Abstract
Introduction. Prognosis is an essential component of informed consent for medical decision making. Research shows that physicians display discrepancies in their prognostication, leading to variable, inaccurate, optimistic, or pessimistic prognosis. Factors driving these discrepancies and the supporting evidence have not been reviewed systematically. Methods. We undertook a scoping review to explore the literature on the factors leading to discrepancies in medical prognosis. We searched Medline (Ovid) and Embase (Ovid) databases for peer-reviewed articles from 1970 to 2017. We included articles that discussed prognosis variation or discrepancy and where factors influencing prognosis were evaluated. We extracted data outlining the participants, methodology, and prognosis discrepancy information and measured factors influencing prognosis. Results. Of 4,723 articles, 73 were included in the final analysis. There was significant variability in research methodologies. Most articles showed that physicians were pessimistic regarding patient outcomes, particularly in early trainees and acute care specialties. Accuracy rates were similar across all time periods. Factors influencing prognosis were clustered in 4 categories: patient-related factors (such as age, gender, race, diagnosis), physician-related factors (such as age, race, gender, specialty, training and experience, attitudes and values), clinical situation-related factors (such as physician-patient relationship, patient location, and clinical context), and environmental-related factors (such as country or hospital size). Discussion. Obtaining accurate prognostic information is one of the highest priorities for seriously ill patients. The literature shows trends toward pessimism, especially in early trainees and acute care specialties. While some factors may prove difficult to change, the physician’s personality and psychology influence prognosis accuracy and could be tackled using debiasing strategies. Exposure to long-term patient outcomes and a multidisciplinary practice setting are environmental debiasing strategies that may warrant further research.
Highlights
Literature on discrepancies in physician’s prognostication is heterogeneous and sparse.
Literature shows that physicians are mostly pessimistic regarding patient outcomes.
Literature shows that a physician’s personality and psychology influence prognostic accuracy and could be improved with evidence-based debiasing strategies.
Medical specialty strongly influences prognosis, with specialties exposed to acutely ill patients being more pessimistic, whereas specialties following patients longitudinally being more optimistic.
Physicians early in their training were more pessimist than more experienced physicians.
Keywords: prognosis, Bias, Physician, Ethics, Attitudes
Graphical Abstract.
This is a visual representation of the abstract.
Prognosis is the prediction of the patient’s disease trajectory and outcome and is the basis of many life-or-death decisions. Physicians’ and patients’ medical decisions are strongly influenced by prognosis,1–5 and arriving at an accurate prognosis is therefore essential to make informed treatment and care decisions.6–11 Prejudiced and pessimistic predictions may lead to premature or unnecessary cessation of life support in individuals who may have otherwise recovered.12,13 By contrast, optimistic predictions may lead to inappropriate treatments and do not prepare patients or their loved ones for death.14
Despite the need for patients and their families to be presented with realistic outcomes,15,16 the literature shows that physicians exhibit discrepancies in their prognosis.14,17 These discrepancies in prognostication can be measured either as a variation (such as comparing one physician’s prediction to another), an inaccuracy in comparison to a gold standard (such as outcomes in the literature), or an inaccuracy in comparison to a true measured prognosis (such as survival).
Discrepancies in prognosis can be analyzed based on an understanding of how individuals make judgments. Cognitive psychologists have shown that while decisions can be made under an analytical, slow, and resource-intensive mode, called type 2 processes, the majority of our decision making occurs under an intuitive, fast, and mostly unconscious mode, called type 1 processes.18,19 These cognitive short cuts involved in type 1 processes, otherwise known as heuristics, can be useful abbreviated ways of thinking, but they are more error prone. When these cognitive short cuts lead to systematic errors, they are called biases.19,20 Biases and heuristics are amplified in medical decision making, where decisions have more uncertain outcomes and are more emotionally laden.20 Most physician errors in clinical judgment can be attributed to their thought processes, as opposed to organizational errors, technical errors, or patient-related errors.20 These systematic cognitive errors can influence prognostic judgment toward prognosis discrepancy.21,22 Physicians may also integrate their personal biases into their predictions. For example, they may believe that relaying a favorable prognosis may improve outcomes by further cultivating a patient’s optimism and hope,23 a phenomenon known as positive iatrogenesis, while relaying a negative prognosis may be more damaging to not only a patient’s hope but also their outcomes.24
There is a substantial body of research on physicians’ decision making, but there is currently no exhaustive review on determinant factors influencing prognostic judgment toward prognosis discrepancy. The aims of this article are 1) to review the literature to identify the factors leading to prognosis discrepancies in medicine, 2) to list and categorize these factors, and 3) to explore and describe the prognosis discrepancy models presented in the literature.
Methods
Research Question and Scoping Review Methodology
We undertook a scoping review of the current literature on physicians’ medical prognostication to investigate the determinant factors leading to prognosis discrepancies. The scoping review is a structured review with a rigorous and transparent methodology25 that allows for the integration of information from various studies conducted using varying methodologies.26 We chose to use the scoping review methodology as developed by Arksey and O’Malley27 and refined in recent publications,28 notably in the context of medical ethics.29–32 This methodology allows us to access this broader topic while keeping a specific focus on the determinant factors influencing prognosis.
Data Sources and Search Strategy
We conducted an electronic literature search the Medline (Ovid) and Embase (Ovid) databases to comprehensively sample the literature in the fields of medicine, psychology, sociology, and ethics. A librarian was consulted to help construct, update, and run the search strategy. Because there are no current MeSH terms for factors leading to prognosis discrepancy, we used the following MeSH terms to create specific search strategies for each database: 1) prognosis: “prognosis” or “decision-making” or “clinical decision-making” or “withholding treatment” or “resuscitation orders”; 2) factors leading to discrepancy: “bias” or “attitude” or “attitude of health personnel” or “attitude to death” or “attitude to health” or “catastrophization” or “optimism” or “pessimism” or “precipitating factors” or “heuristics” or “observer variation” or “prejudice”; 3) physicians: “physicians” or “practice patterns, physicians.” Bibliographies of relevant studies and reviews were hand searched to identify any additional studies for inclusion but were not included in the initial search results.
Study Selection Criteria
All searches were downloaded to a reference database (Endnote X9.3.1, Clavirate Analytics) for an initial screening of titles and abstracts by 2 team members (AF and JP) after removal of duplicates. After the initial screening, if it was unclear whether the article titles and their respective abstracts met our selection criteria, the full text of these publications was retrieved. The 2 reviewers independently appraised these publications to identify those that reported on prognostication discrepancies and/or factors leading to prognostication discrepancy. Discrepancies between the reviewers were resolved through discussion.
We included all peer-reviewed articles published from January 1, 1970, to December 31, 2017. The following inclusion criteria were applied: 1) the article discussed prognosis discrepancy among physicians or 2) factors influencing prognosis were evaluated or measured. Exclusion criteria were developed by AF and confirmed by JP and ER (Figure 1). They consisted of 1) articles written in languages not spoken by the authors (English or French), 2) unrelated article topics or nonphysician populations, 3) nonclinically related papers (i.e., discussing decision making outside of the context of medical prognostication), 4) non-peer-reviewed material such as books and dissertations, 5) reviews, and 6) commentaries, editorials, letters, and opinion pieces. Due to the nature and intent of the scoping review, no restrictions relating to study design or scientific merit were imposed on the studies to avoid potentially excluding relevant data. While reviews were not included, their bibliographies were reviewed to identify relevant studies that may have otherwise been missed in our search.
Figure 1.
Literature sampling and screening process.
