Dinkel J., Faivre S., Soto-Ares G., Veber B., Cottenceau V., Masson F., Tourdias T., André E., Audibert G., Schmitt E., Ibarrola D., Dailler F., Vanhaudenhuyse A., Tshibanda L., Payen J.-F., Le Bas J.-F., Krainik A., Bruder N., Girard N., Laureys S., Benali H., Puybasset L. Assessment of white matter injury and outcome in severe brain traumaa prospective multicenter cohort. Anesthesiology. 2012;117(6):1300–1310. doi: 10.1097/ALN.0b013e3182755558. [DOI] [PubMed] [Google Scholar]
- Gennarelli T.A., Thibault L.E., Adams J.H., Graham D.I., Thompson C.J., Marcincin R.P. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 1982;12(6):564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Kalmar K., Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004;85(12):2020–2029. doi: 10.1016/J.APMR.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Gnanadesikan R., Kettenring J.R. Robust estimates, residuals, and outlier detection with multiresponse data. Biometrics. 1972;28(1):81. doi: 10.2307/2528963. [DOI] [Google Scholar]
- Goodrich-Hunsaker N.J., Abildskov T.J., Black G., Bigler E.D., Cohen D.M., Mihalov L.K., Bangert B.A., Taylor H.G., Yeates K.O. Age- and sex-related effects in children with mild traumatic brain injury on diffusion magnetic resonance imaging properties: a comparison of voxelwise and tractography methods. J. Neurosci. Res. 2018;96(4):626–641. doi: 10.1002/JNR.24142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M.P., Somerville L.H., Ances B.M., Andersson J., Barch D.M., Bastiani M., Bookheimer S.Y., Brown T.B., Buckner R.L., Burgess G.C., Coalson T.S., Chappell M.A., Dapretto M., Douaud G., Fischl B., Glasser M.F., Greve D.N., Hodge C., Jamison K.W., Jbabdi S., Kandala S., Li X., Mair R.W., Mangia S., Marcus D., Mascali D., Moeller S., Nichols T.E., Robinson E.C., Salat D.H., Smith S.M., Sotiropoulos S.N., Terpstra M., Thomas K.M., Tisdall M.D., Ugurbil K., van der Kouwe A., Woods R.P., Zöllei L., Van Essen D.C., Yacoub E. Extending the human connectome project across ages: imaging protocols for the lifespan development and aging projects. Neuroimage. 2018;183:972–984. doi: 10.1016/j.neuroimage.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim E., Caverzasi E., Papinutto N., Lewis C.E., Jing R., Charles O., Zhang S., Lin A., Graham S.J., Schweizer T.A., Bharatha A., Cusimano M.D. Investigating microstructural abnormalities and neurocognition in sub-acute and chronic traumatic brain injury patients with normal-appearing white matter: a preliminary diffusion tensor imaging study. Front. Neurol. 2017;8(MAR):97. doi: 10.3389/fneur.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer P.J., Leech R., Ham T.E., Bonnelle V., Sharp D.J. Individual prediction of white matter injury following traumatic brain injury. Ann. Neurol. 2013;73(4):489–499. doi: 10.1002/ANA.23824. [DOI] [PubMed] [Google Scholar]
- Hill, C. S., Coleman, M. P., & Menon, D. K. (2016). Traumatic Axonal Injury: Mechanisms and Translational Opportunities. In Trends in Neurosciences (Vol. 39, Issue 5, pp. 311–324). Elsevier Ltd. https://doi.org/10.1016/j.tins.2016.03.002. [DOI] [PMC free article] [PubMed]
- Hulkower M.B., Poliak D.B., Rosenbaum S.B., Zimmerman M.E., Lipton M.L. A Decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am. J. Neuroradiol. 2013;34(11):2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E.B., Schwerin S.C., Avram A.V., Juliano S.L., Pierpaoli C. Diffusion MRI and the detection of alterations following traumatic brain injury. J. Neurosci. Res. 2018;96(4):612–625. doi: 10.1002/jnr.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M., Makani S., Johnson G., Cohen B.A., Silver J.A., Gonen O., Grossman R.I. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Izzy S., Compton R., Carandang R., Hall W., Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: do they exist in traumatic brain injury, too? Neurocrit. Care. 2013;19(3):347–363. doi: 10.1007/S12028-013-9925-Z. [DOI] [PubMed] [Google Scholar]
- Jafari M., Ansari-Pour N. Why, when and how to adjust your P values? Cell J. (Yakhteh) 2019;20(4):604. doi: 10.22074/CELLJ.2019.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V.E., Stewart W., Smith D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly A.E., Bălăeţ M., Azor A., Friedland D., Sandrone S., Graham N.S.N., Zimmerman K., Sharp D.J. Detecting axonal injury in individual patients after traumatic brain injury. Brain. 2021;144(1):92–113. doi: 10.1093/brain/awaa372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E., Oliphant, T., & Peterson, P. (2001). SciPy: Open Source Scientific Tools for Python.
