Abstract
Although BALB/c mice develop lesions when infected with Leishmania mexicana, the mechanisms which are responsible for susceptibility to this parasite have not been elucidated. In contrast, susceptibility of BALB/c mice to Leishmania major has been shown to depend on the early production of interleukin-4 (IL-4) by T cells which react to the parasitic LACK antigen. Here, we demonstrate that the lesions induced by L. mexicana are delayed compared to those induced by L. major but rapidly develop at later time points. Interestingly, while LACK-tolerant BALB/c-derived IE-LACK transgenic mice were resistant to L. major, they were susceptible to L. mexicana and developed lesions similar to those observed in wild-type BALB/c mice. The latter result was observed despite the fact that (i) LACK was expressed by L. mexicana, (ii) splenocytes from BALB/c mice were able to stimulate LACK-specific T-cell hybridoma cells when incubated with live L. mexicana promastigotes, and (iii) LACK-specific T cells contributed to IL-4 production in L. mexicana-infected BALB/c mice. Thus, in contrast to what was observed for L. major-infected mice, LACK-specific T cells do not play a critical role in determining susceptibility to L. mexicana. Although BALB/c mice are susceptible to both L. major and L. mexicana, the mechanisms which are responsible for susceptibility to these parasites are likely to be different.
Mice have been widely used as an animal model to study human leishmaniasis. In many cases, the outcome of the disease is different depending on the strains which are infected. In the case of mice infected with Leishmania major, it has been shown that CBA, C3H, C57BL/6, and B10.D2 mice develop cutaneous lesions that spontaneously heal, while BALB/c, SWR/J, DBA/2, and A/Jax mice develop nonhealing cutaneous lesions which are susceptible to dissemination (4, 20). Several studies have shown that the early production of interleukin-4 (IL-4), which is induced in the draining lymph nodes (LNs) of BALB/c mice 16 h after infection with L. major, is one of the critical events leading to the development of a counterprotective antiparasite Th2 response (5, 10, 12–14). IL-4 is produced by CD4+ T cells which express a highly restricted Vβ4 Vα8 receptor repertoire (11). These cells recognize a single parasite determinant derived from the antigen LACK (leishmania receptor for activated C kinase, for its homology with the mammalian receptors for activated C kinase [RACK]), a protein which is conserved among different Leishmania spp. and which accounts for 0.03% of the proteins expressed by Leishmania promastigotes or amastigotes (14, 17, 18). The critical role of the LACK-specific T cells in determining susceptibility to L. major is further demonstrated by the observation that BALB/c mice which have been made tolerant to LACK are resistant to infection (9, 11).
Although studies have shown that IL-4 is critical for susceptibility to Leishmania mexicana (23–25), it is not known whether LACK-specific T cells play a critical role in the animal model for this infection. To address this issue, we have compared the courses of L. mexicana and L. major infections in wild-type (WT) BALB/c mice and in BALB/c-derived IE-LACK transgenic mice which are tolerant to LACK as the result of its constitutive expression in the thymus. In contrast to what was observed for L. major-infected mice, we found that LACK-specific T cells do not seem to play a critical role in determining the susceptibility of BALB/c mice to L. mexicana infection.
BALB/c male mice (8 to 12 weeks old) were purchased from Banting & Kingman Universal Limited (Hull, United Kingdom). IE-LACK transgenic mice have been previously described (9). L. mexicana (strain MHOM/BZ/82/BEL21) and L. major (strain WHOM/IR/−173) promastigotes were grown in RPMI medium (Life Technologies, Merelbeke, Belgium) supplemented with 10% fetal calf serum (Life Technologies), penicillin G (100 U/ml), and streptomycin (100 μg/ml). Promastigote lysates were prepared by 10 cycles of freezing (in liquid nitrogen) and thawing (in a water bath at 37°C), and their endotoxin contents were lower than 0.20 pg/106 promastigotes as determined by the Limulus amebocyte lysate-Coatest endotoxin assay (Chromogenix, Mölndal, Sweden). The LACK-derived peptide, previously identified as an immunodominant epitope in BALB/c mice (amino acids 161 to 172, of sequence SLEHPIVVSGSW) (17), was obtained from Chiron Mimotopes (Paris, France) and from P. Gourlet (Laboratory of Biochemistry, Faculty of Medicine, Université Libre de Bruxelles, Brussels, Belgium). The LACK-specific T-cell hybridoma LMR16.2, producing IL-2 upon recognition of the immunodominant L. major LACK epitope in an I-Ad major histocompatibility complex class II-restricted fashion, was used as previously described (17). IL-2 and IL-4 levels were determined by enzyme-linked immunosorbent assays using commercially available kits (duoset from Genzyme Diagnostics, Cambridge, Mass.). The limit of detection was 5 pg/ml for both cytokines.
