Graphical abstract
Abbreviations: 8-oxodG, 8-oxo-2́deosyguanosine; ABCG, ATP Binding Cassette Subfamily G Member; ADAM10, α-secretase; ADRB3, adrenoceptor Beta 3; APP, amyloid-β precursor protein; ARF, auxin response factor; ARHGAP24, Rho GTPase Activating Protein 24; ARH-I, aplysia ras homology member I; ATF6, activating transcription factor 6; ATP2A3, ATPase Sarcoplasmic/Endoplasmic Reticulum Ca2+ Transporting 3; BCL2L14, apoptosis facilitator Bcl-2-like protein 14; CDH1, cadherin-1; CDKN, cyclin dependent kinase inhibitor; CPT, carnitine palmitoyltransferase; CREBH, cyclic AMP-responsive element-binding protein H; DANT2, DXZ4 associated non-noding transcript 2, distal; DAPK1, death-associated protein kinase 1; DNMT, DNA methyltransferase; DOT1L, disruptor of telomeric silencing 1-like; EWASs, epigenome-wide association studies; EZH2, Enhancer of zeste homolog 2; FAS, Fas cell Surface Death Receptor; GDNF, glial cell line-derived neurotrophic factor; GFAP, glial fibrillary acid protein; GSTP1, Glutathione S-transferases P1; HAT, histone acetylases; HDAC, histone deacetylases; HSD11B2, 11 beta-hydroxysteroid dehydrogenase type 2; IGFBP3, insulin-like growth factor-binding protein 3; IGT, impaired glucose tolerance; KCNK3, potassium two pore domain channel subfamily K Member 3; lncRNA, long non-coding RNA; MBD4, methyl-CpG binding domain 4; MGMT, O-6-methylguanine-DNA methyltransferase; NAFLD, Non-alcoholic fatty liver disease; ncRNA, non-coding RNA; oAβ-induced-LTP, oligomeric amyloid-beta induced long term potentiation; OCT1, Organic cation transporter 1; OGG1, 8-Oxoguanine DNA Glycosylase; PAI-1, plasminogen activator inhibitor 1; PHOSPHO1, Phosphoethanolamine/Phosphocholine Phosphatase 1; PLIN1, perilipin 1; POE3A, RNA polymerase III; PPAR, peroxisome proliferator-activated receptor; PPARGC1A, PPARG coactivator 1 alpha; PRKCA, Protein kinase C alpha; PTEN, phosphatase and tensin homologue; RASSF1A, Ras association domain family member 1; SAH, S -adenosyl-l-homocysteine; SAM, S-adenosyl-methionine; SD, sleep deprivation; SOCS3, suppressor of cytokine signaling 3; SREBP-1C, sterol-regulatory element binding protein-1C; TBX2, t-box transcription factor 2; TCF7L2, transcription factor 7 like 2; TET, ten-eleven translocation proteins; TNNT2, cardiac muscle troponin T; TPA, 12-O-tetradecanoylphorbol-13-acetate
Keywords: Bioactive compounds, Oxidative stress, Gut microbiota modulation, Personalized nutrition, DNA methylation, Histone modifications
Highlights
-
•
NCDs represent growing global health and economic burden.
-
•
Polyphenols have been associated with minimizing oxidative stress and inflammation.
-
•
The gut microbiota and microbial metabolites are mediators of epigenetic changes.
-
•
Polyphenols can modulate gene expression and modify epigenetic alterations in NCDs.
-
•
Polyphenols represent a great step toward designing new drugs and managing NCDs.
Abstract
Chronic Non-Communicable Diseases (NCDs) have been considered a global health problem, characterized as diseases of multiple factors, which are developed throughout life, and regardless of genetics as a risk factor of important relevance, the increase in mortality attributed to the disease to environmental factors and the lifestyle one leads. Although the reactive species (ROS/RNS) are necessary for several physiological processes, their overproduction is directly related to the pathogenesis and aggravation of NCDs. In contrast, dietary polyphenols have been widely associated with minimizing oxidative stress and inflammation. In addition to their antioxidant power, polyphenols have also drawn attention for being able to modulate both gene expression and modify epigenetic alterations, suggesting an essential involvement in the prevention and/or development of some pathologies. Therefore, this review briefly explained the mechanisms in the development of some NCDs, followed by a summary of some evidence related to the interaction of polyphenols in oxidative stress, as well as the modulation of epigenetic mechanisms involved in the management of NCDs.
1. Introduction
Non-communicable diseases (NCDs) are complex conditions associated with cardiovascular diseases, chronic respiratory diseases, diabetes, cancers, and mental illness as a result of a combination of genetic, physiological, behaviors, and environmental factors (https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases). Globally, seven of the top 10 causes of death in 2019 were from NCDs, being responsible for more than 40 of the 55 million deaths worldwide, representing more than 70 % of all deaths. More than 15 million people die from an NCD between the ages of 30 and 69 years and disproportionately affects low/middle-income countries where occur more than three-quarters of global NCD deaths (https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-ncd-mortality-rate). The percentage of premature deaths from NCDs in the Americas represents about 15 % in countries such as Brazil, Mexico, and the USA. In Europe, in countries like Italy, Spain, and France around 10 % die prematurely from NCDs. On the other hand, countries located in Africa, Southeast Asia, and the eastern Mediterranean account for more than 20 % of premature deaths from NCDs (WHO, 2020). Therefore, NCDs are recognized as a major global challenge and the United Nations has set a goal of reducing premature deaths from NCDs by one-third by 2030 through prevention and treatment (https://sdgs.un.org/goals).
A high-quality diet involving increased consumption of fruits, vegetables, whole grains, and seeds is related to a lower risk of NCDs since these foods are rich in polyphenolic antioxidant compounds. In this sense, polyphenols have been continuously explored for their therapeutic properties (Dominguez et al., 2021). In addition, the epigenetic abnormality is also involved in the pathogenesis of NCDs, and the increasing knowledge of dietary polyphenols as well as their impact on the intestinal microbiota, and their role in epigenetic modulation, make them strong candidates for the treatment and prevention of NCDs (Neri-Numa et al., 2020, Shock et al., 2021). In this context, this review aims to provide an overview of the interplay of polyphenols in oxidative stress, as well as the modulation of epigenetic mechanisms involved in NCDs.
2. A brief overview of the non-communicable diseases (NCDs), reactive species of oxygen and nitrogen (ROS and RNS), and antioxidant modulation
Over the last two decades, several studies have focused on understanding the pathophysiology of oxidative/nitrosative stress related to NCDs (Assi, 2017) as well as searching for effective solutions to mitigate the rising NCDs rates to improve the health span and prevent NCD-driven declines in average lifespan as a strategy to reduce healthcare system costs (United Nations, Department of Economic and Social Affairs, 2019).
Oxidative stress is closely related to the aforementioned diseases once that homeostatic redox imbalance may damage biomacromolecules and modify important proteins, triggering the pathogenesis of NCDs (Neri-Numa et al., 2020, Ruiz et al., 2021). Under physiological conditions, cells require a preferentially reducing environment for the occurrence of several biochemical reactions as well as the creation of the electrochemical gradient necessary for electron flow and energy transfer (Lushchak and Storey, 2021, Ruiz et al., 2021). All these processes comprise redox homeostasis which is determined by the combination of all oxidation–reduction reactions that occur in the cell, involving a regulatory network of molecules that control the rate and amplitude of generation and elimination of reactive species (Ruiz et al., 2021). In other words, the cells have a self-protection system against oxidative and/or nitrosative damages since reactive species of oxygen and nitrogen (ROS and RNS) are inescapable by-products of metabolism being essential for several cellular physiological functions (e.g.: cell signaling, proliferation, differentiation, senescence or death) (Al Shahrani et al., 2017, Ruiz et al., 2021). However, when the redox homeostasis is disturbed and there is an overproduction of pro-antioxidants agents, the organisms lose the ability in detoxifying reactive intermediates (Lushchak and Storey, 2021, Neri-Numa et al., 2020).
Although not the main focus of this review, it is important to point out that are differences in the redox status between individuals as well as in different cells or tissues, at the systems level (Meng et al., 2021). So, it does not seem unreasonable to predict that susceptibility to the impacts of oxidative stress will vary amongst those individuals who express polymorphic genes involved in redox signaling and detoxification of reactive species. In a pathophysiological view, the non-detoxified ROS and RNS containing one or more unpaired electrons are more reactive and are involved in cellular dysfunctions, impairment of the intestinal microbiota is trigged beyond several molecular mechanisms which will lead to protein denaturation and enzyme inactivation as well as mutations, genetic instability, and epigenetic modifications (Kumar Saravana et al., 2020, Neri-Numa et al., 2020). For example, redox-active molecules regulate both activities and expression of key enzymes involved in DNA methylation, histone methylation, acetylation, and chromatin remodeling, consequently controlling gene expression and/or enzymatic activity of specific metabolic and redox pathways (Kumar Saravana et al., 2020) (Fig. 1). The redox signaling in human health and disease was nicely illustrated in a recent review (please see: (Zuo et al., 2022).
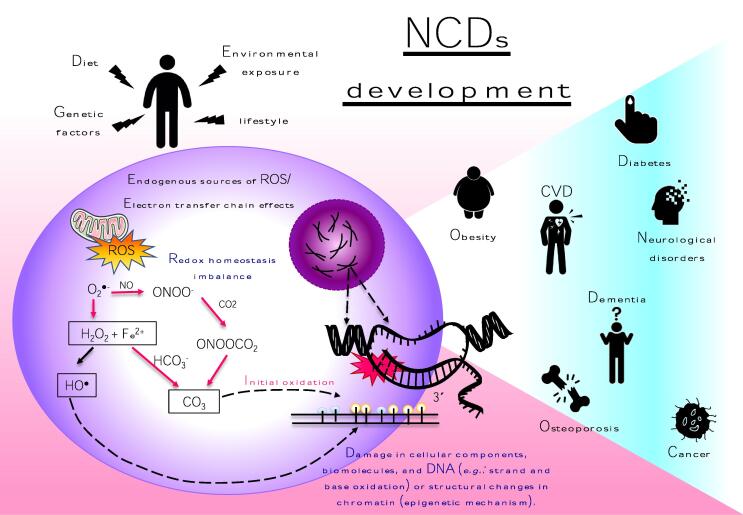
Fig. 1.
Schematic representation of redox homeostasis imbalance in chronic non-communicable diseases (NCDs). ROS/RNS generation results from endogenous process of mitochondrial oxidative metabolism and cellular response to external factors plus an inefficient antioxidant mechanism (nonenzymatic and/or enzymatic antioxidants) that can induce inflammation directly by acting on transcription factors (e.g.: nuclear factor κB) and indirectly by modulating other processes such as cellular senescence, mitochondrial dysfunction and microRNA production which in turn have been implicated in the development several NCDs suchs as metabolic sindromes, cardiovascular diseases (CVD), osteoporosis, neurodegenerative diseases and cancer. ROS, reactive oxygen species; RNS, reactivve nitrogen species; O2•-superoxide anion;H2O2,hydrogen peroxide;•OH, hydroxil radical;RO•, alkoxyl radical;ROO•, peroxyl radical;CO3,carbonate radical anion.
Despite the oxidative stress producers playing an important role in the pathogenesis of potentially severe conditions, they can be substantially reduced by antioxidant modulation which may act both in the prevention and complementary therapy of NDCs. (Neri-Numa et al., 2020). Antioxidants are the first line of defense against the detrimental effects of ROS and RNS, and they are essential to homeostasis maintenance via different mechanisms of action being categorized as endogenous (enzymatic and non-enzymatic) and exogenous (dietary components, i.e. vitamins C and E, carotenoids, polyphenols, and minerals), working synergistically and together with each other (Azat Aziz et al., 2019). Although this review addresses only exogenous antioxidants (polyphenols, in this case), in a general way, antioxidants can neutralize reactive species as well their by-products by accepting or donating electrons to eliminate the unpaired condition of the radical making them less active, less dangerous, and long-lived substances than those radicals that have been neutralized (Azat Aziz et al., 2019). The rationale behind the exogenous antioxidant protective effects is the autoxidation postponement by inhibiting ROS or RNS generation or by interrupting propagation of the reactive species through chelating metal ions, preventing the formation of peroxides, breaking autoxidation chain reaction, and the elimination of singlet oxygen (Santos-Sánchez et al., 2019).
Polyphenols draw attention due to their chemical and physical properties since they can act as antioxidant and/or pro-oxidant properties, depending on their related structure and/or the cellular redox context, which may include increased levels of oxidant-eliminating proteins or reduced levels of oxidized proteins and lipids; however in this text, we will keep the focus only on the antioxidant properties of these compounds have many phenolic hydroxyl groups (Phenyl-OH or for aromatics, Aryl-OH) which are planar and electron-rich being able to act reducing or inhibiting reactive species (e.g.: singlet oxygen quenchers, superoxide radical scavengers and metal chelators over hydroxyl and peroxyl radicals, superoxide anions and peroxynitrites) through hydrogen-atom transfer and/or single-electron transfer (Quideau et al., 2011, Xu et al., 2018).
Although polyphenols are readily ionized (owing to their proneness for electronic delocalization), their bioactivity relies heavily on the position of hydroxyl groups and relative ease of substituent modification. As well, the antioxidant potential of polyphenols depends on the number arrangement of the hydroxyl groups since a large number of methylations will exhibit fewer antioxidants (Quideau et al., 2011, Stockert and Hill, 2018). In general, the antioxidant capacity of natural phenolics is linked with more than one hydroxyl group in the ortho position of the aromatic ring and, what is attributed to the bond dissociation energy (BDE) of O-H which is typically used to evaluate the activity of an antioxidant to neutralize reactive species. It means that the weaker the O-H BDE, the faster the reaction of antioxidants with the free radical (Xu et al., 2018).
