Abstract
Objectives:
This study seeks to examine neighborhood characteristics, physical activity, and health status and their roles in promoting healthy cognitive aging.
Methods:
Using data from the REasons for Geographic And Racial Difference in Stroke (REGARDS) study (N=10,289, mean age=73.4 years), we used multilevel linear regression to examine the relationships between walkable neighborhoods (both objectively measured and subjective perceptions), walking behavior, physical activity, health status and cognitive function.
Results:
Engaging in any moderate physical activity (β=0.47, p<0.001), having better health status (β=0.02, p<0.001), living in neighborhoods with greater street connectivity (β=0.15, p<0.05), and positive perceptions of neighborhood traffic (p<0.01) and parks (p<0.05), were associated with higher cognitive function. Residence in socioeconomically disadvantaged neighborhoods (β=−0.01, p<0.01) was negatively associated with cognitive function.
Discussion:
Both perceived and objective features of walkable environments may have consequences for cognitive health, and can inform the development of health promoting communities.
Keywords: Cognition, physical activity, neighborhoods, older adults, walkability
Background and Objectives
Dementia is a common and disabling brain disorder among older adults that has consequences for independence, functional decline, institutionalization, and mortality (Langa et al., 2001). Physical activity has been identified as one of the few potentially modifiable risk factors for dementia (Livingston et al, 2020), which may improve cognitive function or delay cognitive decline in older adults (Carvalho et al., 2014). Those engaging in physical activity in midlife have faster processing speeds, better executive function, and better memory later in life (Chang et al., 2010). Additionally, low-to-moderate physical activity has been shown to result in lower likelihood of dementia and cognitive impairment in late life (Chang et al., 2010; Sofi et al., 2010). However, the majority of adults do not meet the recommended physical activity guidelines (Tucker et al., 2011).
A large body of research has focused on the factors at the individual level that shape older adults’ decisions to engage in physical activity, including self-efficacy, and expectation of positive outcomes (eg, bone and muscle strength, mood improvement) and negative outcomes (eg, injury/fall, exacerbation of chronic conditions, heart attack, chest pain, or shortness of breath) (Gallagher et al., 2012). But characteristics in the surrounding environment can influence physical activity because they set the stage for opportunities for activity to occur. Neighborhood context has a profound effect on overall health and mortality, such that cumulative exposure to neighborhood disadvantage was found to be associated with functional decline and mortality of older adults over time (Clarke et. al., 2014). Understanding the environmental factors that influence physical activity and general health in older adults, as a potential pathway to prevent cognitive decline, is critical for building age-friendly and health promoting communities. In this study we seek to examine the relationship between neighborhood characteristics and cognitive function, focusing on both perceived and observed environmental factors relevant for physical activity, and examine the role of walking behavior and physical activity as potential mechanisms by which residence in walkable neighborhoods is associated with better cognitive function.
Background
Literature has suggested that cognitive function may be shaped by differential access to local resources that promote healthy behaviors within the neighborhood context (Besser et al., 2017; Clarke et al., 2015). Neighborhood environments that promote physical activity (e.g., recreational centers, gyms, parks, walking paths) are potential resources for preventing cognitive decline with age (Clarke et al., 2015; Aneshensel et al., 2011). This is important, since walking is the most common form of exercise among older adults (Bassett et al., 2002; Belza et. al., 2004; Gallagher et al., 2012; Hughes et al., 2008). Older adults tend to engage in physical activity within one to three blocks of their home (Chaudhury et al., 2016), which emphasizes the importance of local neighborhood environments that promote and encourage physical activity. One study found that neighborhood-level factors, such as neighborhood income, population density and walking trails, paths & parks, accounted for more of the variation in walking activity than individual-level factors (Fisher et al., 2004). Features of the built environment, including green space and open spaces for recreation, have been found to be positively associated with walking activity (King, 2008; Li et al., 2004). Neighborhood walkability, captured through an objective measure of intersection density, block length, and proximity to amenities (Walkscore) has been shown to be associated with increased physical activity in older adults (Hirsch et al., 2017). Street connectivity, measured by intersections per square mile, ratio of straight-line distance of network distance, or average block length (Handy et. al., 2002), has particularly been a focus of many studies concerning the relationship between walkable environments and physical activity. Street connectivity is said to lend an ease and efficiency to walking behavior by way of shorter block lengths, fewer barriers to direct travel (e.g. cul-de-sacs), safety through alternate route options and increased intersections (Saelens et.al., 2003). A 2008 study examining the built environment, adiposity, and physical activity in older adults found that living in areas with high street connectivity was associated with higher walking prevalence and meeting the CDC’s recommended physical activity guidelines (Li et. al., 2008).
While neighborhood characteristics, both observed (objective) and subjectively perceived by residents, have been cited as key determinants of social and physical functioning (Bowling and Stafford, 2007), there may be differences in the way that these characteristics impact health. A considerable body of research has demonstrated that perceptions of neighborhood resources are distinct from objectively measured characteristics (Bowling & Stafford, 2007; Orstad et al., 2017). Objective measures aim to capture an unbiased, more static assessment of the environment, while perceptions of such environments rely heavily upon personal (age, gender, values, experiences) and social (economic conditions, culture, norms) factors (Orstad et al., 2017). Perceptions of the neighborhood environment, including perceptions of quality, safety, and proximity to recreation facilities, have also been shown to be associated with self-rated health (Wen et al., 2006), local park use (Leslie et al., 2010), and walking activity (Leslie et al., 2010; Li et al., 2005).
There is also some debate about whether objective or subjective characteristics are more salient for determining health behaviors (especially physical activity), with many studies highlighting that the association between objective features of the neighborhood environment and physical activity cannot be understood in direct terms, but require important consideration of the role of subjective perceptions of available resources (Kim et al. 2010; Leslie et al., 2010; Orstad et al. 2017). Studies have shown that differences in walking behavior may be influenced by factors related to perceived mobility (Gallagher et al., 2012) and socioeconomic status (Leslie et al., 2010). It has been shown that more socioeconomically disadvantaged neighborhoods are objectively more walkable (i.e., greater street connectivity) (King & Clarke, 2014); yet, despite being highly walkable, residents in such neighborhoods do not have high physical activity levels (Griffin et al., 2008; Ross & Mirowsky, 2001). Thus, there may be unobserved benefit of walkable neighborhoods that extends beyond simply promoting physical activity.