Data Content Extraction
After the retrieval of all relevant articles, we identified the predominant themes in the literature through an inductive process to develop a content extraction chart (AF; Supplemental Material). The content extraction chart was first reviewed with an interdisciplinary health ethics research group. The feedback from this initial review resulted in modifications such as the inclusion of additional details regarding article methodology and location of study, after which the chart was piloted with 10 articles for further precision and validation by JP and ER. The content extraction chart was refined (and then reapplied to the entire sample) during the data extraction phase. The final content extraction chart was divided into the following sections:
Article demographic information (title, authors, year, country of authors, country of study)
Study objectives and hypotheses
Study characteristics (research design, sampling, population, clinical context, measured outcomes)
Prognosis discrepancy type (interphysician variation, inaccuracy compared with a gold standard, inaccuracy compared with an actual outcome)
Prognosis discrepancy direction (optimist, pessimist, accurate, variation, or not measured)
Factors influencing prognosis (all studied and measured factors as well as those confirmed to influence prognosis)
Because there is no consensus on the categorization of quantitative study designs, especially regarding survey studies,33 we used different references33–36 to create an integrative categorization of quantitative studies applicable to 1) experimental studies, 2) observational studies, and 3) survey studies (Figure 2).
Figure 2.
Integrative categorization of quantitative methodology study designs.
We defined the prognosis discrepancy as optimistic if the article showed that physicians were more optimistic than the actual or gold standard prognosis, pessimistic if the article showed that physicians were more pessimistic than actual or gold standard prognosis, and accurate if the article showed that there was no over- or underestimation of outcomes (physicians were accurate) or that there was no interphysician variation. We defined the prognosis discrepancy as variation if the article measured the presence of discrepancy but did not measure if outcomes were over- or underestimated (for example interphysician variation) and the direction as not measured if the article did not measure prognosis discrepancy or stated only qualitative discrepancies.
Ethics
Ethics approval was not required for this work as it was a scoping review of the published literature and did not involve the collection of data from research participants.
Results
Out of 4,723 articles sampled, 73 met inclusion criteria and were included in the final analysis (Figure 1). Overall, results showed significant variability in the research designs and sampling methodologies, with no standard approach to study prognostic discrepancies. Most articles showed that physicians were pessimistic in their prognosis. This tendency remained over time. Factors leading to prognosis discrepancies can be grouped into 4 categories, including patient-related factors, physician-related factors, clinical situation–related factors, and environment-related factors.
Characteristics of Studies
Research designs
There was variability in research design methodologies to address physicians’ discrepancies in prognostication, including qualitative methodologies37 as well as quantitative methodologies ranging from randomized controlled trials38,39 to cross-sectional observational surveys.40,41
Research design was inconsistently and incompletely (41/73) reported with a lot of variation in the terminology used to describe each type of research design. For example, the constant-variable vignette method (Figure 2) was described as a “hypothetical vignette study,”42“case-based questionnaire survey,”43“prospective postal survey,”44“survey-based study,”45“survey study,”14,46“vignette questionnaire,”47 and “vignette study.”48–50 Some studies (3/73) did not report their research design.51–53
Population
The majority of articles (56/73) studied physician specialists, and the other studies assessed discrepancy among general practitioners (2/73). Some studies involved both specialists and general practitioners (14/73). One study did not specify the physician’s field of practice (1/73).54 The specialties studied were numerous and included internal medicine, intensive care, emergency, anesthesia, psychiatry, and pediatrics as well as surgical specialties such as general surgery, urology, neurosurgery, orthopedics, and obstetrics/gynecology, offering a good representation of several areas of medicine.
Clinical context
Prognostication was studied in 1) acute settings (24/73) where patients were in intensive care or in the emergency department, undergoing surgery, starting dialysis, and requiring transplant; (2) palliative settings (12/73), such as hospice care, terminally ill patients, patients with advanced cancer, and patients requiring placement of feeding tube; (3) perinatal settings (24/73), such as fetal surgery, premature infants at the limits of viability, trisomy 18 newborns and infants with hypoplastic left heart syndrome; and (4) chronic care settings (13/73), such as patient rehabilitation, patients in a vegetative state, patients with severe osteoarthritis, patients with breast, prostate, or lung cancer, and children with severe mental retardation (Figure 5).
Figure 5.
Clinical context of prognosis discrepancy studies.
Measured Outcomes
Measured outcomes to evaluate prognosis included measures of survival such as life expectancy (44/73), survival to a predefined period (from 1 mo to 10 y; 11/73), survival to discharge from hospital (6/73), or survival free of severe morbidity or disability (7/73).
Some studies also evaluated the length of hospitalization (2/73), length of sick leave (1/73), improved clinical or functional status (7/73), pain relief (2/73), physical and intellectual morbidity (20/73), and neurodevelopmental outcomes (1/73). Certain studies also measured predictions of quality of life (7/73). Some studies were more general in their outcome estimations, asking physicians to rate prognosis on a scale (1/73) or select between good or bad prognosis (3/73).
Physician Discrepancies
The direction of the discrepancy and prognosis discrepancy by specialty are shown in Figures 3 and 4.
Figure 3.

Type of physicians’ prognosis discrepancy. Optimist (18/73 articles): article showed physicians were more optimistic than the actual or gold standard prognosis. Pessimist (39/73 articles): article showed that physicians were more pessimistic than actual or gold-standard prognosis. Both optimist and pessimist (5/73 articles): article showed that physicians were both more pessimistic and optimistic than actual or gold-standard prognosis. Variation (10/73 articles): article measured the presence of discrepancy but did not measure if outcomes were over- or underestimated (for example. interphysician variation). Accurate (8/73 articles): article showed that there was no over- or underestimation of outcomes (physicians were accurate) or that there was no interphysician variation. Not measured (3/73 articles): article did not measure prognosis discrepancy or stated only qualitative discrepancies.
Figure 4.
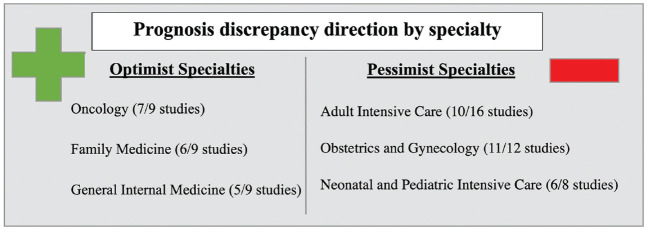
Prognosis discrepancy direction by specialty. Denominator represents the total articles studied for each specialty.
Prognosis discrepancy over time
The articles span from 1972 to 2017. Of the 73 articles, 12 were published from 1972 to 1989, 13 from 1990 to 1999, 25 from 2000 to 2009, and 25 from 2000 to 2017. Overall, the prognosis discrepancy directions (optimist, pessimist, accurate, variation, or not measured) were similar for each time period. Articles from the most recent period (2010–2017) described physicians as being primarily pessimistic regarding their prognosis.
Factors influencing prognosisPatient-related factors
Sociodemographic biases
A patient’s sociodemographic characteristics had no specific pattern of influence on the physician’s prognosis. One study found physicians to be more optimistic toward female patients and more discrepant in either optimistic or pessimistic directions toward African American and Latino patients.55 However, this finding was not reproduced in an experimental study using simulated patients with cancer identifying as White or Black, which showed no influence of race on physicians’ prognosis.38 Similarly, a survey study of neurologists and neurosurgeons14 and another study in an oncology setting56 found that physicians had a similar prognosis regardless of the patient’s race, language, or culture. In addition, while a previous study57 found no influence of a patient’s marital status on prognosis, 2 studies by Grulke et al. reported that physicians in stem cell transplant gave better survival prognostic estimates when their patient lived in a partnership, although partnership was not correlated to actual survival.58,59
Age and health status biases
Numerous studies showed that survival prognosis followed clinical determinants of outcomes.