It is also important to point out that polyphenols are not restricted in only their antioxidant capacity but also in their interaction with proteins involved in the transcription and expression of genes related to metabolism, proliferation, inflammation, and cell growth (Sharma & Padwad, 2020). Moreover, all these health-modulating effects are closely linked with bioavailability, intestinal absorption, and metabolism in the gastrointestinal tract (Stockert & Hill, 2018). Thus, considering that there are some preliminary results on the role of some plant-based bioactive compounds in oxidative/nitrosative stress modulation, glucose and lipid metabolism as well as cardiovascular function, in the following sections, this review will provide up-to-date data on intervention studies investigating the modulatory effect of polyphenols in some NCDS from the point of view of redox and epigenetic.
3. The interplay between oxidative stress, NCDs, and epigenetic mechanisms
Pathogenesis of NCDs is closely associated with genetic susceptibility and environmental exposures (e.g.: diet, physical activity, environment pollution, stress, and bacterial infections) (Ferrari et al., 2019). This set factor directly affects our genes and can lead to mutations and/or modification of gene expression patterns, dramatically altering cellular metabolism and causing a drop in basal metabolism (Breton et al., 2021, Xiao and Loscalzo, 2020). Additionally, epigenetic mechanisms have been seen as an interesting bidirectional link between oxidative stress and genetic in the consolidation of several chronic diseases (Neri Numa & Pastore, 2020). Firstly, because oxidative stress can impair the proliferative capacity of a cell as well as directly induce DNA damage, and second, genetic changes (e.g.: mutations and polymorphisms) may influence gene expression, and thus those functions regulated by the mutated gene (Neri Numa & Pastore, 2020). These contributions to oxidative stress may be partly mediated through changes in epigenetic marks such as DNA methylation, histone modifications, and microRNAs which in turn play an important role gene transcription (Kietzmann et al., 2017). A brief overview of the three major mechanisms of epigenetic regulation is described in Fig. 2.
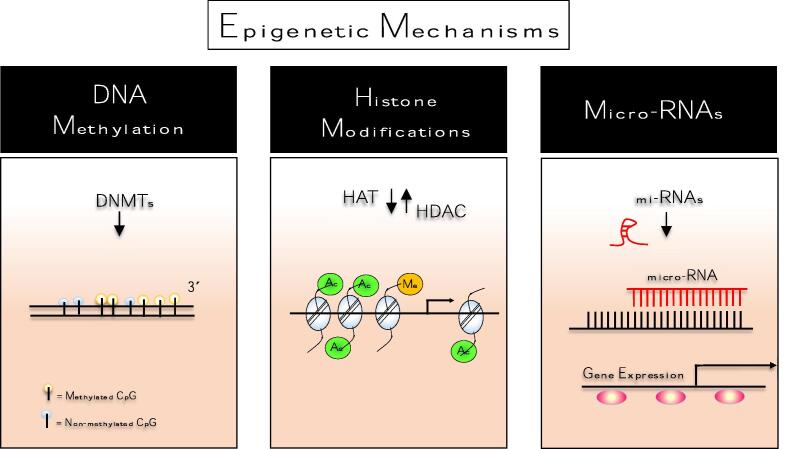
Fig. 2.
Schematic mechanism of epigenetic regulation. Epigenetics is the study of changes in gene expression, involving structural changes in chromatin regardless of changes in the DNA sequence. In general, epigenetic mechanisms act to change the accessibility of chromatin for transcriptional regulation by modifications of the DNA and by nucleosome modification or rearrangement from parental to daughter cells. There are three major mechanisms of epigenetic regulation includes (1) DNA cytosine methylation; (2) chromatin remodeling, mostly achieved via histone modification such as methylation, acetylation, phosphorylation, and ubiquitination, or incorporation of histone variants, and (3) small non-coding RNA (miRNAs) regulation. Epigenetic modifications may be reversible and occur naturally in normal development, health as well as in aging or NCDs development once several factors including environmental factors, lifestyle, upbringing, or emotions may interfere with its regulation. Below is a brief overview of the concept of epigenetic mechanisms. DNA methylation: is defined as a covalent linkage of methyl groups on the C5 position of cytosine residues in DNA, typically in CpG context, catalyzed by DMNTs enzymes using a SAM donor. Histone modifications: refer to covalent post-translational modifications of N-terminal ((including acetylation, phosphorylation, methylation, and ubiquitination) tails of four core histones (H3, H4, H2A, and H2B. Among these alterations, histone acetylation and methylation have received prominence. They are regulated by two groups of enzymes: HAT and HDAC, respectively. The HAT/HDAC system plays a key role in modifying the chromatin structure, which is directly related to the control of the transcriptional process and the gene expression. miRNA: induce degradation of targeted mRNAs by sequence-specific base pairing towards their 3́unstranslated regions within the RNA-induced silencing complex (RISC) which further inhibits the translation. CpG,cytosine-phosphate-guanine; DNMT,DNA methyltransferase;HAT,histone acetyltransferase;HDAC,histone deacetylase;mi-RNA,micro RNA;SAM,S-adenosyl-methionine.
Basically, oxidative and/or nitrosative stress directly suppress DNA methylation through oxidizing DNA, increasing ten-eleven translocation proteins (TET) mediated hydroxymethylation and altering DNA methyltransferases binding which is responsible for the production of methyl donor S-adenosylmethionine (Ionescu-Tucker & Cotman, 2021). ROS and RNS can also damage DNA by hydroxylating pyrimidines and 5-methylcytosine (5mC), which can interfere with 5-hydroxymethylcytosine (5hmC) epigenetic signals (Ionescu-Tucker and Cotman, 2021, Scaccia et al., 2020). Oxidative stress also may affect post-translational histone modifications, changing chromatin structure, gene expression, gene stability, and replication (Ionescu-Tucker & Cotman, 2021). Frequently, these events are indirect, as ROS impair metabolic efficiency, reducing levels of metabolites such as acetyl-CoA, Fe, NAD+, and ketoglutarate that are essential for histone-modifying enzymes (Ionescu-Tucker and Cotman, 2021, Scaccia et al., 2020).
In the scenario of type 2 diabetes mellitus (T2DM) for example, epigenetic alterations include numerous genes (Scaccia et al., 2020). Retinopathy is a classic complication of diabetes that presents various several epigenetic modifications amongst methylation and histone acetylation; where it is also demonstrated the importance of DNA methylation/hydroxymethylation machinery in the regulation of Rac1-mediated oxidative stress (Rac Family Small GTPase 1) (Duraisamy et al., 2018, Scaccia et al., 2020). During glucose homeostasis, 8-oxo-2́deosyguanosine (8-oxodG) residues are removed by 8-oxoguanine DNA glycosylase (OGG1) mostly via the short-patch pathway base excision repair (BER) (Banda et al., 2017, Scaccia et al., 2020). However, under glycotoxic conditions, hydroxyl radical (OH) overproduction affects DNA methylation by oxidation of guanosine to 8-oxodG, resulting in the accumulation of 8-oxodG which suppress the methylation of adjacent cytosines (Banda et al., 2017, Davison et al., 2021, Scaccia et al., 2020). This event triggers a cascade of hypomethylation and transcriptional activation responsible for the recruitment of OGG1/TET1 complex proteins to 8-oxodG, facilitating the conversion of 5mC through to 5caC to sites of ROS-induced damage (Davison et al., 2021, Zhou et al., 2016). Another example is cardiovascular disorders, such as atherosclerosis, in which hydrogen peroxide (H2O2) is associated with aberrant DNA methylation in atherosclerosis by altering the binding of DNMTs to chromatin. Also, epigenetics silencing of superoxide dismutase (SOD2) decreases the production of H2O2 by selective promoter hypermethylation impaired redox signaling, resulting in vascular smooth muscle cell (SMC) proliferation (Gorabi et al., 2020).
Several studies have proposed a strong connection between the misregulation of ROS (by mitochondrial dysfunction and/or age, or both) and neurodegenerative disorders such as Alzheimeŕs, Parkinsońs, and Huntingtońs diseases (Liu et al., 2017, Zuo et al., 2015). In Alzheimeŕs disease (AD), it is believed that the accumulation of reactive species may disrupt the homeostatic balance between anti- and pro-inflammatory cytokines which in turn plays a critical role in the amyloid-beta (Aβ) plaque assembly, resulting in cognitive depletion with impaired speech, vision, behavior, and eventually death (Liu et al., 2017). Epigenetically, ROS alters Aβ-production in the progression of AD by methylation and acetylation. In general, patients with AD not only present a global decrease in DNA methylation and CpG oxidation but also amyloid-β precursor protein (APP)-related mutations, followed by reduced levels of 5hmC and 5mC (Zuo et al., 2015).
Finally, cancer is unquestionably the most studied NCD. Several studies are uncovering the role of ROS on epigenetic modulation and carcinogenesis (García-Guede et al., 2020). Excess of reactive species in addition to triggering a series of changes in the signaling pathways responsible for the control of cell cycle machinery (e.g.: MAPK, NF-κB, STAT3, or PPARγ), may trigger an unbalanced expression in both levels of histones (HDAC1, HMT1, and HATI) and epigenetic regulation (DNMT1, DNMT3A, and MBD4) in cancer target cells (Arfin et al., 2021, García-Guede et al., 2020, Mahalingaiah et al., 2017). DNA hypermethylation has been used as a prognosis biomarker in bladder cancers. ARF, GSTP1, CDH1, and CDKN2A are examples of epigenetically silenced genes by CpG promoter hypermethylation in several molecular pathways contributing to the aggressiveness of urothelial carcinoma once malignant phenotypes present more hypermethylated loci than non-muscle-invasive tumors (Boonla, 2021).
In light of this, the relationship between NCDs and epigenetic changes has attracted much attention in recent years. Therefore, elucidating the physiological/metabolic mechanisms of oxidative stress involved in NCDs, as well as understanding the relationship of polyphenols in the interaction of epigenetic modulation and gut microbiota on these metabolic disorders are essential to advance in the field of epigenetics. Following, this review will address the interplay of polyphenols in the gut microbiota in epigenetic changes and how the relationship between these events could explain the beneficial effects of polyphenols on NCDs.
4. Cross-link of phenolic compounds on the gut microbiota and epigenetic changes
It is increasingly evident the importance of understanding the effects of the consumption of certain bioactive compounds on gut microbiota regulation and its relationship with the health of the host (Farias et al., 2019). Only a small part (5–10 %) of ingested polyphenols are absorbed in the small intestine and, when they reach the colon, they are metabolized by the microorganisms present, leading to the production of short-chain fatty acids (SCFA) and the modulation of the gut microbiota, which in turn play an important role in the prevention/treatment of various NCDs (de Paulo Farias et al., 2021).
Recently, it has been pointed out that there is a mutual relationship between microbiota/host and how this relationship can regulate gene expression, mainly due to the production of metabolites that are generated after colonic fermentation of bioactive compounds such as polyphenols (Gadecka & Bielak-Zmijewska, 2019). Indeed, the gut microbiota and microbial metabolites may be important mediators of diet-epigenome interactions. Changes in the gut microbiota result in changes in the release, metabolism, bioavailability, and metabolic effects of bioactive compounds, epigenetic mechanisms, and their relationship to health (Gerhauser, 2018). However, it is important to emphasize that there is a reciprocal relationship since the host epigenome also influences the composition of the colonic microbial (Shock et al., 2021).
Some studies have shown that metabolites generated by fermentation of polyphenols and other bioactive compounds can alter the activity of important epigenetic enzymes, including histone acetyltransferases (HATs), histone deacetylases (HDACs), DNA methyltransferases (DNMTs), and DNA demethylases, which are responsible for DNA methylation or histone methylation and/or acetylation (Shock et al., 2021). Likewise, the ingestion of foods such as green tea, soy, fruits, and some vegetables rich in polyphenols that are fermented in the colon can modulate the gut microbiota and alter miRNA expression (Gerhauser, 2018). Some of the major phenolic metabolites resulting from microbial metabolism have been recently reported by Cortés-Martín et al. (2020) (Table 1). Although some stilbenes-derived metabolites such as dihydroresveratrol, dihydropiceid, 3,4′-dihydroxy-trans-stilbene and 3,4′-dihydroxydihydro-stilbene (lunularin) have been reported by Cortés-Martín and colleagues, little information on their epigenetic effects is available in the literature. Likewise, catechol (1,2-dihydroxyphenol), a metabolite derived from hydroxycinnamate (e.g.: p-coumaric acid, chlorogenic acid, ferulic acid, sinapic acid, etc.), also has little information in the literature regarding its epigenetic effects. Some effects of polyphenols on the gut microbiota, as well as their metabolites on changes in epigenetic mechanisms, can be seen in Table 1.
Table 1.
