Abstract
Food-producing animals, including dairy cattle, are potential reservoirs of antimicrobial resistance. However, there is limited data on antimicrobial use and the selection of resistant bacteria. Therefore, we investigated the association between antimicrobial use and resistance to mastitis pathogens using 2016 data from milk samples collected from cows with mastitis in 134 dairy farms in Chiba Prefecture, one of the principal dairy production prefectures in Japan. We recorded the antimicrobial use and isolation of methicillin-resistant staphylococci (MRS) and extended-spectrum beta-lactamase (ESBL)-producing coliforms (E. coli and Klebsiella spp.), and used the antimicrobial treatment incidence (ATI; the theoretical number of animals per 1000 animal-days subjected to antimicrobial treatment) to indicate antimicrobial use on each farm. The farms in which MRS or ESBL-producing coliforms were isolated from at least one mastitic milk sample were classified as antimicrobial resistance (AMR)-positive, and those in which neither MRS nor ESBL-producing coliforms were isolated were classified as AMR-negative. The AMR-positive farms showed a significantly higher ATI (median 45.17) than AMR-negative farms (median 38.40). The results indicate that high antimicrobial usage is associated with AMR in staphylococci and coliforms isolated from mastitic milk on dairy farms in Chiba Prefecture.
Keywords: Antimicrobial use, Antimicrobial resistance (AMR), Defined daily dose (DDD), Antimicrobial treatment incidence (ATI), Dairy cattle, Japan, Methicillin-resistant staphylococci (MRS), Extended-spectrum beta-lactamase (ESBL)-Producing coliforms, Mastitis
Antimicrobial use; Antimicrobial resistance (AMR); Defined daily dose (DDD); Antimicrobial treatment incidence (ATI); Dairy cattle; Japan; Methicillin-resistant staphylococci (MRS); Extended-spectrum beta-lactamase (ESBL)-producing coliforms; Mastitis.
1. Introduction
Antimicrobial resistance (AMR) is a global public health concern. Although its development and transmission is complex and not yet fully understood, antimicrobial use is a major driver of AMR (O'Neill, 2016; WHO, 2015). Hence, antimicrobial stewardship is a coordinated program used in human and veterinary medicine. A correlation between antimicrobial use and resistance in food-producing animals, including dairy cattle, has been reported (Oliver, 2011; Chantziaras, 2014). Bacteria are subjected to antimicrobial treatments on dairy farms, and the subsequent selection pressure may result in the selection and dissemination of resistant bacteria (Acar et al., 2006; Silbergeld et al., 2008). The loss of efficacy of antimicrobials due to the presence of resistant bacteria, as seen in human medicine, will also occur in veterinary medicine (European Medicine Agency, 2015). Therefore, it is important to verify if there is association between antimicrobial use and selection of resistant bacteria on dairy farms for both clinical and public health reasons.
Mastitis is one of the primary causes of antimicrobial use in dairy cattle (Mitchell et al., 1998). S. aureus and streptococci are frequently isolated from bovine intramammary infections (Olde Riekerink et al., 2008). Antimicrobial therapy is an approach commonly employed to reduce the incidence of mastitis in dairy farms (Erskine et al., 2003), and various antimicrobials have been approved for mastitis therapy in most countries (Ruegg, 2021). Relatively narrow-spectrum antimicrobials that target gram-positive organisms such as Streptococci and Staphylococci, are the most approved; however, in some countries, broad-spectrum antimicrobials such as 3rd and 4th-generation cephalosporins and some quinolones are approved (Ruegg, 2021). While no 3rd and 4th-generation cephalosporins are approved for mastitis therapy in Japan, β-lactams, aminoglycosides, 14- and 16-membered macrolides, and tetracyclines are approved for intramammary use, and quinolones are approved for parenteral use (Ministry of Agriculture, Forestry and Fisheries, 2014; Shinozuka et al., 2018). However, antimicrobial treatments resulting in failure of bacteriological cure are common in staphylococcal mastitis. AMR is considered as a reason for low cure rates. Resistance to various antimicrobials is commonly seen in bovine staphylococcal mastitis isolates (Barkema et al., 2006).
