1. Introduction
1.1. Depression and inflammation – research on older adults is scant
In recent years, a connection between depression and inflammation has been established, with a range of immunological changes, both cellular and humoral, presenting during depressive states (Beydoun et al., 2016; Haapakoski et al., 2015; Wium-Andersen et al., 2013). Furthermore, there seems to be a dose-response relationship between depression and inflammation, in the sense that the more severe the depression, the higher the level of systemic inflammation markers, most notably expressed as elevated levels of C-reactive protein (CRP) in peripheral blood (Kohler-Forsberg et al., 2017). Accordingly, CRP has been suggested as a marker of depression severity and depression subtypes, as well as an indicator of specific symptom profiles (Jokela et al., 2016). Furthermore, inflammation has been suggested as a target for treatment with immunomodulatory drugs (Alexopoulos and Morimoto, 2011; Kohler et al., 2014).
However, the research populations are predominantly younger adults, mainly in clinical settings, and there are few community-based studies providing comparative analyses of age-groups, or focusing specifically on the older population. For those that do, the results are inconsistent, as some demonstrate an association between CRP and depression (Bondy et al., 2021; Sonsin-Diaz et al., 2020; White et al., 2017), while others do not (Baune et al., 2012; Bremmer et al., 2008; Eurelings et al., 2015; Penninx et al., 2003). Thus, it is still unclear whether the inflammation in depression unfolds to the same extent in depressed older adults as in younger adults, and how the severity of the depression relates to inflammation in different age groups.
1.2. Confounding factors in older adults
One reason why results from studies on younger adults cannot be extrapolated to older adult, is the age-related changes in the immune system known as immunosenescence (Pawelec, 2018). Immunosenescence is characterized by two key features: a weakening of the inflammatory response and a low-grade inflammation (Fulop et al., 2017). The low-grade inflammation, called inflammaging, manifests for instance as an age-dependent increase in systemic CRP and certain cytokines, such as IL-6 and TNF (Franceschi et al., 2000). Inflammaging can start in early adulthood, and as such be a life style indicator, but the process seems to gain momentum and significance from the age of 60 and onwards (Malaguarnera et al., 2001).
Besides immunosenescence, other confounding factors to consider in older people are the high prevalence of somatic disorders (Salive, 2013), and the age-linked increase in BMI, both independent contributors to inflammation (Choi et al., 2013). Consequently, inflammatory findings in depressed younger adults, on whom most studies are undertaken, are not necessarily relevant to older individuals (Bugge et al., 2018).
1.3. Aim of the study
The aim of this large community-based study is to investigate the cross-sectional association between different levels of depression and systemic inflammation, as we compare individuals of two age groups; those 60 years and older versus those between 40 and 60 years. Depression is estimated by using the subscale for depression of the Hospital Anxiety and Depression Scale (HADS-D), and systemic inflammation by measuring serum CRP. Two levels of depression were analysed: depression, i.e. all levels of depression, and moderate-severe depression. The statistical analyses also included covariates previously found to be associated with elevated levels of CRP.
2. Material and methods
2.1. Study population
The Tromsø Study is a community-based, prospective cohort study of inhabitants in the municipality of Tromsø, Norway. The study design includes repeated population surveys to which total birth cohorts and random samples are invited. The study was initiated in 1974 (Tromsø 1), with repeated health surveys in 1979 - 80 (Tromsø 2), 1986 - 87 (Tromsø 3), 1994 - 95 (Tromsø 4), 2001 (Tromsø 5), 2007 - 08 (Tromsø 6) and 2015 - 16 (Tromsø 7).
Our study consists of participants from the latest survey, Tromsø 7. 21,083 individuals ≥40 years participated, i.e. 64.7 % of eligible inhabitants at the time (all inhabitants ≥40 years were invited to participate).
The survey encompassed clinical interviewing and examination, biometric data, biological sampling, including CRP, and self-administered questionnaires, including HADS (The-Tromsø-Study, 2022) .
Participants in the Tromsø Study were informed that data would be treated in strict confidence. Written informed consent was obtained from all participants.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/the participants were approved by the Norwegian Data Protection Authority and the Regional Committee for Medical and Health Research Ethics (REC North, reference no. 2014/940 and 2020/88232).
2.2. The Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a self-rating scale, originally developed for the assessment of psychological distress in non-psychiatric patients (Zigmond and Snaith, 1983). It consists of two, seven item subscales; one for anxiety, HADS-A, and one for depression, HADS-D. HADS has been extensively validated in various populations, including the general population above 60 years of age (Djukanovic et al., 2017; Grønli et al., 2022; Mykletun et al., 2001). A Norwegian version of the HADS scale was used in the present study (Leiknes et al., 2016).
Using the subscale of HADS for depression, HADS-D, two categories of self-rated depression were analysed: depression, i.e. all levels of depression, defined as HADS-D ≥ 8, and moderate-severe depression, i.e. depression of a more serious nature, defined as HADS-D ≥ 11. Studies have shown that these cut-off levels represent a good balance between sensitivity and specificity when screening for depression in general/HADS-D ≥ 8, or more severe depression/HADS-D ≥ 11 (Leiknes et al., 2016; Olsson et al., 2005).