Effect of phenolic compounds and their metabolites on the gut microbiota and epigenome regulation.
| Compound | Effect on gut microbiota | Major phenolic metabolites | Epigenetic effects of metabolites | References |
|---|---|---|---|---|
| Elagitannins and ellagic acid | ↑ Bifidobacterium and Lactobacillus, and ↓ B. fragilis, Clostridia and Enterobacteriaceae in vitro. | Urolithins (A, B, C, D, M5, M6 and M7) | Urolithin A: ↑ the O2 generation activity, ↑ acetylations of Lys-9 residues of histone H3 within chromatin surrounding the promoter region of gp91-phox gene, regulated protein levels of p22phox and gp91phox in U937 cells. | (Kikuchi et al., 2021, Li et al., 2015) |
| Isoflavones | Stimulated the growth of B. bifidum DNG6, L. lactisSt66, L. plantarum 10.960 and L. rhamnose and inhibited pathogenic bacteria (E. coli and S. aureus) in vitro. | O-desmethylangolensin and equol | Equol: ↓ methylation of the cytosine phosphate guanine (CpG) islands in the BRCA1 and BRCA2 promoters in MCF-7 and MDA-MB-231 cells and ↑ BRCA1 and BRCA2 proteins expression in nuclei and cytoplasm in cell lines MCF-7, MDA-MB-231 and MCF-10a by immunohistochemistry. | (Bosviel et al., 2012, Chen et al., 2022) |
| Lignans | ↑ Ruminococcaceae and Bacteroidetes (Bacteroides and Rikenellaceae), ↓ Coriobacteriaceae, especially Collinsella, and Streptococcus in premenopausal women. | Pinoresinol, secoisolariciresinol, matairesinol, lariciresinol, isolariciresinol, and syringaresinol | Pinoresinol: ↑ rate and the expression of collagen type I (Col‑I), ALP, osteopontin (OPN), runt‑related transcription factor 2 (Runx2) and bone morphogenetic protein‑2 (BMP‑2), ↑ ALP activity and Alizarin red size, and ↑ cAMP, PKA and phosphorylated cAMP response element‑binding protein (CREB) levels. | (Corona et al., 2020, Jiang et al., 2019) |
| Flavonoids in general, e.g. flavonols (quercetin) | ↓ Verrocomicrobia and ↑ microbiome diversity and abundance of Actinobacteria, Cyanobacteria and Firmicutes. | Acids and aldehydes phenolic | Chlorogenic acid: ↓ proliferation, colony formation, invasion, and metastasis of HepG2 cells both in vitro and in vivo by down-regulating DNMT1 protein expression, ↑ p53 and p21 activity, ↓ cell proliferation and metastasis, in addition inactivated ERK1/2 and reduced MMP-2 and MMP-9 expression in HepG2 cells. | (Nie et al., 2019, Liu et al., 2020) |
| Stilbenes, e.g. resveratrol | ↑ Bacteroides, Lachnospiraceae_NK4A136_group, Blautia, Lachnoclostridium, Parabacteroides and Ruminiclostridium_9, and ↓ relative abundance of Firmicutes. | Dihydroresveratrol, dihydropiceid, 3,4′-dihydroxy-trans-stilbene and 3,4′-dihydroxydihydro-stilbene (lunularin) | – | (Wang et al., 2019) |
| Hydroxycinnamates (p-coumaric acid, chlorogenic acid, ferulic acid, sinapic acid, etc.) | Reversed dysbiosis of intestinal microbiota, ↓ growth of Desulfovibrionaceae, Ruminococcaceae, Lachnospiraceae, Erysipelotrichaceae and ↑ growth of Bacteroidaceae, Lactobacillaceae. | Catechol (1,2-dihydroxyphenol) | – | (Wang et al., 2019) |
Ingestion of yogurt containing phenolic extract of jabuticaba seeds (Myrciaria jaboticaba) rich in ellagitannins increased bacterial abundance in male Wistar rats, mainly due to the increase in the phylum Bacteroidetes. In addition, the drink containing ellagitannins also reduced the relative abundance of bacteria that are microorganisms considered harmful to the health of the host, such as Pseudomonas, Escherichia, and Shigella (do Carmo et al., 2021). Urolithins are phenolic metabolites formed by the biotransformation of ellagitannins and ellagic acid by intestinal bacteria, mainly Bifidobacterium, Pseudocatenulatum, Lactobacilli, and Gordonibacter. These compounds have been extensively studied due to their promising effects in remodeling the epigenome and their beneficial health effects (Selma et al., 2017). A study by Li and collaborators demonstrated that urolithin A, the predominant isoform among the five urolithins in humans, could be a potential therapeutic target to control obesity in relation to thermogenesis activation. According to the authors, this metabolite can control the composition of miRNAs in brown adipocyte tissue, since it was able to increase the expression of miR-124-3p levels, which resulted in increased adipocyte differentiation, in the biogenesis mitochondrial and ATP synthesis (Li et al., 2022). When evaluating the effect of some metabolites on the epigenetic modulation of the mechanisms involved in inflammation, Kiss and colleagues observed that urolithins A and B at a concentration of 5 μM were able to reduce the activity of HATs by more than 50 %, but did not reduce the activity of HDACs (Kiss et al., 2012).
Likewise, soy flavonoids can also affect the gut microbiota and exert beneficial health effects. When evaluating the modulatory effects of soy milk containing isoflavones on the gut microbiota of male BALB/c mice. Dai and collaborators observed that there was an increase in bacterial taxa such as Bacteroides, Lactobacillus, Odoribacter, and Alistipes, while three taxa from the families Parabacteroides and Ruminococcaeae were drastically reduced. In addition, an increase in the levels of SCFAs and isoflavone metabolites, including O-desmethylangolensin and equol, was also observed (Dai et al., 2019).
Importantly, only 30 to 50 % of the intestinal microbiota can metabolize daidzein to equol, while 80 to 90 % of the population produces O-DMA as the main phenolic metabolite. In a study carried out to determine the antioxidant potential of daidzein metabolites, it was observed that O-DMA has the ability to stimulate catalase and total superoxide dismutase (CuZn‑ and Mn‑SOD) activity and mRNA and protein expression in cells HepG2 (Choi & Kim, 2014). Likewise, another study carried out by Dagdemir et al. (2013) reported that equol, produced exclusively by the action of the intestinal microbiota under daidzein, at a concentration of 12.8 µM, has the potential to decrease trimethylated transcriptional repression markers, such as H3K9me3 and H3K27me3, in addition to modulating the expression of EZH2 protein. Finally, the authors concluded such compounds and other flavonoids present in soy tend to modify transcription through demethylation and acetylation of histones in breast cancer cell lines.
Some flavonoids such as catechins and epicatechins also undergo cleavage of the O-heterocycle and dehydroxylation by the intestinal microbiota, resulting in the formation of various phenolic acids (Gerhauser, 2018). In this sense, a study carried out to investigate the potential epigenetic mechanisms of some phenolic acids in breast cancer reported that treatment with p-coumaric acid and epigallocatechin-3-gallate (EGCG) significantly reduced the viability of four breast cancer cell lines (BT-20, BT-549, MDA-MB-231, and MDA-MB-436). It has also been reported that these compounds can interact with the MTAse domain of human DNMT1 and compete directly with its intrinsic inhibitor S -adenosyl-l-homocysteine (SAH). In addition, EGCG was also able to partially demethylate the RASSF1A promoter region in BT-549 cells (Assumpção et al., 2020). These studies corroborate that polyphenols can be fermented by the intestinal microbiota, being transformed into metabolites capable of modulating the intestinal microbiota and interacting with epigenetic machineries such as DNA methylation and histone modifications. Therefore, understanding the interaction of polyphenols in the gut microbiota and their role in epigenetic modulation is an important step toward new therapeutic opportunities in NCDs.
5. Polyphenols for the management of NCDs: nutritional interactions and actions mechanisms
This section will provide an overview of experimental and clinical studies on dietary polyphenols, focusing on the modulation of epigenetic mechanisms in the main diseases involved in metabolic syndrome, neurodegenerative diseases, and cancer.
5.1. Metabolic syndrome – Obesity/Insulin resistance/diabetes/cardiovascular disease
Metabolic syndrome (MS) (synonyms: Syndrome X, The Deadly Quartet, and The Insulin Resistance Syndrome) is a global health problem that involves a set of diverse cardiovascular (e.g.: heart attack, stroke, and atherosclerosis) and endocrine (e.g.: obesity, non-alcoholic fatty liver disease (NAFLD), insulin resistance, and diabetes), and is associated with a high risk of morbidity and mortality (American Heart Association, 2021). Over the years, expert groups have developed clinical criteria for the diagnosis of MS, but there is no consensus. However, the most used protocols are from the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) and the International Diabetes Federation (IDF) which consider a positive diagnosis for MS with three or more factors, in addition to central obesity, differing from within some limits established by the World Health Organization, namely: (i) low HDL cholesterol (1.03 mmol/L; 40 mg/dL; men; 1, 29 mmol/L; 50 mg/dL women); (ii) high blood pressure ≥ 130/85 mmHg, (iv) increased fasting blood glucose (5.6 mmol/L; 100 mg/dL) (International Diabetes Federation (IDF), 2005).
MS is increasing worldwide and affects nearly 20–30 % of the general population, with differences between ethnic groups and economic and geographic areas (Oliveira et al., 2020). In Brazil, a recent study, based on the National Health Survey of Brazil and the NCEP-ATP III, reported that the prevalence of MS was 38.4 %, with waist circumference (65.5 %) and HDL cholesterol (49.4 %) the most recurrent components (Oliveira et al., 2020). In addition, the incidence was higher in (i) women (41.8 %), (ii) individuals with low education (47.5 %), and (iii) the elderly (66.1 %) (Oliveira et al., 2020). It is noteworthy that waist circumference is a biomarker of the amount of visceral fat and central obesity (https://www.who.int/health-topics/obesity). In this sense, obesity, which kills 2.8 million people/year worldwide, is a major component of MS, being a direct precursor of (a) dyslipidemia (2.6 million deaths/year, globally); (b) NAFLD (≤1 million deaths/year, global); (c) hypertension (9.4 million deaths/year, global); (d) cardiovascular diseases (17.9 million deaths/year, globally); (e) insulin resistance and type 2 diabetes (≅1.5 million deaths/year, global) (Sixty-sixth World Health Assembly., 2013, WHO, 2021, World Health Organization (WHO)., 2016, World Health Organization., 2021). MS and its associated comorbidities can be alleviated with increased physical activity, moderate alcohol exposure, and non-smoking (de Oliveira et al., 2021). In addition, dietary changes, such as nutrition rich in polyphenols, can modulate epigenetics to control MS and its associated comorbidities.
MS arises from complex and non-linear interactions between environment, genetics, and epigenetics. Specifically, epigenetic dysregulation has been pointed out as an attractive molecular mechanism, as it plays a significant role in the pathophysiology of metabolic syndromes, involving gene modification via chromatin remodeling, histone modification, noncoding RNA alteration, and methylation (Silva et al., 2019). Epigenome-wide association studies (EWASs) have shown that epigenetic changes in the presence of MS can be global and locus-specific (Ramzan et al., 2021). A total of 44 gene loci are associated with increased risk of MS, including TXNTP; TGA; OSRI; KCNK3; PPARGC1A; ARHGAP24; PRDMG; LPL; ADRB3; POE3A; HSD11B2; SREBF1; PHOSPHO1; TBX2/SOCS3; GNAS; CPT1A and ABCG1; the latter two being widely reported in association with MS (Low et al., 2021). For example, EWASs detected a decrease in the methylation of the CPT1A gene (regulator of mitochondrial fatty acid oxidation), which is correlated with an increased risk of MS (Chitrala et al., 2020). In other EWASs, increased methylation of the ABCG1 gene (at loci: cg06500161) was reported when in the clinical picture of MS (Nuotio et al., 2020). It is noteworthy that locus cg06500161 is an epigenetic link in MS that provides: (i) myocardial infarction and dyslipidemias (Pfeiffer et al., 2015); (ii) insulin resistance (Kriebel et al., 2016); (iii) obesity (Walaszczyk et al., 2018); and (iv) type 2 diabetes (Kulkarni et al., 2015).
In vitro, in vivo, clinical and epidemiological studies show that polyphenols have antioxidant and anti-inflammatory properties, with potential preventive and/or therapeutic effects for MS, influencing pathological and physiological processes through multiple mechanisms, namely: scavenging of free radicals, metal chelation, regulation of enzymatic activity, inhibition of cell proliferation and alteration of signaling transduction pathways (De Oliveira et al., 2020). From the point of view of diet-gene interaction, polyphenols can affect gene expression in two ways: (i) direct activation of transcription factors or (ii) indirect modulation of signaling pathways (Silva et al., 2019). As seen in Table 2 (for complementary information, please see Supplementary Table 1, S1), there are a variety of biologically active polyphenols such as anthocyanin, resveratrol, catechin, epicatechin, quercetin, genistein, curcumin, etc. (Bo et al., 2018). For example, a clinical study showed that juçara (Euterpe edulis Mart.) supplementation, rich in anthocyanin, modified the serum fatty acid profile, contributing to the reduction of MS, through DNA methylation and histone acetylation (Santamarina et al., 2018). In turn, resveratrol has also been used in different in vivo studies for the epigenetic treatment of type 2 diabetes, NAFLD, and hypertension through the regulation of genes such as SIRT-1, ATF6, CREBH, PLIN1, etc. (see more in Table 2). Quercetin was able to remodel chromatin and modify histones, acting on the expression of LSD1, CYP2E1, and SIRT-1, attenuating obesity, hypertension, and diabetes in rats (Nettore et al., 2019). Recent in vivo models have shown that curcumin can decrease inflammatory cytokines (TNF-α and IL-6) in stroke and obesity by modulating genes such as BAX, HMGB1, and TCF7L2 (Tian et al., 2017). Importantly, complex polyphenols act as probiotics, as they are converted into low-molecular-weight metabolites by the intestinal microbial community (Duttaroy, 2021). In these cases, polyphenols significantly altered the intestinal microbiota, including its metabolic by-products, which, consequently, affect the epigenetics of the host, alleviating MS (Shock et al., 2021). For example, epigallocatechin was able to reduce metabolic disturbances in mice fed a high-fat diet by increasing DNMT1 expression and subsequent hypomethylation of CpG in the colon (Remely et al., 2017). Another study showed that trans-resveratrol, in men with metabolic syndrome, significantly altered the composition of the microbiota (mainly Akkermansia muciniphila) and the primary metabolite of dihydroresveratrol, which may have improved insulin sensitivity and reduced fat mass, due to altered expression of the SIRT gene (Walker et al., 2018).
Table 2.