Older patients were attributed higher mortality estimates14 and were more likely to be viewed as receiving futile treatment,6 correlating with higher actual mortality.6 Patients with multiple comorbidities had a less favorable survival prognosis.60 Quality-of-life prognosis was also dependent on the patient’s disease characteristics, such as the presence of sepsis in the context of cardiomyopathy.61 While the studies did not measure the accuracy of the use of those clinical risk factors affecting prognosis, they reported substantial of variability between physicians’ use of such factors in their prognosis.6,14,60 By contrast, with positive clinical determinants of outcomes such as when the patients were physically active and functioned fairly independently, the physician’s prognosis became more optimistic than actual survival.55
Patient population/diagnosis biases
Physicians were found to provide more discrepant prognosis for specific patient populations or primary diagnoses. One study showed that cancer patients, despite similar outcomes, received more optimistic predictions than AIDS patients did.57 In addition, AIDS patients were the least likely to receive accurate predictions. These findings were reproduced in a subsequent study in which the physician’s prognosis discrepancy increased toward more pessimistic predictions when patients had a diagnosis for illnesses other than cancer such as AIDS, chronic heart failure, or stroke.55 Likewise, a third study62 showed that a higher proportion of physicians had pessimistic perceptions of prognosis for malignancies with high cure rates sparking from long-standing achievements of medical research, compared with malignancies with similar cure rates but whose data were derived from more recent research advancements. Furthermore, physicians gave more optimist prognostic estimates for patients with chronic myelogenic leukemia than for patients with acute leukemia and non-Hodgkin lymphomas.59
A physician’s accuracy varied whether they had to make short-term or long-term predictions of survival. In a study of palliative radiation oncologists, the authors described that physicians were overly optimistic for patients who were predicted to die soon, giving them predictions that on average doubled the time to death compared with the actual time survived.63 They were more accurate or even pessimistic if patients had a longer life span of 6 mo or more. In other studies of palliative patients, a physician’s forecast did not differ in accuracy whether the patient had a short-term survival less than 4 week compared with longer-term survival of more than 4 week.64
Physician-related factors
Physician’s sociodemographic factors
The investigation of the influence of a physician’s sociodemographic factors on prognosis generated contrasting results. With regard to gender, 2 studies45,61 showed that female physicians were more optimistic in their prognosis than their male counterparts were. However, 2 other studies41,65 reported that female physicians were more pessimistic in their outcome predictions for preterm infants and infants with trisomy 18, whereas another study66 found no significant difference between female and male general practitioners’ predictions.
Physician race was also a notable factor influencing prognosis. For example, neonatologists who were non-White were most pessimistic regarding trisomy 18 infants’ neurodevelopmental outcome predictions.45 A practitioner’s personal illness history could also positively affect their prediction. For example, physicians with a personal history of cardiomyopathy were more optimistic when predicting their patient’s survival from cardiomyopathy.61
Physician’s training and medical knowledge
In general, younger and less experienced physicians were found to be more pessimistic and less accurate in their predictions. Many studies demonstrated that the more experienced the physician, measured either as years in practice, specialty training for a certain population, or frequency of encountering patients of a certain pathology, the less likely they were to make discrepant or pessimist predictions of their patient’s survival.55,57,61,65,67–69 More experienced physicians, as evidenced by objective factors such as the number of hospice referrals they made, produced more accurate predictions of their patients’ survival times55 and overall prognosis.57
The level of training of physicians was also found to be a factor influencing prognosis. Results showed that physicians early in their training (residents, house officers, fellows) exhibited 2 major patterns: they could be either more pessimistic6,62,69 and inaccurate,69,70 or, on the contrary, have similar predictions as staff attending physicians.61,71 Physicians who were up to date on the current literature and national guidelines were also more accurate in their prognosis.41,44 Physicians who would follow up on their patients longitudinally would also have more accurate prognosis.72
A physician’s specialty was a major factor responsible for the variability of prognosis.14,40,43,44,46,57,60,61,65,67,69,73–78 For a similar patient population, physicians from 2 different specialties could be at odds in their estimates of prognosis. For example, surgeons gave more pessimistic estimates of survival than nonsurgeons did,14 and oncologists tended to be less pessimistic in comparison with other nononcologic medical subspecialties,57 including internal medicine generalists73 and urologists.46 Their differing prognosis lead to either more57 or less73 accurate assessment of patient survival. For extremely premature infants, pediatricians were more accurate than obstetricians in their predictions of survival, with both significantly more pessimistic in their predictions of survival and rates of life without disability.78 In contrast, another study showed that obstetricians were more accurate than neonatologists in their estimates of survival and survival without severe disability, with both specialties reporting more pessimistic survival prognosis with at least a 22% underestimation of survival in comparison with outcomes in the literature.40 Neonatologists were much more pessimistic than pediatric pulmonologists for the outcomes of patients with trisomy 18 and thus were more likely to recommend comfort care, palliative care, or hospice care for that population.44
Physician’s psychology, attitudes, and values
A study of pediatricians’ survival predictions for premature newborns compared physicians’ predictions according to their self-rated attitude of being an optimist or pessimist.74 Optimists’ estimates of survival were accurate and comparable with actual survival rates, while pessimists’ estimates of survival consistently underestimated actual survival rates. The optimistic or pessimistic attitudes were similarly found to impact prognosis in a study of Italian neonatologists.49
Physicians were also found to be influenced in their prognosis by an ego bias in which they systematically judged the prognosis of their own patient to be better than that of similar patients in general.79 This ego bias could also be reversed, where physicians thought that their patients would have poorer prognosis than general survival rates, similar to what has been found in a study of critical care physicians.79
Clinical situation-related factors
Clinical context
The clinical environment in which physicians worked influenced predictions of outcomes. Physicians became more pessimistic if they had been on service for many days.6 Physicians were more pessimistic when a more severe patient case scenario was presented first.61 As well, physicians were more pessimistic when the survival question was framed as “living at least” instead of “dying within” that interval.61 The passage of time did not lessen the disagreement between physicians regarding an individual patient’s prognosis, and physicians maintained their initial prognosis estimate even after a few days of treating the patient,80 a phenomenon cognitive psychologists call anchoring bias. Pediatricians reported being influenced in their prognosis and treatment recommendation by parental wishes.3,44
While having access to prognostic scores influenced physicians toward optimism if the prognosis score was good or pessimism if the score was poor, there remained a wide variable range of predictions despite the use of these predictive tools.14,39
Patient location
Physicians were also influenced in their prognosis by where the patient was located, either prior to or during hospitalization.6,78,80 Prognosis were more pessimistic if the patient was previously in a nursing facility or long-term acute care facility and more optimistic if the patient was previously at home.6 Patients treated in medical intensive care had more prognosis of futility than those admitted to cardiac intensive care.6 In addition, the longer the length of a patient’s admission, the more the predictions would tend toward futility of care.6 While these factors may reflect poorer or better health condition linked to more negative or positive outcomes, there was substantial variation in physicians’ assessments and a poor correlation with actual survival.
