Abstract
Fibronectin-binding protein I (SfbI) represents a major adhesin of Streptococcus pyogenes. Mice were intranasally immunized with recombinant proteins spanning different portions of SfbI to identify the minimal fragment able to elicit a protective response against a lethal challenge with S. pyogenes. The strongest cellular responses and the highest levels of antigen-specific secretory immunoglobulin A (IgA) were detected in mice immunized with the fibronectin-binding region of SfbI. In contrast, animals vaccinated with a polypeptide spanning the aromatic and proline-rich regions showed the highest titers and fastest IgG response in serum. Vaccination with either SfbI without a membrane anchor and signal peptide or a polypeptide encompassing its fibronectin-binding regions resulted in efficient protection against heterologous challenge (60% and 80%, respectively), whereas the use of a polypeptide lacking this region conferred marginal protection (10%) with respect to the control group (0%). These results demonstrate that the fibronectin-binding region of SfbI is a promising candidate antigen for developing anti-S. pyogenes vaccines.
Streptococcus pyogenes is a human pathogen that can cause different diseases, ranging from localized infections like pharyngitis to highly invasive diseases, such as sepsis, necrotizing fasciitis, and toxic shock-like syndrome. Severe sequelae, such as rheumatic fever, rheumatic heart diseases, and acute poststreptococcal glomerulonephritis, have often been observed following streptococcal infections (19). Although infections caused by S. pyogenes can be treated with antibiotics, an increase in the incidence of streptococcal infections and especially of their sequelae has been observed throughout the world (7, 14). Therefore, there is an urgent need to develop a vaccine that can confer protective immunity without leading to cross-reactions with host tissues. Several potential vaccine candidates include the M protein, a major virulence factor of S. pyogenes (1, 2, 13); the C5a peptidase, a surface-bound peptidase which cleaves mouse and human C5a chemotaxins (6); and the extracellular cysteine protease, which cleaves human fibronectin and converts interleukin 1β (IL-1β) precursor to biologically active IL-1β (8).
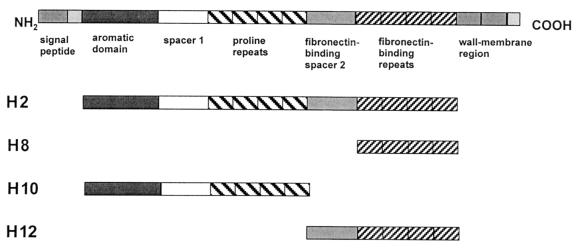
We have recently shown that intranasal immunization with the fibronectin-binding protein I (SfbI) induces protection against homologous or heterologous lethal challenge with S. pyogenes (3). SfbI is a multifunctional protein that can mediate bacterial attachment to host cells and the subsequent colonization of the upper respiratory tract, as well as bacterial internalization into nonphagocytic cells (4, 5, 9, 12, 15–17). In addition, SfbI binds to the Fc fragment of human immunoglobulin, (Ig) interfering with Fc-receptor-mediated phagocytosis and antibody-dependent cell cytotoxicity by macrophages (10). The advantages of the SfbI protein as a candidate antigen for inclusion in vaccine formulations against S. pyogenes include (i) the high conservation of its functional domains, (ii) its surface localization, (iii) its expression by a large number of clinical isolates from different serotypes (73%), and (iv) the lack of cross-reactivity with host tissues (15–18). SfbI comprises an NH2-terminal signal peptide which is followed by an aromatic domain, a region containing proline-rich repeats which is flanked by nonrepetitive spacer sequences (the latter of them with fibronectin-binding activity), a second fibronectin-binding region encompassing different repeats, and a typical cell wall and membrane anchor region in the COOH terminus (Fig. 1).
FIG. 1.
Schematic structure of the SfbI protein and the recombinant derivatives used in this work.
The instability of the SfbI protein observed during protein purification and/or storage may constitute a problem during the scale-up process. Previous studies demonstrated that truncated portions of SfbI were significantly more stable. Therefore, the objective of this study was to identify the minimal region of SfbI which retains the capacity to confer protective immunity against S. pyogenes. To achieve this aim, purified recombinant polypeptides encompassing different regions of the SfbI protein, which were generated and purified as previously described (3, 12), were used to immunize mice that were subsequently challenged with a heterologous, virulent S. pyogenes strain. The immune responses stimulated by the different fragments were then characterized.