AMR has also been commonly observed in coliforms isolated from mastitic cows (Lehtolainen et al., 2003). Broad-spectrum antimicrobials, such as quinolones or 3rd or 4th-generation cephalosporins, are commonly used to treat coliform mastitis (including E. coli and Klebsiella spp). in some countries (Erskine et al., 2002; Suojala et al., 2013; Shinozuka et al., 2018). Resistance to 3rd generation cephalosporins or quinolones in E. coli in dairy farms has been reported (Duse et al., 2016; Boireau et al., 2018; Yu et al., 2020). Moreover, the prevalence of extended-spectrum beta-lactamase (ESBL)-producing coliforms in dairy farms has been studied in several countries (Dahmen et al., 2013; Ali et al., 2016; Taniguchi et al., 2021; Weber et al., 2021).
Herd-level association has been reported between antimicrobial use and AMR in bovine mastitis pathogens, such as Staphylococcus aureus (Saini et al., 2012), gram-negative bacteria (Saini et al., 2013), and non-aureus staphylococci (Nobrega et al., 2018). However, there is little information on the association between antimicrobial use and AMR in mastitis pathogens in Japan. This study evaluated the herd-level association between antimicrobial use and methicillin-resistant staphylococci (MRS) and ESBL-producing coliforms isolated from mastitic milk in dairy farms using data from 134 dairy farms in Chiba Prefecture, Japan.
2. Materials and methods
2.1. Dairy farms subjected to the analysis in this study
As of February 2016, 720 dairy farms housing 25,100 cows operated in Chiba Prefecture (one of the 47 prefectures in Japan) (Ministry of Agriculture, Forestry and Fisheries, 2017). Chiba Prefectural Agricultural Mutual Aid Association (NOSAI Chiba) has eight veterinary clinics in five districts of Chiba Prefecture (Figure 1). NOSAI Chiba had contracts with 596 of the 720 dairy farms in the prefecture at the end of 2016, providing them with veterinary services, including mastitis treatment. Of these 596 farms, 442 farms maintained data on antimicrobial use and the number of cows in 2016. Of these 442 farms, 134 farms that were subjected to mastitic milk bacterial isolation and drug resistance identification in 2016 were analyzed in this study. NOSAI is a nationwide agricultural insurance scheme that provides contracted dairy and beef cattle, horse, and breeding pig farms with veterinary services and life insurance for dead and culled animals. This study was approved by the Animal Research Ethics Committee of the NOSAI Chiba (approval number CNS140102).
Figure 1.
Location of Chiba Prefecture and its five jurisdictional districts Black open circles indicate the location of NOSAI Chiba Veterinary Clinics. Number of farms analyzed in each district is shown in parentheses.
2.2. Creation of a database
We constructed a database by entering for each farm data on antimicrobial use, isolation of resistant bacteria and herd size: data on antimicrobial use included the weight of active ingredient (in mg) aggregated from the treatment records for twenty-three microbial agents (grouped into nine classes) and administration routes (injection, intramammary, oral, or intrauterine) (Table 1). The intramammary products for dry-cow therapy (DCT) were entered separately from those with the same constituents for lactating cows. We calculated the data on hard-size (average number of cows kept on the farm) by summing the number of cows over two years of age present on the farm at the beginning of every 21-day period (i.e., the average length of the bovine estrous cycle) of the year and dividing the sum by 18 (the number of cycles in a year).
Table 1.
