SUMMARY
Objective
To investigate the relationship between risk of Benign Paroxysmal Positional Vertigo (BPPV) recurrence and hypothyroidism treated with hormone replacement therapy (HRT).
Methods
797 patients with idiopathic BPPV were divided into two groups: 250 patients with recurrence of BPPV (R-BPPV) and 547 patients without recurrence (NR-BPPV). Regarding patients with thyroid disease on HRT, we collected serum test results of thyroid-stimulating hormone (TSH), free triiodothyronine f-T3, free thyroxine f-T4, thyroglobulin antibodies (TG-Ab) and thyroid peroxidase antibodies (TPO-Ab).
Results
Hypothyroidism in long-term HRT was found in 61/250 (24.4%) patients of the R-BPPV group vs 79/547 (14.4%) of the NR-BPPV–group (p = 0.0006). Hashimoto thyroiditis (HT) was associated with recurrence (p < 0.0001). A significant correlation was found between recurrence and level of serum TPO-Ab (p = 0.0117) and TG-Ab (p = 0.0025), but not with mean serum TSH, f-T3 and f-T4.
Conclusions
We assume that patients with hypothyroidism in HRT have an increased risk of BPPV recurrence, which is particularly strong for patients with HT and positive thyroid antibodies, suggesting an association between autoimmunity and recurrent vertigo.
KEY WORDS: BPPV, recurrence vertigo, hypothyroidism, thyroid autoimmunity, vertigo comorbidities
RIASSUNTO
Obiettivo
Analisi dell’associazione tra la ricorrenza della Vertigine parossistica posizionale benigna (BPPV) e l’ipotiroidismo in corso di terapia ormonale sostitutiva (TOS).
Metodi
797 pazienti affetti da BPPV idiopatica sono stati suddivisi in due gruppi: 250 con vertigine ricorrente (R-BPPV) e 547 (NR-BPPV) non ricorrente. Nei pazienti ipotiroidei sono stati analizzati i valori ematici tiroidei: ormone tireotropo (TSH), frazioni libere di tetraiodotironina (f-T4) e triiodiotironina (f-T3), anticorpi anti tireoperossidasi (TPO-Ab) ed anti tireoglobulina (TG-Ab).
Risultati
L’ipotiroidismo è emerso in 61/250 (24,4%) pazienti del gruppo R-BPPV vs 79/547 (14,4%) del gruppo NR - BPPV (p = 0,0006). La ricorrenza è risultata statisticamente associata alla tiroidite di Hashimoto (HT) (p < 0,0001) ed alla positività anticorpale di Ab-TPO (p = 0,0117) e Ab-TG (p = 0,0025); le concentrazioni sieriche medie di TSH, f-T3 e f-T4 non sono risultate statisticamente significative.
Conclusioni
Nei pazienti affetti da ipotiroidismo in TOS è stato dimostrato un aumentato rischio di ricorrenza di BPPV, particolarmente evidente nei pazienti con HT e dosaggio anticorpale tiroideo positivo, suggerendo l’associazione tra autoimmunità e ricorrenza della vertigine.