Physician-patient relationship
A stronger physician-patient relationship was associated with lower prognostic accuracy.57 Indeed, physicians who had the longest professional relationship with their patients were more erroneous.57 The more recent their examination of the patient, the more pessimistic and less accurate their prediction would become.57 Physicians who had seen many similar cases in the last year of practice tended to be more pessimistic.14 Physicians judged the prognosis of their own patients to be different than that of critically ill patients in general.79
Environment-related factors
Hospital related
The type and size of medical practice were shown to affect prognosis, with physicians from academic hospitals being less pessimistic than their community counterparts62 and physicians from larger cities being more accurate in their predictions than those working in smaller towns.67 Other studies found no impact of the nature of the physician’s practice72 or the characteristics of the hospital on their estimates.3,41,75,81
Practice related
Physicians felt that the medico-legal environment in which they practiced influenced their willingness to resuscitate premature infants,3 and physicians presented with a litigious family would change their opinion to favor active treatment when they had initially offered a grim prognosis.42
Geographical
The geographical locations of physicians’ practices influenced their prognosis. In one study, physicians in Italy, France, Spain, and the United States were found to be overall more pessimistic than those practicing in Sweden or Japan regarding the survival of premature newborns, whereas all these countries have similar level of neonatal intensive care.49 The heterogeneity of prognosis remained significant even within countries49,82 with, for example, more pessimistic predictions from physicians of working in the Western areas of the United States in comparison with physicians in the Northeast or Southern regions.14
Discussion
Prognosis is a crucial component of medicine and is likely to affect decision making of both the physician and the patient. Improvement of prognosis accuracy to reduce error and improve decision making necessitates an understanding of what factors influence prognosis. Prognosis has been the object of several studies, but no literature review has attempted to review and analyze this literature to identify different factors influencing prognosis.
Overall, this scoping review of the literature on physician medical prognostication and the factors leading to prognosis discrepancies showed that the literature on prognosis discrepancy is heterogeneous and sparse. The articles in this review adapted many different types of research designs. Using a detailed content extraction strategy, we found that only a few studies appropriately reported their research design, and many of the articles were actually not designed to identify as their main outcome the factors influencing prognosis. This may explain why different studies examining a specific factor would find different and sometimes contradictory outcomes. Thus, we must be careful not to overstate our observations. Future studies should aim at developing standard approaches to identify factors influencing prognosis. Our categorization of quantitative studies (Figure 2) could potentially be used in future studies as a basis to enhance the selection and reporting of the methodological approach adopted by investigators. A body of research with similar methodology and population (real-life or simulated data, evaluation of similar outcomes such as survival or quality of life) would yield stronger noncontradictory results to further our understanding our prognostication biases. Thus, there is a real opportunity to advance the science of prognostication.
This review showed that almost half of physicians’ discrepancies of prognosis were pessimistic in comparison with actual survival or rates in the literature, whereas a quarter of articles showed that physicians were, on the contrary, optimistic in their prognosis. Only a minority of articles showed that physicians were accurate in their prognosis. The discrepancies in prognostication that lead physicians to overestimate or underestimate survival, outcomes of disease, and quality of life could be attributed to many identified factors. These factors could be categorized into 4 categories, namely, factors related to the patient, the treating physician, the clinical situation, and the environment (Table 1).
Table 1.
Clinical-Related Factors Influencing Prognosis
| Confirmed Factors | Unconfirmed Factors | |
|---|---|---|
| Patient-related factors | ||
| Patient’ssociodemographics | Gender55 | Level of education Income Insurance status/ability to pay Parental age Language Culture Religion Having minor children Living alone Living distance from home to hospital |
| Age6,14,43,60 | ||
| Race55 | ||
| Partnership status58,59 | ||
| Patient population | Baseline health status55 | Nursing home resident |
| Gestational age at birth40 | ||
| Birth weight3 | ||
| Parental parity/fertility3 | ||
| Patient’s diseasecharacteristics | Diagnosis55,57–59,61,62,98 | Disease duration Presence of heart rate at 24 wk of gestation |
| Disease characteristics6,14,43,58–61 | ||
| Treatment characteristics58,59 | ||
| Short-term or long-term actual survival63 | ||
| Physician-related factors | ||
| Physician’ssociodemographics | Gender41,45,61,65 | Being a parent |
| Age62 | ||
| Race45,55 | ||
| Personal illness history61 | ||
| Physician’s medicalknowledge and training | Specialty14,40,43,44,46,57,60,61,65,67,69,73–78 | Years of training Board certification Prestige of medical school Educational intervention |
| Level of training6,62,70,79 | ||
| Experience6,62,70,79 | ||
| Knowledge of current outcomes41,44 Averaged prognosis versus individual prognosis70 | ||
| Exposure to follow-up of patients72 | ||
| Physician’s psychology,attitudes, and values | Ethical values49 | Importance of religion/being
religious Political orientation Having a misconception regarding the prognosis of patients with a certain malignancy Physician’s preference for rehabilitation setting Empathy Views on abortion Personal beliefs Fear of litigation |
| Optimist or pessimist attitude49,74 | ||
| Physician’s baseline opinion of best judgment (to resuscitate or not)42 | ||
| Ego bias79 | ||
| Clinical-related factors | ||
| Clinical context | Clinical case order of presentation61 | Discussion with colleagues |
| Number of similar cases seen in the last year14 | ||
| Physician day on clinical service6 | ||
| Parental wishes for their child3,44 | ||
| Access to prognostic score tools14,39 | ||
| Patient location | Location prior to admission to hospital6 | Length of palliative consultations Proportion of case mix with palliative intent |
| In-hospital location6,78,80 | ||
| Inpatient versus outpatient6 | ||
| Length of hospitalization6 | ||
| Hospitalization day of patient6,78 | ||
| Physician-patientrelationship | Recentness of patient-physician contact57 | Frequency of patient-physician contact |
| Duration of patient-physician contact57 | ||
| Physician’s own patient79 | ||
| Environment-related factors | ||
| Hospital related | Hospital size and type62 | Number of births/deliveries at the hospital Presence of fetal monitors and ultrasoundequipment |
| Practice related | Medico-legal environment3,42 | University affiliation Compensation structure (billing/salary) Number of physicians in group practice Multidisciplinary group or not Clinical context (intensive care unit or clinic visit) |
| Geographic factors | Geographic location (country/region/state)14,48,49,82 Town size67 |
City or rural area Study center/institution |
Over the past 50 years covered by this review, the sheer accumulation of scientific evidence, improved clinical knowledge, and technologies could be expected to improve prognostic accuracy, but the situation seems more complex, as there appears to be ongoing challenges, defying this common assumption given the persistence of prognosis discrepancies. This could be related to the fact that our review was not designed to assess whether prognostic accuracy improved over time and across specialties given the heterogeneity of research designs and the wide-ranging clinical contexts studied. It may also be related to the fact that lack of knowledge does not seem to be a major contributing factor. For example, educational interventions,83 in which physicians were given improved access to accurate prognostic information, did not improve their prognosis. There may be more insidious or complex factors contributing to prognosis discrepancy stemming from the fact that prognosis is a human judgment. Likewise, this review identified multiple factors related to a physician’s personality and psychology that may influence their prognosis such as their ethical values, an optimist or pessimist attitude toward life, their baseline opinion of what is the best course of action for a given patient population, or the psychological distortion based on ego bias that their own patients may fare better than their colleague’s patients. However, other personality factors such as religion, political orientation, views on abortion, and other personal beliefs were not found to influence prognosis.