Antimicrobial use in 134 farms by antimicrobial agent and administration route (2016).
| Antimicrobial class | Antimicrobial agent | Administration route | Number of antimicrobial treatment incidence (ATI) (proportion in percentage) |
|||||
|---|---|---|---|---|---|---|---|---|
| All farms (n = 134) | AMR (+) farms (n = 47) | AMR (-) farms (n = 87) | ||||||
| Ttracycline | Oxytetracycline | intramammary | 0.6 | (1.4%) | 1 | (2.3%) | 0.3 | (0.9%) |
| injection | 0.3 | (0.7%) | 0.4 | (0.9%) | 0.2 | (0.6%) | ||
| Chlortetracycline | intrauterine | 0.05 | (0.1%) | 0.05 | (0.1%) | 0.05 | (0.1%) | |
| Amphenicols | Florfenicol | injection | 0.02 | (0.04%) | 0.02 | (0.03%) | 0.02 | (0.04%) |
| Penicillins | Amoxicillin | injection | 0.0005 | (0.001%) | 0.0001 | (0.0002%) | 0.0007 | (0.002%) |
| Ampicillin | injection | 3.2 | (7.7%) | 3.7 | (8.1%) | 3 | (7.5%) | |
| intrauterine | 0.2 | (0.5%) | 0.2 | (0.5%) | 0.2 | (0.5%) | ||
| peroral | 0.1 | (0.3%) | 0.1 | (0.3%) | 0.1 | (0.3%) | ||
| Dicloxacillin | intramammary | 0.2 | (0.4%) | 0.2 | (0.4%) | 0.2 | (0.4%) | |
| Mecillinam | injection | 0.07 | (0.2%) | 0.06 | (0.1%) | 0.08 | (0.2%) | |
| Penicillin | intramammary | 12 | (27.6%) | 12 | (26.5%) | 11 | (28.3%) | |
| injection | 0.7 | (1.8%) | 0.7 | (1.5%) | 0.8 | (1.9%) | ||
| Sulfonamides | Sulfadimethoxine | injection | 0.03 | (0.08%) | 0.05 | (0.1%) | 0.02 | (0.06%) |
| Sulfamonomethoxine | peroral | 0.006 | (0.01%) | 0.01 | (0.03%) | 0.002 | (0.006%) | |
| Macrolide | Erythromycin | intramammary | 0.02 | (0.06%) | 0.03 | (0.07%) | 0.02 | (0.05%) |
| Tilmicosin | peroral | 0.1 | (0.3%) | 0.1 | (0.2%) | 0.1 | (0.3%) | |
| injection | 0.007 | (0.02%) | 0.007 | (0.01%) | 0.007 | (0.02%) | ||
| Tylosin | injection | 0.2 | (0.5%) | 0.3 | (0.6%) | 0.1 | (0.4%) | |
| Aminoglycoside | Dihydrostreptomycin | intramammary | 11 | (27.1%) | 12 | (25.9%) | 11 | (27.8%) |
| injection | 0.1 | (0.3%) | 0.1 | (0.3%) | 0.1 | (0.3%) | ||
| Kanamycin | intramammary | 0.2 | (0.4%) | 0.2 | (0.5%) | 0.1 | (0.3%) | |
| injection | 0.02 | (0.05%) | 0.03 | (0.06%) | 0.02 | (0.04%) | ||
| Cephalosporin | Cefapirin | intramammary | 0.005 | (0.01%) | 0.01 | (0.03%) | 0 | (0.0%) |
| Cefazolin | intramammary | 10 | (24%) | 11 | (23.8%) | 9.6 | (24.2%) | |
| Cefazolin | injection | 1.4 | (3.2%) | 1.6 | (3.4%) | 1.2 | (3.1%) | |
| Cefuroxime | intramammary | 1.2 | (2.8%) | 1.7 | (3.8%) | 0.9 | (2.2%) | |
| Trimetoprim | Ormetoprim | peroral | 0.006 | (0.02%) | 0.01 | (0.03%) | 0.003 | (0.007%) |
| Quinolones | Danofloxacin | injection | 0.001 | (0.003%) | 0.004 | (0.008%) | 0 | (0.0%) |
| Enrofloxacin | injection | 0.1 | (0.3%) | 0.2 | (0.4%) | 0.1 | (0.3%) | |
| Orbifloxacin | injection | 0.07 | (0.2%) | 0.08 | (0.2%) | 0.07 | (0.2%) | |
| Used for DCT | 18.44 | (43.8%) | 19.41 | (42.1%) | 17.92 | (44.9%) | ||
| Total | 42.06 | (100.0%) | 46.06 | (100.0%) | 39.90 | (100.0%) | ||
2.3. Quantification of antimicrobial use on dairy farms
Antimicrobial treatment incidence (ATI) was used to measure the antimicrobial use in each dairy farm. The ATI presents the theoretical number of animals per 1000 animal-days subjected to antimicrobial treatment, assuming that the antimicrobial products are used in a cow of standard weight according to the dosage specified in the SPC (summary of the product characteristics). We first calculated the number of defined daily doses (DDDs) of the antimicrobial agent a (weight of biomass subjected to treatment with an antimicrobial agent a in kg-days) by dividing the weight of the active ingredient by the DDD of the antimicrobial agent. The DDDs used are available from the Japanese DDD values of antimicrobial agents (DDDjp) (Fujimoto et al., 2021). The ATI for antimicrobial agent α (ATIα) was calculated by dividing the number of DDDs by the average number of cows on the farm and the standard weight of dairy cows (635 kg), as in Eq. (1).