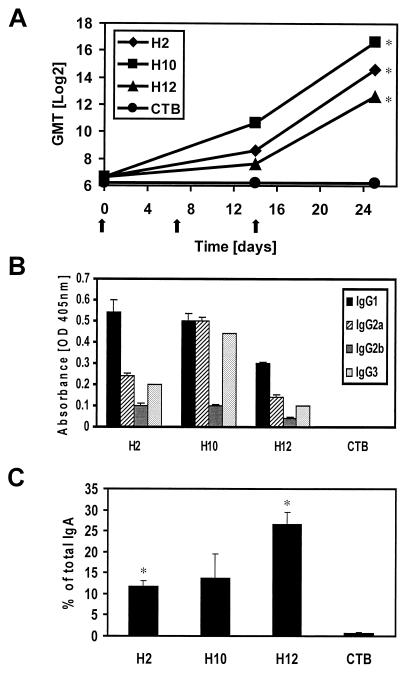
Antigen-specific serum antibody responses after intranasal immunization with the SfbI derivatives.
Intranasal immunization with a polypeptide spanning the SfbI protein without signal peptide and cell-wall and membrane anchor regions (H2) or polypeptides encompassing distinct regions (H10 or H12) resulted in the stimulation of efficient antigen-specific IgG responses in serum at day 25 after immunization (Fig. 2A). The highest titers and similar IgG response kinetics were observed for mice immunized with H2 and H10, with high titers even after the first boost (day 14). Although H12-specific IgG titers were low after the first boost in H12-immunized mice, high titers were observed at day 25 after vaccination. The stimulation of a different T-helper subpopulation may have a dramatic impact on vaccine efficacy. Thus, the major IgG isotype patterns stimulated by the different antigens were also investigated. While IgG1 was the dominant isotype in mice immunized with H2 or H12 (Th2-like pattern), animals immunized with H10 showed equal amounts of IgG1 and IgG2a, followed by IgG3 (mixed Th1-Th2-type pattern) (Fig. 2B).
FIG. 2.
Humoral immune responses stimulated by the SfbI derivatives. Mice (n = 5) were intranasally immunized with 510 pmol of the corresponding polypeptide together with 180 pmol of CTB. (A) Kinetics of the fragment-specific serum IgG responses. Results are expressed as the reciprocal log2 of the geometric mean endpoint titer (GMT) of five mice per group; immunizations are indicated by arrows. The obtained results are statistically significant (Student's t test) when compared with values for the control group (CTB alone) at P < 0.05 (∗). The standard errors of the mean (SEM) were in all cases lower than 5% of the values. (B) Isotype profiles of the antigen-specific IgG antibodies present in the serum of vaccinated mice. Results are the averages of triplicate samples. SEM are indicated by vertical lines. (C) Antigen-specific IgA antibodies in lung washes of mice. Results are expressed as the percent antigen-specific IgA antibodies with respect to total IgA. The obtained results are statistically significant when compared with values for the control group (CTB alone) at P < 0.05 (∗). SEM are indicated by vertical lines.
Antigen-specific mucosal antibody responses after intranasal immunization with the SfbI derivatives.
The elicitation of a strong local mucosal response seems to play an important role in protection against many microbial pathogens. In our experimental model, results of previous studies using SfbI also suggested that the stimulation of secretory antibodies is critical to achieve full protection against S. pyogenes (3). Thus, the ability of SfbI derivatives to trigger the elicitation of antigen-specific antibodies in the respiratory mucosa was also evaluated. The obtained results (Fig. 2C) show that the fragment encompassing both fibronectin-binding regions (H12) was the most efficient at stimulating fragment-specific mucosal IgA, followed by the H2 and H10 derivatives.
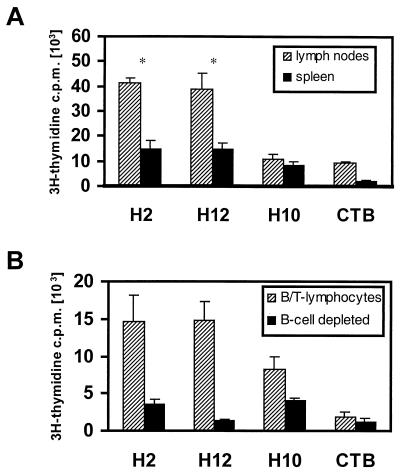
Antigen-specific cell-mediated immune responses after intranasal immunization with the SfbI derivatives.