- Jones D.K., Travis A.R., Eden G., Pierpaoli C., Basser P.J. PASTA: Pointwise assessment of streamline tractography attributes. Magn. Reson. Med. 2005;53(6):1462–1467. doi: 10.1002/mrm.20484. [DOI] [PubMed] [Google Scholar]
- Kampfl A., Schmutzhard E., Franz G., Pfausler B., Haring H.P., Ulmer H., Felber S., Golaszewski S., Aichner F. Prediction of recovery from post-traumatic vegetative state with cerebral magnetic-resonance imaging. Lancet (London, England) 1998;351(9118):1763–1767. doi: 10.1016/S0140-6736(97)10301-4. [DOI] [PubMed] [Google Scholar]
- Kim N., Branch C.A., Kim M., Lipton M.L., Valdes-Sosa P.A. Whole brain approaches for identification of microstructural abnormalities in individual patients: comparison of techniques applied to mild traumatic brain injury. PLoS One. 2013;8(3):e59382. doi: 10.1371/journal.pone.0059382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen K.M., Greenwood R., Powell J.H., Leech R., Hawkins P.C., Bonnelle V., Patel M.C., Counsell S.J., Sharp D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(2):449–463. doi: 10.1093/BRAIN/AWQ347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreilkamp B.A.K., Weber B., Richardson M.P., Keller S.S. Automated tractography in patients with temporal lobe epilepsy using TRActs Constrained by UnderLying Anatomy (TRACULA) NeuroImage. Clin. 2017;14:67–76. doi: 10.1016/J.NICL.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Gupta R.K., Husain M., Chaudhry C., Srivastava A., Saksena S., Rathore R.K.S. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 2009;23(7):675–685. doi: 10.1080/02699050903014915. [DOI] [PubMed] [Google Scholar]
- Li J., Li X.Y., Feng D.F., Gu L. Quantitative evaluation of microscopic injury with diffusion tensor imaging in a rat model of diffuse axonal injury. Eur. J. Neurosci. 2011;33(5):933–945. doi: 10.1111/j.1460-9568.2010.07573.x. [DOI] [PubMed] [Google Scholar]
- Lindemer E.R., Salat D.H., Smith E.E., Nguyen K., Fischl B., Greve D.N. White matter signal abnormality quality differentiates mild cognitive impairment that converts to Alzheimer’s disease from nonconverters. Neurobiol. Aging. 2015;36(9):2447–2457. doi: 10.1016/j.neurobiolaging.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Dikranian K., Song S.K., Bayly P.V., Holtzman D.M., Brody D.L. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp. Neurol. 2007;205(1):116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei C., Lee C., Planich M., Ramprasad M., Ravi N., Trainor D., Urban Z., Kim M., Jones R.J., Henin A., Hofmann S.G., Pizzagalli D.A., Auerbach R.P., Gabrieli J.D.E., Whitfield-Gabrieli S., Greve D.N., Haber S.N., Yendiki A. Using diffusion MRI data acquired with ultra-high gradient strength to improve tractography in routine-quality data. Neuroimage. 2021;245 doi: 10.1016/J.NEUROIMAGE.2021.118706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalanobis, P. C. (1936). On the generalised distance in statistics. Proceedings of the National Institute of Sciences of India, 49–55.