All HADS-D questionnaires with three missing items or more were excluded from the study. Questionnaires with one or two missing items were included; for these questionnaires the scores were based on the sum of completed items multiplied by 7/6 or 7/5, correspondingly.
2.3. C-reactive protein (CRP) measured with the high-sensitivity technique
CRP is an acute phase protein playing a key role in infection, immunity and inflammation (Sproston and Ashworth, 2018). In peripheral blood it is a reliable indicator of inflammation, and as such, one of the most widely used biomarkers in medicine. CRP, in particular when detected with the high-sensitive technique, has repeatedly shown to correlate with inflammation in depression in younger adults, even when adjusting for various risk factors (Valkanova et al., 2013; Wium-Andersen et al., 2013), as well as being an indicator of depression severity and treatment response (Chamberlain et al., 2019; Yang et al., 2019). Additionally, CRP is associated with increased all-cause mortality (Proctor et al., 2015).
It should be noted that though the immune response in older people is somewhat slower and weaker compared to younger people, the elevation of CRP is a reliable indicator of trauma, infections, mortality etc. even in older adults (Alfaddagh et al., 2020; Ticinesi et al., 2017).
Participants with CRP >10 mg/L (N = 484) were excluded due to the risk of an active infection or highly inflammatory conditions eventually distorting the results (Lelubre et al., 2013; Pepys and Hirschfield, 2003).
A CRP value of 3 mg/L is broadly accepted as a cut-off point between normal and elevated serum levels, i.e. the majority of healthy adults, irrespective of age, have a serum concentration below 3 mg/L (Kalogeropoulos et al., 2010; Kushner et al., 2006; Tang et al., 2018). Accordingly, CRP ≥3 mg/L was defined as elevated, signifying the presence of a low-grade, systemic inflammation (Osimo et al., 2019).
CRP in serum was analysed using the high-sensitivity technique, at the Department of Laboratory Medicine, University Hospital of North Norway, Tromsø, Norway. The analyses were executed by employing a particle enhanced immunoturbidimetric assay on a Modular P autoanalyzer (Roche Diagnostics, Mannheim, Germany), with a detection limit of 0.12 mg/L (Hafner et al., 1997; Roche-Diagnostics, 2019).
2.4. Covariates
Participants completed questionnaires about their health, diseases and medication, as well as various life style factors and socio-economic status. Based on previous studies, and using a stepwise selection process with a mixed elimination approach, the following variables were examined as covariates: Age, sex, obesity (defined as BMI ≥30), daily smoking, having a partner or close friend, higher education (i.e. a college or university degree) and current somatic disease, including angina pectoris, congestive heart failure, atrial fibrillation, hypertension, cerebral insult, diabetes, kidney disease (any), chronic obstructive pulmonary disease, asthma, cancer (any), rheumatoid arthritis, osteoarthritis and migraine.
Underweight individuals (N = 153), i.e. individuals with BMI <18.5, were excluded from the study, as underweight is known to compromise the immune system, thereby leading to aberrant inflammatory markers (Brown et al., 2008).
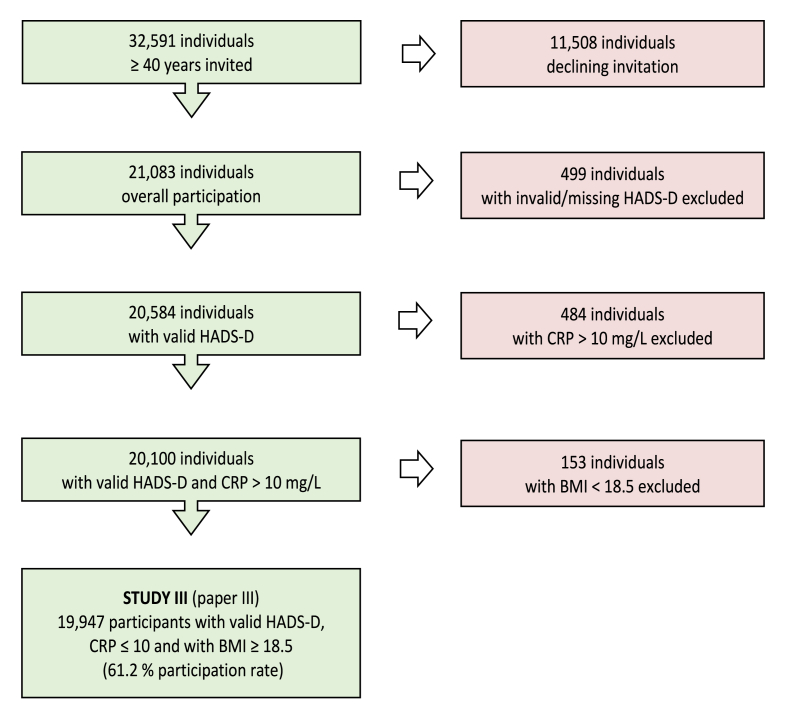
The present analyses are based on data for participants with valid HADS-D,
CRP <10 mg/L, and BMI ≥18.5. 19,947 participants were included in the final analyses, i.e. 61.2 % participation rate (Fig. 1).
Fig. 1.
Flow-chart inclusion/exclusion.
2.5. Statistical analyses
IBM Statistical Package for the Social Sciences, Version 26 (SPSS Inc., Chicago, Illinois, USA) software was applied in the statistical analysis.