Generation of antigen-specific effector cells in response to vaccination was evaluated for H2-, H10-, and H12-immunized mice. At day 25 after immunization, cells were isolated from the spleen and lymph nodes from immunized mice and restimulated in vitro for 4 days with the corresponding antigen as previously described (11). Cells isolated from mice immunized with the H2 or H12 fragment showed comparable proliferative responses to the homologous antigen, responses which were significantly higher (P < 0.05) than those of cells from H10-vaccinated animals (Fig. 3A). A higher frequency of antigen-specific precursors was also observed in cells isolated from lymph nodes in comparison with cells isolated from the spleens of all immunization groups (P < 0.05). To determine the nature of the stimulated cellular responses, cells from spleens of immunized mice were depleted of B cells, and proliferation was measured after 4 days of antigenic restimulation. The obtained results showed that cellular responses were significantly impaired (P < 0.05) after depletion of B cells (Fig. 3B). This consistent impairment seems to result from the elimination of the main responding population, since other antigen-presenting cells (e.g., macrophages and dendritic cells) were not depleted during the preparation.
FIG. 3.
Cellular immune responses stimulated by the SfbI derivatives. (A) Antigen-specific proliferative responses of spleen and lymph node cells to the specific polypeptide. The obtained results are statistically significant (Student's t test) when values for the H2 and H12 groups are compared with values for the H10 group at P < 0.05 (∗). (B) Effect of B-cell depletion on antigen-specific proliferative responses of spleen cells. Proliferation was assessed after 4 days of culture in the presence of 20 μg of the corresponding antigen/ml by measuring the incorporation of [3H]thymidine (11). Results are expressed as the mean counts per minute (c.p.m.) of triplicate samples; standard deviation is indicated by vertical lines.
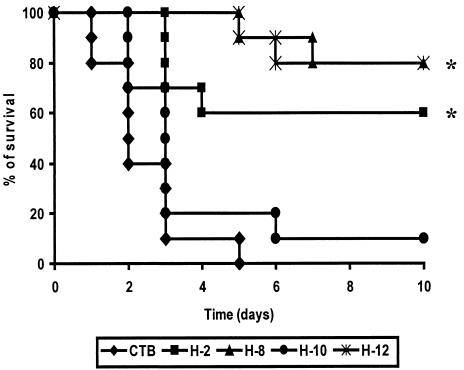
Determination of protective immunity induced by the SfbI derivatives.
BALB/c mice vaccinated with the SfbI derivative H2, H10, or H12 coadministered with the cholera toxin B subunit (CTB) as an adjuvant or with CTB alone were intranasally challenged with the virulent S. pyogenes strain NS192 (a heterologous blood isolate from the Australian Northern Territories) (Fig. 4). All mice from the control group died within 5 days, with a mean survival time of approximately 2.4 days. The highest level of protection (80%) was observed after immunization with the fibronectin-binding regions (fibronectin-binding spacer and fibronectin-binding repeats; H12), followed by 60% protection induced by the H2 fragment. In contrast, the construct lacking both fibronectin-binding regions (H10) showed no protective capacity, with 90% lethality on day 6 after challenge and a mean survival time comparable to that of the control group (∼2.4 days).
FIG. 4.
Survival of mice intranasally immunized with the SfbI derivatives coadministered with CTB against a lethal challenge with a heterologous S. pyogenes strain. Animals (n = 10) were vaccinated and challenged with 108 CFU of S. pyogenes strain NS192, following standard protocols (3), and mortality was recorded daily. The obtained results are statistically significant when compared with values for the control group (CTB alone) at P < 0.05 (∗). There is no statistically significant difference among the groups vaccinated with H2, H8, and H12.
To discriminate between the potential contribution of the fibronectin-binding repeats and that of the fibronectin-binding spacer, mice were also vaccinated with a polypeptide encompassing the fibronectin-binding repeats alone (H8). Immunization with the H8 fragment induced the same level of protection as that with H12 (80%). This suggests that the fibronectin-binding repeats are the minimal region of the tested fragments which is required to stimulate a protective response against lethal respiratory challenge with S. pyogenes. This is further supported by the fact that fibronectin-binding spacer-specific antibodies could not be detected after immunization with the H2 fragment (data not shown). Whether smaller fragments, e.g., a single fibronectin-binding repeat, can also confer protective immunity remains to be elucidated.