- McGinn M.J., Povlishock J.T. Pathophysiology of traumatic brain injury. Neurosurg. Clin. 2016;27(4):397–407. doi: 10.1016/J.NEC.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Mesulam M.-M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann. Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Moe H.K., Follestad T., Andelic N., Håberg A.K., Flusund A.M.H., Kvistad K.A., Saksvoll E.H., Olsen Ø., Abel-Grüner S., Sandrød O., Skandsen T., Vik A., Moen K.G. Traumatic axonal injury on clinical MRI: association with the Glasgow Coma Scale score at scene of injury or at admission and prolonged posttraumatic amnesia. J. Neurosurg. 2020;1(aop):1–12. doi: 10.3171/2020.6.JNS20112. [DOI] [PubMed] [Google Scholar]
- Moen K.G., Brezova V., Skandsen T., Håberg A.K., Folvik M., Vik A. Traumatic axonal injury: the prognostic value of lesion load in corpus callosum, brain stem, and thalamus in different magnetic resonance imaging sequences. J. Neurotrauma. 2014;31(17):1486–1496. doi: 10.1089/neu.2013.3258. [DOI] [PubMed] [Google Scholar]
- Newcombe V.F.J., Williams G.B., Nortje J., Bradley P.G., Harding S.G., Smielewski P., Coles J.P., Maiya B., Gillard J.H., Hutchinson P.J., Pickard J.D., Carpenter T.A., Menon D.K. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br. J. Neurosurg. 2007;21(4):340–348. doi: 10.1080/02688690701400882. [DOI] [PubMed] [Google Scholar]
- Newcombe V.F.J., Williams G.B., Scoffings D., Cross J., Carpenter T.A., Pickard J.D., Menon D.K. Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. J. Neurol. Neurosurg. Psychiatry. 2010;81(5):552–561. doi: 10.1136/JNNP.2009.196246. [DOI] [PubMed] [Google Scholar]
- Nolan, A. L., Petersen, C., Iacono, D., Donald, C. L. Mac, Mukherjee, P., Van Der Kouwe, A., Jain, S., Stevens, A., Diamond, B. R., Wang, R., Markowitz, A. J., Fischl, B., Perl, D. P., Manley, G. T., Keene, C. D., Diaz-Arrastia, R., & Edlow, B. L. (2020). Tractography-Pathology Correlations in Traumatic Brain Injury: A TRACK-TBI Study. J Neurotrauma. 2021 Jun 15;38(12):1620-1631. doi: 10.1089/neu.2020.7373. [DOI] [PMC free article] [PubMed]
- O’Phelan K.H., Otoshi C.K., Ernst T., Chang L. Common patterns of regional brain injury detectable by diffusion tensor imaging in otherwise normal-appearing white matter in patients with early moderate to severe traumatic brain injury. J. Neurotrauma. 2018;35(5):739. doi: 10.1089/NEU.2016.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordóñez-Rubiano E.G., Johnson J., Enciso-Olivera C.O., Marín-Muñoz J.H., Cortes-Lozano W., Baquero-Herrera P.E., Ordóñez-Mora E.G., Cifuentes-Lobelo H.A. Reconstruction of the ascending reticular activating system with diffusion tensor tractography in patients with a disorder of consciousness after traumatic brain injury. Cureus. 2017;9(9) doi: 10.7759/CUREUS.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen T.W., de Tisi J., Vos S.B., Winston G.P., Duncan J.S., Wang Y., Taylor P.N. Multivariate white matter alterations are associated with epilepsy duration. Eur. J. Neurosci. 2021;53(8):2788. doi: 10.1111/EJN.15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlbarg V., Puybasset L., Tollard E., Lehéricy S., Benali H., Galanaud D. Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: a diffusion tensor imaging study using voxel-based approaches. Hum. Brain Mapp. 2009;30(12):3924–3933. doi: 10.1002/HBM.20817/FORMAT/PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Sullivan E.V., Hedehus M., Lim K.O., Adalsteinsson E., Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn. Reson. Med. 2000;44(2):259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rappaport M., Hall K.M., Hopkins K., Belleza T., Cope D.N. Disability rating scale for severe head trauma: coma to community. Arch. Phys. Med. Rehabil. 1982;63(3):118–123. [PubMed] [Google Scholar]
- Ropper A.H. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. New Engl. J. Med. 1986;314(15):953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- RStudio Team. (2020). R: A Language and Environment for Statistical Computing.