An overview of the beneficial effects of polyphenols in non-communicable diseases (NCDs).
| Polyphenol/Substrate/ Metabolite | Experimental model/Condition | Epigenetic mechanism | Consequence | Main outcomes | References |
|---|---|---|---|---|---|
| Metabolic Syndrome | |||||
| Quercetin | Obesity (In vitro: PPARγ, C/EBPα and 14–3-3ε, H3K9, LSD1, H3K4 antibodies, and oligonucleotides) | Chromatin remodeling and histone modifications | ↓ LSD1 for the 5′ region of the c/EBPα and PPARγ genes. | ↓ Body weight improved dyslipidemia and glucose tolerance. ↓ Hepatic accumulation of lipids. | (Nettore et al., 2019) |
| Epicatechin | Hyperglycemia (in vitro: THP-1 cells pretreated with epicatechin and exposed to 25 mM glucose for a total of 24 h, 5 µM for 4 h) | DNA methylation |
↑Acetilação H3K9 e H3K4. ↓ Dimethylation H3K9 ↓HDAC4 levels and TNF-α release. |
EC induces increased levels of NF-κB in human monocytes when there is diabetes. | (Cordero-Herrera, Chen, Ramos, & Devaraj, 2017) |
| Resveratrol/Quercetin | Hypertension (In vivo: Male rats aged 6 months, diet with a mixture of 50 mg/kg/day resveratrol + 0.95 mg/kg/day quercetin. Systolic pressure was measured using the tail cuff method) | Histone modification | Modulation of the SIRT1 and SIRT3 genes. Regulation of endothelial nitric oxide synthase, nitric oxide, and superoxide dismutase. |
↓ Blood pressure in rats resistência à insulina, ↓ Insulin resistance |
(Castrejón-Téllez et al., 2020) |
| Curcumin | Stroke (In vivo: Male rats received daily intraperitoneal injections of curcumin (50 mg/kg) for 5 days) | Histone modification |
↓ TNF-α and IL-6 levels in the brain. ↑ SIRT1 expression. ↓ Ac-p53 e Bax expression. |
↓ Stroke-induced brain injury. ↓ Inflammation and mitochondrial dysfunction after cerebral ischemia. |
(Miao et al., 2016) |
| Genistein | Diabetes (In vivo: 40 female rats were fed a diet containing 1 mg/kg/day of genistein for 8 weeks. After that, pancreas tissue was removed and used for Western blotting and Hematoxylin-Eosin staining) | Histone modification |
↓ Nf-κB and IL-1β expression. ↑ SIRT1 expression |
↓ Inflammatory changes in the pancreas. ↓ Pancreatic injury. Improved glucose homeostasis |
(Yousefi et al., 2017) |
| Resveratrol | Diabetes type 2 (clinical trial: use of western blot, 192 patients, 40 – 500 mg for 72 h) | Histone acetylation at lysine residue 56 (H3K56ac) |
↑ TAS levels, ↓ Percentage of H3K56ac. |
SIRT-1 can affect redox homeostasis. ↓ Body fat percentage | (Bo et al., 2018) |
| Neurodegenerative disorders | |||||
| Flavonoid-rich (BBJ) blueberry juice | Exercise-induced protected dopaminergic neurons against MPP + or MPTP-induced toxicity: Fischer 344/Brown (male) Norway hybrid rats aged 3 months, treated with BBJ (20 % solution) for 4 weeks. | NE | ↓ 6-OHDA-induced DA cell death, ↓ rotational behavior induced by amphetamine; |
BBJ consumption + resulted in a greater reduction in amphetamine-induced rotational behavior and protected against the loss of striatal DA terminals. | (Castro et al., 2022) |
| Curcumin | Modulation of Synaptic Plasticity Related model: C57BL/6J (male) mice aged 8 weeks, treated with curcumin by intraperitoneal and monitored each 2 h. | DNA methylation, ↓ HAT3, ↓ HAT4. | ↓ TREM-1 expression, ↓ p300 activity in the TREM-1 promoter region |
Epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. | (Yuan et al., 2012) |
| Extra virgin olive oil derived (OLE) oleuropein aglycone | Transgenic hemizygous CRND8 male and female mice following 3 age groups of mice treated with OLE-fed mice for 8 experimental weeks | ↑ HA3, ↑ HA4 and, ↓HDAC2. | ↓ β42 deposits in the brain of young and middle-aged TgCRND8 mice glutaminylcyclase-catalyzed pE3-Aβ generation reduces enzyme expression and interferes both with Aβ42 and pE3-Aβ aggregation. | Improvement of synaptic function in a murine model of Alzheimer’s disease. | (Luccarini et al., 2015) |
| Anthocyanin-rich blueberry (Vaccinium corymbossum) extract | Modulation of Synaptic Plasticity Related model: Wistar (male) rats, treated with anthocyanin (2 % w/w) diet treated for 6 weeks. | NE |
↑ ERK1, Akt Ser473, mTOR Ser2448 and BDNF protein levels, ↓ α-E-catenin, JNK1, and p38 protein levels. |
Activation of ERK-CREB-BDNF pathway; ↑ Spatial and psychomotor performances in aged rats; modulation of key synaptic proteins. |
(Vauzour et al., 2021) |
| Luteolin-7-O-glucoside (LUT-7G) | Neuroprotection PD models:SH-SY5Y cells and C57BL6 (male) mice aged 20 weeks treated with LUT-7G treated for 15 days. |
NE | ↑ Bcl-2/Bax ratio, ↓ Expression of cleaved caspase 3 ↑ ERα and Erβ expression, ↑ ERK1/2/STAT3/c-Fos activatipn, ↑Muscle strength, ↓ MPTP-induced gliosis in substantia nigra, ↑ TH positive nerve fibers in striatum. |
Protection of dopaminergic neurons against MPP + or MPTP-induced toxicity, Activation ER-mediated signaling pathway. |
(Qin et al., 2019) |
| Grape juice (Vitis labrusca) (GJ) | Randomized human clinical trial - Aquatic exercise + GJ (400 mL/daily) consumption: male/female PD patients, aged over 48 years old, treated for 4 weeks. | ↑ HAT4 | ↑ BDNF levels, | GP + Aquatic exercise resulted in amelioration of functional capacity and mobility in PD patients. | (Oliveira et al., 2020) |
| Cancer | |||||
| Genistein + Sulforophane + sodium butyrate | Breast cancer cell line (MDA-MB-231 and MCF-7) treated with Genistein (5 µM) + Sulforophane (15 µM) + sodium butyrate (2,5 µM) for 72 h | ↓ DNMT3A, ↓ DNMT3B, ↓ HDAC1, ↓ HDAC6, ↓ HDAC11 | ↓ EZH2, ↓ SUV39H1 ↓ KAT2A, ↓ KAT2B, ↓ EP300, ↓ CBP |
↑ apoptosis, ↓ cell cycle |
(Sharma & Tollefsbol, 2022) |
| Curcumim | Gastric cancer cell line (HGC-27, MGC-803, MKN-1 and SGC-7901) treated with 30 – 50 µM for 24 h / K19-Wnt1/C2mE transgenic mice (Gan) Six-week-old treated with curcumin (0.005 % (w/v)) for 3 times a week for 48 weeks | ↓ DMNT1 | ↑ RB1 | ↓ cell proliferation, ↑ apoptosis, ↓ cell cycle, ↓ inflammation |
(Cao et al., 2020) |
| Quercetin | Acute myeloid leukemia (HL60 and U937) treated with 50 – 75 µM for 72 h /Human xenograft acute myeloid leukemia (AML) models - female mice (NOD.CB17-Prkdcscid/J lineage) 8–11-week-old treated with quercetin (120 mg/kg every 4 days for 20 days | ↓ DNMT1, ↓ DNMT3A, ↓ HDACs | ↑ DAPK1, ↑ BCL2L1, ↑ BAX, ↑ APAF1, ↑ BNIP3L | ↑ apoptosis |
(Alvarez et al., 2018) |
| Hippophae rhamnoide Polyphenols | Colorectal (HCT116, HT29, and FHC) treated with 40–120 µM for 48 h /tumor xenograft model - BALB/c female nude mice (five weeks old) were treated with Hippophae rhamnoide extract (50 mg/kg/day for 21 days) | ↑ miR −195-5p, ↑ miR − 497-5p, ↓ miR-1247-3p | ↓ CCND1, ↓ CCND2 ↓ CCND3, ↓ CCNE1 ↓ BCL-2, ↑CASP2 |
↑ apoptosis, ↓ cell cycle |
(Wu et al., 2021) |
| Apigenin | Breast cancer cell line (MDA-MB-231) treated with 40 µM for 96 h / tumor xenograft model - female athymic nude mice (BALB/c-nu) aged 6 weeks treated with apigenin (25 mg/kg/day for 8 weeks) | ↓ HDAC, ↑ HAT | ↓ CDK1, ↓ CCNA2, ↓ CCNB1, ↑ CDKN1A | ↓ cell proliferation, ↓ tumor growth | (Tseng et al., 2016) |
| Genistein | Head and neck squamous cell carcinoma (HNSCC) - Phase II clinical trial – 39 patients (300 mg/day for 3 weeks) | ↑ Global Hypermethylation | ↓ LINE-1 | ↑ genomic stability | (Rozek et al., 2019) |
Symbol: ↔ sample did not affect the parameter; ↓ sample induced a significant reduction; ↑ sample induced significant increase. NE not evaluated. 6-OHDA, 6-hydroxydopamine; AKT Ser473, anti-phospho-AKT (ser473) antibody; APAF1, apoptotic protease activating factor-1; BAX, BCL2 associated X; BCL2, B-cell leukemia/lymphoma 2 protein; BCL2L1, BCL2 Like 1; BDNF, brain-derived neurotrophic factor; BNIP3L, BCL2 interacting protein 3 like; C/EBPα, CCAAT-enhancer-binding proteins; CASP2, caspase 2; CBP, CREB-binding protein; CCNA2, cyclin A2; CCNB1, cyclin B1; CCND1, cyclin D1; CCND2, cyclin D2; CCND3, cyclin D3; CCNE1, cyclin E1; CDK1, cyclin dependent kinase 1; CDKN1A, cyclin dependent kinase inhibitor 1A; DAPK1, death-associated protein kinase 1; DNMT, DNA methyltransferase; EP300, E1A binding protein P300; ERK1, extracellular signal-related kinase; EZH2, Enhancer of zeste homolog 2;HAT, histone acetylases; HDAC, histone deacetylases; KAT2A, Lysine acetyltransferase 2A; KAT2B, Lysine Acetyltransferase 2B; LINE-1, long interspersed nuclear element-1; LSD1, lysine demethylase 1A; NF-κB, factor nuclear kappa B; P53, tumor protein 53; PPAR, peroxisome proliferator-activated receptor; RB1, RB Transcriptional Corepressor 1; SIRT, sirtuin; SUV39H1, Histone-lysine N-methyltransferase; TNF-α, tumor necrosis dactor alfa; TREM1, triggering receptor expressed on myeloid cells.
These results indicate that polyphenols may have a potential role in innovative MS prevention strategies. However, several challenges in this field of research must be overcome, such as understanding the mechanisms underlying (metabolic, cellular, and molecular) the beneficial effects of polyphenol consumption (Silva et al., 2019). One should also consider the genetic heterogeneity of animals and humans, tissue specificity, and the prominent role of the gut microbiota for a more comprehensive study relating polyphenols, epigenetics, and MS. Therefore, future research is extremely necessary, for more detailed elucidation of the effects of polyphenols on epigenetic dynamics.
5.2. Neurodegenerative disorders
Neurodegenerative diseases (NDDs) comprise a heterogeneous group of disorders (e.g.: Alzheimeŕs disease, Parkinson's, and Huntingtońs diseases plus amyotrophic lateral sclerosis) affecting millions of people worldwide. In general, is characterized by loss of function and eventual death of nerve cells in the brain or peripheral nervous system as a result of mitochondrial dysfunction, neurotoxic proteins deposition, and excitotoxicity (Arruda et al., 2020). The most common NDDs are Alzheimer's disease (AD) and Parkinson's disease (PD) (Monzio Compagnoni et al., 2020). In 2021, as many as 6.2 million Americans were living with Alzheimer’s disease, which is projected to be nearly 14 million people by 2060 (Alzheimer’s Association, 2022). Whereas, approximately 1.2 million people in the United States will be living with Parkinsońs disease by 2030 (Marras et al., 2018).
The pathophysiology of NDDs is complex and not yet completely understood; however, between several downstream degenerative events, prenatal adverse environments, imbalanced redox status, toxicological exposures, and neuroinflammation appear to be the key triggering factors in neurodegeneration, including neurotoxic protein accumulation impairs insulin signals and downregulate neurotrophins expression (Arruda et al., 2020, Kundakovic and Jaric, 2017).
Interestingly, new evidence has addressed the dysregulation of neuroepigenetics related to the same chemical modifications of DNA, and histones but in neurons (Marshall & Bredy, 2016). In the same way, particular attention has been devoted to the gut microbiome, which is deeply involved in nutrient absorption and lipid metabolism, representing a pillar of the gut microbiome-brain axis besides being activation and upregulation of cellular and molecular pathways in NDDs (Milošević et al., 2021). But how does dysregulation of epigenetic mechanism interface with synapse-specific mechanisms of neural plasticity?
In the brain, DNA methylation, for example, acts by silencing gene expression which is essential in the mediation of memory acquisition and storage (Hwang et al., 2017). In adulthood, it also acts in developing and modifying brain function through DNA methyl-transferases such as DNMT1, DNMT3A, and DNMT3B (Brabson et al., 2021). Some DNA methylation by-products play an important role in the maintenance of genomic stability, and the reasoning behind this is a series of oxidative modifications (e.g.: DNA methylation and demethylation processes) in the nervous system (Christopher et al., 2017). For example, DNA demethylation is mediated by Ten-eleven translocation proteins (TETs; family of dioxygenases) by initiating 5-methylcystosine (5-mC) oxidation to generate 5-hydroxymethylcytocine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) (Brabson et al., 2021, Christopher et al., 2017).