Frequencies were used to describe the population characteristics. Test score reliability for the HADS-D scale was assessed by estimating Cronbach’s alpha. Possible multicollinearity between the covariates was tested by creating a correlation matrix, as well as applying linear regression to calculate estimates of variance inflation (VIF).
To test possible associations between systemic inflammation/elevated CRP, i.e. the dependent variable, and depression/moderate-severe depression and age/age-groups, i.e. the two primary independent variables, and the various covariates, we used binary logistic regression to calculate odds ratios. First, two logistic regression models were analysed: an adjusted model, including depression (HADS-D ≥ 8) and moderate-severe depression (HADS ≥11), as well as 10 years strata and sex as covariates, and a multi-adjusted model, including depression and moderate-severe depression, as well as 10 years strata, sex, obesity, daily smoking, current somatic disease, higher education and having a partner/close friend as covariates. Second, the aforementioned adjusted and multi-adjusted models were utilized to compare two age groups; those 60 years and older versus those between 40 and 60 years. In these analyses, the covariate 10 years age strata was removed from both models.
3. Results
The characteristics of the participants with normal and elevated CRP are presented in Table 1. Participants aged 40–59 years had a higher proportion of depression and moderate-severe depression, than participants aged 60 and above. Mean HADS-D, however, was not significantly different between the two age groups (Table 1).
Table 1.
Population characteristics of participants aged ≥60 years versus 40–59 years, and with normal versus elevated CRP.
| N = 19,947 | Age ≥60 years |
Age 40–59 years |
P-value X2/t-test |
Normal CRP 0–2.99 mg/L |
Elevated CRP 3–10 mg/L |
P-value X2/t-test |
|---|---|---|---|---|---|---|
| N | 8010 | 11,937 | 0.00 | 17,216 | 2731 | 0.00 |
| HADS-D, mean (SD) | 2.84 (2.6) | 2.81 (2.8) | 0.40 | 2.8 (2.7) | 3.1 (3.0) | 0.00 |
| Depressiona, N (%) | 470 (5.9) | 880 (7.4) | 0.00 | 1088 (6.3) | 262 (9.6) | 0.00 |
| Moderate-severe depressionb, N (%) | 96 (1.2) | 225 (1.9) | 0.00 | 247 (1.4) | 74 (2.7) | 0.00 |
| Age, mean (SD) | 68.7 (6.8) | 49.3 (5.7) | 0.00 | 56.8 (11.2) | 58.8 (11.6) | 0.00 |
| Age ≥60 years, N (%) | – | – | – | 6767 (39.3) | 1243 (45.5) | 0.00 |
| Elevated CRP 3–10 mg/L, N (%) | 1243 (15.5) | 1488 (12.5) | 0.00 | – | – | – |
| Women, N (%) | 4100 (51.2) | 6333 (53.1) | 0.01 | 8888 (51.6) | 1545 (56.6) | 0.00 |
| BMI, mean (SD) | 27.4 (4.3) | 27.3 (4.5) | 0.53 | 26.9 (4.1) | 30.3 (5.3) | 0.00 |
| Obesity, N (%) | 1864 (23.3) | 2823 (23.6) | 0.54 | 3388 (19.7) | 1299 (47.6) | 0.00 |
| Daily smoking, N (%) Missing = 173 |
968 (12.1) | 1743 (14.6) | 0.00 | 2171 (12.7) | 540 (20.0) | 0.00 |
| Current somatic disease, N (%) Missing = 780 |
4883 (61.0) | 4224 (35.4) | 0.00 | 7506 (45.4) | 1601 (61.1) | 0.00 |
| Higher education, N (%)c Missing = 319 |
2917 (36.4) | 6840 (57.3) | 0.00 | 8673 (51.2) | 1084 (40.5) | 0.00 |
| Partner or close friend, N (%) Missing = 230 |
7718 (96.4) | 11,597 (97.2) | 0.81 | 16,718 (98.1) | 2597 (96.9) | 0.00 |
HADS-D ≥ 8, i.e. all levels of depression.
HADS-D ≥ 11.
University or college degree.
The group of participants with elevated CRP had a higher proportion of people aged 60 and above, as well as a higher mean age, than the group of participants with normal CRP. Furthermore, participants with elevated CRP had a higher mean HADS-D, and a higher proportion of depression and moderate-severe depression, than participants with normal CRP.
Cronbach’s alpha for HADS-D was 0.73, indicating satisfactory internal consistency. For the regression models, low correlation coefficients and VIFs (range 1.02–1.16) signified that multicollinearity between the covariates was not a concern.
In general, participants with depression, i.e. all levels of depression, and moderate-severe depression, were significantly more likely than non-depressed participants to have an elevated CRP, even when adjusting for several covariates (Table 2). Moreover, a 10-year increase in age produced a 16 % increase in the odds for an elevated CRP in the adjusted model, and an 11 % increase in the odds for an elevated CRP in multi-adjusted model, regardless of depression level (Table 2).
Table 2.