One of the most frequently studied factors influencing prognosis was medical specialty. Physicians in specialties that see patients solely when they are acutely ill (i.e., intensive care physicians, emergency physicians, neonatologists) tended to be pessimistic, whereas physicians in specialties that follow patients longitudinally also when they are well (such as general practitioners, general internal medicine, and pediatrics) were instead optimistic in their prognosis. The pessimism in prognostication by acute care specialties could be explained by the lack of exposure of those physicians to patients in their well or recovered state, leading them to see only the worst-case scenarios. This is especially the case in more subjective evaluations of well-being and quality of life. If a physician sees their patients in only their most disabled state, when they are in pain and in a medicalized context, it becomes challenging for them to visualize a patient enjoying their life despite their impairments. There is therefore a rift between a physician’s predicted poor quality of life with impairments and how patients actually rate their quality of life.84 In this review, 1 article showed that physicians having long-term follow-up of their patients would improve their prognostic accuracy.72 While this factor was sparsely studied, the exposure to longitudinal patient outcomes acts as a positive feedback loop for physicians in acute care settings whose prognosis is essential to life-or-death decision making and thus may be a worthwhile avenue for further study.85
The limited follow-up may also explain why physicians early in their training (residents, fellows), who take care of patients mostly in acute settings without the opportunity to follow them over time or interact with them when they are well, may be biased toward thinking that all the patients they come across in these settings have unfavorable outcomes. As reflected in this review, physicians early in their training were more pessimistic or inaccurate, while physicians with more experience, training, and knowledge of current literature had less discrepant prognosis. The impact that difficult and negative patient outcomes experienced by physicians during medical training has on shaping physicians’ minds brings fascination and interest, as reflected by the worldwide success of the medical novel book The House of God by author Samuel Shem, a satire of the impact of such negative experiences on the personalities of physicians in training.86–88 Follow-up opportunities for exposure to patients in their recovered or well state may mitigate this effect.89
It is interesting to note that a stronger doctor-patient relationship was associated with less accurate prognosis. As such, physicians became pessimistic the more they knew their patients over time or when they had recently examined them, whereas physicians with less contact or long-term knowledge of the patient gave a more accurate prognosis. This may be because, as the physician has a stronger personal involvement in the doctor-patient relationship, the predictions become more (positively or negatively) emotionally laden and therefore less objective. This could also be a case of reverse ego bias, in which physicians believe their own patients fare worse than their colleague’s patients do.79
While many of the factors identified in the articles of this review cannot be modified in practice, such as the patient’s characteristics or environmental factors, other factors such as the physician-related factors and the clinical situation-related factors may be tempered to improve prognosis accuracy. These modifiable factors related to the physician’s attitudes and values may be even more important to address now that it has been demonstrated that physicians do not recognize the importance of these factors and their own potential biases on their clinical judgment. Physicians tend to think that their prognosis is influenced by the patient’s characteristics and their clinical experience, instead of their work environment, their attitudes, or their personality.90,91 That being the case, debiasing interventions in health care settings have been shown to be effective.92
Debiasing strategies can aim to modify a person’s more deeply entrenched attitudes and habits. As such, self-awareness of one’s attitudes and biases toward a patient population are part of the strategies used in cognitive debiasing methodologies that could be used to lessen the impact of patient-related factors on prognosis discrepancies.93 Such strategies have been studied to reduce bias in diagnosis94–96 but not yet applied to prognostic judgment. Physicians could benefit from educative and self-reflective approaches such as the ouR-HOPE model.97 This model draws from research on the neurological prognosis of infants to foster reflection about biases and implicit assumptions about quality of life. The application of such strategies to reduce prognostic biases should be further studied in order to implement them into current clinical practice as well as the medical curriculum for new generations of physicians.
Debiasing strategies can also aim to modify the environment to decrease high-risk situations of bias.93 With respect to our results, a multidisciplinary practice environment, one that may include obtaining a second opinion from more experienced physicians or from other allied health care professionals, could help mitigate prognosis discrepancies by increasing the number of individuals developing a prognostic judgment, which would serve as a cognitive strategy to reduce bias.70,94,95
Conclusion
Obtaining accurate prognostic information to improve decision making and reduce error is a high priority for seriously ill patients. Improvement of prognosis accuracy necessitates an understanding of the factors influencing prognosis. Many factors influencing physicians’ prognosis including patient-related factors, physician-related factors, clinical situation-related factors, and environment-related factors have been highlighted in the current body of literature. This scoping review identified significant heterogeneity of study designs in the literature on the subject of factors leading to prognosis discrepancies. This observation emphasizes the need for further studies focused on studying explicitly factors influencing prognosis (as a main outcome) such that the methodological rigor of studies be increased.
Clinical strategies to reduce the impact of factors leading to biases in prognostication could include increased exposure to long-term outcomes of acutely ill patients, adoption of an attitude of humility toward patient self-evaluation of quality of life, use of proven debiasing strategies, and a multidisciplinary practice environment in which second opinions are openly sought.
While some factors may prove difficult to act upon, evidence-based debiasing strategies can help mitigate important and modifiable factors, such as physicians’ attitudes.
Supplemental Material
Supplemental material, sj-xlsx-1-mpp-10.1177_23814683221145158 for Factors Influencing Physician Prognosis: A Scoping Review by Amaryllis Ferrand, Jelena Poleksic and Eric Racine in MDM Policy & Practice
Acknowledgments
We would like to thank members of the Pragmatic Health Ethics Research Unit for helpful feedback on a previous version of this article as well as Patrice Dupont for literature search and methodological advice.
Footnotes
This work was presented as a poster presentation at the Montreal Clinical Research Institute Scientific Forum, Mont-Gabriel, Quebec, 2018.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Amaryllis Ferrand’s doctoral research is supported by a Vanier Canada Graduate scholarship. Eric Racine’s research is supported by a career award of the Fonds de recherche du Québec-Santé. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
ORCID iD: Amaryllis Ferrand  https://orcid.org/0000-0002-3078-354X
https://orcid.org/0000-0002-3078-354X
Supplemental Material: Supplementary material for this article is available on the MDM Policy & Practice Web site at https://journals.sagepub.com/home/mpp.
Contributor Information
Amaryllis Ferrand, Pragmatic Health Ethics Research Unit, Montreal Clinical Research Institute, Montreal, QC, Canada; Faculty of Medicine, Department of Biomedical Sciences, University of Montreal, Montreal, QC, Canada; Jewish General Hospital, Division of Neonatal-Perinatal Medicine, Department of Pediatrics, McGill University, Montreal, QC, Canada.
Jelena Poleksic, Pragmatic Health Ethics Research Unit, Montreal Clinical Research Institute, Montreal, QC, Canada; Faculty of Medicine, University of Western Ontario, London, ON, Canada.
Eric Racine, Pragmatic Health Ethics Research Unit, Montreal Clinical Research Institute, Montreal, QC, Canada; Departments of Medicine and Social and Preventive Medicine, University of Montreal, Montreal, Canada; Biomedical Ethics Unit, McGill University, Montreal, QC, Canada.