Diseases induced by L. mexicana and L. major in BALB/c mice.
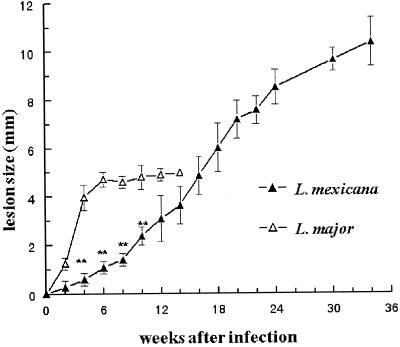
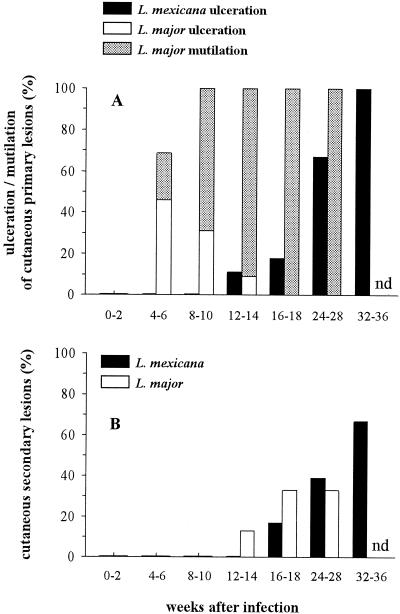
Although BALB/c mice are known to be susceptible to both L. major and L. mexicana (1), the diseases induced by these two species have not been compared under the same experimental conditions. In our study, L. mexicana or L. major promastigotes were injected into the left footpad of BALB/c mice. Each mouse received 107 stationary-phase promastigotes in a final volume of 25 μl of RPMI medium. The contralateral right footpad received an identical volume of RPMI medium, without parasites, as an internal control. Footpad thicknesses were measured with a metric caliper, and the difference between both measurements corresponded to lesion size. As shown in Fig. 1, while L. mexicana induced slowly progressive primary lesions which reached 10 to 11 mm after 34 weeks, L. major induced the rapid development of lesions which were 4 to 5 mm thick at 4 weeks postinfection (wpi). The cutaneous lesions induced by L. major became ulcerated more frequently and earlier than those induced by L. mexicana. Likewise, L. major, but not L. mexicana, induced spontaneous losses (mutilations) of necrotic footpad lesions which could not be measured after 14 weeks (Fig. 2A). While both L. mexicana and L. major induced secondary lesions which appeared in the contralateral posterior or anterior footpads, the eyelids, the nostrils, the ears, and the base of the tail, these lesions developed later in mice infected with L. mexicana than in those infected with L. major (Fig. 2B). Thus, while BALB/c mice were susceptible to both L. major and L. mexicana infections, the courses of disease induced by these two parasites were markedly different.
FIG. 1.
Sizes of primary footpad lesions of BALB/c mice infected with L. mexicana or L. major. Results are from one representative experiment performed with 28 (L. mexicana) and 15 (L. major) animals and are expressed as means ± SEM. ∗∗, P < 0.001 (Student's t test, comparing L. mexicana and L. major infections). Three other experiments using 15, 16, and 12 animals infected with L. mexicana displayed similar results. This was also the case for another experiment including 10 mice infected with L. major.
FIG. 2.
Frequencies of ulcerated cutaneous primary lesions and mutilations of footpads (A) and cutaneous secondary lesions (B) during the course of L. mexicana and L. major infections in BALB/c mice. Results are from the same experiment described for Fig. 1. nd, not determined.
Stimulation of a LACK-specific T-cell hybridoma by BALB/c splenocytes incubated with L. mexicana live promastigotes.