| (1) |
The overall antimicrobial use on each farm was then calculated by summing the ATI for the antimicrobial agents used on that farm, as in Eq. (2).
| (2) |
2.4. Isolation of MRS and ESBL-producing coliforms
Mastitis cows were detected by farmers during milking by visual observation of abnormal milk (flakes, fibrin clots, or abnormal milk color) and/or inflammatory changes in the udder (swelling, hardness, heat, pain, or redness). Before treatment, milk samples were collected in sterilized tubes from the affected quarter(s) by trained farmers or veterinarians. The samples were stored at 4 °C or frozen at −20 °C and immediately subjected to a bacterial isolation test. Bacteriological examinations were outsourced to the Sanritsu Zelkova Veterinary Laboratory (Kanagawa, Japan). After one predominant bacterium was isolated from the milk sample, identification, and antimicrobial susceptibility testing of staphylococci and coliforms (E. coli and Klebsiella. spp) were conducted using a MicroScan WalkAway Plus System (Beckman Coulter, USA), an automated identification system for most gram-positive cocci and gram-negative rods. The broth microdilution method was applied for susceptibility testing according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2012). The identification of multidrug-resistant bacteria was based on the CLSI guidelines and criteria. Staphylococcus isolates were identified as MRS or MRSA (methicillin-resistant Staphylococcus aureus) using the detection of oxacillin and cefoxitin resistance with minimum inhibitory concentration (MIC) interpretive criteria ≥4 μg/mL for oxacillin and ≥8 μg/mL for cefoxitin in S. aureus and S. lugdunensis, and ≥0.5 μg/mL for oxacillin in coagulase-negative staphylococci (CNS), except S. lugdunensis. For cases that were difficult to detect using oxacillin resistance only (MICs 0.5–2.0 μg/mL), mecA gene detection was conducted using PCR assay. Based on the National Institute of Infectious Diseases guidelines (NIID, 2019), mA1 (5'-TGCTATCCACCCTCAAACAGG-3’) and mA2 (5’AACGTTGTAACCACCCCAAGA-3’) primers were used for mecA PCR with initial denaturation conditions of 94 °C for 1 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 50 °C for 30 s, and elongation at 72 °C for 2 min. Coliform (E. coli and Klebsiella spp.) isolates were identified as ESBL-producing using the broth microdilution method, followed by testing the hydrolysis of chromogenic oxyimino-cephalosporin HMRZ-86 using the Cica Beta Test (Kanto Chemical, Tokyo, Japan).