- Salat D.H., Tuch D.S., Hevelone N.D., Fischl B., Corkin S., Rosas H.D., Dale A.M. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann. N. Y. Acad. Sci. 2005;1064:37–49. doi: 10.1196/ANNALS.1340.009. [DOI] [PubMed] [Google Scholar]
- Sarica A., Cerasa A., Vasta R., Perrotta P., Valentino P., Mangone G., Guzzi P.H., Rocca F., Nonnis M., Cannataro M., Quattrone A. Tractography in amyotrophic lateral sclerosis using a novel probabilistic tool: a study with tract-based reconstruction compared to voxel-based approach. J. Neurosci. Methods. 2014;224:79–87. doi: 10.1016/J.JNEUMETH.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Schweitzer A.D., Niogi S.N., Whitlow C.T., Tsiouris A.J. Traumatic brain injury: imaging patterns and complications. Radiographics. 2019;39(6):1571–1595. doi: 10.1148/RG.2019190076/ASSET/IMAGES/LARGE/RG.2019190076.FIG11F.JPEG. [DOI] [PubMed] [Google Scholar]
- Setsompop K., Cohen-Adad J., Gagoski B.A., Raij T., Yendiki A., Keil B., Wedeen V.J., Wald L.L. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage. 2012;63(1):569–580. doi: 10.1016/j.neuroimage.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., Scott G., Leech R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 2014;10(3):156–166. doi: 10.1038/NRNEUROL.2014.15. [DOI] [PubMed] [Google Scholar]
- Sherer M., Nakase-Thompson R., Yablon S.A., Gontkovsky S.T. Multidimensional assessment of acute confusion after traumatic brain injury. Arch. Phys. Med. Rehabil. 2005;86(5):896–904. doi: 10.1016/J.APMR.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/J.NEUROIMAGE.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith D.H., Nonaka M., Miller R., Leoni M., Chen X.H., Alsop D., Meaney D.F. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J. Neurosurg. 2000;93(2):315–322. doi: 10.3171/jns.2000.93.2.0315. [DOI] [PubMed] [Google Scholar]
- Snider S.B., Bodien Y.G., Bianciardi M., Brown E.N., Wu O., Edlow B.L. Disruption of the ascending arousal network in acute traumatic disorders of consciousness. Neurology. 2019;93(13):E1281–E1287. doi: 10.1212/WNL.0000000000008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England) 1974;2(7872):81–84. doi: 10.1016/S0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Teasdale G., Maas A., Lecky F., Manley G., Stocchetti N., Murray G. The glasgow coma scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844–854. doi: 10.1016/S1474-4422(14)70120-6. [DOI] [PubMed] [Google Scholar]
- Turgeon A.F., Lauzier F., Simard J.F., Scales D.C., Burns K.E.A., Moore L., Zygun D.A., Bernard F., Meade M.O., Dung T.C., Ratnapalan M., Todd S., Harlock J., Fergusson D.A. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581–1588. doi: 10.1503/CMAJ.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata S., Ueda K., Sugihara G., Yassin W., Aso T., Fukuyama H., Murai T. Corpus callosum pathology as a potential surrogate marker of cognitive impairment in diffuse axonal injury. J. Neuropsychiatr. Clin. Neurosci. 2016;28(2):97–103. doi: 10.1176/APPI.NEUROPSYCH.15070159/ASSET/IMAGES/LARGE/APPI.NEUROPSYCH.15070159F3.JPEG. [DOI] [PubMed] [Google Scholar]
- van der Kouwe A.J.W., Benner T., Salat D.H., Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke, W., Leemans, A., & Emsell, L. (2016). DTI Analysis Methods: Voxel-Based Analysis. Diffusion Tensor Imaging: A Practical Handbook, 183–203. https://doi.org/10.1007/978-1-4939-3118-7_10.