To determine if antigen-presenting cells could process the L. mexicana LACK antigen and present it to T cells, splenocytes from BALB/c mice were incubated in 96-well plates (Nunc, Roskilde, Denmark) with the LACK-specific T-cell hybridoma LMR16.2 and L. mexicana or L. major live or lysed promastigotes. In some wells, splenocytes (5 × 105/well) were incubated with either live L. mexicana or L. major promastigotes for 6 h at 37°C. Other splenocyte-containing wells received L. mexicana or L. major lysate (at the corresponding parasite/splenocyte ratios used for live parasites) or the LACK-derived peptide (at concentrations ranging from 0.2 to 25.6 μM). The LMR16.2 hybridoma cells (105/well) were added to each well. Twenty hours later, cellular supernatants were harvested for subsequent measurement of IL-2 levels.
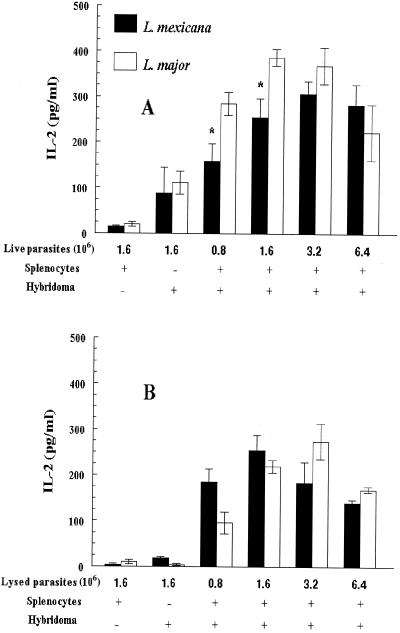
LACK peptide (data not shown) and both L. major and L. mexicana promastigotes (Fig. 3) induced the LMR16.2 hybridoma cells to secrete IL-2 in a dose-dependent manner. The fact that splenocytes incubated with L. mexicana promastigotes could stimulate the LMR16.2 T-cell hybridoma recognizing specifically the L. major LACK antigen suggested that the antigenic determinant which was recognized by these LACK-specific cells was conserved between the L. mexicana and L. major LACK proteins.
FIG. 3.
IL-2 secretion by LMR16.2 hybridoma cells in response to L. major and L. mexicana promastigotes. Splenocytes from BALB/c mice were incubated with LMR16.2 hybridoma cells and with live (A) or lysed (B) L. mexicana or L. major promastigotes. Results are expressed as means ± SEM of IL-2 levels released in culture supernatants for three experiments. ∗, P < 0.05 (Student's t test, comparing L. mexicana and L. major).
Interestingly, while the L. mexicana and L. major lysed promastigotes did not differ in the ability to stimulate LMR16.2 hybridoma cells (Fig. 3B), L. mexicana live promastigotes induced less IL-2 than L. major live promastigotes (Fig. 3A). Though slight, such differences were significant and might be related to differences in the proportion of metacyclic-like promastigotes (the parasitic form infecting cells) within the L. major and L. mexicana stationary-phase populations (22) or to differences in the intracellular development of both parasites, interfering with antigen presentation (19). Indeed, the tight binding of L. mexicana amastigotes to the membrane vacuole of infected macrophages, which is not seen with L. major amastigotes (2), and the sequestration of major histocompatibility complex class II molecules at this point of the membrane (rendering difficult their egress from the parasitophorous vacuole) (21), as well as the production of large amounts of cysteine proteases by L. mexicana (8, 16) but not L. major, are likely to reduce antigen processing and presentation of the L. mexicana LACK antigen.
Molecular identification of the LACK epitope in L. mexicana.
In order to confirm that the antigenic epitope recognized by the LACK-specific cells was conserved between the L. mexicana and L. major LACK proteins, cDNA was prepared from RNA of L. mexicana promastigotes, and the sequence corresponding to the immunodominant LACK epitope was amplified by PCR using the following primers: LM12 (TCCATCCGCATGTGGGACCTG) and LM13 (CCCGTTCACGTTCCATACTTTG) (17). The nucleotide sequence of the L. mexicana PCR products, determined using standard procedures, was identical to that of the corresponding L. major region (data not shown). This confirms that the parasite LACK antigen is well conserved among the different studied Leishmania species, including L. major, Leishmania amazonensis, Leishmania donovani, Leishmania chagasi (17), and L. mexicana.
Susceptibility to L. mexicana of BALB/c mice made tolerant to LACK protein.