PAROLE CHIAVE: vertigine parossistica posizionale benigna (VPPB), vertigine ricorrente, ipotiroidismo, autoimmunità tiroidea, comorbidità nella VPPB
Introduction
Hypothyroidism is one of the most common disorders of the endocrine system. It can refer to insufficient synthesis of thyroxine (T4) and triiodothyronine (T3), released into the bloodstream in response to stimulation by pituitary thyroid stimulating hormone (TSH), or to the dysfunction of hormone-free biologically active forms (f-T3, f-T4) related to peripheral receptors 1. The prevalence of hypothyroidism is approximately 1-3% in Western general population 2. In iodine sufficient areas of the world, the most frequent cause of hypothyroidism is chronic autoimmune thyroiditis, also known as Hashimoto’s thyroiditis (HT). Hypothyroidism is also common following radioiodine treatment, hemithyroidectomy or total thyroidectomy, and neck radiation or surgery for cancer therapy. Furthermore, suboptimal iodine status can result in goitre, thyroid nodules and hypothyroidism 1. Thyroid hormone replacement with levothyroxine is the standard treatment for patients with hypothyroidism 3. The most typical clinical manifestations of hypothyroidism include fatigue, lethargy, bradycardia, weight gain and cold intolerance, but it has also been recently associated with ENT disorders, such as hearing loss 4, tinnitus 5 and vestibular disorders 6. Among the different vestibular pathologies, the association between hypothyroidism and benign paroxysmal positional vertigo (BPPV) has recently been investigated 7. BPPV is the most common peripheral vestibular disease accounting for approximately 17-42% of diagnoses of dizziness in specialised clinics. It can be described as a sudden and abnormal sensation of motion and/or rotational vertigo, lasting less than one minute, accompanied by characteristic nystagmus. In this disorder, otoliths detach from the utricle and enter in the semicircular canals to cause vertigo 8. Symptoms are triggered by positional changes of the head and can range from mild dizziness to debilitating episodes that may induce nausea or vomiting.
BPPV can be classified as primary or idiopathic (50-70%) and secondary to trauma or viral infections, Meniere’s disease, migraine, otologic and non-otologic surgery, and prolonged bed rest 9. In idiopathic cases, no apparent cause of BPPV is identified in the patient’s history. Most episodes of BPPV, even if untreated, recover spontaneously in 2 to 6 weeks 10. However, clinical resolution is more often obtained by executing one of more manoeuvres in 50-70% of cases 11. Later recurrences of BPPV are common. Choi et al. defined recurrent BPPV as the reappearance of positional nystagmus after at least 2 weeks from the execution of repositioning manoeuvres 12.
Previous papers by our group 13,14 and other authors 15 showed that risk of recurrence increases for patients with a comorbidity compared to healthy patients, and multiple associated diseases further increase the risk of recurrence of idiopathic BPPV. Among the associated pathologies, hypothyroidism and its autoimmune pathogenesis has gained increasing emphasis, but there are still few studies investigating the relationship between thyroid function or pathology and BPPV pathogenesis or recurrence. Moreover, not all studies on the topic have consistent results and large-scale clinical trials to clarify the associations are needed 16.
The aim of this study was to investigate the relationship between hypothyroidism, thyroid autoimmunity and BPPV recurrence by measurement of thyroid hormones and serum thyroid autoantibodies.
Materials and methods
Patients consecutively referred to the Vestibular Service, in the day clinic, reporting vertigo and diagnosed with idiopathic BPPV, were recruited for the study. We collected data on 797 patients aged from 12 to 87 years (mean 62.5 ± 14.3 years): 506 females (63.5%) and 291 males (36.5%).
We retrospectively divided all patients in two groups: non-recurrent BPPV (NR-BPPV), reporting first occurrence of vertigo, and recurrent (R-BPPV) defined as the reappearance of positional nystagmus after at least 2 weeks from its resolution 12. We excluded patients with previous head trauma, whiplash injuries, or “persistent” BPPV, namely no remission of symptoms or nystagmus after 2 weeks or 5 repositioning manoeuvres. We excluded patients with hyperthyroidism and/or thyroid eye-related disease from the study.
All patients were evaluated based on accurate clinical history and bedside-examination by an experienced examiner. Posterior canal (PC)-BPPV was diagnosed in case of geotropic torsional nystagmus with specific latency, frequency, duration and “reduction effect” (nystagmus reduction after repetition of the manoeuvre) by the Dix-Hallpike or Semont manoeuvre. On the other hand, horizontal canal (HC)-BPPV diagnosis was based on the observation of horizontal direction-changing positional nystagmus with the McClure-Pagnini manoeuvre. We treated patients with specific repositioning manoeuvres and reassessed them after 7 days, until the resolution of symptoms and nystagmus disappeared. In case of recurrence of symptoms, patients were re-evaluated in the day clinic and treated again.