References
- 1. Munro M, Yu VY, Partridge JC, Martinez AM. Antenatal counselling, resuscitation practices and attitudes among Australian neonatologists towards life support in extreme prematurity. Aust N Z J Obstet Gynaecol. 2001;41(3):275–80. [DOI] [PubMed] [Google Scholar]
- 2. Bottoms SF, Paul RH, Iams JD, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. Am J Obstet Gynecol. 1997;176:960–6. DOI: 10.1016/s0002-9378(97)70386-7 [DOI] [PubMed] [Google Scholar]
- 3. Sanders MR, Donohue PK, Oberdorf MA, Rosenkrantz TS, Allen MC. Perceptions of the limit of viability: neonatologists’ attitudes toward extremely preterm infants. J Perinatol. 1995;15:494–502. [PubMed] [Google Scholar]
- 4. Gripp S, Moeller S, Bolke E, et al. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol. 2007;25:3313–20. DOI: 10.1200/JCO.2006.10.5411 [DOI] [PubMed] [Google Scholar]
- 5. Schoenborn NL, Bowman TL, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neville TH, Wiley JF, Holmboe ES, et al. Differences between attendings’ and fellows’ perceptions of futile treatment in the intensive care unit at one academic health center: implications for training. Acad Med. 2015;90:324–30. DOI: 10.1097/ACM.0000000000000617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xafis V, Wilkinson D, Sullivan J. What information do parents need when facing end-of-life decisions for their child? A meta-synthesis of parental feedback. BMC Palliat Care. 2015;14:19. DOI: 10.1186/s12904-015-0024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Veatch RM. Abandoning informed consent. Hastings Cent Rep. 1995;25:5–12. [PubMed] [Google Scholar]
- 9. Minkoff H, Zafra K, Amrita S, Wilson TE, Homel P. Physician morality and perinatal decisions. Eur J Obstet Gynecol Reprod Biol. 2016;206:36–40. DOI: 10.1016/j.ejogrb.2016.08.042 [DOI] [PubMed] [Google Scholar]
- 10. Tysdahl C, Tysdahl T, Wendt J, Wendt L, Feltman DM. Helping families navigate center variability in antenatal counseling for extremely early births. Pediatrics. 2019;144:e20191625. DOI: 10.1542/peds.2019-1625 [DOI] [PubMed] [Google Scholar]
- 11. Bernat JL. Ethical aspects of determining and communicating prognosis in critical care. Neurocrit Care. 2004;1:107–17. DOI: 10.1385/ncc:1:1:107 [DOI] [PubMed] [Google Scholar]
- 12. Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56:766–72. DOI: 10.1212/wnl.56.6.766 [DOI] [PubMed] [Google Scholar]
- 13. Backes CH, Soderstrom F, Agren J, et al. Outcomes following a comprehensive versus a selective approach for infants born at 22 weeks of gestation. J Perinatol. 2019;39:39–47. DOI: 10.1038/s41372-018-0248-y [DOI] [PubMed] [Google Scholar]
- 14. Zahuranec DB, Fagerlin A, Sanchez BN, et al. Variability in physician prognosis and recommendations after intracerebral hemorrhage. Neurology. 2016;86:1864–71. DOI: 10.1212/WNL.0000000000002676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson WG, Cimino JW, Ernecoff NC, et al. A multicenter study of key stakeholders’ perspectives on communicating with surrogates about prognosis in intensive care units. Ann Am Thorac Soc. 2015;12:142–52. DOI: 10.1513/AnnalsATS.201407-325OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evans LR, Boyd EA, Malvar G, et al. Surrogate decision-makers’ perspectives on discussing prognosis in the face of uncertainty. Am J Respir Crit Care Med. 2009;179:48–53. DOI: 10.1164/rccm.200806-969OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow E, Harth T, Hruby G, Finkelstein J, Wu J, Danjoux C. How accurate are physicians’ clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? A systematic review. Clin Oncol (R Coll Radiol). 2001;13:209–18. DOI: 10.1053/clon.2001.9256 [DOI] [PubMed] [Google Scholar]
- 18. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58–64. DOI: 10.1136/bmjqs-2012-001712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124–31. DOI: 10.1126/science.185.4157.1124 [DOI] [PubMed] [Google Scholar]
- 20. Stiggelbout AM, De Vries MC, Scherer LD. Medical decision making. In: Keren G, Wu G, eds. The Wiley Blackwell Handbook of Judgment and Decision Making. 1st ed. Chichester (UK): John Wiley & Sons; 2015. p 775–99. [Google Scholar]
- 21. Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Making. 2015;35:539–57. DOI: 10.1177/0272989X14547740 [DOI] [PubMed] [Google Scholar]
- 22. Crupi V, Elia F, Apra F, Tentori K. Double conjunction fallacies in physicians’ probability judgment. Med Decis Making. 2018;38:756–60. DOI: 10.1177/0272989X18786358 [DOI] [PubMed] [Google Scholar]
- 23. Delvecchio Good MJ, Good BJ, Schaffer C, Lind SE. American oncology and the discourse on hope. Cult Med Psychiatry. 1990;14:59–79. [DOI] [PubMed] [Google Scholar]
- 24. Miyaji NT. The power of compassion: truth-telling among American doctors in the care of dying patients. Soc Sci Med. 1993;36:249–64. [DOI] [PubMed] [Google Scholar]
- 25. Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5:371–85. DOI: 10.1002/jrsm.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. DOI: 10.1111/j.1471-1842.2009.00848.x [DOI] [PubMed] [Google Scholar]
- 27. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. DOI: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 28. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. DOI: 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saigle V, Seguin M, Racine E. Identifying gaps in suicide research: a scoping review of ethical challenges and proposed recommendations. IRB. 2017;39:1–9. [PubMed] [Google Scholar]
- 30. Saigle V, Dubljević V, Racine E. The impact of a landmark neuroscience study on free will: a qualitative analysis of articles using libet and colleagues’ methods. AJOB Neurosci. 2018;9:29–41. DOI: 10.1080/21507740.2018.1425756 [DOI] [Google Scholar]
- 31. Bogossian A, Gorter JW, Racine E. Protocol for a scoping review about ethics in transition programmes for adolescents and young adults with neurodisabilities. BMJ Open. 2018;8:e020914. DOI: 10.1136/bmjopen-2017-020914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ewusi-Boisvert E, Racine E. A critical review of methodologies and results in recent research on belief in free will. Neuroethics. 2018;11:97–110. DOI: 10.1007/s12152-017-9346-3 [DOI] [Google Scholar]
- 33. Stoop I, Harrison E. Classification of surveys. In: Gideon L, ed. Handbook of Survey Methodology for the Social Sciences. New York: Springer, 2012. p 7–21. [Google Scholar]
- 34. Jacoby A, Smith M, Eccles M. A qualitative study to explore influences on general practitioners’ decisions to prescribe new drugs. Br J Gen Pract. 2003;53:120–5. [PMC free article] [PubMed] [Google Scholar]
- 35. Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods approaches. 5th ed. Thousand Oaks (CA): SAGE; 2017. p 304. [Google Scholar]
- 36. Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126:2234–42. DOI: 10.1097/PRS.0b013e3181f44abc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blumenthal-Barby JS, Krieger H, Wei A, et al. Communication about maternal-fetal surgery for myelomeningocele and congenital diaphragmatic hernia: preliminary findings with implications for informed consent and shared decision-making. J Perinat Med. 2016;44:645–53. DOI: 10.1515/jpm-2015-0039 [DOI] [PubMed] [Google Scholar]