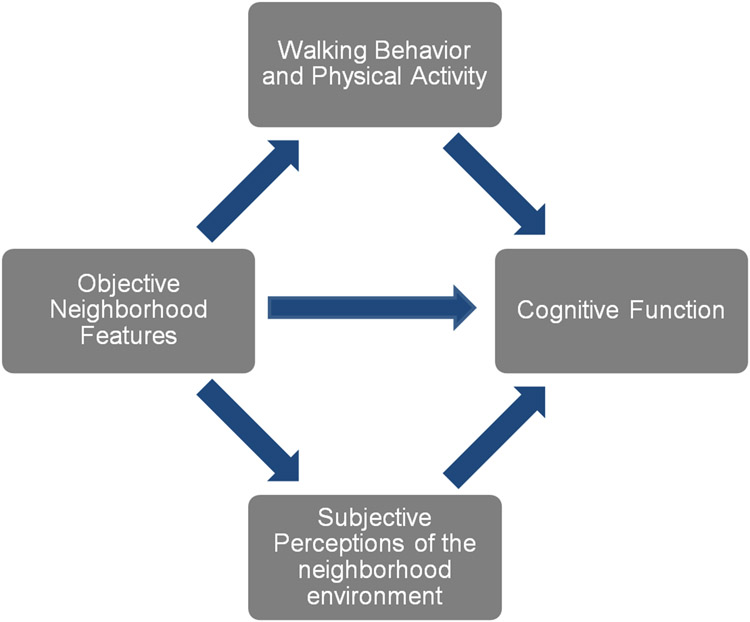
In order to understand how to promote health for the prevention and delay of cognitive impairment at a population level, it is important to understand the role of neighborhood context, both objectively and subjectively assessed, for physical activity, health and cognition. Existing research, however, has yet to examine the objective and subjective neighborhood environments with respect to physical activity, as they shape cognitive health. As detailed in Figure 1., we hypothesize that the cognitive benefit of walking behavior and physical activity, as posited by the literature, is a function of the availability of objective neighborhood features for a walkable environment; and that the cognitive impact of these objective neighborhood features operate through subjective perceptions of the neighborhood environment as well.
Figure 1.
Potential Pathways of the relationships between Cognition, Walkable Environments, and Physical Activity
Research Design and Methods
Data
This is a cross-sectional study design using data from the REasons for Geographic and Racial Difference in Stroke (REGARDS) study, a nationwide, prospective cohort study examining regional and racial influences on stroke incidence and mortality. Over 30,000 Black and White community-dwelling adults (mean age of 65) were recruited between 2003 and 2007. Potential participants were randomly selected from well-characterized commercially available lists of U.S. residents (Genesys, Daly City, CA), stratified by age, race, sex, and geographic region (Howard et al., 2005). Because the largest stroke-related racial disparities are between Black and White adults, REGARDS only enrolled those reporting race as either non-Hispanic Black (42%) or White (58%). The cohort excluded those with a history of any condition that could prevent long-term participation (Howard et al., 2005). The cohort consists of 30,239 U.S. residents, with 16,934 from eight Stroke Belt states (eg, Alabama, Georgia, Louisiana) and 13,305 from the remaining 40 contiguous states and the District of Columbia.
Socio-demographic, behavioral, and lifestyle information, along with health history were collected at enrollment via computer assisted telephone interview and an in-home visit (Howard et al., 2005). Cognitive function was assessed biennially (since 2006) via well validated telephone screening instruments. A second in-home data collection was conducted between 2013 and 2016 with almost 70% of surviving participants (N=16,164) (Long et al., 2019). At this visit, updated information was obtained on sociodemographic factors, health status, and health history. Participants were also given a self-administered questionnaire (leave-behind questionnaire) that included questions on neighborhood perceptions and physical activity, which they were invited to complete following the visit and return via mail. The current project included data from this second wave of data collection, including data from the leave-behind questionnaire and the cognitive telephone screener administered around the time of the in-home visit. There were 10,288 participants who completed the leave-behind questionnaire following the second in home visit, and who had complete data on physical activity, neighborhood perceptions, and all covariates of interest. Because of the known association between stroke and cognitive function, we excluded participants with a known history of stroke before this assessment.
Measures
Cognitive function was assessed using the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) Word List Learning (WLL), and Word List Delayed Recall (WLD), and Animal Fluency Test (AF) (Moms et al. 1989; Morris et al. 1987). These cognitive measures are consistent with the Vascular Cognitive Impairment Harmonization Standards (Hachinski et al., 2006) and have been validated for Black and White individuals (Lucas et al., 2005). The CERAD world list tasks measure episodic memory. The AF assesses semantic memory and executive function, with scores based on the number of animals generated in 1 minute. The testing protocol also included the well-validated measures of letter fluency (LF), and delayed memory recall items from the National Institute of Neurological Disorders and Stroke and the Canadian Stroke Network (NINDS-CSN) neuropsychological battery (Hachinski et al., 2006; Morris et al., 1987).
We captured overall cognitive status using factor scores from a measurement model of cognitive function estimated with AF, LF, WLL, WLD, and the NINDS-CDS word recall items (Finlay et al., 2020). The measurement model fit the data very well (factor loadings range from .43-.80, Comparative Fit index and Tucker Lewis Index >.95), with correlated residuals in the memory items accounting for common residual error across these cognitive tests. Standardized factor scores were output for each participant and used in all subsequent analyses.
A measure of Cognitive impairment was also used to conduct a sensitivity analysis excluding those with cognitive impairment based on scores from the Six Item Screener (SIS) (Callahan et al., 2002), which was not included in our composite measure of cognitive function but was administered at similar times to the other tests in our measure. The SIS is a test of global cognitive function derived from the widely used Mini-Mental State Exam, consisting of a 3-item word recall and 3-item temporal orientation (score range=0-6). In community samples, a score ≤4 indicates cognitive impairment (Callahan et al., 2002; Wilber et al., 2005); Steffens et al., 2006) with 74.2% sensitivity and 80.2% specificity for clinically confirmed cognitive impairment no dementia (CIND) and dementia.