Previous histories of hypothyroidism on hormone replacement therapy (HRT), such as autoimmune thyroiditis, goitre and thyroidectomy, were investigated in both the R -BPPV and NR-BPPV groups. We collected laboratory data on TSH (normal values: 0·35-2.80 μIU/mL), f-T3 (n.v. 2.3-4.2 pg/ml), f-T4 (n.v. 8.5-15.5 pg/ml), Thyroglobulin antibodies TG-Ab (n.v. < 4.1 IU/ml) and thyroid peroxidase antibodies TPO-Ab (n.v. < 5.6 IU/ml).
In accordance with AACE/ATA guidelines 17, we considered mild or subclinical hypothyroidism patients with TSH concentrations above the reference range with f-T3 and f-T4 concentrations within normal range; overt or clinical hypothyroidism high TSH with f-T4 and/or f-T3 concentrations below the reference range. Autoimmunity pattern was defined in the presence of serum antibody titres (TG-Ab and/or TPO-Ab) above the reference range.
We analysed the clinical characteristics of BPPV (number of recurrences, canal involved, number and type of manoeuvres, duration of therapy) to establish possible differences in patients with thyroid disease compared to other idiopathic BPPV patients. Moreover, data were evaluated statistically in order to establish the relationship between BPPV recurrence and thyroid disease in patients taking hormone replacement therapy, and the risk of recurrence in both subclinical/overt hypothyroidism related to different etiopathogeneses.
Statistical analysis was performed using commercially available software (Excel–Microsoft Corporation, Redmond, Washington, USA). Continuously distributed data were summarised as the mean and median, and categorical variables with frequencies and percentages. The χ² test and odds ratios (OR) with 95% confidence intervals were used for non-parametric variables; Student t-test was performed for parametric variables. A p value less than 0.05 was considered as significant.
Results
The group of non-recurrent (NR-BPPV) consisted of 547/797 (68.6%) patients, whereas 250/797 (31.4%) had recurrent vertigo (R-BPPV) (Tab. I).
Table I.
Thyroid functional pattern.
| R-BPPV | NR-BPPV | p value | OR | |
|---|---|---|---|---|
| n = 250 | n = 547 | |||
| History of thyroid dysfunction in HRT | 61 (24.4%) | 79 (14.4%) | 0.0006 | 1.92 |
| Patients with altered thyroid hormones | 22 (8.8%) | 21 (3.8%) | 0.0039 | 3.01 |
| Subclinical hypothyroidism | 10 (4%) | 6 (1%) | 0.0067 | 3.76 |
| Overt hypothyroidism | 12 (4.8%) | 15 (2.7%) | > 0.05 | |
| Autoimmunity | 49 (19.6%) | 35 (6.4%) | < 0.0001 | 3.57 |
Comparison between recurrent benign paroxysmal positional vertigo (R -BPPV) and non-recurrent (NR-BPPV). Statistical significance was set at p < 0.05. HRT: hormone replacement therapy. BPPV: benign paroxysmal positional vertigo.
140/797 (17.6%) patients suffered from hypothyroidism treated with HRT. In detail, 61/250 (24.4%) patients with R-BPPV vs 79/547 (14.4%) with NR-BPPV reporting a history of thyroid dysfunction. Thyroid disease requiring HRT, regardless of aetiology, can be considered a risk factor for recurrence vertigo (p = 0.0006, OR = 1.92) (Tab. I).
Laboratory evaluation of thyroid gland functional activity showed that in the R-BPPV group, 22/250 (8.8%) patients had hypothyroidism: 10/22 with a subclinical pattern and 12/22 with overt hypothyroidism. In the NR-BPPV group, 21/549 (3.8%) subjects showed hypothyroidism, of whom 6/21 had subclinical and 15/21 overt. Statistical analysis showed that hypothyroidism was more frequently encountered in the group of recurrent BPPV (p = 0.0039, OR = 3.01), although a significant association was present only with subclinical hypothyroidism (p = 0.0067, OR = 3.76) and not with overt hypothyroidism (p > 0.05) (Tab. I). Mean serum TSH, free-T3 and free-T4 concentrations, on the other hand, were not significantly different between the two groups (p > 0.05) (Tab. II).