- 38. Barnato AE, Mohan D, Downs J, et al. A randomized trial of the effect of patient race on physicians’ intensive care unit and life-sustaining treatment decisions for an acutely unstable elder with end-stage cancer. Crit Care Med. 2011;39:1663–9. DOI: 10.1097/CCM.0b013e3182186e98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sacks GD, Dawes AJ, Ettner SL, et al. Impact of a risk calculator on risk perception and surgical decision making: a randomized trial. Ann Surg. 2016;264:889–95. [DOI] [PubMed] [Google Scholar]
- 40. Chan KL, Kean LH, Marlow N. Staff views on the management of the extremely preterm infant. Eur J Obstet Gynecol Reprod Biol. 2006;128:142–7. DOI: 10.1016/j.ejogrb.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 41. Power ML, Henderson Z, Behler JE, Schulkin J. Attitudes and practices regarding late preterm birth among American obstetrician-gynecologists. J Womens Health (Larchmt). 2013;22:167–72. DOI: 10.1089/jwh.2012.3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ballard DW, Li Y, Evans J, Ballard RA, Ubel PA. Fear of litigation may increase resuscitation of infants born near the limits of viability. J Pediatr. 2002;140:713–8. DOI: 10.1067/mpd.2002.124184 [DOI] [PubMed] [Google Scholar]
- 43. Hasenbein U, Kuss O, Bäumer M, Schert C, Schneider H, Wallesch CW. Physicians’ preferences and expectations in stroke rehabilitation—results of a case-based questionnaire survey. Disabil Rehabil. 2002;24:954–60. DOI: 10.1080/0963828021000007888 [DOI] [PubMed] [Google Scholar]
- 44. Hurley EH, Krishnan S, Parton LA, Dozor AJ. Differences in perspective on prognosis and treatment of children with trisomy 18. Am J Med Genet A. 2014;164A:2551–6. DOI: 10.1002/ajmg.a.36687 [DOI] [PubMed] [Google Scholar]
- 45. Jacobs AP, Subramaniam A, Tang Y, et al. Trisomy 18: a survey of opinions, attitudes, and practices of neonatologists. Am J Med Genet A. 2016;170:2638–43. [DOI] [PubMed] [Google Scholar]
- 46. Kim SP, Gross CP, Nguyen PL, et al. Specialty bias in treatment recommendations and quality of life among radiation oncologists and urologists for localized prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:163–9. DOI: 10.1038/pcan.2014.3 [DOI] [PubMed] [Google Scholar]
- 47. Kua JH, Parker G, Lee C, Jorm AF. Beliefs about outcomes for mental disorders: a comparative study of primary health practitioners and psychiatrists in Singapore. Singapore Med J. 2000;41:542–7. [PubMed] [Google Scholar]
- 48. Kuehlmeyer K, Palmour N, Riopelle RJ, Bernat JL, Jox RJ, Racine E. Physicians’ attitudes toward medical and ethical challenges for patients in the vegetative state: comparing Canadian and German perspectives in a vignette survey. BMC Neurol. 2014;14:119. DOI: 10.1186/1471-2377-14-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paterlini G, Tagliabue P. Decision making in neonatologia. Minerva Pediatr. 2010;62:121–3. [PubMed] [Google Scholar]
- 50. Clarke MG, Ewings P, Hanna T, Dunn L, Girling T, Widdison AL. How accurate are doctors, nurses and medical students at predicting life expectancy? Eur J Intern Med. 2009;20:640–4. DOI: 10.1016/j.ejim.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 51. Brannen AL, II, Godfrey LJ, Goetter WE. Prediction of outcome from critical illness: a comparison of clinical judgment with a prediction rule. Arch Intern Med. 1989;149:1083–86. [PubMed] [Google Scholar]
- 52. Parkes CM. Accuracy of predictions of survival in later stages of cancer. Br Med J. 1972;2:29–31. DOI: 10.1136/bmj.2.5804.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Durham G, Durham J. General practitioners’ ability to predict outcome for elderly patients admitted to acute medical beds. N Z Med J. 1990;103:585–7. [PubMed] [Google Scholar]
- 54. Knaus WA, Harrell FE, Jr, Lynn J, et al. The SUPPORT prognostic model. Objective estimates of survival for seriously ill hospitalized adults. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1995;122:191–203. DOI: 10.7326/0003-4819-122-3-199502010-00007 [DOI] [PubMed] [Google Scholar]
- 55. Alexander M, Christakis NA. Bias and asymmetric loss in expert forecasts: a study of physician prognostic behavior with respect to patient survival. J Health Econ. 2008;27:1095–108. DOI: 10.1016/j.jhealeco.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 56. Butow PN, Sze M, Eisenbruch M, et al. Should culture affect practice? A comparison of prognostic discussions in consultations with immigrant versus native-born cancer patients. Patient Educ Couns. 2013;92:246–52. DOI: 10.1016/j.pec.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 57. Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–72. DOI: 10.1136/bmj.320.7233.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grulke N, Bailer H. Facing haematopoietic stem-cell transplantation: do patients and their physicians agree regarding the prognosis? Psycho-Oncol. 2010;19:1035–43. DOI: 10.1002/pon.1671 [DOI] [PubMed] [Google Scholar]
- 59. Grulke N, Bunjes D, Larbig W, Kächele H, Bailer H. Physicians’ prognostic estimates of survival for patients undergoing allogeneic hematopoietic stem cell transplantation. J Psychosom Res. 2008;65:61–6. DOI: 10.1016/j.jpsychores.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 60. Turgeon AF, Lauzier F, Burns KE, et al.; Canadian Critical Care Trials Group. Determination of neurologic prognosis and clinical decision making in adult patients with severe traumatic brain injury: a survey of Canadian intensivists, neurosurgeons, and neurologists. Crit Care Med. 2013;41:1086–93. DOI: 10.1097/CCM.0b013e318275d046 [DOI] [PubMed] [Google Scholar]
- 61. Quartin AA, Calonge RO, Schein RM, Crandall LA. Influence of critical illness on physicians’ prognoses for underlying disease: a randomized study using simulated cases. Crit Care Med. 2008;36:462–70. DOI: 10.1097/01.CCM.0B013E3181611F968 [DOI] [PubMed] [Google Scholar]
- 62. Goldvaser H, Milman Y, Dujovni T, et al. Perception of prognosis of cancer patients by non-oncologists. Int J Clin Pract. 2016;70:1027–32. [DOI] [PubMed] [Google Scholar]
- 63. Chow E, Davis L, Panzarella T, et al. Accuracy of survival prediction by palliative radiation oncologists. Int J Radiat Oncol Biol Phys. 2005;61:870–3. DOI: 10.1016/j.ijrobp.2004.07.697 [DOI] [PubMed] [Google Scholar]
- 64. Bruera E, Miller MJ, Kuehn N, MacEachern T, Hanson J. Estimate of survival of patients admitted to a palliative care unit: a prospective study. J Pain Symptom Manage. 1992;7:82–6. DOI: 10.1016/0885-3924(92)90118-2 [DOI] [PubMed] [Google Scholar]
- 65. Wilkinson DJ, de Crespigny L, Lees C, et al. Perinatal management of trisomy 18: a survey of obstetricians in Australia, New Zealand and the UK. Prenat Diagn. 2014;34:42–9. DOI: 10.1002/pd.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ericson Sjöström M, Wallin I, Strandhagen E, Baigi A, Hensing G, Björkelund C. GP and patient predictions of sick-listing duration: how well do they correspond? A prospective observational study. Scand J Prim Health Care. 2014;32:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wilson AL, Wellman LR, Fenton LJ, Witzke DB. What physicians know about the prognosis of preterm newborns. Am J Dis Child. 1983;137:551–4. DOI: 10.1001/archpedi.1983.02140320027004 [DOI] [PubMed] [Google Scholar]