Walking Behavior and physical activity were self-reported in the leave-behind questionnaire by a number of questions assessing walking behavior, moderate physical activity, and vigorous physical activity. Walking behavior was assessed with the question: “During the last 7 days, on how many days did you walk for at least 10 minutes at a time?”. For those participants indicating any walking behavior, a follow up question asked “How much time did you spend walking on one of those days” captured in hours and minutes. We created two measures: a binary indicator for any walking behavior vs. no walking in the past 7 days (0= 0 days, 1 = more than 0 days); and a measure of the duration of walking (minutes per day). Participants who did not spend any days walking were assigned a value of 0 minutes per day. Moderate physical activity was assessed by a similar set of questions: “During the last 7 days, on how many days did you do moderate physical activities like gardening, cleaning, bicycling at a regular pace, swimming, or other fitness activities. Think only about those physical activities that you did for at least 10 minutes at a time. Do not include walking.” This was followed by a question asking “How much time did you usually spend doing moderate physical activities on one of those days?” We created a binary indicator for any moderate physical activity (vs. none) and a measure of duration (minutes per day). Lastly, the same questions were asked about vigorous physical activity starting with “During the last 7 days, on how many days did you do vigorous physical activities like heavy lifting, heavier garden or construction work, chopping wood, aerobics, jogging/running, or fast bicycling? Think only about those physical activities that you did for at least 10 minutes at a time.” Again, we created a binary variable capturing any vigorous physical activity (vs. none) as well as duration (minutes/day).
Physical Health Status was assessed using the 12-item Short-Form Health Survey (SF-12) (Ware, 1995). We used the physical component summary (PCS) score that captures physical health-related domains including general health, physical functioning, physical limitations in social roles and activities, and body pain. Scores were standardized to have a population mean of 50 and standard deviation of 10, with higher scores indicating better self-reported physical health.
Subjective Perceptions of the Neighborhood Environment.
We used two indicators of the subjective neighborhood environment in the leave-behind questionnaire that capture perceptions of neighborhoods that may be related to walkability: problems with traffic and quality of parks. Participants were asked a series of questions surrounding perceptions of their neighborhood using the following prompt: “Now we would like to ask you some questions about what it is like to live in your neighborhood. By neighborhood we mean the area around where you live and around your house. It may include places you shop, religious or public institutions, or a local business district. It is the general area around your house or apartment where you might perform routine tasks, such as shopping, going to the park, or visiting with neighbors.” Problems with traffic was assessed with the question asking “Think about your neighborhood as a whole… how much of a problem is heavy traffic or speeding cars in your neighborhood?” Likewise, the same was asked regarding parks, “Think about your neighborhood as a whole… how much of a problem is a lack of parks or playgrounds in your neighborhood?” Reponses to both questions were reported on a four-point scale: 1=Very Serious Problem, 2=Somewhat Serious Problem, 3=Minor Problem, and 4=Not Really a Problem.
Objectively Measured Features of the Neighborhood Environment.
We used four observed indicators of the neighborhood environment that are relevant for both walkability and cognitive function (Aneshensel et al., 2011; Angevaren et al., 2010; Besser et al., 2017; Lang et al., 2008): street connectivity, the number of parks, rural/urban classification, and neighborhood socioeconomic disadvantage. Using Geographic Information Systems (GIS), REGARDS participants’ residential addresses were geocoded and assigned to a census tract, as a proxy for neighborhood. Census tracts have a population of about 4,000 people, and have been shown to roughly map to neighborhoods (Aneshensel et al., 2011). Street Connectivity (based on 2010 Census data) was captured using the Gamma index (Berrigan et al., 2010), which is a ratio of the total number of street lengths in the tract to the maximum possible number of streets in the tract (range 0-1). For analysis, the Gamma index was multiplied by 10 to reflect a unit change per 0.1, consistent with all other variables. Data on the number of open parks in each participant’s census tract neighborhood were obtained from the National Neighborhood Data Archive (Clarke et al., 2020), which is based on a national database of publicly owned local, state, and national parks derived from websites and satellite imagery (Trust for Public Land, 2018). For analysis, this variable was top-coded at 16 parks and log transformed to address positive skew. Rural Urban Commuting Area (RUCA) codes use data from the 2010 decennial census and the 2006-10 American Community Survey to classify census tracts as rural vs. urban based on geography and work commuting flows between places (Morrill et al., 1999). The use of work commuting data strongly differentiates rural areas according to their economic integration with urban areas and other rural areas (Hart et al., 2005). Based on previous work with the REGARDS data (Howard et al., 2017), we use RUCA categories to classify census tracts as urban (coded 1) or rural (including large rural, and small/isolated rural) (coded 0).
We used a measure of neighborhood socioeconomic disadvantage (Melendez et al., 2020) that is derived from a principal components analysis and is related to multiple health and aging outcomes (Clarke et al., 2014). The index is an average of four census indicators (2010 Census) at the census tract: percent of female headed families with children; percent of households with public assistance income or food stamps; percent of families with income below the federal poverty level; percent of population age 16+ unemployed). The index ranges from 0 to 100 with a higher score indicating higher neighborhood disadvantage.
Covariates.
We included key covariates that are related to both cognitive function and neighborhood context, including race (1=Black, 0=White), age (in years, and centered at the minimum age 55 for analyses), gender (1=Female, 0=Male), and highest level of education (1=<High School (HS), 2=HS Graduate, 3=Some College, 4=College Degree or higher). Data for all covariates came from the REGARDS baseline assessment.
Statistical Analysis
To account for the clustering of participants within census tracts we used multilevel linear regression to examine the relationships between neighborhood characteristics, physical activity, health status and cognitive function. In a set of nested multilevel models, we first regressed the cognition measure on objectively measured features of walkable environments, adjusting for potential confounders (sociodemographic variables), and then added subjective perceptions of the neighborhood environment, followed by physical activity and health status. Analyses were performed in Stata Version 17.1. Statistical significance was assessed with a two-tailed alpha of .05. Sobel test calculations were used to assess the significance of any mediating effect of physical activity, physical health status, and subjective perceptions of the neighborhood environment in the pathways between neighborhood walkability and cognitive function (MacKinnon, & Dwyer 1993; MacKinnon et. al., 1995). We also conducted a sensitivity analysis to determine whether cognitive impairment (as measured by the SIS) had any bearing on the participant’s reporting of neighborhood perceptions and physical activity by excluding the 454 participants who met the threshold for cognitive impairment.