2.5. Statistical analysis
Farms in which either MRS or ESBL-producing coliforms were isolated from at least one mastitic milk sample were classified as AMR-positive, and those with neither MRS nor ESBL-producing coliforms were classified as AMR-negative. The Wilcoxon rank-sum test was used to compare the average herd size and ATI between AMR-positive and AMR-negative farms. Statistical analysis was conducted using R Statistical Software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results and discussion
3.1. Antimicrobial use
The average antimicrobial use on 134 dairy farms, measured using ATI, was 42.06 (Table 1), of which, approximately 84% of antimicrobials were intramammary-administered to dry cows (44%) and lactating cows (40%). This was followed by injection (14%) and oral administration (2%). The most frequently used antimicrobial was penicillin for intramammary use with the ATI of 12, followed by dihydrostreptomycin for intramammary use (ATI of 11), cefazolin for intramammary use (ATI of 10), ampicillin for injection (ATI of 3.2), cefazolin for injection (ATI of 1.4), cefuroxime for intramammary use (ATI of 1.2), penicillin for injection (ATI of 0.7), oxytetracycline for intramammary use (ATI of 0.6) and oxytetracycline for injection (ATI of 0.3), ampicillin for intrauterine use (ATI of 0.2).
Antimicrobial use on dairy farms has been previously assessed using ATI in the USA and Belgium (de Campos et al., 2021; Stevens et al., 2016). Our results revealed that the dairy farms in Chiba Prefecture analyzed in this study used twice as much antimicrobials as those in Wisconsin, USA and Flanders, Belgium, where the overall ATI antimicrobial use was 17.2 in 2017 and 20.8 in 2012–2013, respectively (de Campos et al., 2021; Stevens et al., 2016).
3.2. Antimicrobial resistance
To our knowledge, this study is the first to reveal an association between antimicrobial use assessed using ATI and AMR in pathogens isolated from mastitic milk in Japan. Of the 134 farms studied, MRS was isolated from 40, and ESBL-producing coliforms were isolated from 7 farms. Neither MRS nor ESBL-producing coliforms were isolated from 87 of the 134 farms. In terms of herd size (the number of cows on the farm), there was no significant difference between AMR-positive and AMR-negative farms, with the mean number of cows 38.2 and 36.2, respectively.
Of the 210 CNS isolates, 83 (39.5%) were methicillin-resistant (Table 2). The proportion of MRS in bovine mastitic milk was higher than previously reported in other countries (Fessler et al., 2010; Gindonis et al., 2013; Kim et al., 2019; Sampimon et al., 2011; Sawamt et al., 2009). Although MRS is a suspected reservoir of the mecA gene in staphylococci (Hanssen et al., 2007), whether there was a clonal expansion of MRS is unclear because the genetic diversity of the mecA gene was not analyzed in this study. Further studies involving genetic analysis of MRS are needed to reveal the distribution of methicillin-resistant genes in milk from cows with mastitis in dairy farms in Chiba Prefecture.
Table 2.
Number of mastitic milk samples from which Staphylococci, Escherichia coli, or Klebsiella spp. were isolated from cows with clinical mastitis (n = 1010) in Chiba Prefecture, Japan, dairy farms.
| Bacterial species | Number of isolates | Number of multidrug-resistant isolates |
|---|---|---|
| Coagulase-negative staphylococci (CNS) | 210 | 83∗ |
| Staphylococcus aureus | 110 | 0∗ |
| Escherichia coli (E. coli) | 65 | 6∗∗ |
| Klebsiella spp. | 13 | 2∗∗ |
Number of methicillin-resistant isolates.
Number of extended-spectrum beta-lactamase (ESBL)-producing isolates.
The proportions of ESBL-producing coliforms of the coliform isolates (6 of 65 E coli and 2 of 15 Klebsiella spp) (Table 2). were considerably higher than previously reported in Japan and elsewhere. Ohnishi et al. (2013) reported that 65 CTX-M-2/15/14 ESBL-producing Enterobacteriaceae were isolated from 258,888 mastitic milk samples from Japanese dairy farms between 2007 and 2011. This proportion was lower (0.4%) in France (Dahmen et al., 2013). Locatelli et al. (2010) reported that only one out of 140 Klebsiella isolates from milk samples between 2008 and 2009 contained CTX-M1 ESBL-producing bacteria. Freitag et al. (2017) reported that 12/878 (1.4%) of unrelated E. coli from mastitis cases in Germany were ESBL-producing, implying that the frequency of ESBL-producing coliforms is higher in dairy farms in Chiba Prefecture than in other prefectures in Japan and other countries.