Relationship between inflammation (CRP ≥3 mg/L) and two levels of depression – depression (i.e. all levels of depression) and moderate-severe depression.
| Adjusted model |
Multi-adjusted model |
||||||
|---|---|---|---|---|---|---|---|
| ORa | (95 % CI) | P-value | OR | (95 % CI) | P-value | ||
| Depressionb | 1.62 | (1.40–1.86) | 0.00 | 1.31 | (1.11–1.53) | 0.00 | |
| 10 years age strata | 1.16 | (1.12–1.20) | 0.00 | 1.11 | (1.07–1.16) | 0.00 | |
| Sex (male = 1) | 0.81 | (0.75–0.88) | 0.00 | 0.80 | (0.74–0.88) | 0.00 | |
| Obesity | 3.60 | (3.29–3.94) | 0.00 | ||||
| Daily smoking | 1.80 | (1.60–2.02) | 0.00 | ||||
| Current somatic disease | 1.47 | (1.34–1.62) | 0.00 | ||||
| Higher educationc | 0.85 | (0.77–0.93) | 0.00 | ||||
| Having a partner/close friend | 0.74 | (0.56–0.97) | 0.03 | ||||
| Constant | 0.12 | 0.00 | 0.11 | 0.00 | |||
| Hosmer-Lemeshow test |
X2 = 2.70 Sig. = 0.85 |
X2 = 6.46 Sig. = 0.60 |
|||||
| Moderate-severe depressiond | 2.00 | (1.54–2.61) | 0.00 | 1.50 | (1.11–2.04) | 0.01 | |
| 10 years age strata | 1.16 | (1.12–1.20) | 0.00 | 1.11 | (1.07–1.16) | 0.00 | |
| Sex (male = 1) | 0.81 | (0.75–0.88) | 0.00 | 0.81 | (0.74–0.88) | 0.00 | |
| Obesity | 3.61 | (3.30–3.95) | 0.00 | ||||
| Daily smoking | 1.80 | (1.61–2.02) | 0.00 | ||||
| Current somatic disease | 1.48 | (1.35–1.62) | 0.00 | ||||
| Higher educationb | 0.84 | (0.77–0.93) | 0.00 | ||||
| Having a partner/close friend | 0.73 | (0.56–0.96) | 0.03 | ||||
| Constant | 0.12 | 0.00 | 0.11 | 0.00 | |||
| Hosmer-Lemeshow test | X2 = 1.71 Sig. = 0.94 | X2 = 12.87 Sig. = 0.12 | |||||
Odds ratio.
HADS-D ≥ 8.
University or College Degree.
HADS-D ≥ 8.
Participants 60 years and older with depression and moderate-severe depression had higher odds of elevated CRP than participants without depression when adjusting for sex, but not when adjusting for several covariates (Table 3). Participants between 40 and 60 years with depression and moderate-severe depression had higher odds of elevated CRP, even when adjusting for several covariates, than participants without depression (Table 3).
Table 3.
Relationship between inflammation (CRP ≥3 mg/L), depression (HADS-D ≥ 8, i.e. all levels of depression) and m oderate-severe depression (HADS-D ≥ 11).
| Age ≥60 years |
Age 40–59 years |
|||||
|---|---|---|---|---|---|---|
| ORa | (95 % CI) | P-value | OR | (95 % CI) | P-value | |
| Depression, adjusted modelb | 1.29 | (1.01–1.64) | 0.04 | 1.85 | (1.55–2.20) | 0.00 |
| Depression, multi-adjusted modelc | 0.99 | (0.75–1.30) | 0.91 | 1.51 | (1.24–1.84) | 0.00 |
| Moderate-severe depression, adjusted model | 2.06 | (1.31–3.25) | 0.00 | 1.95 | (1.41–2.70) | 0.00 |
| Moderate-severe depression, multi-adjusted model | 1.50 | (0.87–2.58) | 0.14 | 1.50 | (1.03–2.17) | 0.03 |
Odds ratio.
Adjusted model: Adjusted for sex.
Multi-adjusted model: Adjusted for sex, obesity, daily smoking, ongoing somatic disease, higher education, partner or close friend.
4. Discussion
To our knowledge, this study is the largest cross-sectional community-based study of inflammation in depression with comparative analyses of age-groups, with a total of 19,949 participants, of which 8010 were aged 60 and older. The main finding is that, when adjusting for the covariates (as defined in 2.4), depression and moderate-severe depression was significantly associated with an elevated level of CRP in participants younger than 60 years, but not in those people aged 60 or above. The former is in accordance with most previous clinical studies and community-based studies. As for the latter, comparable studies are few and the findings conflicting. For instance, two Dutch population studies reported no correlation between CRP and depression (Bremmer et al., 2008; Tiemeier et al., 2003), whilst two studies from the English Longitudinal Study of Aging (ELSA) reported the opposite (Gallagher et al., 2017; White et al., 2017). However, all of these studies have some significant limitations, of which missing data on CRP is perhaps one of the most prominent, ranging from 17 to 42 %. There is also a considerable variation in the prevalence of depression. For instance, Bremmer and colleagues reports a prevalence of 14.8 %, whereas Tiemeier and co-workers reports 2.7 %, despite the latter population being younger (mean age 61 versus 76) with a higher proportion of women, thus expected to have a higher rate of depression. Both research groups used the 20-item Center for Epidemiological Studies Depression scale (CED-S), with 16 points as cut-off for depression. Such methodological issues are common in this field of research, making comparisons and interpretations of the studies a precarious undertaking. Besides missing data and a substantial variety in the rates of depression and depressive symptoms, the issues include e.g. a large span in mean age, different cut-off scores for CRP, various methods of statistical structuring and analysis, and insufficient covariates analyses. For example, a mean age of 61 years (Stewart et al., 2009) versus 85 years (van den Biggelaar et al., 2007) are likely to reflect very different levels of immunosenescence in the two populations, with potentially dissimilar effects on the levels of CRP. Another example; despite multimorbidity affecting the majority of people over 65 years (Salive, 2013), some studies include no chronic diseases as covariates, others only include one or two (Smith et al., 2018).