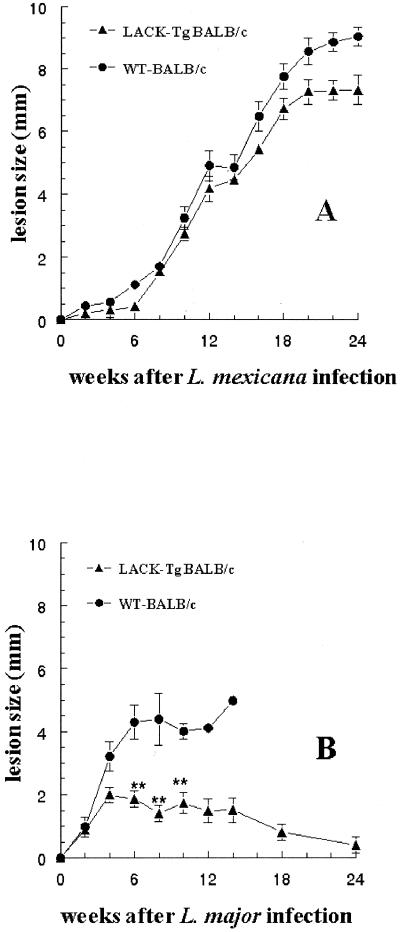
Since LACK-specific T cells play a critical role in determining susceptibility to L. major, we sought to determine if this was also the case for L. mexicana infection. To this end, IE-LACK transgenic and WT BALB/c mice were injected with 107 promastigotes. In agreement with previous results (9), the lesions induced by L. major were much smaller in IE-LACK transgenic mice than in WT BALB/c mice (Fig. 4B). In contrast, the lesions induced by L. mexicana were similar in transgenic and nontransgenic animals (Fig. 4A). Likewise, parasite loads in the footpads and in the draining popliteal LNs (measured after tissue homogenization by staining released amastigotes with acridine orange [Sigma, Bornem, Belgium], as previously reported for Plasmodium and Leishmania promastigotes [3, 15]), as well as the frequencies of both necrotic and secondary cutaneous lesions, were similar for IE-LACK transgenic and WT BALB/c mice (Table 1). Thus, despite the fact that BALB/c splenocytes were able to stimulate LACK-specific T cells when incubated with L. mexicana promastigotes, IE-LACK transgenic mice tolerant to the LACK antigen were as susceptible as WT BALB/c mice to L. mexicana infection. This might be related to the reduced ability of L. mexicana live promastigotes to stimulate LACK-specific T cells, as shown in our experiment with LMR16.2 hybridoma cells. Moreover, since the processing of LACK by infected macrophages requires the destruction of the internalized parasites (6, 19), differences in the innate immune responses to L. mexicana and L. major and in the subsequent early production of cytokines activating the parasiticidal function of macrophages may also account for this result.
FIG. 4.
Sizes of primary footpad lesions during the course of L. mexicana (A) or L. major (B) infection in IE-LACK (LACK-TgBALB/c) and WT BALB/c mice. Results are expressed as means ± SEM. Twelve male mice per group were inoculated. ∗∗, P < 0.001 (Student's t test, comparing IE-LACK and WT animals).
TABLE 1.
Parasitic loads and frequencies of lesions in mice infected with L. mexicana
| wpi | Mouse groupc | Parasitic loada (103/mg [mean ± SEM]) in:
|
Ulcerationsb (no. present/ total) (%) | Secondary lesionsb (no. present/ total) (%) | |
|---|---|---|---|---|---|
| Foot pad | LN | ||||
| 2 | IE-LACK | 200 ± 19 | 18 ± 1 | 0/12 (0) | 0/12 (0) |
| WT | 125 ± 63 | 15 ± 4 | 0/12 (0) | 0/12 (0) | |
| 6 | IE-LACK | 185 ± 27 | 48 ± 4 | 0/10 (0) | 0/10 (0) |
| WT | 97 ± 19 | 20 ± 3 | 0/10 (0) | 0/10 (0) | |
| 14 | IE-LACK | 835 ± 91 | 152 ± 7 | 2/7 (29) | 0/7 (0) |
| WT | 685 ± 108 | 148 ± 19 | 2/6 (33) | 0/6 (0) | |
| 24 | IE-LACK | 3,456 ± 132 | 1,456 ± 196 | 4/4 (100) | 2/4 (50) |
| WT | 2,169 ± 303 | 1,483 ± 74 | 3/3 (100) | 2/3 (67) | |
Determinations of parasitic loads at each time point were performed for two or three animals infected at the same time as those from the experiment described for Fig. 4.
Data on ulcerations and secondary lesions were derived from the mice from the experiment described for Fig. 4.