Although the H12 polypeptide was less efficient than H10 in triggering serum responses, it proved to be the most efficient for the stimulation of secretory IgA. This suggests that high levels of antigen-specific mucosal IgA against the fibronectin-binding regions of SfbI represent a good correlate for vaccine protection. Since the fibronectin-binding repeats mediate bacterial attachment to respiratory cells via binding to fibronectin (15, 17), we can speculate that the immune response stimulated by H12 interferes with bacterial colonization and subsequent disease development.
Acknowledgments
We thank B. Karge and A. Müller for outstanding technical help.
This work was supported in part by the DFG grant GU 482/2-1.
REFERENCES
- 1.D'Alessandri R, Plotkin G, Kluge R M, Wittner M K, Fox E N, Dorfman A, Waldman R H. Protective studies with group A streptococcal M protein vaccine. III. Challenge of volunteers after systemic or intranasal immunization with type 3 or type 12 group A streptococcus. J Infect Dis. 1978;138:712–718. doi: 10.1093/infdis/138.6.712. [DOI] [PubMed] [Google Scholar]
- 2.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzmán C A, Talay S R, Molinari G, Medina E, Chhatwal G S. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with the fibronectin-binding protein SfbI. J Infect Dis. 1999;179:901–906. doi: 10.1086/314655. [DOI] [PubMed] [Google Scholar]
- 4.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanski E, Horwitz P A, Caparon M G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Y, Carlson B, Kondagunta A, Cleary P P. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A streptococcus. Infect Immun. 1997;65:2080–2087. doi: 10.1128/iai.65.6.2080-2087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson D R, Stevens D L, Kaplan E L. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992;166:374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- 8.Kapur V, Maffei J T, Greer R S, Li L L, Adams G J, Musser J M. Vaccination with streptococcal extracellular cysteine protease (interleukin-1β-convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 9.LaPenta D, Rubens C, Chi E, Cleary P P. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina E, Molinari G, Rohde M, Haase B, Chhatwal G S, Guzmán C A. Fc-mediated nonspecific binding between fibronectin-binding protein I of Streptococcus pyogenes and human immunoglobulins. J Immunol. 1999;163:3396–3402. [PubMed] [Google Scholar]
- 11.Medina E, Talay S R, Chhatwal G S, Guzmán C A. Fibronectin-binding protein I of Streptococcus pyogenes is a promising adjuvant for antigens delivered by mucosal route. Eur J Immunol. 1998;28:1069–1077. doi: 10.1002/(SICI)1521-4141(199803)28:03<1069::AID-IMMU1069>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Molinari G, Talay S R, Valentin Weigand P, Rohde M, Chhatwal G S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polly S M, Waldman R H, High P, Wittner M K, Dorfman A. Protective studies with a group A streptococcal M protein vaccine. II. Challenge of volunteers after local immunization in the upper respiratory tract. J Infect Dis. 1975;131:217–224. doi: 10.1093/infdis/131.3.217. [DOI] [PubMed] [Google Scholar]
- 14.Stevens D L. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1995;1:69–78. doi: 10.3201/eid0103.950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talay S R, Valentin Weigand P, Jerlstrom P G, Timmis K N, Chhatwal G S. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;60:3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talay S R, Valentin Weigand P, Timmis K N, Chhatwal G S. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol Microbiol. 1994;13:531–539. doi: 10.1111/j.1365-2958.1994.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 17.Talay, S. R., A. Zock, M. Rohde, G. Molinari, M. Oggioni, G. Pozzi, C. A. Guzmán, and G. S. Chhatwal. Cooperative binding of human fibronectin to SfbI protein triggers streptococcal invasion into respiratory epithelial cells. Cell. Microbiol., in press. [DOI] [PubMed]
- 18.Valentin-Weigand P, Talay S R, Kaufhold A, Timmis K N, Chhatwal G S. The fibronectin binding domain of the Sfb protein adhesin of Streptococcus pyogenes occurs in many group A streptococci and does not cross-react with heart myosin. Microb Pathog. 1994;17:111–120. doi: 10.1006/mpat.1994.1057. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Community control of rheumatic heart disease in developing countries: 1 a major public health problem. WHO Chron. 1980;34:336–345. [PubMed] [Google Scholar]