The absence of an adjusted correlation between depression and elevated levels of CRP in individuals aged 60 and older, signify that inflammation is not an intrinsic part of the pathophysiology of depression in a general population of older adults. Theoretically, there might be smaller subgroups of geriatric depression for whom inflammation are a key factor, but in terms of severity, we did not find any association between neither depression in general (HADS-D ≥ 8), nor moderate-severe depression (HADS-D ≥ 11), with an elevated CRP.
An alternative or complementary explanation is that the inflammatory processes triggered by depression in older persons, are eventually blunted by immunosenescent mechanisms, even to the extent that no significant changes in systemic inflammatory markers (e.g. CRP) are detected (Shaw et al., 2013). A similar problem may arise because of the non-specific nature of CRP. CRP is elevated in inflammation of virtually all causes (with the exception of a few autoimmune diseases). In older people, there are generally several potent contributors to an elevated CRP, due to the high prevalence of somatic diseases, obesity, degenerative processes, senescent cells etc. Consequently, a possible contribution to a systemic elevation of CRP stemming from depression - expectedly modest in size - might be obscured by other inflammation sources.
Though we could not identify a correlation between CRP and depression, it is possible that more sensitive, and possibly age-specific, inflammatory markers are better at detecting inflammation in depressed older adults. So far, the results from, for instance, multiplex immunoassays studies on cytokines in depression diverge (Bugge et al., 2019; Himmerich et al., 2019; Sonsin-Diaz et al., 2020), and there have yet not been identified reliable immuno-markers, or patterns of immune-markers, in depression (Najjar et al., 2013; Yuan et al., 2019).
Furthermore, depression is a heterogenous disorder, with important clinical differences between depression in the geropsychiatric population and depression of younger people (Hegeman et al., 2012). For instance, later life depression presents with higher rates of somatic symptoms, anxiety and cognitive impartment, compared to depression in younger individuals (Gottfries, 1998; Hegeman et al., 2012; Wu et al., 2021). Given that the HADS-D used in our study does not include somatic symptoms, it is conceivable that some of the depressed older adults are not identified in our study, or that their depression level is inaccurately estimated. That being said, the prevalence of depression in participants 60 years and older in this study (5,9 %) does not deviate from prevalence estimates of geropsychiatric depression in general populations (CDC, 2021). On a related note, it is worth mentioning that some studies on the general population points to an association between specific depressive symptoms and CRP (Frank et al., 2021; Jokela et al., 2016). It is not known whether this holds true for geriatric depression, but as we did not undertake any analyses on single factors of the HADS-D, in cannot be ruled out that such correlations exist. Then again, it is uncertain whether individual symptoms hold adequate sensitivity and specificity for the identification of a depressive disorder in older adults.
The main strengths of our study are the number of participants and the inclusion of most relevant covariates, including a broad range of somatic disorders. It is worth mentioning that alcohol consumption and psychotropic drugs were amongst the covariates excluded from the final analyses, as they did not turn out to be impacting factors in the stepwise selection process of covariates. Pertaining to alcohol, this could be explained by the J-shaped relation between alcohol and inflammation, i.e. moderate drinkers have been shown to have a lower level of inflammation compared to occasional drinkers, abstainers and heavy drinkers (Bektas et al., 2016). An analogous case could be made for psychotropic drugs, their effects on inflammation varying both between and within groups of drugs (Baumeister et al., 2016).
The participation rate of this study (61.2 %), as well as the recruitment from a single community, calls for caution in generalization of the results. The inclusion of important confounding variables is another strong point of the study, but the presence of unrecognized covariates contributing to residual confounding, cannot be ruled out. Besides, several covariates are based on questionnaires, which by itself involves an increased risk of misclassification.
Just like all community-based studies, our study runs the risk of a selection bias, as it is well documented that women, healthier people, people living with a partner, and people from higher socio-economic classes are more likely to attend population surveys (Galea and Tracy, 2007). In our study, the sex distribution was 52 % women, and 48 % men. The statistical analyses showed that being male is associated with lower odds of systemic inflammation (Table 2). This is, however, in concordance with most other studies, demonstrating that various populations of men tend to have lower CRP-levels than women (Khera et al., 2009; Valentine et al., 2009; Choi et al., 2013).
We do not know who the non-attenders of our study are, but from the Tromsø 6 survey, we learned that attenders were older, with a higher proportion of married/cohabitants, compared to the non-attenders (Eggen et al., 2013). A study of non-attenders aged 55 years and younger in The Tromsø 2 survey, revealed that the non-attenders had a higher prevalence of psychiatric disorders, particularly substance abuse, compared with attenders (Hansen et al., 2001). It is reasonable to assume that these characteristics are, at least to some extent, also present in our study, though it is unclear what the effects, if any, might be on the overall outcome of the study. In any case, it is to be noted that the difference in attendance rate between the age groups was less than 1 %; 61.7 % in individuals ≤60 years and 60.8 % for individuals aged 40–59 years.