Table II.
Laboratory testing in thyroid disorders.
| Laboratory | R-BPPV | NR-BPPV | p value |
|---|---|---|---|
| n = 61 | n = 79 | ||
| TSH (n.v. 0·35-2.80 μIU/ml) | 1.8 ± 0.9 | 1.4 ± 0.9 | > 0.05 |
| f-T3 (n.v. 2.3-4.2 pg/ml) | 2.9 ± 0.6 | 2.5 ± 0.4 | > 0.05 |
| f-T4 (n.v. 8.5-15.5 pg/ml) | 10.8 ± 4.3 | 8.8 ± 2.6 | > 0.05 |
| TPO-Ab (n.v. < 5.6 IU/ml) | 249.6 ± 393 | 107.1 ± 213.8 | 0.0117 |
| TG-Ab (n.v. < 4.1 IU/ml) | 132.1 ± 162 | 22.3 ± 54.3 | 0.0025 |
Hormonal dosages and thyroid autoantibodies: comparison between the two groups of BPPV patients suffering from thyroid diseases. Statistical significance was set at p < 0.05. TSH: thyroid stimulating hormone; f-T3: triiodothyronine; f-T4: free thyroxine; TG-Ab: Thyroglobulin antibodies; TPO-Ab: thyroid peroxidase antibodies; BPPV: benign paroxysmal positional vertigo; R-BPPV: recurrent BPPV; NR-BPPV: non-recurrent BPPV.
Previous history of thyroid disorders such as goitre, hypothyroidism and autoimmune thyroiditis was investigated in both the R-BPPV and NR-BPPV groups. In relation to the different causes of hypothyroidism, an autoimmune pathogenesis was detected in 84/140 (60%) patients: 49/250 (19.6%) R-BPPV vs 35/547 (6.4%) NR-BPPV (Tab. I). The association between autoimmune chronic thyroiditis pattern and BPPV recurrence was significant (p < 0.0001, OR = 3.57). In particular, the presence of high antibody serum titres increased the risk of recurrence by 3.57 times (Table I), and mean TPO-Ab and TG-Ab levels were significantly higher in the R-BPPV group (p = 0.0117) (Tab. II).
Multinodular goitre was the cause of HRT in 22.4% (31/140) of cases: 6/250 (2.4%) with R-BPPV and 25/574 (4.3%) with NR-BPPV, with no significant difference (p> 0.05). In all, 17.8% (25/140) of patients were taking HRT following total thyroidectomy: 9/250 (3.6%) in the group of R-BPPV and 16/574 (2.7%) in NR-BPPV, with no significant difference (p > 0.05) (Fig. 1).
Figure 1.
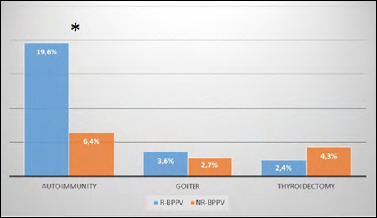
Percentage distribution of NR-BPPV and R-BPPV patients based on different causes of hypothyroidism. *: statistical significance.