Results
Descriptive statistics for the study sample are presented in Table 1. The mean cognitive factor score was 0.3 (range, −7.8 – 10.6) following a normal distribution. The average participant was age 73.4 years (SD=8.3). About 57% of the sample was female, and about 31% Black. More than half the sample had at least some college education. The majority of respondents lived in urban areas, with almost 20% residing in a rural area. The average park count per neighborhood was almost 1, with most participants having between 0 and 16 parks within their census tract. Regarding subjective perceptions of features related to neighborhood walkability, the majority of respondents generally reported heavy traffic and a lack of adequate parks as “Not really a problem.” Almost 13% of participants, however, reported traffic as a “Somewhat serious problem,” and about 13% reported lack of parks as a “Somewhat serious problem” or worse.
Table 1.
Sample Descriptive Characteristics: REgards, 2013-2016 (n=10,289)
| Measure | Mean or % (N) | Std. deviation | Range |
|---|---|---|---|
| Cognitive Factor Score | 0.3 (10,289) | 2.4 | −7.8 – 10.6 |
| Any Walking: | |||
| Yes | 80% (8,194) | ||
| No | 20% (2,095) | -- | -- |
| Walking (mins/day) | 88.7 (10,118) | 99.8 | 0-480 |
| Any Moderate PA: | |||
| Yes | 77% (7,937) | -- | -- |
| No | 23% (2,352) | -- | -- |
| Moderate PA (mins/day) | 88.7 (10,065) | 88.4 | 0-360 |
| Any Vigorous PA: | |||
| Yes | 34% (3,547) | -- | -- |
| No | 66% (6,742) | -- | -- |
| Vigorous PA (mins/day) | 27.3 (10,101) | 51.4 | 0-240 |
| Physical Health Status (SF-12) | 45.3 (9,678) | 10.7 | 10.6 – 65.4 |
| Age (years) | 73.4 (10,289) | 8.3 | 55 - 101 |
| Race: | |||
| Black | 31% (3,231) | -- | -- |
| White | 69% (7,058) | -- | -- |
| Sex: | |||
| Female | 57% (5,846) | -- | -- |
| Male | 43% (4,443) | -- | -- |
| Education: | |||
| <HS | 7% (727) | -- | -- |
| HS | 24% (2,401) | -- | -- |
| Some college | 26% (2,706) | -- | -- |
| College + | 43% (4,454) | -- | -- |
| Objectively Measured Neighborhood Features | |||
| Socioeconomic Disadvantage (%) | 19.8 (10,274) | 13.1 | 0 – 73.5 |
| Urbanicity: | |||
| Urban | 82% (8,394) | -- | -- |
| Rural | 18% (1,895) | -- | -- |
| Count Open Parks (natural log) | 0.8 (7,015) | 0.7 | 0 – 2.8 |
| Street Connectivity (in 0.1 units) | 4.1 (10,289) | 0.5 | 3.2 – 6.7 |
| Subjectively Perceived Neighborhood Features | |||
| Heavy Traffic: | |||
| Very Serious Problem | 3% (292) | -- | -- |
| Somewhat Serious Problem | 10% (1,039) | -- | -- |
| Minor Problem | 32% (3,199) | -- | -- |
| Not Really a Problem | 55% (5,599) | -- | -- |
| Lack of Adequate Parks: | |||
| Very Serious Problem | 5% (462) | -- | -- |
| Somewhat Serious Problem | 7% (733) | -- | -- |
| Minor Problem | 20% (2,070) | -- | -- |
| Not Really a Problem | 68% (6,813) | -- | -- |
SF-12 = 12-item Short-Form Health Survey; HS=High School ; RUCA=Rural-Urban Commuting Areas
Results from the multilevel regression models are presented in Table 2. About 8% of the unadjusted variation in cognitive function exists between census tracts (intraclass correlation, ICC=0.082) (Model A). Model B adjusts for individual characteristics. For every one-year increase in age, there is a 0.11 decrease in cognitive score (p<0.001). Females have an average 0.37 higher cognitive score than men (p<0.001), while Black adults have a 1.11 lower cognitive score compared to White adults (p<0.001). Lastly, there was an education gradient, in that cognitive scores increased at each level higher of education (p<0.001).
Table 2.
Multilevel Linear Regression Models for Cognitive Function, Walkable Neighborhoods, Walking Behavior, and Physical Activity: REgards, 2013-2016
| Unconditional Model |
+ Individual factors |
+ Objective NB features |
+ Subjective NB features |
+ Physical Activity |
+ Physical Health Status |
|
|---|---|---|---|---|---|---|
| Model A | Model B | Model C | Model D | Model E | Model F | |
| Measure | ß-Coefficients | |||||
| Fixed effects | ||||||
| Intercept | 0.33*** | 3.16 *** | 2.59*** | 2.61*** | 2.00*** | 1.12*** |
| Individual factors (Level 1) | ||||||
| Age (centered at 55) | −0.11*** | −0.11*** | −0.11*** | −0.10*** | −0.10*** | |
| Female (ref=Male) | 0.37*** | 0.41*** | 0.43*** | 0.43*** | 0.46*** | |
| Black (ref=White) | −1.11*** | −1.01*** | −1.01*** | −1.02*** | −1.02*** | |
| Education: (ref=College+) | ||||||
| <HS | −2.20*** | −2.06*** | −2.02*** | −1.94*** | −1.86*** | |
| HS | −1.49*** | −1.38*** | −1.34*** | −1.28*** | −1.22*** | |
| Some college | −0.88*** | −0.79*** | −0.76*** | −0.70*** | −0.68*** | |
| Any Walking (ref=none) | 0.14* | 0.10 | ||||
| Walking (mins/day) | −0.0003 | −0.0005 | ||||
| Any Moderate PA (ref=none) | 0.48*** | 0.47*** | ||||
| Moderate PA (mins/day) | −0.0003 | −0.0003 | ||||
| Any Vigorous PA (ref=none) | 0.20* | 0.15 | ||||
| Vigorous PA (mins/day) | −0.002* | −0.002* | ||||
| Physical Health Status (SF-12) | 0.02*** | |||||
| Objectively Measured Neighborhood Features (Level 2) | ||||||
| Socioeconomic Disadvantage (%) | −0.01*** | −0.01*** | −0.01*** | −0.01** | ||
| Urban (ref=Rural) | 0.16* | 0.13 | 0.13 | 0.11 | ||
| Count of open parks (natural log) | 0.08* | 0.06 | 0.06 | 0.06 | ||
| Street connectivity (in 0.1 units) | 0.13* | 0.15** | 0.17** | 0.15* | ||
| Subjectively Perceived Neighborhood Features (Level 1) | ||||||
| Heavy Traffic: (ref=Not Really a Problem) | ||||||
| Very Serious Problem | −0.56*** | −0.54*** | −0.47** | |||
| Somewhat Serious Problem | −0.29** | −0.29** | −0.23** | |||
| Minor Problem | −0.12* | −0.13* | −0.11* | |||
| Lack of Parks: (ref=Not Really a Problem) | ||||||
| Very Serious Problem | −0.43** | −0.40** | −0.34* | |||
| Somewhat Serious Problem | −0.31** | −0.31** | −0.26* | |||
| Minor Problem | −0.11 | −0.11 | −0.09 | |||
| Variance Components | ||||||
| Intercept (tract-level) | 0.479 | 0.042 | 4.44e-12 | 1.07e-08 | 3.72e-12 | 4.54e-12 |
| ICC | 0.082 | 0.010 | 1.09e-12 | 2.65e-09 | 9.38e-13 | 1.17e-12 |
p<0.05 = *, p<0.01=**, p<0.001***
Note: ICC=Intraclass Correlation Coefficient; HS=High School; NB=Neighborhood; Table values are ß-Coefficients
Model C of Table 2 adds the objectively measured features of neighborhood walkability. For every tenth of a unit increase in street connectivity, cognitive scores were 0.13 units higher (p<0.05), net of covariates. Socioeconomic disadvantage was negatively associated with cognitive function; for every 10% increase in concentrated disadvantage, the cognitive score was 0.10 lower (p<0.001). Compared to participants who lived in rural areas, those in urban areas had a 0.16 higher cognitive score (p<0.05). For every unit increase in the natural log of parks, cognitive scores were 0.08 units higher (p<0.05).