3.3. Association between antimicrobial use and resistance
Table 3 shows the ATIs of AMR-positive and AMR-negative farms and the Wilcoxon rank-sum test results. The overall ATI of AMR-positive farms was significantly higher than that of AMR-negative farms, with median ATI values of 45.17 and 38.40, respectively (P = 0.023). The ATIs of antimicrobials intramammary-administered for lactating cows and those administered by injection in AMR-positive farms were also significantly higher than those in AMR-negative farms, with median values of 15.29 vs. 11.37 (P = 0.045) and 6.67 vs. 4.79 (P = 0.021) respectively. This suggests that under field conditions the antimicrobial use is positively associated with AMR in bovine mastitis pathogens.
Table 3.
Antimicrobial treatment incidence (ATI) medians in antimicrobial resistance (AMR)-positive and AMR-negative farms.
| Number of antimicrobial treatment incidence (ATI) |
||||
|---|---|---|---|---|
| AMR-positive farms (n = 47) | AMR-negative farms (n = 87) | p-value | ||
| Administration route | Intramammary (for dry cow therapy) | 20.09 | 19.17 | 0.22 |
| Intramammary (for lactating cow) | 15.29a | 11.37b | 0.045 | |
| Injection | 6.67a | 4.79b | 0.021 | |
| Oral | 0.02 | 0.00 | 0.23 | |
| Intrauterine | 0.06 | 0.00 | 0.29 | |
| Antimicrobial class | Tetracyclines | 0.93a | 0.34b | 0.00016 |
| Amphenicol | 0 | 0 | 0.27 | |
| Penicillins | 17.30a | 14.62b | 0.04 | |
| Sulfonamides | 0.01a | 0b | 0.018 | |
| Macrolides | 0.04 | 0 | 0.071 | |
| Aminoglycosides | 12.40 | 11.29 | 0.19 | |
| Cephalosporins | 12.51 | 9.74 | 0.051 | |
| Trimetoprim | 0 | 0 | 0.33 | |
| Quinolones | 0.12 | 0.05 | 0.065 | |
| Total | 45.17a | 38.4b | 0.023 | |
AMR-positive farms: farms in which methicillin-resistant staphylococci (MRS) or extended-spectrum β-lactamase (ESBL)-producing coliforms (E. coli and Klebsiella spp.) were detected in at least one mastitic milk sample.
AMR-negative farms: farms in which neither MRS nor ESBL-producing coliforms were detected in the mastitic milk samples.
a - b: significant difference between AMR-positive and AMR-negative farms (p < 0.05, Wilcoxon rank-sum test).
There is an ongoing debate on whether antimicrobial use is a risk factor in selecting resistance to S. aureus in dairy farms (Schnitt and Tenhagen, 2020). A Canadian study revealed a positive correlation between intramammary and systemically administered penicillin treatments and emergence of resistant S. aureus in dairy farms (Saini et al., 2012). In contrast, Oliver et al. (2011) concluded no association between antimicrobial use in adult dairy cows and resistant veterinary and human pathogens. studies in Germany and the UK have suggested an association between the use of 3rd or 4th generation cephalosporins and the presence of ESBL-producing E. coli (Gonggrijp et al., 2016; Snow et al., 2012), whereas a study in the Netherlands concluded no association between antimicrobial use and ESBL-producing E. coli (Santman-Berends et al., 2017).
Our results did not reveal a significant correlation between the presence of resistant bacteria (MRS or ESBL-producing coliforms) and the use of intramammary antimicrobial used for DCT, whereas a previous Canadian study reported that the herd-level use of intramammary-administered penicillin for DCT (penicillin-novobiocin combination) was positively associated with AMR in S. aureus isolated from bovine mastitic milk using a multivariable logistic regression model (Saini et al., 2012), possibly because most dairy farms in Chiba Prefecture practice blanket dry-cow therapy (BDCT) for most cows regardless of their antimicrobial resistance status. Forty-five of 47 AMR-positive farms and 82 of 87 AMR-negative farms practiced BDCT.