Clinical characteristics of BPPV in patients with thyroid dysfunction are reported in Table III: the affected ear was the right one in 76/140 (54.3%) patients and the left in 60/140 (42.8%); 4/140 patients (2.8%) had bilateral disease. The canal involved was posterior in 127/140 (90.7%) and horizontal in 13/140 (9.3%): 69.2% had the apogeotropic type and 30.8% the geotropic type. Multicanalar involvement occurred in 9/140 patients (6.4%) with a double involvement of horizontal and posterior canals, in one or both inner ears. The analysis of clinical management of T-BPPV patients showed that this group required a mean of 1.9 ± 1.5 manoeuvres. In 86/140 (61.5%) patients, resolution was achieved with a single manoeuvre, while 54/140 (38%) needed more than one manoeuvre. Comparison between thyroid dysfunctional BPPV and other idiopathic BPPV patients showed that the posterior semicircular canal was more involved in the first group with a significant difference (p = 0.0087). There was no difference as to side involved and prognosis, or mean number of manoeuvres, resolution rate after one manoeuvre and multicanalar involvement (Tab. III).
Table III.
Clinical characteristics of study patients.
| BPPV tot. | Thyroid dysfunctional BPPV | Other BPPV | P value |
|---|---|---|---|
| N = 797 | N =140 | N = 657 | |
| Bilateral | 4 (2.8%) | 8 (1.2%) | > 0.05 |
| Side affected | Right: 76 (54.3%) | Right: 363 (55.2%) | > 0.05 |
| Left: 60 (42.8%) | Left: 286 (43.5%) | ||
| Posterior canal | 127 (90.7%) | 536 (81.6%) | 0.0087 |
| Horizontal canal | 13 (9.3%) | 121 (18.4%) | 0.0087 |
| Apogeotropic: 9/13(69.2%) | Apogeotropic: 66/121 (54.5%) | ||
| Geotropic: 4/13 (30.8%) | Geotropic: 55/121 (45.4%) | ||
| No. manoeuvres | 1.9 ± 1.5 | 1.8 ± 1.3 | > 0.05 |
| Single episode resolution after 1 manoeuvre | 86 (61.5%) | 401 (61%) | > 0.05 |
| Multicanalar Involvement | 9 (6.4%) | 39 (5.9%) | > 0.05 |
Comparison between patients with thyroid dysfunctional BPPV and patients with other type of idiopathic BPPV. Statistical significance was set at p < 0.05. BPPV: benign paroxysmal positional vertigo.
Discussion
This retrospective analysis, performed on a large sample of idiopathic BPPV patients, showed that 17.6% (140/797) had hypothyroidism and were undergoing long-term treatment with HRT. Papi et al. described a similar prevalence result in a multicentre case-control study with a percentage of 21% in the BPPV group 18. In literature, the relationship between thyroid disease and inner ear dysfunction has been attributed to abnormal thyroid functioning resulting in changes of the endolymphatic ionic composition of the labyrinth, through altered expression of ion transporters such as sodium iodide symporter (NIS) and pendrin 19, identified both in the inner ear and thyroid. It has been suggested that the volume or compositional changes of the endolymph of the vestibular labyrinth can induce BPPV 20. Therefore, reduced levels of thyroid hormones were also related to cardiovascular compromise, endothelial dysfunction and changes in blood pressure, thus reducing blood flow in the inner ear 21. The combination of the aforementioned pathogenic mechanisms can bring about the absorption or precipitation of otoconial debris, inducing BPPV 7.
Similarly, we can hypothesise that altered thyroid function may be somewhat related to the recurrence of BPPV. From our results, clinical history for hypothyroidism on treatment with HRT was significantly (p = 0.0006) more frequent in patients with R-BPPV (61/250; 24.4%) than in NR-BPPV (79/547; 14.4%). This result underscores that a history of hypothyroidism, on its own, represents a risk factor for development of the recurrence of BPPV, regardless of its aetiopathogenesis.
Nevertheless, considering laboratory examination for thyroid function, no certain association was found between TSH, f-T4 and/or f-T3 levels and recurrence, since only subclinical hypothyroidism showed an increased risk of R-BPPV (OR = 3.76). Conversely, we did not find a significantly positive association between recurrence and overt hypothyroidism. Furthermore, the mean values of f-T3, f-T4 and TSH were not significantly different between the two groups (R-BPPV and NR-BPPV), showing that reduced levels of hormones in the bloodstream alone does not influence the clinical course of benign positional vertigo. In line with these results, Chiarella et al. showed a significant correlation between vestibular disease and serum TPO-Ab but not with TSH 22, hypothesising that the association between vestibular lesions and HT was not influenced by the thyroid functional status.