The fourth column (Model D; Table 2), adds subjective perceptions of the neighborhood environment. Heavy traffic was negatively associated with cognitive function; participants perceiving traffic as a “Very Serious Problem,” “Somewhat Serious Problem,” or “Minor Problem” had cognitive scores that were 0.56 (p<0.001), 0.29 (p<0.01), and 0.12 (p<0.05) lower, respectively, than those who perceived traffic as “Not Really a Problem.” A lack of adequate parks was negatively associated with cognitive function; participants perceiving the lack of parks in their neighborhood as a “Very Serious Problem,” “Somewhat Serious Problem,” or “Minor Problem” had cognitive scores that were 0.43 (p<0.01), 0.31 (p<0.01), and 0.11 lower, respectively, than those who did not perceive a lack of parks as a problem. The association between urban/rural residence and cognition was attenuated, and resulted in a loss of statistical significance after the addition of subjective perceptions of neighborhood features to the models. Using a Sobel test for mediation, we found that both subjective perceptions of traffic and parks significantly mediated the relationship between objective street connectivity and cognitive function (p<0.001, Table 3).
Table 3.
Sobel Test for Mediation Significance: REgards, 2013-2016
| Walking Behavior |
Moderate Physical Activity |
Vigorous Physical Activity |
Physical Health Status |
Traffic | Parks | |
|---|---|---|---|---|---|---|
| Sobel Test Statistic (Street Connectivity) | −0.43 | −1.02 | 0.41 | 0.82 | −4.43*** | 3.31*** |
| (Subjective Traffic Perceptions) | −0.39 | −0.41 | −0.52 | 4.52*** | ||
| (Subjective Parks Perceptions) | 1.33 | 2.40* | 0.54 | 3.40*** |
p<0.05 = *, p<0.01=**, p<0.001***
The fifth column (Model E; Table 2) adds two measures each of walking behavior, moderate physical activity, and vigorous physical activity, first as binary (0= 0 days, 1 = more than 0 days) measures and then as minutes per day. Compared to participants who do not walk at all, those who do walk have a 0.14 higher cognitive score (p<0.05). Those who engage in moderate physical activity have a 0.48 higher cognitive score (p<0.001) compared to those who do not. Participants who engage in vigorous physical activity have a 0.20 higher cognitive score (p<0.05), compared to those who do not. Duration of walking and moderate physical activity was not significantly associated with cognitive function. There was however, a 0.002 (p<0.05) decrease in cognitive function for every one-minute increase in vigorous physical activity per day. The Sobel test found that physical activity, of any kind, did not mediate the relationship between the objective measure of street connectivity and cognitive function. Walking behavior and vigorous physical activity also did not mediate the relationship between subjective perceptions of traffic & parks and cognitive function, however, moderate physical activity did mediate the relationship between subjective perception of parks and cognitive function (p<0.05, Table 3).
The sixth column (Model F; Table 2) added both the physical activity measures and physical health status. For every unit increase in the SF-12 physical component summary score, there was a 0.02 (p<0.001) increase in cognitive function. When adjusting for physical health status, the associations between walking behavior & vigorous physical activity were no longer statistically significantly associated with cognitive function, while moderate physical activity and physical health status remained positively and significantly associated with cognitive function. The Sobel test indicated that physical health status did not mediate the relationship between the objective measure of street connectivity and cognitive function, but physical health status did mediate the relationship between subjective perceptions of both traffic and parks with cognitive function (p<0.001, Table 3).
Results from multilevel regression models for sensitivity analysis are presented as supplementary material at the end of the manuscript, Table 2b. After excluding about 4% of individuals from our analytic sample that met the threshold for cognitive impairment, the results were similar to the results in Table 2. The most noticeable differences were found among the coefficients for education (the category of less than high school education), which were attenuated in the sensitivity analysis. All other differences were very minor.
Discussion & Implications
This study used cross-sectional data from a large nation-wide study of aging Americans to examine the pathways in the relationship between the neighborhood environment and cognitive function, focusing specifically on walkable environments and walking behavior & physical activity as potentially modifiable risk factors for dementia (Livingston et al., 2020). Consistent with prior literature, we found positive associations between moderate physical activity and cognitive function (Carvalho et al., 2014; Chang et al., 2010; Sofi et al., 2010), net of individual sociodemographic characteristics that could influence both physical activity and cognition. However, we did not find that the relationships between walking behavior and vigorous physical activity and cognition persisted after adjusting for physical health status. This suggests that physical function may be a common cause of both walking behavior and cognitive function, rendering this association spurious. Alternatively, physical function may lie on the causal pathway between walking behavior and cognition, whereby greater walking behavior leads to increased functional health, which in turn promotes cognitive health. The use of cross-sectional data prevents a full understanding of these pathways but suggests complex relationships between neighborhood context, health behaviors, physical function and cognitive health.