Alterations in global methylation of histones associated with neuropathogenesis occur mostly at H3 and H4 levels of lysine residues (e.g.: H3K4, H3K36, and H3K9), and the main consequence culminates in transcriptional activation (Griñán-Ferré et al., 2018, Wu et al., 2022). While acetylation of histones by HDAC2 is implicated not only in the regulation of synaptic plasticity but also in triggering permanent changes in neurons resulting in a number of learning and memory impairments (Wu et al., 2022). Similarly, deletion of HDAC4 in the brain results in impaired hippocampal-dependent learning, and long-term memory formation (Christopher et al., 2017). Further, histone methylation has been recognized as a pathogenic mechanism in both AD and cognitive deficits where it was observed an increase in both KMT2 and H3K9me2 (Wu et al., 2022). Thus, due to their multifactorial causes, there are still no available treatments for NDDs and it places a huge burden on healthcare systems as they are a common cause of morbidity and cognitive impairment in elderly people and/or neurodegeneration carriers who require increasingly complex and prolonged care (Peplow et al., 2022).
Epidemiological studies have correlated diet, physical activity, and NDDs incidence, indicating that diet is an important modifiable risk factor; since the role of dietary patterns and lifestyle variables in both preventing and slowing down a progression to full-blown diseases is evident (SACN, 2021, WHO/FAO, 2003). Mediterranean diet, for example, is implicated in improving metabolic and lipid profiles in not only NDDs but also in all kinds of chronic diseases (D’Innocenzo et al., 2019, Romanos-Nanclares et al., 2020). The mechanism for these salutary effects resides in the interaction of a plethora of bioactive constituents (e.g.: phenolic compounds, sulforaphane, vitamins, MUFA, PUFA, etc.) which may modulate free radical damages and mitigate aging biomarkers in a manner favorable for the longevity and disease prevention (Alasalvar et al., 2020).
Polyphenols, in particular, may exert neuromodulation affects both directly, affecting brain functions and protecting them against oxidative stress and inflammatory injury, or indirectly, modulating the composition of the intestinal microbiota and the metabolites produced since both actions determine the production of neurotransmitters and neuropeptides capable of influencing brain functions (Di Meo et al., 2020, Filosa et al., 2018). Moreover, depending on bioavailability and absorption rates, the polyphenol's active metabolites may directly modulate the synthesis of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF) or glial cell line-derived neurotrophic factor (GDNF) (Di Meo et al., 2020). Likewise, emerging evidence points to a number of polyphenols that display the ability to reverse epigenetic regulation involved in NDDs (Russo et al., 2017).
By analyzing a group of 5,209 participants aged 28–62 from the Framingham Heart Study Offspring cohort, it was shown that a higher long-term flavonoid intake is associated with a lower risk of AD and related dementias (ADRD) in US adults (Shishtar et al., 2020). This evidence corroborates another prospective cohort study reporting a positive correlation between a lower risk of mortality among Parkinsońs disease patients and the consumption frequency of flavonoid-rich foods, especially anthocyanins and flavan-3-ols (Zhang et al., 2022). Additionally, we can cite epigallocatechin-3-gallate (EGCG), the major catechin found in green tea (Camellia sinensis), and also the resveratrol, a flavonoid commonly found in berries which mitigation of AD and other neurodegenerative processes are not only limited to their antioxidant and anti-inflammatory action but also include disruption of amyloid β protein production, activation of α-secretase (ADAM10), and inhibition of β-secretase (BACE-1) as well as activation of sirtuin 1 (SIRT1) and vitagenes followed by the miRNA upregulation (Folch et al., 2018, Lukiw, 2012). Table 2 shows additional data reported in the literature concerning the biological effects of several plant-based polyphenols and phenolic-rich extracts/fractions on NDDs.
Although polyphenols present a great variety of structures able to modulate several brain functions, there is still a lack of information related to physiological, biochemical, and molecular aspects, in both epidemiological studies as in experimental (in vitro, in vivo, and ex vivo) models and/or human clinical trials. Another concern is dedicated to the bioavailability of flavonoids and their optimal intake, which should be investigated to elucidate the mechanisms related to the action of these compounds in the modulation of the microbiota, DNA methylation, and their effect on preventing neurodegeneration onset or slowing the progression of the disease. Therefore, scientific research and technological developments still need to advance, aiming to correlate the full potential of polyphenols to design new adjuvant therapies for NDDs.
5.3. Epigenetic targets in cancer modified by polyphenols
Cancer is a generic term for a large group of diseases that can affect any part of the body characterized by unregulated cell growth and spread of abnormal cells (https://www.who.int/cancer/en/). Cancer is the second leading cause of death globally, accounting for an estimated 5.5 million deaths in males and 4.4 million deaths in females in 2020. Lung, colorectal, liver, stomach, pancreas, and prostate cancer are the most common types of cancer in men, while breast, ovarian, lung, colorectal, cervical, and stomach cancer are the most common among women (https://gco.iarc.fr/). Conceptually, all cancers arise as a result of changes in the DNA sequence caused by the accumulation of genetic mutations and epigenetic alterations. Studies have shown that multiple genomic changes contribute to the progression of cancer and it has been incontestable the aberrant pattern in the epigenetic processes leads to altered expression of several genes involved in cell cycle, cell proliferation, cell motility, and apoptosis (Irshad & Husain, 2021).
The main epigenetic mechanisms that occur during cancer progression are DNA methylation, histone modifications, and post-transcriptional gene regulation by miRNAs and lncRNAs. These processes affect transcript stability, DNA folding, nucleosome positioning, chromatin compaction, and complete nuclear organization of the genetic material (Özyalçin & Sanlier, 2020). For instance, ovarian cancer (OC) consists of a complex, heterogeneous group of invasive cancers that originate from different tissues. Tumor suppressor genes such as BRCA1, RASSF1A, MLH1, CDH1, CDKN2A, CDKN2B, DAPK, and APC have been identified as hypermethylated with associated loss of expression in ovarian cancer (Koukoura et al., 2019). In addition, prostate cancer is considered to be a highly heterogeneous cancer and the hypermethylation of GSTP, HOXD3, MGMT, CDKN2A, CDH1, and APC genes are the most commonly identified epigenetic alteration (Sugiura et al., 2021). Additionally, histone modifications in chromatin have important effects on gene regulation and carcinogenesis (Özyalçin & Sanlier, 2020). Recent intensive investigations in cancer focus on altering the expression of HATs or HDACs and have found that they contribute to tumorigenesis (M. Sharma & Tollefsbol, 2022). Furthermore, to the activity of DNMTs and histone modifications, ncRNAs such as miRNA and lncRNAs play important roles in a variety of biological processes, and dysregulation of the expression of these ncRNAs is highly associated with cancer development (Zhang et al., 2020). In breast cancer, the hypermethylation of some suppressor tumor genes such as APC, RARB, GSTP1, DAPK, and SFN may be related to the dysregulation of ncRNAs (Sher et al., 2020). Therefore, the epigenetic changes mediated mainly by DNMTs, are a promising strategy for developing anticancer agents due to their capacity to reverse hypomethylation in oncogenes and hypermethylation in tumor suppressor genes (Koukoura et al., 2019). This feature is important due to emerging evidence that hypermethylation-induced transcriptional silencing of tumor suppressor genes constitutes a frequent epigenetic defect in many human cancers (Sugiura et al., 2021). Recently, studies have shown that polyphenols play an important role in reversing aberrant epigenetic changes in several cancers polyphenols are known to increase apoptosis and suppress tumor growth in several cancer strains. In addition, polyphenolic compounds have good activity in the reversal of mutant genes and synergy in the effectiveness of conventional cancer treatments (Irshad & Husain, 2021).
Resveratrol is a phytoalexin present in some plants and not only modifies signaling pathways that affect gene expression but also impacts epigenetic mechanisms. Several genes and miRNAs associated with ovarian cancer were modulated by resveratrol by increasing ARH-I expression triggering autophagy and inhibiting cell migration in ovarian cancer (Ferraresi et al., 2017). Treatment of breast cancer cell lines with resveratrol demonstrated that DNMT inhibition increases ATP2A3 gene expression, triggering apoptosis and intracellular Ca2+ changes (Izquierdo-Torres et al., 2019). Also, the authors demonstrate that resveratrol decreases HDAC activity, decreases the abundance of nuclear HDAC2 in breast cancer cells, increases HAT activity, and causes enrichment of H3K27Ac. Therefore, the resveratrol-mediated activity of HDAC and HAT enzymes can create a favorable environment for transcription due to the acetylation gain associated with transcriptional activation.
Hesperetin is a common citrus flavanone widely distributed among citrus fruits with great antitumor potential and its ability to reduce histone methylation (H3K79me3) in gastric, breast, lung, liver, and colon cancer cells in vitro has been demonstrated (Wang et al., 2021). Furthermore, the authors implemented gastric tumor cells in mice and demonstrated that the reduction of methylation in H3K79 can be explained by the degradation of DOT1L expression through CBP-mediated acetylation and consequently a reduction in cell migration and invasion.
Recently, evidence has shown that polyphenols exert their antiproliferative and pro-apoptotic effects through the regulation of one or more ncRNAs, leading to inhibition of cancer cell growth, induction of apoptosis, or enhancement of conventional cancer therapeutic efficacy (Zhang et al., 2020). Resveratrol acts as a potent regulator of miRNAs decreasing the overexpression of miR-196b/miR-1290 and elevating IGFBP3 expression in the ALL cell lines triggering apoptosis, antiproliferation, cell cycle arrest, and inhibition of migration (Zhou et al., 2017). Pterostilbene is a stilbenoid chemically related to resveratrol and demonstrated to suppress the cell viability and induced apoptosis of endometrial cancer cells by down-regulation of miR-663b and indirectly increasing the expression of its target, the BCL2L14 gene (Wang et al., 2017).
Recent reports have demonstrated the contribution of polyphenols as potentiation of several drugs in the treatment of cancer, acting mainly on the gene expression of several signaling pathways and epigenetic mechanisms (Hidetomo Kikuchi et al., 2019). An example is the use of the flavone apigenin to potentiate the anticancer effect of cisplatin in lung cancer by inhibiting HDAC and consequently increasing the degree of histone acetylation and increasing the expression of CKDN1A and BBC3 genes, inducing apoptosis and cell cycle arrest in these cells (Yan et al., 2020).
The polyphenols also act to reverse hypomethylation in oncogenes, such as pterostilbene, that exert anticancer action by remodeling DNA methylation and gene expression. The treatment with PTS decrease the OCT1 transcription factor with an increase in activity of DNMT3B and was accompanied by PRKCA promoter and TNNT2 and DANT2 enhancer hypermethylation, and consequently, gene silencing in breast cancer cell line (Beetch et al., 2021).
In addition to the direct contribution of polyphenols to cancer, recent studies demonstrate an indirect influence of polyphenols from the modulation of the composition and metabolism of the intestinal microbiota from epigenetic regulation (Haque et al., 2021). These microorganisms present in the gut are able to transform polyphenols into SCFA such as butyrate, propionate, and acetate that can participate in the modulation of epigenetic processes (Laborda-Illanes et al., 2020). For example, the use of butyrate in combination with sulforaphane and genistein has been shown to increase apoptosis and reduce the viability of breast cancer cells, from epigenetic modulation involved in DNA methylation and histone modifications, such as DNMTs, HDACs, and HATs (Sharma & Tollefsbol, 2022). Table 2 shows additional data reported in the literature concerning the biological effects of several plant-based polyphenols on cancer.
The most recent research covering the antitumor potential of various polyphenols targeting epigenetic regulatory pathways has been shown on this topic. The studies reveal the great potential of polyphenols for cancer prevention and treatment with their individual and/or combined with anticancer drugs to improve anticancer activity. The benefits of polyphenols in epigenetic modulation in tumors are evident, and great progress has been made in experimental models, however, human studies involving the effects of polyphenols in epigenetic modulation in cancer remain insufficient.
6. Concluding remarks and future perspectives
This review highlighted some findings of research developed in the field of polyphenols as antioxidants and their interplay with microbiota followed by epigenetic mechanism modulation, focusing on the management of NCDs. Although many recent data are encouraging, caution is needed when referring to polyphenols as effective therapeutic agents in the treatment of some NCDs, since studies related to the mechanism underlying their protective effects are still incipient. Implementation of technological strategies to overcome the drawbacks of polyphenols extraction and their bioavailability/bioaccessibility and that enable the management of the intestinal microbiota, aiming at obtaining target metabolites, are necessary since few studies address their interaction with other compounds in the diet and/or drug consumption. Likewise, there are still many questions to be answered regarding the role, and mechanism of polyphenols targeting diverse epigenetic landscapes, their parameters as well as signaling pathways, and physiological barriers related to NCDs. Given that, comprehensive studies with age, sex, and ethnicity stratification are also strongly recommended to confirm the main mechanisms of action of polyphenols with regard to regulation of redox status, gene expression, and modulation of epigenetic mechanisms in MS, NDDs, and cancer.
Results presented here emphasize that among the prevention and treatment strategies, the association between nutrition, physical activity, and reduction of sedentary behavior can condition the body to a balance point between health-disease relationship, opening up a range of possibilities in the universe of personalized nutrition. It, consequently, may reduce the impact on public health systems. Thus, we address here only a small slice of all that we can envision of possibilities to identify potential adjuvant therapies (mainly, epigenetic regulators) in a faster, safer, and more economical way. It is now up to future studies to seek ways to overcome the drawbacks of bioavailability and translate them into criteria for the technological design of various innovative diets and food products to promote well-being, as well as to prevent and treat NCDs.