Among different causes of hypothyroidism, chronic autoimmune thyroiditis or HT was reported as the most frequent thyroid disease in our sample, and its prevalence was higher among R–BVVP (49/250; 19.6%) patients compared to NR-BVVP (35/547; 6.4%) with a significant difference between the two groups. TPO-Ab and TG-Ab are the most common HT autoantibodies identified and are associated with complement-mediated cytotoxicity against thyrocytes 23.
Further interesting data emerged concerning the antibody dosage of the recruited patients with a diagnosis of HT. Both antibody titres were increased in patients with recurrence compared to the NR-BVVP group: mean TPO-Ab titre was 249.6 vs 107 IU/ml, while mean TG-Ab titre was 132.1 IU/ml vs 22.3 IU/ml. This result confirms the evidence of a positive linkage between thyroid antibodies and vestibular disease as previously demonstrated by Papi et al. 18: these authors showed that the association of BPPV with elevated anti-thyroid antibodies (OR 25.6) was stronger than the association of BPPV with hypothyroidism (OR 12.9). Moreover, the same group of authors found BPPV in 18% of patients affected by HT and normal thyroid hormonal pattern, compared to 2% of healthy control subjects 24. Modugno et al. 25 also hypothesised autoimmune alterations in patients with BPPV: they discovered autoimmune alterations in 48.5%, with a major level of anti-thyroid antibodies in 27.1%, without other ‘risk factors’. The authors postulated a link between HT and vestibular disease, possibly related to mechanical stimulation by immune complexes and possible co-existence of microangioitis in the inner ear. Our experience on recurrent BPPV and thyroid autoimmunity could be related to this hypothesis, with a tendency of vertigo not to heal quickly, compared with other types of idiopathic BPPV. On the other hand, patients with non-autoimmune hypothyroidism, such as post-thyroidectomy or multinodular goitre, had no increased risk of recurrence, suggesting that autoimmune pathogenesis can overload the clinical course of vertigo. In contrast with our results, Sari et al. 16 found that thyroid autoantibody levels in subjects with BPPV did not significantly differ from those in patients with other vestibular diseases and in normal subjects, taking into account that further large-scale studies should be done to clarify the relation.
Regarding the clinical characteristics of BPPV in patients with thyroid dysfunction, the posterior semicircular canal was most frequently involved compared to other idiopathic patients (p = 0.0087). We can hypothesise that the posterior canal can be more easily damaged by a terminal vascularisation ensured by the posterior vestibular artery, since there is a longer distance from the main internal auditory artery without collateral pathway, compared to the horizontal canal. In case of cardiovascular impairment due to hypothyroidism, this mechanism could be exacerbated.
Conclusions
The present study revealed an increased risk of recurrence of BPPV in patients with a history of hypothyroidism on HRT; the association is particularly strong for patients with positive thyroid antibodies dosage. TPO-Ab and TG-Ab levels were significantly higher in the R-BPPV group, demonstrating an association between autoimmunity and recurrence. Therefore, in complete evaluation of recurrent BPPV, we suggest investigating thyroid dysfunction by studying not only TSH, fT3 and fT4, but also the autoantibody thyroid pattern. Finally, a multidisciplinary approach and collaboration between the ENT and endocrinologist specialists is desirable.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LT, TDC, MPP. LT wrote the first draft of the manuscript and others authors commented on previous versions of the manuscript.
Ethical consideration
The study protocol was approved by the local Ethics Committee (44075/2018).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Written informed consent was obtained from all participants included in the study.