Residence in urban (vs. rural) areas and neighborhoods with lower socioeconomic disadvantage were associated with higher cognitive function, consistent with prior work (Aneshensel et al., 2011; Clarke et al., 2012; Clarke et al., 2015; Finlay et al., 2020). We also found that walkable neighborhoods (with more connected streets) have a direct effect estimate on cognitive function, which likely operates through unmeasured variables, such as greater access to neighborhood resources (ie, doctors’ offices, pharmacies, healthy food stores) conducive to cognitive health that tend to exist in more walkable environments. Older adults’ perceptions of neighborhood walkability, including safety from traffic and adequate parks, were also associated with better cognitive function, highlighting the importance of considering both subjective perceptions and objective indicators of the neighborhood context, as other scholars have noted as well (Schipperijn et. al., 2017; Thornton et. al., 2017; Moran et. al., 2014; Chaudhury et. al., 2012). Subjective perceptions of traffic and parks in these walkable neighborhoods operated as an underlying suppression effect in the relationship between street connectivity and cognition, and after accounting for these perceptions, the effect estimate for street connectivity was somewhat larger.
Perceptions of neighborhoods are important to consider since perceived neighborhood walkability may be more important for older adults’ cognitive health and behaviors than objectively measured indicators of the built and social environment. Indeed, recent work suggests that subjective perceptions of neighborhood quality play a more important role in explaining cognitive health than objective indicators (Lee and Waite, 2018). Our work suggests that neighborhood perceptions account for some of the effects of objectively measured features. These results have implications for the design of age-friendly and health promoting communities, which may be easier to modify at the neighborhood level than individual perceptions or behavior.
While we did find moderate physical activity to be associated with cognition after accounting for physical health status, the Sobel test for mediation of moderate physical activity between cognition and objectively measured street connectivity did not reach statistical significance, preventing any conclusions about mediating mechanisms. We also found no evidence that the association between cognition and objectively measured street connectivity operates through physical health status. We did find, however, evidence that the effect estimate of objective street connectivity operates, in part, through subjective perceptions of traffic and subjective perceptions of parks, pointing to the importance of considering residents’ perceptions of their neighborhood resources for potential cognitive benefit.
Limitations
The strengths of this study include a large racially diverse cohort of older adults living across both rural and urban areas in the United States. The use of both objective and subjectively measured neighborhood built-environment data is also a strength, while we acknowledge that there is limited available data examining subjective perceptions. Because these data are cross-sectional, we cannot rule out reverse causality; cognitive function may impact perceptions of neighborhood resources, frequency of walking behavior, and physical activity. However, a sensitivity analysis, excluding the 454 participants with cognitive impairment, yielding similar results. Our sample was also limited to those who participated in the second wave of the REGARDS data collection and who completed the self-administered questionnaire. However, the withdrawal mechanism in REGARDS has been shown to be random rather than systematically related to social, behavioral, or clinical assessments, indicating little evidence of selection bias (Long et al., 2019). Although the cognitive function tests were administered by telephone, which could be a limitation to those with sensory impairments, word list, and verbal fluency have been shown to be measured reliably and precisely over the telephone in middle aged and older adults (Rapp et al., 2012; Wilson et al., 2010). Lastly, the physical activity measures were self-reported and may be prone to measurement error. It is possible that other measures of physical activity (eg, captured using accelerometer data) would more accurately capture the mediating pathway between street connectivity and cognitive function. Nonetheless, our results point to the importance of other aspects of walkable environments that may promote cognitive health among older adults aging in place.
Implications
This study highlights the importance of both perceived and objective features of neighborhood environments for the cognitive health of older adults aging in place. Findings may inform the development of walkable, age-friendly communities that may not only improve physical health, but cognitive health as well. While built environment features may promote cognitively-supportive health behaviors, such as physical activity, it is important to consider the quality of such resources and how they are perceived by older adults themselves. Addressing the neighborhood context, particularly with respect to walkable environments, may be one way to mitigate cognitive impairment and cognitive decline with age.
Supplementary Material
References
- Aneshensel CS, Ko MJ, Chodosh J, & Wight RG (2011). The urban neighborhood and cognitive functioning in late middle age. Journal of Health and Social Behavior, 52(2), 163–179. 10.1177/0022146510393974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevaren M, Vanhees L, Nooyens ACJ, Wendel-Vos CGW, & Verschuren WMM (2010). Physical activity and 5-year cognitive decline in the doetinchem cohort study. Annals of Epidemiology, 20(6), 473–479. 10.1016/j.annepidem.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Bassett DR, Fitzhugh EC, Crespo CJ, King GA, & McLaughlin JE (2002). Physical activity and ethnic differences in hypertension prevalence in the United States. Preventive Medicine, 34(2), 179–186. 10.1006/pmed.2001.0969 [DOI] [PubMed] [Google Scholar]
- Belza B, Walwick J, Shiu-Thornton S, Schwartz S, Taylor M, & LoGerfo J (2004). Older Adult Perspectives on Physical Activity and Exercise: Voices From Multiple Cultures. Preventing Chronic Disease - Pubic Health Research, Practice, and Policy, 1(4), 1–12. [PMC free article] [PubMed] [Google Scholar]
- Berrigan D, Pickle LW, & Dill J (2010). Associations between street connectivity and active transportation. International Journal of Health Geographics, 9(20). 10.1186/1476-072X-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser LM, McDonald NC, Song Y, Kukull WA, & Rodriguez DA (2017). Neighborhood Environment and Cognition in Older Adults: A Systematic Review. American Journal of Preventive Medicine, 53(2), 241–251. 10.1016/j.amepre.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A, & Stafford M (2007). How do objective and subjective assessments of neighbourhood influence social and physical functioning in older age? Findings from a British survey of ageing. Social Science and Medicine, 64(12), 2533–2549. 10.1016/j.socscimed.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, & Hendrie HC (2002). Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care, 40(9), 771–781. 10.1097/00005650-200209000-00007 [DOI] [PubMed] [Google Scholar]