Finally, the cross-sectional design of the study does not allow for any hypothesis about the direction of the associations between CRP and depression, and thus, precludes information about causality.
In conclusion, we did not find an adjusted correlation between depression, and moderate-severe depression, with systemic inflammation measured by CRP, in individuals 60 years and older. This finding indicates that inflammation is not a prominent part of the pathophysiology of depression in most older adults, and thus, it does not support previous theories about inflammation as an assessment tool or treatment target in geriatric depression.
Further studies should include purposely adapted approaches to target depressive symptomatology and inflammatory changes in the older population.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Erlend Bugge, Email: erlend.bugge@unn.no.
Rolf Wynn, Email: rolf.wynn@unn.no.
Tom Eirik Mollnes, Email: t.e.mollnes@medisin.uio.no.
Solveig Klæbo Reitan, Email: solveig.reitan@ntnu.no.
Maria Lapid, Email: lapid.maria@mayo.edu.
Ole Kristian Grønli, Email: ole.k.gronli@unn.no.
Data availability
Data will be made available on request.
References
- Alexopoulos G.S., Morimoto S.S. The inflammation hypothesis in geriatric depression. Int. J. Geriatr. Psychiatr. 2011;26(11):1109–1118. doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaddagh A., Martin S.S., Leucker T.M., Michos E.D., Blaha M.J., Lowenstein C.J., Jones S.R., Toth P.P. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol. 2020;4 doi: 10.1016/j.ajpc.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D., Ciufolini S., Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology (Berl) 2016;233(9):1575–1589. doi: 10.1007/s00213-015-4044-5. [DOI] [PubMed] [Google Scholar]
- Baune B.T., Smith E., Reppermund S., Air T., Samaras K., Lux O., Brodaty H., Sachdev P., Trollor J.N. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37(9):1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Bektas A., Sen R., Ferrucci L. Does a bit of alcohol turn off inflammation and improve health? Age Ageing. 2016;45(6):747–748. doi: 10.1093/ageing/afw146. [DOI] [PubMed] [Google Scholar]
- Beydoun M.A., Beydoun H.A., Dore G.A., Canas J.A., Fanelli-Kuczmarski M.T., Evans M.K., Zonderman A.B. White blood cell inflammatory markers are associated with depressive symptoms in a longitudinal study of urban adults. Transl. Psychiatry. 2016;6(9):e895. doi: 10.1038/tp.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy E., Norton S.A., Voss M., Marks R.B., Boudreaux M.J., Treadway M.T., Oltmanns T.F., Bogdan R. Inflammation is associated with future depressive symptoms among older adults. Brain Behav Immun Health. 2021;13 doi: 10.1016/j.bbih.2021.100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer M.A., Beekman A.T., Deeg D.J., Penninx B.W., Dik M.G., Hack C.E., Hoogendijk W.J. Inflammatory markers in late-life depression: results from a population-based study. J. Affect. Disord. 2008;106(3):249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Brown R.F., Bartrop R., Birmingham C.L. Immunological disturbance and infectious disease in anorexia nervosa: a review. Acta Neuropsychiatr. 2008;20(3):117–128. doi: 10.1111/j.1601-5215.2008.00286.x. [DOI] [PubMed] [Google Scholar]
- Bugge E., Wynn R., Mollnes T.E., Reitan S.K., Gronli O.K. Cytokine profiles and diagnoses in elderly, hospitalized psychiatric patients. BMC Psychiatr. 2018;18(1):315. doi: 10.1186/s12888-018-1900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge E., Wynn R., Mollnes T.E., Reitan S.K., Lapid M.I., Gronli O.K. Changes in cytokines during treatment of elderly, hospitalized psychiatric patients - a naturalistic study. Psychoneuroendocrinology. 2019;108:135–139. doi: 10.1016/j.psyneuen.2019.06.014. [DOI] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2021. Depression Is Not a Normal Part of Growing Older.https://www.cdc.gov/aging/depression/index.html January 6, 2021. Retrieved 06.02.2022 from. [Google Scholar]
- Chamberlain S.R., Cavanagh J., de Boer P., Mondelli V., Jones D.N.C., Drevets W.C., Cowen P.J., Harrison N.A., Pointon L., Pariante C.M., Bullmore E.T. Treatment-resistant depression and peripheral C-reactive protein. Br. J. Psychiatry. 2019;214(1):11–19. doi: 10.1192/bjp.2018.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Joseph L., Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes. Rev. 2013;14(3):232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- Djukanovic I., Carlsson J., Arestedt K. Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65-80 years old? A psychometric evaluation study. Health Qual. Life Outcome. 2017;15(1):193. doi: 10.1186/s12955-017-0759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen A.E., Mathiesen E.B., Wilsgaard T., Jacobsen B.K., Njolstad I. The sixth survey of the Tromso Study (Tromso 6) in 2007-08: collaborative research in the interface between clinical medicine and epidemiology: study objectives, design, data collection procedures, and attendance in a multipurpose population-based health survey. Scand. J. Publ. Health. 2013;41(1):65–80. doi: 10.1177/1403494812469851. [DOI] [PubMed] [Google Scholar]