Ethical approval
This article does not contain studies with human participants or animals performed by any of the authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the National Council for Scientific and Technological Development, CNPq-Brazil (grant number 406820/2018-0 and 142316/2019-9), São Paulo Research Foundation, FAPESP-Brazil (grant number 2020/08761-4 and 2020/15163-6) and Coordination for the Improvement of Higher Education Personnel, CAPES-Brazil (Finance Code 001) for their financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2022.100155.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Al Shahrani M., Heales S., Hargreaves I., Orford M. Oxidative stress: Mechanistic insights into inherited mitochondrial disorders and Parkinson’s disease. Journal of Clinical Medicine. 2017;6(11):100. doi: 10.3390/jcm6110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasalvar C., Salvadó J.S., Ros E. Bioactives and health benefits of nuts and dried fruits. Food Chemistry. 2020;314 doi: 10.1016/j.foodchem.2020.126192. [DOI] [PubMed] [Google Scholar]
- Alvarez M.C., Maso V., Torello C.O., Ferro K.P., Saad S.T.O. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clinical Epigenetics. 2018;10(1):1–11. doi: 10.1186/s13148-018-0563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2022). More Than Normal Aging: Understanding Mild Cognitive Impairment.
- American Heart Association. (2021, May). About Metabolic Syndrome. What Is Metabolic Syndrome?
- Arfin, S., Jha, N. K., Jha, S. K., Kesari, K. K., Ruokolainen, J., Roychoudhury, S., Rathi, B., & Kumar, D. (2021). Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, Vol. 10, Page 642, 10(5), 642. 10.3390/ANTIOX10050642. [DOI] [PMC free article] [PubMed]
- Arruda H.S., Neri-Numa I.A., Kido L.A., Maróstica Júnior M.R., Pastore G.M. Recent advances and possibilities for the use of plant phenolic compounds to manage ageing-related diseases. Journal of Functional Foods. 2020;75 doi: 10.1016/J.JFF.2020.104203. [DOI] [Google Scholar]
- Assi, M. (2017). The differential role of reactive oxygen species in early and late stages of cancer. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 313(6), R646–R653. 10.1152/ajpregu.00247.2017. [DOI] [PubMed]
- Assumpção, J. H. M., Takeda, A. A. S., Sforcin, J. M., & Rainho, C. A. (2020). Effects of propolis and phenolic acids on triple-negative breast cancer cell lines: Potential involvement of epigenetic mechanisms. Molecules 2020, Vol. 25, Page 1289, 25(6), 1289. doi: 10.3390/MOLECULES25061289. [DOI] [PMC free article] [PubMed]
- Azat Aziz, M., Shehab Diab, A., & Abdulrazak Mohammed, A. (2019). Antioxidant categories and mode of action. In Antioxidants. IntechOpen. 10.5772/intechopen.83544.
- Banda, D. M., Nuñez, N. N., Burnside, M. A., Bradshaw, K. M., & David, S. S. (2017). Repair of 8-oxoG:A mismatches by the MUTYH glycosylase: Mechanism, metals & medicine. Free Radical Biology & Medicine, 107, 202. 10.1016/J.FREERADBIOMED.2017.01.008. [DOI] [PMC free article] [PubMed]
- Beetch M., Boycott C., Harandi-Zadeh S., Yang T., Martin B.J.E., Dixon-McDougall T., Ren K., Gacad A., Dupuis J.H., Ullmer M., Lubecka K., Yada R.Y., Brown C.J., Howe L.A.J., Stefanska B. Pterostilbene leads to DNMT3B-mediated DNA methylation and silencing of OCT1-targeted oncogenes in breast cancer cells. The Journal of Nutritional Biochemistry. 2021;98 doi: 10.1016/J.JNUTBIO.2021.108815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo S., Togliatto G., Gambino R., Ponzo V., Lombardo G., Rosato R., Cassader M., Brizzi M.F. Impact of sirtuin-1 expression on H3K56 acetylation and oxidative stress: A double-blind randomized controlled trial with resveratrol supplementation. Acta Diabetologica. 2018 doi: 10.1007/s00592-017-1097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonla, C. (2021). Oxidative stress, epigenetics, and bladder cancer. In Cancer (pp. 67–75). Elsevier. 10.1016/B978-0-12-819547-5.00007-9.
- Bosviel R., Durif J., Déchelotte P., Bignon Y.J., Bernard-Gallon D. Epigenetic modulation of BRCA1 and BRCA2 gene expression by equol in breast cancer cell lines. British Journal of Nutrition. 2012;108(7):1187–1193. doi: 10.1017/S000711451100657X. [DOI] [PubMed] [Google Scholar]
- Brabson J.P., Leesang T., Mohammad S., Cimmino L. Epigenetic regulation of genomic stability by vitamin C. Frontiers in Genetics. 2021;12:640. doi: 10.3389/FGENE.2021.675780/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton C.V., Landon R., Kahn L.G., Enlow M.B., Peterson A.K., Bastain T.…Fry R. Exploring the evidence for epigenetic regulation of environmental influences on child health across generations. Communications Biology. 2021;4(1):769. doi: 10.1038/s42003-021-02316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D., Jia Z., Wu Y., Su T., Zhao D., Wu M., Tsukamoto T., Oshima M., Jiang J., Cao X. Demethylation of the RB1 promoter concomitant with reactivation of TET2 and TET3 impairs gastric carcinogenesis in K19-Wnt1/C2mE transgenic mice. Life Sciences. 2020;263 doi: 10.1016/J.LFS.2020.118580. [DOI] [PubMed] [Google Scholar]
- Castrejón-Téllez V., Villegas-Romero M., Rubio-Ruiz M.E., Pérez-Torres I., Carreón-Torres E., Díaz-Díaz E., Guarner-Lans V. Effect of a resveratrol/quercetin mixture on the reversion of hypertension induced by a short-term exposure to high sucrose levels near weaning and a long-term exposure that leads to metabolic syndrome in rats. International Journal of Molecular Sciences. 2020 doi: 10.3390/ijms21062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro S.L., Tapias V., Gathagan R., Emes A., Brandon T.E., Smith A.D. Blueberry juice augments exercise-induced neuroprotection in a Parkinson’s disease model through modulation of GDNF levels. IBRO Neuroscience Reports. 2022;12:217–227. doi: 10.1016/J.IBNEUR.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Sun J., Liang Z., Xu H., Du P., Li A., Meng Y., Reshetnik E.I., Liu L., Li C. The bioavailability of soy isoflavones in vitro and their effects on gut microbiota in the simulator of the human intestinal microbial ecosystem. Food Research International. 2022;152 doi: 10.1016/J.FOODRES.2021.110868. [DOI] [PubMed] [Google Scholar]
- Chitrala K.N., Hernandez D.G., Nalls M.A., Mode N.A., Zonderman A.B., Ezike N., Evans M.K. Race-specific alterations in DNA methylation among middle-aged African Americans and Whites with metabolic syndrome. Epigenetics. 2020 doi: 10.1080/15592294.2019.1695340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.J., Kim G.H. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Molecular Medicine Reports. 2014;9(1):328–332. doi: 10.3892/MMR.2013.1752/HTML. [DOI] [PubMed] [Google Scholar]
- Christopher, M. A., Kyle, S. M., & Katz, D. J. (2017). Neuroepigenetic mechanisms in disease. Epigenetics & Chromatin 2017 10:1, 10(1), 1–18. 10.1186/S13072-017-0150-4. [DOI] [PMC free article] [PubMed]
- Cordero-Herrera I., Chen X., Ramos S., Devaraj S. (−)-Epicatechin attenuates high-glucose-induced inflammation by epigenetic modulation in human monocytes. European Journal of Nutrition. 2017 doi: 10.1007/s00394-015-1136-2. [DOI] [PubMed] [Google Scholar]
- Corona G., Kreimes A., Barone M., Turroni S., Brigidi P., Keleszade E., Costabile A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microbial Cell Factories. 2020;19(1):1–14. doi: 10.1186/S12934-020-01341-0/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés-Martín A., Selma M.V., Tomás-Barberán F.A., González-Sarrías A., Espín J.C. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Molecular Nutrition & Food Research. 2020;64(9):1900952. doi: 10.1002/MNFR.201900952. [DOI] [PubMed] [Google Scholar]
- D’Innocenzo S., Biagi C., Lanari M. Obesity and the Mediterranean diet: A review of evidence of the role and sustainability of the Mediterranean Diet. Nutrients. 2019;11(6):1306. doi: 10.3390/nu11061306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagdemir, A., Durif, J., Ngollo, M., Bignon, Y. J., & Bernard-Gallon, D. (2013). Histone lysine trimethylation or acetylation can be modulated by phytoestrogen, estrogen or anti-HDAC in breast cancer cell lines. doi: 10.2217/Epi.12.74, 5(1), 51–63. 10.2217/EPI.12.74. [DOI] [PubMed]
- Dai S., Pan M., El-Nezami H.S., Wan J.M.F., Wang M.F., Habimana O., Lee J.C.Y., Louie J.C.Y., Shah N.P. Effects of lactic acid bacteria-fermented soymilk on isoflavone metabolites and short-chain fatty acids excretion and their modulating effects on gut microbiota. Journal of Food Science. 2019;84(7):1854–1863. doi: 10.1111/1750-3841.14661. [DOI] [PubMed] [Google Scholar]
- Davison G.W., Irwin R.E., Walsh C.P. The metabolic-epigenetic nexus in type 2 diabetes mellitus. Free Radical Biology and Medicine. 2021;170:194–206. doi: 10.1016/J.FREERADBIOMED.2020.12.025. [DOI] [PubMed] [Google Scholar]
- de Oliveira W.Q., Neri-Numa I.A., Arruda H.S., Lopes A.T., Pelissari F.M., Barros F.F.C., Pastore G.M. Special emphasis on the therapeutic potential of microparticles with antidiabetic effect: Trends and possible applications. Trends in Food Science and Technology. 2021;111(February):442–462. doi: 10.1016/j.tifs.2021.02.043. [DOI] [Google Scholar]
- de Paulo Farias D., de Araújo F.F., Neri-Numa I.A., Pastore G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Research International. 2021;145 doi: 10.1016/j.foodres.2021.110383. [DOI] [PubMed] [Google Scholar]
- Di Meo F., Valentino A., Petillo O., Peluso G., Filosa S., Crispi S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. International Journal of Molecular Sciences. 2020;21(7) doi: 10.3390/IJMS21072564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo, M. A. V., Fidelis, M., de Oliveira, P. F., Feitoza, L. Q., Marques, M. J., Ferreira, E. B., Oh, W. Y., Shahidi, F., Hellström, J., Almeida, L. A., Novaes, R. D., Granato, D., & Azevedo, L. (2021). Ellagitannins from jabuticaba (Myrciaria jaboticaba) seeds attenuated inflammation, oxidative stress, aberrant crypt foci, and modulated gut microbiota in rats with 1,2 dimethyl hydrazine-induced colon carcinogenesis. Food and Chemical Toxicology, 154, 112287. 10.1016/J.FCT.2021.112287. [DOI] [PubMed]
- Dominguez L.J., Di Bella G., Veronese N., Barbagallo M. Impact of mediterranean diet on chronic non-communicable diseases and longevity. Nutrients. 2021;13(6) doi: 10.3390/NU13062028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisamy A.J., Mishra M., Kowluru A., Kowluru R.A. Epigenetics and Regulation of Oxidative Stress in Diabetic Retinopathy. Investigative Opthalmology & Visual Science. 2018;59(12):4831. doi: 10.1167/iovs.18-24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy, A. K. (2021). Polyphenols and their impacts on the host epigenome and the gut microbiome. In Evidence-Based Nutrition and Clinical Evidence of Bioactive Foods in Human Health and Disease. 10.1016/b978-0-12-822405-2.00002-5.
- Farias, D. de P., de Araújo, F. F., Neri-Numa, I. A., & Pastore, G. M. (2019). Prebiotics: Trends in food, health and technological applications. Trends in Food Science and Technology, 93, 23–35. 10.1016/j.tifs.2019.09.004.