- Carvalho A, Rea IM, Parimon T, & Cusack BJ (2014). Physical activity and cognitive function in individuals over 60 years of age: A systematic review. Clinical Interventions in Aging, 9, 661–682. 10.2147/CIA.S55520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, … Launer LJ (2010). The effect of midlife physical activity on cognitive function among older adults: AGES - Reykjavik study. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 65 A(12), 1369–1374. 10.1093/gerona/glq152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury H, Mahmood A, Michael YL, Campo M, & Hay K (2012). The influence of neighborhood residential density, physical and social environments on older adults’ physical activity: An exploratory study in two metropolitan areas. Journal of Aging Studies, 26(1), 35–43. 10.1016/j.jaging.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury H, Campo M, Michael Y, & Mahmood A (2016). Neighbourhood environment and physical activity in older adults. Social Science and Medicine, 149, 104–113. 10.1016/j.socscimed.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Ailshire JA, House JS, Morenoff JD, King K, Melendez R, & Langa KM (2012). Cognitive Function in the Community Setting: The Neighborhood as a Source of “Cognitive Reserve”? Journal of Epidemiology Community Health, 66(8), 730–736. 10.1136/jech.2010.128116.Cognitive [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, Morenoff J, Debbink M, Golberstein E, Elliott MR, & Lantz PM (2014). Cumulative Exposure to Neighborhood Context: Consequences for Health Transitions Over the Adult Life Course. Research on Aging, 36(1), 115–142. 10.1177/0164027512470702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Weuve J, Barnes L, Evans DA, & Mendes de Leon CF (2015). Cognitive decline and the neighborhood environment. Annals of Epidemiology, 25(11), 849–854. 10.1016/j.annepidem.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, Melendez R, and Chenoweth M (2020). National Neighborhood Data Archive (NaNDA): Parks by Census Tract, United States, 2018. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2020-February-27. 10.3886/E117921V1 [DOI] [Google Scholar]
- Finlay J, Esposito M, Tang S, Gomez-Lopez I, Sylvers D, Judd S, & Clarke P (2020). Fast-food for thought: Retail food environments as resources for cognitive health and wellbeing among aging Americans? Health and Place, 64(February), 102379. 10.1016/j.healthplace.2020.102379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KJ, Li F, Michael Y, & Cleveland M (2004). Neighborhood-Level Influences on Physical Activity among Older Adults: A Multilevel Analysis. Journal of Aging and Physical Activity, 12(1), 45–63. 10.1123/japa.12.1.45 [DOI] [PubMed] [Google Scholar]
- Gallagher NA, Clarke PJ, Ronis DL, Cherry CL, Nyquist L, & Gretebeck KA (2012). Influences on neighborhood walking in older adults. Research in Gerontological Nursing, 5(4), 238–250. 10.3928/19404921-20120906-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin SF, Wilson DK, Wilcox S, Buck J, & Ainsworth BE (2008). Physical Activity Influences in a Disadvantaged African American Community and the Communities’ Proposed Solutions. Health Promotion Practice, 9(2), 180–190. 10.1177/1524839906296011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, … Leblanc GG (2006). National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke, 37(9), 2220–2241. 10.1161/01.STR.0000237236.88823.47 [DOI] [PubMed] [Google Scholar]
- Handy SL, Boarnet MG, Ewing R, & Killingsworth RE (2002). How the built environment affects physical activity: Views from urban planning. American Journal of Preventive Medicine, 23(2 SUPPL. 1), 64–73. 10.1016/S0749-3797(02)00475-0 [DOI] [PubMed] [Google Scholar]
- Hart LG, Larson EH, & Lishner DM (2005). Rural definitions for health policy and research. American Journal of Public Health, 95(7), 1149–1155. 10.2105/AJPH.2004.042432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Winters M, Clarke PJ, Ste-Marie N, & McKay HA (2017). The influence of walkability on broader mobility for Canadian middle aged and older adults: An examination of Walk Score™ and the Mobility Over Varied Environments Scale (MOVES). Preventive Medicine, 95, S60–S67. 10.1016/j.ypmed.2016.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G, Kleindorfer DO, Cushman M, Long DL, Jasne A, Judd SE, … Howard VJ (2017). Contributors to the Excess Stroke Mortality in Rural Areas in the United States. Stroke, 48(7), 1773–1778. 10.1161/STROKEAHA.117.017089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley LV, Gomez CR, Go RC, Prineas RJ, … Howard G (2005). The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology, 25(3), 135–143. 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- Hughes JP, McDowell MA, & Brody DJ (2008). Leisure-Time Physical Activity Among US Adults 60 or More Years of Age: Results From NHANES 1999-2004. Journal of Physical Activity and Health, 5, 347–358. [DOI] [PubMed] [Google Scholar]
- Kim J, Liu J, Colabianchi N, & Pate RR (2010). The effect of perceived and structural neighborhood conditions on adolescents’ physical activity and sedentary behaviors. Archives of Pediatrics and Adolescent Medicine, 164(10), 935–942. 10.1001/archpediatrics.2010.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D (2008). Neighborhood and individual factors in activity in older adults: Results from the neighborhood and senior health study. Journal of Aging and Physical Activity, 16(2), 144–170. 10.1123/japa.16.2.144 [DOI] [PubMed] [Google Scholar]
- King KE, & Clarke PJ (2014). A disadvantaged advantage in walkability: Findings from socioeconomic and geographical analysis of national built environment data in the United States. American Journal of Epidemiology, 181(1), 17–25. 10.1093/aje/kwu310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, & Melzer D (2008). Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: Analyses from the english longitudinal study of ageing. Journal of the American Geriatrics Society, 56(2), 191–198. 10.1111/j.1532-5415.2007.01557.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Chernew ME, Kabeto MU, Herzog AR, Ofstedal MB, Willis RJ, … Fendrick AM (2001). National estimates of the quantity and cost of informal caregiving for the elderly with dementia. Journal of General Internal Medicine, 16(11), 770–778. 10.1046/j.1525-1497.2001.10123.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Harmer PA, Cardinal BJ, Bosworth M, Acock A, Johnson-Shelton D, & Moore JM (2008). Built Environment, Adiposity, and Physical Activity in Adults Aged 50-75. American Journal of Preventive Medicine, 35(1), 38–46. 10.1016/j.amepre.2008.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LD, George H, Long DM, Judd S, Manly JJ, McClure LA, … Glymour MM (2019). An investigation of selection bias in estimating racial disparity in stroke risk factors. American Journal of Epidemiology, 188(3), 587–597. 10.1093/aje/kwy253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, & Waite LJ (2018). Cognition in Context: The Role of Objective and Subjective Measures of Neighborhood and Household in Cognitive Functioning in Later Life. Gerontologist, 58(1), 159–169. 10.1093/geront/gnx050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie E, Cerin E, & Kremer P (2010). Perceived neighborhood environment and park use as mediators of the effect of area socio-economic status on walking behaviors. Journal of Physical Activity and Health, 7(6), 802–810. 10.1123/jpah.7.6.802 [DOI] [PubMed] [Google Scholar]