- Eurelings L.S., Richard E., Eikelenboom P., van Gool W.A., Moll van Charante E.P. Low-grade inflammation differentiates between symptoms of apathy and depression in community-dwelling older individuals. Int. Psychogeriatr. 2015;27(4):639–647. doi: 10.1017/S1041610214002683. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging - an evolutionary perspective on immunosenescence. Molecular and Cellular Gerontology. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. <Go to ISI>://WOS:000088671200022. [DOI] [PubMed] [Google Scholar]
- Frank P., Jokela M., Batty G.D., Cadar D., Steptoe A., Kivimaki M. Association between systemic inflammation and individual symptoms of depression: a pooled analysis of 15 population-based cohort studies. Am. J. Psychiatr. 2021;178(12):1107–1118. doi: 10.1176/appi.ajp.2021.20121776. [DOI] [PubMed] [Google Scholar]
- Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S., Tracy M. Participation rates in epidemiologic studies. Ann. Epidemiol. 2007;17(9):643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Gallagher D., Kiss A., Lanctot K., Herrmann N. Depression with inflammation: longitudinal analysis of a proposed depressive subtype in community dwelling older adults. Int. J. Geriatr. Psychiatr. 2017;32(12):e18–e24. doi: 10.1002/gps.4645. [DOI] [PubMed] [Google Scholar]
- Gottfries C.G. Is there a difference between elderly and younger patients with regard to the symptomatology and aetiology of depression? Int. Clin. Psychopharmacol. 1998;13(Suppl. 5):S13–S18. doi: 10.1097/00004850-199809005-00004. [DOI] [PubMed] [Google Scholar]
- Grønli O., Bramness J., Wynn R., Høye A. Depressive symptoms in the general population: the 7th Tromsø Study. Journal of Affective Disorders Reports. 2022;8 doi: 10.1016/j.jadr.2022.100322. [DOI] [Google Scholar]
- Hafner G., Thomas L., Larrea L.B.d., Galanti L., llardo C., Scheurmann T., Röddiger R., Zawta B. Determination of C-reactive protein by an improved turbidimetric assay. J. Lab. Med. 1997;21 file:///C:/Users/eb0512unn/Downloads/10.1515_labm.1997.21.12.659.pdf. [Google Scholar]
- Hansen V., Jacobsen B.K., Arnesen E. Prevalence of serious psychiatric morbidity in attenders and nonattenders to a health survey of a general population : the Tromso Health Study. Am. J. Epidemiol. 2001;154(10):891–894. doi: 10.1093/aje/154.10.891. [DOI] [PubMed] [Google Scholar]
- Hegeman J.M., Kok R.M., van der Mast R.C., Giltay E.J. Phenomenology of depression in older compared with younger adults: meta-analysis. Br. J. Psychiatry. 2012;200(4):275–281. doi: 10.1192/bjp.bp.111.095950. [DOI] [PubMed] [Google Scholar]
- Himmerich, H., Patsalos, O., Lichtblau, N., Ibrahim, M. A. A., & Dalton, B. (2019). Cytokine research in depression: principles, challenges, and open questions. Front. Psychiatr., 10. https://doi.org/ARTN 3010.3389/fpsyt.2019.00030. [DOI] [PMC free article] [PubMed]
- Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M., Virtanen M., Batty G.D., Kivimaki M. Inflammation and specific symptoms of depression. JAMA Psychiatr. 2016;73(1):87–88. doi: 10.1001/jamapsychiatry.2015.1977. [DOI] [PubMed] [Google Scholar]
- Kalogeropoulos A., Georgiopoulou V., Psaty B.M., Rodondi N., Smith A.L., Harrison D.G., Liu Y., Hoffmann U., Bauer D.C., Newman A.B., Kritchevsky S.B., Harris T.B., Butler J., Health A.B.C.S.I. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J. Am. Coll. Cardiol. 2010;55(19):2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler-Forsberg O., Buttenschon H.N., Tansey K.E., Maier W., Hauser J., Dernovsek M.Z., Henigsberg N., Souery D., Farmer A., Rietschel M., McGuffin P., Aitchison K.J., Uher R., Mors O. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 2017;62:344–350. doi: 10.1016/j.bbi.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Khera A., Vega G.L., Das S.R., Ayers C., McGuire D.K., Grundy S.M., de Lemos J.A. Sex differences in the relationship between C-reactive protein and body fat. J. Clin. Endocrinol. Metab. 2009;94(9):3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatr. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Kushner I., Rzewnicki D., Samols D. What does minor elevation of C-reactive protein signify? Am. J. Med. 2006;119(2) doi: 10.1016/j.amjmed.2005.06.057. 166 e117-128. [DOI] [PubMed] [Google Scholar]
- Leiknes K.A., Dalsbø T.K., Siqveland J. 2016. Psychometric assessment of the Norwegian version of the Hospital Anxiety and Depression Scale (HADS). Norwegian Institute of Public Health, report 2016. [Google Scholar]
- Lelubre C., Anselin S., Zouaoui Boudjeltia K., Biston P., Piagnerelli M. Interpretation of C-reactive protein concentrations in critically ill patients. BioMed Res. Int. 2013 doi: 10.1155/2013/124021. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera L., Ferlito L., Imbesi R.M., Gulizia G.S., Di Mauro S., Maugeri D., Malaguarnera M., Messina A. Immunosenescence: a review. Arch. Gerontol. Geriatr. 2001;32(1):1–14. doi: 10.1016/s0167-4943(00)00086-8. [DOI] [PubMed] [Google Scholar]