- Ferraresi A., Phadngam S., Morani F., Galetto A., Alabiso O., Chiorino G., Isidoro C. Resveratrol inhibits IL-6-induced ovarian cancer cell migration through epigenetic up-regulation of autophagy. Molecular Carcinogenesis. 2017;56(3):1164–1181. doi: 10.1002/mc.22582. [DOI] [PubMed] [Google Scholar]
- Ferrari, L., Pavanello, S., & Bollati, V. (2019). Molecular and epigenetic markers as promising tools to quantify the effect of occupational exposures and the risk of developing non-communicable diseases. La Medicina Del Lavoro, 110(3), 168. 10.23749/MDL.V110I3.8538. [DOI] [PMC free article] [PubMed]
- Filosa S., Di Meo F., Crispi S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regeneration Research. 2018;13(12):2055. doi: 10.4103/1673-5374.241429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J., Ettcheto M., Petrov D., Abad S., Pedrós I., Marin M., Olloquequi J., Camins A. Review of the advances in treatment for Alzheimer disease: Strategies for combating β-amyloid protein. Neurología (English Edition) 2018;33(1):47–58. doi: 10.1016/j.nrleng.2015.03.019. [DOI] [PubMed] [Google Scholar]
- Gadecka, A., & Bielak-Zmijewska, A. (2019). Slowing Down Ageing: The Role of Nutrients and Microbiota in Modulation of the Epigenome. Nutrients 2019, Vol. 11, Page 1251, 11(6), 1251. 10.3390/NU11061251. [DOI] [PMC free article] [PubMed]
- García-Guede Á., Vera O., Ibáñez-de-Caceres I. When oxidative stress meets epigenetics: implications in cancer development. Antioxidants. 2020;9(6):468. doi: 10.3390/antiox9060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhauser C. Impact of dietary gut microbial metabolites on the epigenome. Philosophical Transactions of the Royal Society B: Biological Sciences. 2018;373(1748) doi: 10.1098/RSTB.2017.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorabi A.M., Penson P.E., Banach M., Motallebnezhad M., Jamialahmadi T., Sahebkar A. Epigenetic control of atherosclerosis via DNA methylation: A new therapeutic target? Life Sciences. 2020;253 doi: 10.1016/j.lfs.2020.117682. [DOI] [PubMed] [Google Scholar]
- Griñán-Ferré C., Corpas R., Puigoriol-Illamola D., Palomera-Ávalos V., Sanfeliu C., Pallàs M. Understanding epigenetics in the neurodegeneration of Alzheimer’s disease: SAMP8 mouse model. Journal of Alzheimer’s Disease. 2018;62(3):943–963. doi: 10.3233/JAD-170664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S., Raina R., Afroze N., Hussain A., Alsulimani A., Singh V., Mishra B.N., Kaul S., Kharwar R.N. Microbial dysbiosis and epigenetics modulation in cancer development – A chemopreventive approach. Seminars in Cancer Biology. 2021 doi: 10.1016/j.semcancer.2021.06.024. [DOI] [PubMed] [Google Scholar]
- Hwang J.Y., Aromolaran K.A., Zukin R.S. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nature Reviews. Neuroscience. 2017;18(6):347. doi: 10.1038/NRN.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Diabetes Federation (IDF) The IDF consensus worldwide definition of the metabolic syndrome. Obesity and Metabolism. 2005;2(3):47–49. doi: 10.14341/2071-8713-4854. [DOI] [Google Scholar]
- Ionescu-Tucker A., Cotman C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiology of Aging. 2021;107:86–95. doi: 10.1016/J.NEUROBIOLAGING.2021.07.014. [DOI] [PubMed] [Google Scholar]
- Irshad R., Husain M. Natural products in the reprogramming of cancer epigenetics. Toxicology and Applied Pharmacology. 2021;417 doi: 10.1016/j.taap.2021.115467. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Torres E., Hernández-Oliveras A., Meneses-Morales I., Rodríguez G., Fuentes-García G., Zarain-Herzberg Á. Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. International Journal of Biochemistry and Cell Biology. 2019;113:37–47. doi: 10.1016/j.biocel.2019.05.020. [DOI] [PubMed] [Google Scholar]
- Jiang X., Chen W., Shen F., Xiao W., Guo H., Su H., Xiu J., Sun W. Pinoresinol promotes MC3T3-E1 cell proliferation and differentiation via the cyclic AMP/protein kinase A signaling pathway. Molecular Medicine Reports. 2019;20(3):2143–2150. doi: 10.3892/MMR.2019.10468/HTML. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann T., Petry A., Shvetsova A., Gerhold J.M., Görlach A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. British Journal of Pharmacology. 2017;174(12):1533. doi: 10.1111/BPH.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H., Harata K., Madhyastha H., Kuribayashi F. Ellagic acid and its fermentative derivative urolithin A show reverse effects on the gp91-phox gene expression, resulting in opposite alterations in all-trans retinoic acid-induced superoxide generating activity of U937 cells. Biochemistry and Biophysics Reports. 2021;25 doi: 10.1016/J.BBREP.2020.100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H., Yuan B., Hu X., Okazaki M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. American Journal of Cancer Research. 2019;9(8):1517–1535. www.ajcr.us/ [PMC free article] [PubMed] [Google Scholar]
- Kiss A.K., Granica S., Stolarczyk M., Melzig M.F. Epigenetic modulation of mechanisms involved in inflammation: Influence of selected polyphenolic substances on histone acetylation state. Food Chemistry. 2012;131(3):1015–1020. doi: 10.1016/J.FOODCHEM.2011.09.109. [DOI] [Google Scholar]
- Koukoura O., Sifakis S., Goutsias N., Gkorezi-Ntavela I., Hajiioannou J. In: Epigenetics of Cancer Prevention. Bishayee A., Bhatia D., editors. Academic Press -; Elsevier: 2019. Epigenomics of Ovarian Cancer and Its Chemoprevention; pp. 333–358. [DOI] [Google Scholar]
- Kriebel J., Herder C., Rathmann W., Wahl S., Kunze S., Molnos S., Volkova N., Schramm K., Carstense-Kirberg M., Waldenberger M., Gieger C., Peters A., Illig T., Prokisch H., Roden M., Grallert H. Association between DNA Methylation in whole blood and measures of glucose metabolism: Kora F4 study. PLoS ONE. 2016 doi: 10.1371/journal.pone.0152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni H., Kos M.Z., Neary J., Dyer T.D., Kent J.W., Göring H.H.H., Cole S.A., Comuzzie A.G., Almasy L., Mahaney M.C., Curran J.E., Blangero J., Carless M.A. Novel epigenetic determinants of type 2 diabetes in Mexican-American families. Human Molecular Genetics. 2015 doi: 10.1093/hmg/ddv232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Saravana R.M., Wang Y., Zhang X., Cheng H., Sun L., He S., Hao F. Redox Components: Key Regulators of Epigenetic Modifications in Plants. International Journal of Molecular Sciences. 2020;21(4):1419. doi: 10.3390/ijms21041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M., Jaric I. The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes. 2017;8(3) doi: 10.3390/GENES8030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborda-Illanes A., Sanchez-Alcoholado L., Dominguez-Recio M.E., Jimenez-Rodriguez B., Lavado R., Comino-Méndez I., Alba E., Queipo-Ortuño M.I. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers. 2020;12(9):1–27. doi: 10.3390/cancers12092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang L., Liu H., Ren W., Zhang Z., Xia B. Roles of miR-124-3p/Scd1 in urolithin A-induced brown adipocyte differentiation and succinate-dependent regulation of mitochondrial complex II. Biochemical and Biophysical Research Communications. 2022;606:174–181. doi: 10.1016/J.BBRC.2022.03.112. [DOI] [PubMed] [Google Scholar]
- Li Z., Summanen P.H., Komoriya T., Henning S.M., Lee R.P., Carlson E., Heber D., Finegold S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe. 2015;34:164–168. doi: 10.1016/J.ANAEROBE.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Liu Y., Feng Y., Li Y., Hu Y., Zhang Q., Huang Y., Shi K., Ran C., Hou J., Zhou G., Wang X. Chlorogenic acid decreases malignant characteristics of hepatocellular carcinoma cells by inhibiting DNMT1 expression. Frontiers in Pharmacology. 2020;11:867. doi: 10.3389/FPHAR.2020.00867/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Medicine and Cellular Longevity. 2017;2017 doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low, F. M., Gluckman, P. D., & Hanson, M. A. (2021). Epigenetic and Developmental Basis of Risk of Obesity and Metabolic Disease. In Cellular Endocrinology in Health and Disease, Second Edition. 10.1016/B978-0-12-819801-8.00014-4.
- Luccarini, I., Grossi, C., Rigacci, S., Coppi, E., Pugliese, A. M., Pantano, D., la Marca, G., Ed Dami, T., Berti, A., Stefani, M., & Casamenti, F. (2015). Oleuropein aglycone protects against pyroglutamylated-3 amyloid-ß toxicity: biochemical, epigenetic and functional correlates. Neurobiology of Aging, 36(2), 648–663. 10.1016/J.NEUROBIOLAGING.2014.08.029. [DOI] [PubMed]
- Lukiw W.J. NF-κB-regulated, proinflammatory miRNAs in Alzheimer’s disease. Alzheimer’s Research and Therapy. 2012;4(6):1–11. doi: 10.1186/ALZRT150/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak V.I., Storey K.B. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. EXCLI Journal. 2021;20:956–967. doi: 10.17179/EXCLI2021-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah, P. K. S., Ponnusamy, L., & Singh, K. P. (2017). Oxidative stress-induced epigenetic changes associated with malignant transformation of human kidney epithelial cells. Oncotarget, 8(7), 11127. 10.18632/ONCOTARGET.12091. [DOI] [PMC free article] [PubMed]
- Marras, C., Beck, J. C., Bower, J. H., Roberts, E., Ritz, B., Ross, G. W., Abbott, R. D., Savica, R., Van Den Eeden, S. K., Willis, A. W., & Tanner, C. (2018). Prevalence of Parkinson’s disease across North America. Npj Parkinson’s Disease 2018 4:1, 4(1), 1–7. 10.1038/s41531-018-0058-0. [DOI] [PMC free article] [PubMed]
- Marshall, P., & Bredy, T. W. (2016). Cognitive neuroepigenetics: the next evolution in our understanding of the molecular mechanisms underlying learning and memory? Npj Science of Learning 2016 1:1, 1(1), 1–8. 10.1038/npjscilearn.2016.14. [DOI] [PMC free article] [PubMed]
- Meng J., Lv Z., Zhang Y., Wang Y., Qiao X., Sun C., Chen Y., Guo M., Han W., Ye A., Xie T., Chu B., Shi C., Yang S., Chen C. Precision redox: The key for antioxidant pharmacology. Antioxidants and Redox Signaling. 2021;34(14):1069–1082. doi: 10.1089/ARS.2020.8212/ASSET/IMAGES/LARGE/ARS.2020.8212_FIGURE3.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y., Zhao S., Gao Y., Wang R., Wu Q., Wu H., Luo T. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Research Bulletin. 2016 doi: 10.1016/j.brainresbull.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Milošević M., Arsić A., Cvetković Z., Vučić V. Memorable Food: Fighting Age-Related Neurodegeneration by Precision Nutrition. Frontiers in Nutrition. 2021;8:507. doi: 10.3389/FNUT.2021.688086/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzio Compagnoni, G., Di Fonzo, A., Corti, S., Comi, G. P., Bresolin, N., & Masliah, E. (2020). The role of mitochondria in neurodegenerative diseases: the Lesson from Alzheimer’s disease and Parkinson’s disease. Molecular Neurobiology 2020 57:7, 57(7), 2959–2980. 10.1007/S12035-020-01926-1. [DOI] [PMC free article] [PubMed]
- Neri-Numa I.A., Cazarin C.B.B., Ruiz A.L.T.G., Paulino B.N., Molina G., Pastore G.M. Targeting flavonoids on modulation of metabolic syndrome. Journal of Functional Foods. 2020;73 doi: 10.1016/j.jff.2020.104132. [DOI] [Google Scholar]
- Neri Numa I.A., Pastore G.M. Novel insights into prebiotic properties on human health: A review. Food Research International. 2020;131 doi: 10.1016/j.foodres.2019.108973. [DOI] [PubMed] [Google Scholar]
- Nettore I.C., Rocca C., Mancino G., Albano L., Amelio D., Grande F., Puoci F., Pasqua T., Desiderio S., Mazza R., Terracciano D., Colao A., Bèguinot F., Russo G.L., Dentice M., Macchia P.E., Sinicropi M.S., Angelone T., Ungaro P. Quercetin and its derivative Q2 modulate chromatin dynamics in adipogenesis and Q2 prevents obesity and metabolic disorders in rats. Journal of Nutritional Biochemistry. 2019 doi: 10.1016/j.jnutbio.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Nie J., Zhang L., Zhao G., Du X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. Journal of Applied Microbiology. 2019;127(6):1824–1834. doi: 10.1111/JAM.14441. [DOI] [PubMed] [Google Scholar]
- Nuotio M.L., Pervjakova N., Joensuu A., Karhunen V., Hiekkalinna T., Milani L., Kettunen J., Järvelin M.R., Jousilahti P., Metspalu A., Salomaa V., Kristiansson K., Perola M. An epigenome-wide association study of metabolic syndrome and its components. Scientific Reports. 2020 doi: 10.1038/s41598-020-77506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira G.S., Iraci L., Pinheiro G.S., Casal M.Z., Haas A.N., Pochmann D., Martinez F.G., Elsner V., Dani C. Effect of exercise and grape juice on epigenetic modulation and functional outcomes in PD: A randomized clinical trial. Physiology & Behavior. 2020;227 doi: 10.1016/J.PHYSBEH.2020.113135. [DOI] [PubMed] [Google Scholar]
- Oliveira L.V.A., Dos Santos B.N.S., Machado Í.E., Malta D.C., Velasquez-Melendez G., Felisbino-Mendes M.S. Prevalence of the metabolic syndrome and its components in the Brazilian adult population. Ciencia e Saude Coletiva. 2020 doi: 10.1590/1413-812320202511.31202020. [DOI] [PubMed] [Google Scholar]
- Özyalçin, B., & Sanlier, N. (2020). The effect of diet components on cancer with epigenetic mechanisms. In Trends in Food Science and Technology (Vol. 102, pp. 138–145). Elsevier. 10.1016/j.tifs.2020.06.004.