No statistical differences were seen between IE-LACK transgenic and WT BALB/c mice.
IL-4 secretion by splenocytes of IE-LACK transgenic and WT BALB/c mice infected with L. mexicana.
Although IE-LACK transgenic mice were as susceptible as WT BALB/c mice to L. mexicana, we sought to determine if LACK-specific T cells contributed to IL-4 secretion in L. mexicana-infected mice. To this end, splenocytes of IE-LACK transgenic and WT BALB/c mice infected with 107 L. mexicana promastigotes were harvested at 0, 2, 6, and 14 wpi and incubated in 24-well plates (Nunc) (3 × 106 cells/800 μl/well) at 37°C for 48 h in a 5% CO2 humidified atmosphere, with or without concanavalin A (8 μg/well; Sigma) or L. mexicana extracts (106 lysed parasites/well). Supernatants of unstimulated or stimulated cells were collected for subsequent determination of IL-4 levels. Concanavalin A stimulated splenocytes to produce IL-4 (100 to 390 pg/ml) at each time point for both mouse groups. Neither splenocytes from naive animals nor those from mice which had been infected 2 or 6 weeks earlier produced IL-4 in response to L. mexicana lysates. This agrees with previous studies which have shown that IL-4 transcripts are undetectable in the LNs and the spleens of L. mexicana-infected mice 1 and 7 days after infection (7). In contrast, when mice were analyzed 14 weeks after infection, splenocytes from both IE-LACK transgenic and WT BALB/c mice secreted IL-4 in response to parasite extracts. However, splenocytes from IE-LACK transgenic mice secreted significantly less IL-4 than those from WT BALB/c mice. (Means ± standard errors of the means [SEM] for stimulated cells were as follows: IE-LACK mice, 56 ± 9 pg/ml; WT mice, 179 ± 65 pg/ml [P < 0.05 by Student's t test, n = 3]. The results for unstimulated cells were as follows: IE-LACK mice, 11 ± 8 pg/ml; WT mice, 29 ± 15 pg/ml.) Such a difference suggests that LACK-specific T cells contributed to the production of IL-4 in L. mexicana-infected WT mice.
In conclusion, despite BALB/c splenocytes being able to stimulate LACK-specific T cells, which contribute to the production of IL-4 in L. mexicana-infected mice, and despite the fact that IL-4 was shown to be required for susceptibility to L. mexicana (23), IE-LACK transgenic mice tolerant of LACK antigen were as susceptible as WT BALB/c mice to L. mexicana infection. This indicates that LACK-specific T cells do not play a critical role in determining susceptibility to L. mexicana. This result is in striking contrast to those obtained in the case of BALB/c mice infected with L. major. It is tempting to hypothesize that such a difference preventing the development of an early counterprotective antiparasite Th2 response contributes to the slower development of lesions in L. mexicana-infected animals. It also suggests that the mechanisms which determine susceptibility to these two species are markedly different.
Acknowledgments
F.A.T. was supported successively by Ph.D. fellowships from the Comission de Operaciones para el Fomento Academico y Administrativo (COFA) of the Instituto Politecnico Nacional (Mexico) and ULB. This work was supported by grants of the Belgian Ministry of Scientific Policy (“Action de Recherche Concertée”) and ULB.
We thank C. Truyens for advice and for measuring endotoxin contents and D. Le Ray (Institut of Tropical Medicine, Antwerp, Belgium) for giving us the MHOM/BZ/82/BEL21 strain of L. mexicana. We are indebted to C. Truyens, E. Muraille, and J.-C. Antoine for their critical review of the manuscript and to A. Wathelet, P. Delblandre, K. Mjidi, and A. Ben Messaoud for their diligent technical assistance.