Figures and tables
References
- 1.Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet 2017;390:1550-1562. https://doi.org/10.1016/S0140-6736(17)30703-1 10.1016/S0140-6736(17)30703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giorda CB, Carnà P, Romeo F, et al. Prevalence, incidence and associated comorbidities of treated hypothyroidism: an update from a European population. Eur J Endocrinol 2017;176:533-542. https://doi.org/10.1530/EJE-16-0559 10.1530/EJE-16-0559 [DOI] [PubMed] [Google Scholar]
- 3.Koulouri O, Auldin MA, Agarwal R, et al. Diagnosis and treatment of hypothyroidism in TSH deficiency compared to primary thyroid disease: pituitary patients are at risk of under-replacement with levothyroxine. Clin Endocrinol (Oxf) 2011;74:744-749. https://doi.org/10.1111/j.1365-2265.2011.03984.x 10.1111/j.1365-2265.2011.03984.x [DOI] [PubMed] [Google Scholar]
- 4.Mortensen AH, Fang Q, Fleming MT, et al. Genetic variation in thyroid folliculogenesis influences susceptibility to hypothyroidism-induced hearing impairment. Mamm Genome 2019;30:5-22. https://doi.org/10.1007/s00335-019-09792-6 10.1007/s00335-019-09792-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, Min C, Kim HJ, et al. Low thyroid-stimulating hormone levels are associated with annoying tinnitus in adult women: Korea National Health and Nutrition Examination Surveys. Otol Neurotol 2021;42:e408-e415. https://doi.org/10.1097/MAO.0000000000003030 10.1097/MAO.0000000000003030 [DOI] [PubMed] [Google Scholar]
- 6.Chiarella G, Russo D, Monzani F, et al. Hashimoto thyroiditis and vestibular dysfunction. Endocr Pract 2017;23:863-868. https://doi.org/10.4158/EP161635.RA 10.4158/EP161635.RA [DOI] [PubMed] [Google Scholar]
- 7.Choi HG, Song YS, Wee JH, et al. Analyses of the relation between BPPV and thyroid diseases: a nested case-control study. Diagnostics (Basel) 2021;11:329. https://doi.org/10.3390/diagnostics11020329 10.3390/diagnostics11020329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med 2014;370:1138-1147. https://doi.org/10.1056/NEJMcp1309481 10.1056/NEJMcp1309481 [DOI] [PubMed] [Google Scholar]
- 9.Pérez P, Franco V, Cuesta P, et al. Recurrence of benign paroxysmal positional vertigo. Otol Neurotol 2012;33:437-443. https://doi.org/10.1097/MAO.0b013e3182487f78 10.1097/MAO.0b013e3182487f78 [DOI] [PubMed] [Google Scholar]
- 10.Zucca G, Valli S, Valli P, et al. Why do benign paroxysmal positional vertigo episodes recover spontaneously? J Vestib Res 1998;8:325-329. [PubMed] [Google Scholar]
- 11.Brandt T, Huppert D, Hecht J, et al. Benign paroxysmal positioning vertigo: a long-term follow-up (6-17 years) of 125 patients. Acta Otolaryngol 2006;126:160-163. https://doi.org/10.1080/00016480500280140 10.1080/00016480500280140 [DOI] [PubMed] [Google Scholar]
- 12.Choi SJ, Lee JB, Lim HJ, et al. Clinical features of recurrent or persistent benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 2012;147:919-924. https://doi.org/10.1177/0194599812454642. 10.1177/0194599812454642 Corrigendum. Otolaryngol Head Neck Surg 2013;149:798. https://doi.org/10.1177/0194599813505840 [DOI] [PubMed] [Google Scholar]
- 13.Picciotti PM, Lucidi D, De Corso E, et al. Comorbidities and recurrence of benign paroxysmal positional vertigo: personal experience. Int J Audiol 2016;55:279-284. https://doi.org/10.3109/14992027.2016.1143981 10.3109/14992027.2016.1143981 [DOI] [PubMed] [Google Scholar]