- Peplow P.V., Martinez B., Gennarelli T.A. In: Peplow P., Martinez B., Gennarelli T., editors. Vol. 173. Humana New York; NY: 2022. Prevalence, needs, strategies, and risk factors for neurodegenerative diseases; pp. 3–8. (Neuromethods). [DOI] [Google Scholar]
- Pfeiffer L., Wahl S., Pilling L.C., Reischl E., Sandling J.K., Kunze S.…Waldenberger M. DNA methylation of lipid-related genes affects blood lipid levels. Circulation: Cardiovascular Genetics. 2015 doi: 10.1161/CIRCGENETICS.114.000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Chen Z., Yang L., Shi H., Wu H., Zhang B., Zhang W., Xu Q., Huang F., Wu X. Luteolin-7-O-glucoside protects dopaminergic neurons by activating estrogen-receptor-mediated signaling pathway in MPTP-induced mice. Toxicology. 2019;426 doi: 10.1016/J.TOX.2019.152256. [DOI] [PubMed] [Google Scholar]
- Quideau S., Deffieux D., Douat-Casassus C., Pouységu L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angewandte Chemie International Edition. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- Ramzan F., Vickers M.H., Mithen R.F. Epigenetics, microrna and metabolic syndrome: A comprehensive review. International Journal of Molecular Sciences. 2021 doi: 10.3390/ijms22095047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remely M., Ferk F., Sterneder S., Setayesh T., Roth S., Kepcija T., Noorizadeh R., Rebhan I., Greunz M., Beckmann J., Wagner K.H., Knasmüller S., Haslberger A.G. EGCG prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Oxidative Medicine and Cellular Longevity. 2017 doi: 10.1155/2017/3079148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanos-Nanclares A., Sánchez-Quesada C., Gardeazábal I., Martínez-González M.Á., Gea A., Toledo E. Phenolic acid subclasses, individual compounds, and breast cancer risk in a Mediterranean cohort: The SUN Project. Journal of the Academy of Nutrition and Dietetics. 2020;120(6):1002–1015.e5. doi: 10.1016/J.JAND.2019.11.007. [DOI] [PubMed] [Google Scholar]
- Rozek L.S., Virani S., Bellile E.L., Taylor J.M.G., Sartor M.A., Zarins K.R., Virani A., Cote C., Worden F.P., Mark M.E.P., McLean S.A., Duffy S.A., Yoo G.H., Saba N.F., Shin D.M., Kucuk O., Wolf G.T. Soy isoflavone supplementation increases long interspersed nucleotide element-1 (LINE-1) methylation in head and neck squamous cell carcinoma. Nutrition and Cancer. 2019;71(5):772–780. doi: 10.1080/01635581.2019.1577981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz G.P., Camara H., Fazolini N.P.B., Mori M.A. Extracellular miRNAs in redox signaling: Health, disease and potential therapies. Free Radical Biology and Medicine. 2021;173:170–187. doi: 10.1016/J.FREERADBIOMED.2021.05.004. [DOI] [PubMed] [Google Scholar]
- Russo G.L., Vastolo V., Ciccarelli M., Albano L., Macchia P.E., Ungaro P. Dietary polyphenols and chromatin remodeling. Critical Reviews in Food Science and Nutrition. 2017;57(12):2589–2599. doi: 10.1080/10408398.2015.1062353. [DOI] [PubMed] [Google Scholar]
- SACN. (2021). The Scientific Advisory Committee on Nutrition (SACN) position statement on nutrition and older adults living in the community. [DOI] [PubMed]
- Santamarina, A. B., Jamar, G., Mennitti, L. V., César, H. de C., de Rosso, V. V., Vasconcelos, J. R., Oyama, L. M., & Pisani, L. P. (2018). Supplementation of Juçara berry (Euterpe edulis mart.) modulates epigenetic markers in monocytes from obese adults: A double-blind randomized trial. Nutrients. 10.3390/nu10121899. [DOI] [PMC free article] [PubMed]
- Santos-Sánchez, N. F., Salas-Coronado, R., Villanueva-Cañongo, C., & Hernández-Carlos, B. (2019). Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants [Working Title]. IntechOpen. https://doi.org/10.5772/intechopen.85270.
- Scaccia, E., Bordin, A., Balistreri, C. R., & De Falco, E. (2020). Epigenetics, oxidative states and diabetes. Diabetes: Oxidative Stress and Dietary Antioxidants, 87–96. 10.1016/B978-0-12-815776-3.00009-7.
- Selma, M. V., Beltrán, D., Luna, M. C., Romo-Vaquero, M., García-Villalba, R., Mira, A., Espín, J. C., & Tomás-Barberán, F. A. (2017). Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Frontiers in Microbiology, 8(AUG), 1521. 10.3389/FMICB.2017.01521. [DOI] [PMC free article] [PubMed]
- Sharma, M., & Tollefsbol, T. O. (2022). Combinatorial epigenetic mechanisms of sulforaphane, genistein and sodium butyrate in breast cancer inhibition. Experimental Cell Research, 416(1), 113160. 10.1016/j.yexcr.2022.113160. [DOI] [PubMed]
- Sharma, R., & Padwad, Y. (2020). Perspectives of the potential implications of polyphenols in influencing the interrelationship between oxi-inflammatory stress, cellular senescence and immunosenescence during aging. Trends in Food Science & Technology, 98, 41–52. 10.1016/j.tifs.2020.02.004.
- Sher G., Salman N.A., Khan A.Q., Prabhu K.S., Raza A., Kulinski M., Dermime S., Haris M., Junejo K., Uddin S. Epigenetic and breast cancer therapy: Promising diagnostic and therapeutic applications. Seminars in Cancer Biology. 2020;2–14 doi: 10.1016/j.semcancer.2020.08.009. [DOI] [PubMed] [Google Scholar]
- Shishtar E., Rogers G.T., Blumberg J.B., Au R., Jacques P.F. Long-term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. The American Journal of Clinical Nutrition. 2020;112(2):343–353. doi: 10.1093/AJCN/NQAA079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock T., Badang L., Ferguson B., Martinez-Guryn K. The interplay between diet, gut microbes, and host epigenetics in health and disease. The Journal of Nutritional Biochemistry. 2021;95 doi: 10.1016/J.JNUTBIO.2021.108631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, L. B. A. R., Pinheiro-Castro, N., Novaes, G. M., Pascoal, G. de F. L., & Ong, T. P. (2019). Bioactive food compounds, epigenetics and chronic disease prevention: Focus on early-life interventions with polyphenols. Food Research International, 125(August), 108646. 10.1016/j.foodres.2019.108646. [DOI] [PubMed]
- Sixty-sixth World Health Assembly. (2013). Follow-up to the Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases.
- Stockert A.L., Hill M. Bioactive Components, Diet and Medical Treatment in Cancer Prevention. Springer International Publishing; 2018. Anticancer Potential of Dietary Polyphenols; pp. 25–50. [DOI] [Google Scholar]
- Sugiura M., Sato H., Kanesaka M., Imamura Y., Sakamoto S., Ichikawa T., Kaneda A. Epigenetic modifications in prostate cancer. International Journal of Urology. 2021;28(2):140–149. doi: 10.1111/iju.14406. [DOI] [PubMed] [Google Scholar]
- Tian L., Song Z., Shao W., Du W.W., Zhao L.R., Zeng K., Yang B.B., Jin T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death and Disease. 2017 doi: 10.1038/cddis.2016.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng, T.-H., Chien, M.-H., Lin, W.-L., Wen, Y.-C., Chow, J.-M., Chen, C.-K., Kuo, T.-C., Lee, W.-J., & Library, W. O. (2016). Inhibition of MDA-MB-231 Breast Cancer Cell Proliferation and Tumor Growth by Apigenin Through Induction of G2/M Arrest and Histone H3 Acetylation-mediated p21 WAF1/CIP1 Expression. Inc. Environ Toxicol, 32, 434–444. 10.1002/tox.22247. [DOI] [PubMed]
- United Nations, Department of Economic and Social Affairs, P. D. (2019). W. P. A. 2019: H. (ST/ESA/SER. A. (2019). World Population Ageing 2019: Highlights.
- Vauzour D., Rendeiro C., D’amato A., Waffo-Téguo P., Richard T., Mérillon J.M., Pontifex M.G., Connell E., Müller M., Butler L.T., Williams C.M., Spencer J.P.E. Anthocyanins Promote Learning through Modulation of Synaptic Plasticity Related Proteins in an Animal Model of Ageing. Antioxidants (Basel. Switzerland) 2021;10(8) doi: 10.3390/ANTIOX10081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaszczyk E., Luijten M., Spijkerman A.M.W., Bonder M.J., Lutgers H.L., Snieder H., Wolffenbuttel B.H.R., van Vliet-Ostaptchouk J.V. DNA methylation markers associated with type 2 diabetes, fasting glucose and HbA1c levels: A systematic review and replication in a case–control sample of the Lifelines study. Diabetologia. 2018 doi: 10.1007/s00125-017-4497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, J.M.; Eckardr, P.; Aleman, J.O.; Rosa, J.C.; Liang; Y.; Iizumi, T.; Estheve, S.; Blaser, J.M.; Breslow, J. L. H. P. R. (2018). The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. Journal of Clinical and Translational Research, 4(2), 122–135. [PMC free article] [PubMed]
- Wang, P., Li, D., Ke, W., Liang, D., Hu, X., & Chen, F. (2019). Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. International Journal of Obesity 2019 44:1, 44(1), 213–225. 10.1038/s41366-019-0332-1. [DOI] [PubMed]
- Wang, S. wei, Sheng, H., Zheng, F., & Zhang, F. (2021). Hesperetin promotes DOT1L degradation and reduces histone H3K79 methylation to inhibit gastric cancer metastasis. Phytomedicine, 84, 153499. 10.1016/J.PHYMED.2021.153499. [DOI] [PubMed]
- Wang Y.L., Shen Y., Xu J.P., Han K., Zhou Y., Yang S., Yin J.Y., Min D.L., Hu H.Y. Pterostilbene suppresses human endometrial cancer cells in vitro by down-regulating MIR-663b. Acta Pharmacologica Sinica. 2017;38(10):1394–1400. doi: 10.1038/aps.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Lam K.L., Hu J., Ge S., Zhou A., Zheng B., Zeng S., Lin S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Science & Nutrition. 2019;7(2):579–588. doi: 10.1002/FSN3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/FAO. (2003). WHO Technical Report Series: Diet, Nutrition and the Prevention of Chronic Diseases. [PubMed]
- WHO. (2020). Non-communicable diseases. In Noncommunicable diseases progress monitor 2020. 10.5005/jp/books/11410_18.
- WHO. (2021). Diabetes.
- World Health Organization. (2021). Obesity.
- World Health Organization (WHO). (2016). Raised Cholesterol. World Health Organisation (WHO).
- Wu H., Li C., Cui M., Guo H., Chen S., Du J., Li H., Li Z. Polyphenols from Hippophae rhamnoides suppressed colon cancer growth by regulating miRNA-mediated cell cycle arrest and apoptosis in vitro and in vivo. Journal of Functional Foods. 2021;87 doi: 10.1016/j.jff.2021.104780. [DOI] [Google Scholar]
- Wu Y., Wang R., Liu R., Ba Y., Huang H. The roles of histone modifications in metal-induced neurological disorders. Biological Trace Element Research. 2022 doi: 10.1007/S12011-022-03134-5. [DOI] [PubMed] [Google Scholar]
- Xiao W., Loscalzo J. Metabolic responses to reductive stress. Antioxidants and Redox Signaling. 2020;32(18):1330–1347. doi: 10.1089/ARS.2019.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.Q., Neoh K.-G., Kang E.-T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Progress in Polymer Science. 2018;87:165–196. doi: 10.1016/J.PROGPOLYMSCI.2018.08.005. [DOI] [Google Scholar]
- Yan W., Wu T.H.Y., Leung S.S.Y., To K.K.W. Flavonoids potentiated anticancer activity of cisplatin in non-small cell lung cancer cells in vitro by inhibiting histone deacetylases. Life Sciences. 2020;258 doi: 10.1016/J.LFS.2020.118211. [DOI] [PubMed] [Google Scholar]
- Yousefi, H., Alihemmati, A., Karimi, P., Alipour, M. R., Habibi, P., & Ahmadiasl, N. (2017). Effect of genistein on expression of pancreatic SIRT1, inflammatory cytokines and histological changes in ovariectomized diabetic rat. Iranian Journal of Basic Medical Sciences. doi: 10.22038/ijbms.2017.8585. [DOI] [PMC free article] [PubMed]
- Yuan Z., Syed M.A., Panchal D., Rogers D., Joo M., Sadikot R.T. Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. The International Journal of Biochemistry & Cell Biology. 2012;44(11):2032–2043. doi: 10.1016/J.BIOCEL.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang B., Tian L., Xie J., Chen G., Wang F. Targeting miRNAs by natural products: A new way for cancer therapy. Biomedicine & Pharmacotherapy. 2020;130 doi: 10.1016/J.BIOPHA.2020.110546. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Molsberry, S. A., Yeh, T. S., Cassidy, A., Schwarzschild, M. A., Ascherio, A., & Gao, X. (2022). Intake of Flavonoids and Flavonoid-Rich Foods and Mortality Risk Among Individuals With Parkinson Disease. Neurology, 98(10), e1064–e1076. 10.1212/WNL.0000000000013275. [DOI] [PMC free article] [PubMed]
- Zhou W., Shunqing W., Yi Y., Zhou R., Mao P. MiR-196b/miR-1290 participate in the antitumor effect of resveratrol via regulation of IGFBP3 expression in acute lymphoblastic leukemia. Oncology Reports. 2017;37(2):1075–1083. doi: 10.3892/or.2016.5321. [DOI] [PubMed] [Google Scholar]
- Zhou X., Zhuang Z., Wang W., He L., Wu H., Cao Y., Pan F., Zhao J., Hu Z., Sekhar C., Guo Z. OGG1 is essential in oxidative stress induced DNA demethylation. Cellular Signalling. 2016;28(9):1163–1171. doi: 10.1016/J.CELLSIG.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Zuo J., Zhang Z., Luo M., Zhou L., Nice E.C., Zhang W., Wang C., Huang C. Redox signaling at the crossroads of human health and disease. MedComm. 2022;3(2):e127. doi: 10.1002/mco2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L., Hemmelgarn B.T., Chuang C.C., Best T.M. The Role of Oxidative Stress-Induced Epigenetic Alterations in Amyloid-β Production in Alzheimer’s Disease. Oxidative Medicine and Cellular Longevity. 2015;2015 doi: 10.1155/2015/604658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.