REFERENCES
- 1.Alexander J, Kaye P M. Immunoregulatory pathways in murine leishmaniasis: different regulatory control during Leishmania mexicana mexicana and Leishmania major infections. Clin Exp Immunol. 1985;61:674–682. [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine J C, Prina E, Lang T, Courret N. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;6:392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- 3.Barreca G S, Berlinghieri M C, Foti F, Matera G, Foca A. Microscopic observation of progressive immobilization of leishmania promastigotes in acridine orange stain. J Clin Microbiol. 1997;35:1867–1869. doi: 10.1128/jcm.35.7.1867-1869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley D J. Genetics of susceptibility and resistance in the vertebrate host. In: Peters W, Killick-Kendrick R, editors. The leishmaniases in biology and medicine. London, United Kingdom: Academic Press; 1987. pp. 551–581. [Google Scholar]
- 5.Chatelain R, Varkila K, Coffman R L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- 6.Courret N, Prina E, Mougneau E, Saraiva E M, Sacks D L, Glaichenhaus N, Antoine J C. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur J Immunol. 1999;29:762–773. doi: 10.1002/(SICI)1521-4141(199903)29:03<762::AID-IMMU762>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Guevara-Mendoza O, Une C, Franceschi C P, Orn A. Experimental infection of Balb/c mice with Leishmania panamensis and Leishmania mexicana: induction of early IFN-gamma but not IL-4 is associated with the development of cutaneous lesions. Scand J Immunol. 1997;46:35–40. doi: 10.1046/j.1365-3083.1997.d01-96.x. [DOI] [PubMed] [Google Scholar]
- 8.Ilg T, Fuchs M, Gnau V, Wolfram M, Harbecke D, Overath P. Distribution of parasite cysteine proteinases in lesions of mice infected with Leishmania mexicana amastigotes. Mol Biochem Parasitol. 1994;67:193–203. doi: 10.1016/0166-6851(94)00126-x. [DOI] [PubMed] [Google Scholar]
- 9.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 10.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann K H, Lefrang K, Humborg C, Ledermann B, Solbach W. IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Launois P, Maillard I, Pingel S, Swihart K G, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley R M, MacDonald H R, Louis J A. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 12.Launois P, Ohteki T, Swihart K, MacDonald H R, Louis J A. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1−. Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 13.Launois P, Swihart K G, Milon G, Louis J A. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–3324. [PubMed] [Google Scholar]
- 14.Locksley R M, Pingel S, Lacy D, Wakil A E, Bix M, Fowell D J. Susceptibility to infectious diseases: Leishmania as a paradigm. J Infect Dis. 1999;179(Suppl. 2):S305–S308. doi: 10.1086/513843. [DOI] [PubMed] [Google Scholar]
- 15.Long G W, Jones T R, Rickman L S, Fries L, Egan J, Wellde B, Hoffman S L. Acridine orange diagnosis of Plasmodium falciparum: evaluation after experimental infection. Am J Trop Med Hyg. 1994;51:613–616. doi: 10.4269/ajtmh.1994.51.613. [DOI] [PubMed] [Google Scholar]
- 16.Mottram J C, Brooks D R, Coombs G H. Roles of cysteine proteinases of trypanosomes and Leishmania in host-parasite interactions. Curr Opin Microbiol. 1998;1:455–460. doi: 10.1016/s1369-5274(98)80065-9. [DOI] [PubMed] [Google Scholar]
- 17.Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z E, Waldmann R, Locksley R M, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 18.Pingel S, Launois P, Fowell D J, Turck C W, Southwood S, Sette A, Glaichenhaus N, Louis J A, Locksley R M. Altered ligands reveal limited plasticity in the T cell response to a pathogenic epitope. J Exp Med. 1999;189:1111–1120. doi: 10.1084/jem.189.7.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prina E, Lang T, Glaichenhaus N, Antoine J C. Presentation of the protective parasite antigen LACK by Leishmania-infected macrophages. J Immunol. 1996;156:4318–4327. [PubMed] [Google Scholar]
- 20.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 21.Russell D G, Xu S, Chakraborty P. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages. J Cell Sci. 1992;103:1193–1210. doi: 10.1242/jcs.103.4.1193. [DOI] [PubMed] [Google Scholar]
- 22.Sacks D L, Perkins P V. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 23.Satoskar A, Bluethmann H, Alexander J. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect Immun. 1995;63:4894–4899. doi: 10.1128/iai.63.12.4894-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoskar A, Brombacher F, Dai W J, McInnes I, Liew F Y, Alexander J, Walker W. SCID mice reconstituted with IL-4-deficient lymphocytes, but not immunocompetent lymphocytes, are resistant to cutaneous leishmaniasis. J Immunol. 1997;159:5005–5013. [PubMed] [Google Scholar]
- 25.Stamm L M, Raisanen-Sokolowski A, Okano M, Russell M E, David J R, Satoskar A R. Mice with STAT6-targeted gene disruption develop a Th1 response and control cutaneous leishmaniasis. J Immunol. 1998;161:6180–6188. [PubMed] [Google Scholar]