- 14.Picciotti PM, Di Cesare T, Tricarico L, et al. Is drug consumption correlated with benign paroxysmal positional vertigo (BPPV) recurrence? Eur Arch Otorhinolaryngol 2020;277:1609-1616. https://doi.org/10.1007/s00405-020-05855-6 10.1007/s00405-020-05855-6 [DOI] [PubMed] [Google Scholar]
- 15.Messina A, Casani AP, Manfrin M, et al. Italian survey on benign paroxysmal positional vertigo. Survey italiana sulla vertigine parossistica posizionale. Acta Otorhinolaryngol Ital 2017;37:328-335. https://doi.org/10.14639/0392-100X-1121 10.14639/0392-100X-1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sari K, Yildirim T, Borekci H, et al. The relationship between benign paroxysmal positional vertigo and thyroid autoimmunity. Acta Otolaryngol 2015;135:754-757. https://doi.org/10.3109/00016489.2015.1021932 10.3109/00016489.2015.1021932 [DOI] [PubMed] [Google Scholar]
- 17.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association Thyroid 2012;22:1200-1235. https://doi.org/10.1089/thy.2012.0205 10.1089/thy.2012.0205. Erratum in: Thyroid. 2013;23:251. Erratum in: Thyroid. 2013;23:129 [DOI] [PubMed] [Google Scholar]
- 18.Papi G, Corsello SM, Milite MT, et al. Association between benign paroxysmal positional vertigo and autoimmune chronic thyroiditis. Clin Endocrinol (Oxf) 2009;70:169-170. https://doi.org/10.1111/j.1365-2265.2008.03311.x 10.1111/j.1365-2265.2008.03311.x [DOI] [PubMed] [Google Scholar]
- 19.Mian C, Lacroix L, Alzieu L, et al. Sodium iodide symporter and pendrin expression in human thyroid tissues. Thyroid 2001;11:825-830. https://doi.org/10.1089/105072501316973073 10.1089/105072501316973073 [DOI] [PubMed] [Google Scholar]
- 20.Manzari L. Enlarged vestibular aqueduct (EVA) related with recurrent benign paroxysmal positional vertigo (BPPV). Med Hypotheses 2008;70:61-65. https://doi.org/10.1016/j.mehy.2007.04.032 10.1016/j.mehy.2007.04.032 [DOI] [PubMed] [Google Scholar]
- 21.Jabbar A, Pingitore A, Pearce SH, et al. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 2017;14:39-55. https://doi.org/10.1038/nrcardio.2016.174 10.1038/nrcardio.2016.174 [DOI] [PubMed] [Google Scholar]
- 22.Chiarella G, Tognini S, Nacci A, et al. Vestibular disorders in euthyroid patients with Hashimoto’s thyroiditis: role of thyroid autoimmunity. Clin Endocrinol (Oxf) 2014;81:600-605. https://doi.org/10.1111/cen.12471 10.1111/cen.12471 [DOI] [PubMed] [Google Scholar]
- 23.Blanchin S, Estienne V, Durand-Gorde JM, et al. Complement activation by direct C4 binding to thyroperoxidase in Hashimoto’s thyroiditis. Endocrinology 2003;144:5422-5429. https://doi.org/10.1210/en.2003-0918 10.1210/en.2003-0918 [DOI] [PubMed] [Google Scholar]
- 24.Papi G, Guidetti G, Corsello SM, et al. The association between benign paroxysmal positional vertigo and autoimmune chronic thyroiditis is not related to thyroid status. Thyroid 2010;20:237-238. https://doi.org/10.1089/thy.2009.0319 10.1089/thy.2009.0319 [DOI] [PubMed] [Google Scholar]
- 25.Modugno GC, Pirodda A, Ferri GG, et al. A relationship between autoimmune thyroiditis and benign paroxysmal positional vertigo? Med Hypotheses 2000;54:614-615. https://doi.org/10.1054/mehy.1999.0905 10.1054/mehy.1999.0905 [DOI] [PubMed] [Google Scholar]