- Li F, Fisher KJ, Bauman A, Ory MG, Chodzko-zajko W, Harmer P, … Cleveland M (2005). Neighborhood Influences on Physical Activity in Middle-Aged and Older Adults: A Multilevel Perspective. Journal of Aging and Physical Activity, 13, 87–114. [DOI] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, … Mukadam N (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413–446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Ferman TJ, Willis FB, Petersen RC, & Graff-Radford NR (2005). Mayo’s older African Americans normative studies: Norms for Boston naming test, controlled oral word association, category fluency, animal naming, token test, WRAT-3 reading, trail making test, stroop test, and judgment of line orientation. Clinical Neuropsychologist, 19(2), 243–269. 10.1080/13854040590945337 [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, & Dwyer JH (1993). Estimating mediated effects in prevention studies. Evaluation Review, 17, 144–158. [Google Scholar]
- MacKinnon DP, Warsi G, & Dwyer JH (1995). A simulation study of mediated effect measures. Multivariate Behavioral Research, 30, 41–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez R, Clarke P, Khan A, Gomez-Lopez I, Li M, and Chenoweth M (2020). National Neighborhood Data Archive (NaNDA): Socioeconomic Status and Demographic Characteristics of Census Tracts, United States, 2008-2017. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2020-December-14. 10.3886/E119451V2 [DOI] [Google Scholar]
- Moran M, Van Cauwenberg J, Hercky-Linnewiel R, Cerin E, Deforche B, & Plaut P (2014). Understanding the relationships between the physical environment and physical activity in older adults: A systematic review of qualitative studies. International Journal of Behavioral Nutrition and Physical Activity, 11(1), 1–12. 10.1186/1479-5868-11-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill R, Cromartie J, & Hart G (1999). Metropolitan, urban, and rural commuting areas: Toward a better depiction of the United States settlement system. Urban Geography, 20(8), 727–748. 10.2747/0272-3638.20.8.727 [DOI] [Google Scholar]
- Morris JC, Mohs R, Rogers H, Fillenbaum G, & Heyman A (1987). Consortium to establish a registry for Alzheimer's disease (CERAD) clinical and neuropsychological assessment of Alzheimer's disease. Psychopharmacology bulletin, 24(4), 641–652. [PubMed] [Google Scholar]
- Orstad SL, McDonough MH, Stapleton S, Altincekic C, & Troped PJ (2017). A Systematic Review of Agreement Between Perceived and Objective Neighborhood Environment Measures and Associations With Physical Activity Outcomes. Environment and Behavior, 49(8), 904–932. 10.1177/0013916516670982 [DOI] [Google Scholar]
- Rapp SR, Legault C, Espeland MA, Resnick SM, Hogan PE, Coker LH, … Shumaker SA (2012). Validation of a cognitive assessment battery administered over the telephone. Journal of the American Geriatrics Society, 60(9), 1616–1623. 10.1111/j.1532-5415.2012.04111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CE, & Mirowsky J (2001). Neighborhood Disadvantage, Disorder, and Health. Journal of Health and Social Behavior, 42(3), 258–276. [PubMed] [Google Scholar]
- Saelens BE, Sallis JF, & Frank LD (2003). Environmental correlates of walking and cycling: Findings from the transportation, urban design, and planning literatures. Annals of Behavioral Medicine, 25(2), 80–91. 10.1207/S15324796ABM2502_03 [DOI] [PubMed] [Google Scholar]
- Schipperijn J, Cerin E, Adams MA, Reis R, Smith G, Cain K, … Sallis JF (2017). Access to parks and physical activity: An eight country comparison. Urban Forestry and Urban Greening, 27(April), 253–263. 10.1016/j.ufug.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian N, Spivey BN, Zaheed AB, & Zahodne LB (2020). Psychological distress links perceived neighborhood characteristics to longitudinal trajectories of cognitive health in older adulthood. Social Science and Medicine, 258. 10.1016/j.socscimed.2020.113125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, & Macchi C (2011). Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. Journal of Internal Medicine, 269(1), 107–117. 10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Steffens DC, Snowden M, Fan MY, Hendrie H, Katon WJ, & Unützer J (2006). Cognitive impairment and depression outcomes in the IMPACT study. American Journal of Geriatric Psychiatry, 14(5), 401–409. 10.1097/01.JGP.0000194646.65031.3f [DOI] [PubMed] [Google Scholar]
- Thornton CM, Kerr J, Conway TL, Saelens BE, Sallis JF, Ahn DK, … King AC (2017). Physical Activity in Older Adults: an Ecological Approach. Annals of Behavioral Medicine, 51(2), 159–169. 10.1007/s12160-016-9837-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne SD, Won J, Lee S, Ory MG, Forjuoh SN, Wang S, & Lee C (2016). Using Walk Score™ and Neighborhood Perceptions to Assess Walking Among Middle-Aged and Older Adults. Journal of Community Health, 41(5), 977–988. 10.1007/s10900-016-0180-z [DOI] [PubMed] [Google Scholar]
- Trust for Public Land (2018). Parkscore and Parkserve database: About, methodology, and FAQ. https://www.tpl.org/parkserve/about.
- Tucker JM, Welk GJ, & Beyler NK (2011). Physical activity in U.S. adults: Compliance with the physical activity guidelines for Americans. American Journal of Preventive Medicine, 40(4), 454–461. 10.1016/j.amepre.2010.12.016 [DOI] [PubMed] [Google Scholar]
- Ware JE (1995). The status of health assessment 1994. Annual Review of Public Health, 16(7), 327–354. 10.1146/annurev.pu.16.050195.001551 [DOI] [PubMed] [Google Scholar]
- Wen M, Hawkley LC, & Cacioppo JT (2006). Objective and perceived neighborhood environment, individual SES and psychosocial factors, and self-rated health: An analysis of older adults in Cook County, Illinois. Social Science and Medicine, 63(10), 2575–2590. 10.1016/j.socscimed.2006.06.025 [DOI] [PubMed] [Google Scholar]
- Wilber ST, Lofgren SD, Mager TG, Blanda M, & Gerson LW (2005). An evaluation of two screening tools for cognitive impairment in older emergency department patients. Academic Emergency Medicine, 12(7), 612–616. 10.1197/j.aem.2005.01.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.