- 68. McElrath TF, Norwitz ER, Nour N, Robinson JN. Contemporary trends in the management of delivery at 23 weeks’ gestation. Am J Perinatol. 2002;19:9–15. DOI: 10.1055/s-2002-20176 [DOI] [PubMed] [Google Scholar]
- 69. Poses RM, Bekes C, Copare FJ, Scott WE. The answer to “What are my chances, doctor?” depends on whom is asked: prognostic disagreement and inaccuracy for critically ill patients. Crit Care Med. 1989;17:827–33. DOI: 10.1097/00003246-198908000-00021 [DOI] [PubMed] [Google Scholar]
- 70. Poses RM, Bekes C, Winkler RL, Scott WE, Copare FJ. Are two (inexperienced) heads better than one (experienced) head? Averaging house officers’ prognostic judgments for critically ill patients. Arch Intern Med. 1990;150:1874–8. [PubMed] [Google Scholar]
- 71. Allegretti AS, Hundemer G, Chorghade R, Cosgrove K, Bajwa E, Bhan I. Perspectives of continuous renal replacement therapy in the intensive care unit: a paired survey study of patient, physician, and nurse views. BMC Nephrol. 2015;16:105. DOI: 10.1186/s12882-015-0086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tseng YD, Krishnan MS, Sullivan AJ, Jones JA, Chow E, Balboni TA. How radiation oncologists evaluate and incorporate life expectancy estimates into the treatment of palliative cancer patients: a survey-based study. Int J Radiat Oncol Biol Phys. 2013;87:471–8. DOI: 10.1016/j.ijrobp.2013.06.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rose JH, O’Toole EE, Dawson NV, et al. Generalists and oncologists show similar care practices and outcomes for hospitalized late-stage cancer patients. SUPPORT Investigators. Study to understand prognoses and preferences for outcomes and risks for treatment. Med Care. 2000;38:1103–18. DOI: 10.1097/00005650-200011000-00005 [DOI] [PubMed] [Google Scholar]
- 74. Haywood JL, Morse SB, Goldenberg RL, Bronstein J, Nelson KG, Carlo WA. Estimation of outcome and restriction of interventions in neonates. Pediatrics. 1998;102:e20. DOI: 10.1542/peds.102.2.e20 [DOI] [PubMed] [Google Scholar]
- 75. Coyte PC, Hawker G, Croxford R, Attard C, Wright JG. Variation in rheumatologists’ and family physicians’ perceptions of the indications for and outcomes of knee replacement surgery. J Rheumatol. 1996;23:730–8. [PubMed] [Google Scholar]
- 76. Haywood JL, Goldenberg RL, Bronstein J, Nelson KG, Carlo WA. Comparison of perceived and actual rates of survival and freedom from handicap in premature infants. Am J Obstet Gynecol. 1994;171:432–9. DOI: 10.1016/0002-9378(94)90279-8 [DOI] [PubMed] [Google Scholar]
- 77. Perez EA. Perceptions of prognosis, treatment, and treatment impact on prognosis in non-small cell lung cancer. Chest. 1998;114:593–604. DOI: 10.1378/chest.114.2.593 [DOI] [PubMed] [Google Scholar]
- 78. Morse SB, Haywood JL, Goldenberg RL, Bronstein J, Nelson KG, Carlo WA. Estimation of neonatal outcome and perinatal therapy use. Pediatrics. 2000;105:1046–50. DOI: 10.1542/peds.105.5.1046 [DOI] [PubMed] [Google Scholar]
- 79. Poses RM, McClish DK, Bekes C, Scott WE, Morley JN. Ego bias, reverse ego bias, and physicians’ prognostic. Crit Care Med. 1991;19:1533–9. DOI: 10.1097/00003246-199112000-00016 [DOI] [PubMed] [Google Scholar]
- 80. Poses RM, Bekes C, Copare FJ, Scott WE. What difference do two days make? The inertia of physicians’ sequential prognostic judgments for critically ill patients. Med Decis Making. 1990;10:6–14. DOI: 10.1177/0272989X9001000103 [DOI] [PubMed] [Google Scholar]
- 81. Goldenberg RL, Nelson KG, Dyer RL, Wayne J. The variability of viability: the effect of physicians’ perceptions of viability on the survival of very low-birth weight infants. Am J Obstet Gynecol. 1982;143:678–84. DOI: 10.1016/0002-9378(82)90114-4 [DOI] [PubMed] [Google Scholar]
- 82. Gooi A, Oei J, Lui K. Attitudes of Level II obstetricians towards the care of the extremely premature infant: a national survey. J Paediatr Child Health. 2003;39:451–5. DOI: 10.1046/j.1440-1754.2003.00187.x [DOI] [PubMed] [Google Scholar]
- 83. Connors AF, Jr, Dawson NV, Desbiens NA, et al. A controlled trial to improve care for seriously ill hospitalized patients: the study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274:1591–8. [PubMed] [Google Scholar]
- 84. Saigal S, Stoskopf BL, Feeny D, et al. Differences in preferences for neonatal outcomes among health care professionals, parents, and adolescents. JAMA. 1999;281:1991–7. DOI: 10.1001/jama.281.21.1991 [DOI] [PubMed] [Google Scholar]
- 85. Dupont-Thibodeau A, Hindié J, Bourque CJ, Janvier A. Provider perspectives regarding resuscitation decisions for neonates and other vulnerable patients. J Pediatr. 2017;188:142–7.e3. DOI: 10.1016/j.jpeds.2017.03.057 [DOI] [PubMed] [Google Scholar]
- 86. Jones AH. Images of physicians in literature: medical Bildungsromans. Lancet. 1996;348:734–6. DOI: 10.1016/S0140-6736(96)07315-1 [DOI] [PubMed] [Google Scholar]
- 87. Markel H. A book doctors can’t close. The New York Times, 2009. [Google Scholar]
- 88. Shem S. The House of God. New York: Penguin Random House; 1978. [Google Scholar]
- 89. Sharma N, Lalinde PS, Brosco JP. What do residents learn by meeting with families of children with disabilities? A qualitative analysis of an experiential learning module. Pediatr Rehabil. 2006;9:185–9. DOI: 10.1080/13638490600570606 [DOI] [PubMed] [Google Scholar]
- 90. Racine E, Dion MJ, Wijman CA, Illes J, Lansberg MG. Profiles of neurological outcome prediction among intensivists. Neurocrit Care. 2009;11:345–52. DOI: 10.1007/s12028-009-9225-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121:S2–23. DOI: 10.1016/j.amjmed.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 92. Ludolph R, Schulz PJ. Debiasing health-related judgments and decision making: a systematic review. Med Decis Making. 2018;38:3–13. DOI: 10.1177/0272989x17716672 [DOI] [PubMed] [Google Scholar]
- 93. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65–72. DOI: 10.1136/bmjqs-2012-001713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Graber ML, Kissam S, Payne VL, et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21:535–57. DOI: 10.1136/bmjqs-2011-000149 [DOI] [PubMed] [Google Scholar]
- 95. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19:174. DOI: 10.1186/s12911-019-0901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Daniel M, Carney M, Khandelwal S, et al. Cognitive debiasing strategies: a faculty development workshop for clinical teachers in emergency medicine. MedEdPORTAL. 2017;13:10646. DOI: 10.15766/mep_2374-8265.10646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Racine E, Bell E, Farlow B, et al. The ‘ouR-HOPE’ approach for ethics and communication about neonatal neurological injury. Dev Med Child Neurol. 2017;59:125–35. DOI: 10.1111/dmcn.13343 [DOI] [PubMed] [Google Scholar]
- 98. Hanson LC, Garrett JM, Lewis C, et al. Physicians’ expectations of benefit from tube feeding. J Palliat Med 2008; 11:1130–1134. DOI: 10.1089/jpm.2008.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-xlsx-1-mpp-10.1177_23814683221145158 for Factors Influencing Physician Prognosis: A Scoping Review by Amaryllis Ferrand, Jelena Poleksic and Eric Racine in MDM Policy & Practice