- Mykletun A., Stordal E., Dahl A.A. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br. J. Psychiatry. 2001;179:540–544. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- Najjar S., Pearlman D.M., Alper K., Najjar A., Devinsky O. Neuroinflammation and psychiatric illness. J. Neuroinflammation. 2013;10:43. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Mykletun A., Dahl A.A. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatr. 2005;5:46. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol. Med. 2019;49(12):1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G. Age and immunity: what is "immunosenescence. Exp. Gerontol. 2018;105:4–9. doi: 10.1016/j.exger.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Penninx B.W., Kritchevsky S.B., Yaffe K., Newman A.B., Simonsick E.M., Rubin S., Ferrucci L., Harris T., Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol. Psychiatr. 2003;54(5):566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor M.J., McMillan D.C., Horgan P.G., Fletcher C.D., Talwar D., Morrison D.S. Systemic inflammation predicts all-cause mortality: a glasgow inflammation outcome study. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche-Diagnostics . 2019. Cardiac C-Reactive Protein (Latex) High Sensitive Order Information.https://labogids.sintmaria.be/sites/default/files/files/crphs_2019-01_v11.pdf [Google Scholar]
- Salive M.E. Multimorbidity in older adults. Epidemiol. Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13(12):875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.J., Au B., Ollis L., Schmitz N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: a systematic review and meta-analysis. Exp. Gerontol. 2018;102:109–132. doi: 10.1016/j.exger.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Sonsin-Diaz N., Gottesman R.F., Fracica E., Walston J., Windham B.G., Knopman D.S., Walker K.A. Chronic systemic inflammation is associated with symptoms of late-life depression: the ARIC study. Am. J. Geriatr. Psychiatr. 2020;28(1):87–98. doi: 10.1016/j.jagp.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.C., Rand K.L., Muldoon M.F., Kamarck T.W. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav. Immun. 2009;23(7):936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Liang P., Chen J., Fu S., Liu B., Feng M., Lin B., Lee B., Xu A., Lan H.Y. The baseline levels and risk factors for high-sensitive C-reactive protein in Chinese healthy population. Immun. Ageing. 2018;15:21. doi: 10.1186/s12979-018-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The-Tromsø-Study . 2022. Information about the Tromsø Studies and a Complete Overview of Questionnaires and Variables Can Be Found at the Homepage of the Tromsø Study.https://uit.no/research/tromsostudy [Google Scholar]
- Ticinesi A., Lauretani F., Nouvenne A., Porro E., Fanelli G., Maggio M., Meschi T. C-reactive protein (CRP) measurement in geriatric patients hospitalized for acute infection. Eur. J. Intern. Med. 2017;37:7–12. doi: 10.1016/j.ejim.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Tiemeier H., Hofman A., van Tuijl H.R., Kiliaan A.J., Meijer J., Breteler M.M. Inflammatory proteins and depression in the elderly. Epidemiology. 2003;14(1):103–107. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Valentine R.J., McAuley E., Vieira V.J., Baynard T., Hu L., Evans E.M., Woods J.A. Sex differences in the relationship between obesity, C-reactive protein, physical activity, depression, sleep quality and fatigue in older adults. Brain Behav. Immun. 2009;23(5):643–648. doi: 10.1016/j.bbi.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar A.H., Gussekloo J., de Craen A.J., Frolich M., Stek M.L., van der Mast R.C., Westendorp R.G. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp. Gerontol. 2007;42(7):693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- White J., Kivimaki M., Jokela M., Batty G.D. Association of inflammation with specific symptoms of depression in a general population of older people: the English Longitudinal Study of Ageing. Brain Behav. Immun. 2017;61:27–30. doi: 10.1016/j.bbi.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen M.K., Orsted D.D., Nielsen S.F., Nordestgaard B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatr. 2013;70(2):176–184. doi: 10.1001/2013.jamapsychiatry.102. [DOI] [PubMed] [Google Scholar]
- Wu Y., Levis B., Sun Y., He C., Krishnan A., Neupane D., Bhandari P.M., Negeri Z., Benedetti A., Thombs B.D., Group D.E.S.D.H. Accuracy of the Hospital Anxiety and Depression Scale Depression subscale (HADS-D) to screen for major depression: systematic review and individual participant data meta-analysis. BMJ. 2021;373:n972. doi: 10.1136/bmj.n972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Wardenaar K.J., Bosker F.J., Li J., Schoevers R.A. Inflammatory markers and treatment outcome in treatment resistant depression: a systematic review. J. Affect. Disord. 2019;257:640–649. doi: 10.1016/j.jad.2019.07.045. [DOI] [PubMed] [Google Scholar]
- Yuan N., Chen Y., Xia Y., Dai J., Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry. 2019;9(1):233. doi: 10.1038/s41398-019-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.