- Venables, W. N., & Ripley, B. D. (2002). Modern Applied Statistics with S. https://doi.org/10.1007/978-0-387-21706-2.
- Wang J.Y., Bakhadirov K., Devous M.D., Abdi H., McColl R., Moore C., Marquez De La Plata C.D., Ding K., Whittemore A., Babcock E., Rickbeil T., Dobervich J., Kroll D., Dao B., Mohindra N., Madden C.J., Diaz-Arrastia R. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch. Neurol. 2008;65(5):619–626. doi: 10.1001/ARCHNEUR.65.5.619. [DOI] [PubMed] [Google Scholar]
- Warner M.A., De La Plata C.M., Spence J., Wang J.Y., Harper C., Moore C., Devous M., Diaz-Arrastia R. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J. Neurotrauma. 2010;27(12):2121. doi: 10.1089/NEU.2010.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.T.L., Pettigrew L.E.L., Teasdale G.M. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: guidelines for their use. J. Neurotrauma. 1998;15(8):573–580. doi: 10.1089/NEU.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Xu J., Rasmussen I.A., Lagopoulos J., Haberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J. Neurotrauma. 2007;24(5):753–765. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- Yendiki A., Panneck P., Srinivasan P., Stevens A., Zöllei L., Augustinack J., Wang R., Salat D., Ehrlich S., Behrens T., Jbabdi S., Gollub R., Fischl B. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinf. 2011;5:23. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A., Koldewyn K., Kakunoori S., Kanwisher N., Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh, E. L., Cooper, S. R., Mukherjee, P., Yue, J. K., Lingsma, H. F., Gordon, W. A., Valadka, A. B., Okonkwo, D. O., Schnyer, D. M., Vassar, M. J., Maas, A. I. R., Manley, G. T., Casey, S. S., Cheong, M., Dams-O’Connor, K., Hricik, A. J., Inoue, T., Menon, D. K., Morabito, D. J., … Sinha, T. K. (2014). Diffusion Tensor Imaging for Outcome Prediction in Mild Traumatic Brain Injury: A TRACK-TBI Study. Https://Home.Liebertpub.Com/Neu, 31(17), 1457–1477. https://doi.org/10.1089/NEU.2013.3171. [DOI] [PMC free article] [PubMed]
- Zhang J., Wei R.-L., Peng G.-P., Zhou J.-J., Wu M., He F.-P., Pan G., Gao J., Luo B.-Y. Correlations between diffusion tensor imaging and levels of consciousness in patients with traumatic brain injury: a systematic review and meta-analysis. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-02950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllei L., Jaimes C., Saliba E., Grant P.E., Yendiki A. TRActs constrained by UnderLying INfant anatomy (TRACULInA): an automated probabilistic tractography tool with anatomical priors for use in the newborn brain. Neuroimage. 2019;199:1–17. doi: 10.1016/j.neuroimage.2019.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have inserted a data availability statement in the text