Use of antimicrobials administrated intrammamarily for lactating cows reflects the frequency of treatment for clinical mastitis. Our results indicated a higher incidence of antimicrobial treatment for clinical mastitis in AMR-positive farms than in AMR-negative. This was consistent with the results of previous studies conducted in the USA and Canada (Nobrega et al., 2018; Sani et al., 2012). Nobrega et al. (2018) reported that AMR in non-Aureus staphylococci isolated from milk was associated with systemic but not intramammary administration of antimicrobials. Saini et al. (2012) reported a positive correlation between intramammary and systemically administered penicillin and AMR in mastitis S. aureus isolates.
Our results also revealed that injection-administered antimicrobials could affect AMR in udder pathogens (Table 3). Administered antimicrobials must reach the site of infection in an effective concentration in order form them to be effective. However, attaining and maintaining therapeutic concentrations of most antimicrobials in udder tissue or milk following systemic administration is difficult (Pyörälä, 2009). Furthermore, although the bovine udders have a rich blood supply, the rate of translocation of antimicrobials into milk following parenteral administration is affected by the lipid solubility and plasma-protein binding (Baggot, 2006). It is only the lipid-soluble, non-ionized, and plasma protein-unbound fraction of antimicrobials that can cross the blood-milk barrier to enter milk and disperse into the transcellular fluid. Penicillins do not easily cross biological membranes, as they are predominantly ionized in the plasma and less lipid-soluble. Thus, the therapeutic concentration of penicillin achieved in the udder following systemic administration might lead to the selection of staphylococci that are penicillin- and ampicillin-resistant.
Among the antimicrobial classes, the penicillins, tetracyclines, and sulfonamides were used more frequently in AMR-positive than in AMR-negative farms, with median ATIs of 17.30 vs. 14.62 (P = 0.04), 0.93 vs. 0.34 (P = 0.00016), and 0.01 vs. 0.00 (P = 0.018), respectively.
Penicillins include penicillin, the most used antimicrobials administrated intrammamarily and ampicillin, the most used antimicrobials administrated by injection and intrauterine route. Overuse of penicillins might increase the selective pressure for resistant bacteria, possibly resulting in the occurrence of MRS.
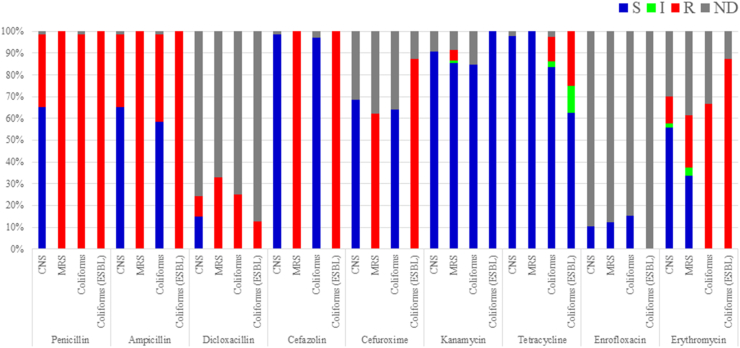
The relationship between tetracycline overuse and emergence of MRSA in pig farms has been reported by Larsen et al. (2016) and van Duijkeren et al. (2008). That is considered to be caused by co-selection by which bacteria acquire tetracycline resistant gene such as tet(K) and methicillin resistant genes simultaneously. However, this might be not the case with the results in this study because the sensitivity tests on bacteria isolated from mastitic milk in this study revealed that none of the 83 MRS and only 2 of the 8 ESBL-producing coliforms (Escherichia coli or Klebsiella spp.) exhibited tetracycline resistance (Figure 2). However, the actual prevalence of AMR in the farms was unclear in this study because the data available was limited to a part of bacteria isolated from mastitic milk. Further studies including genetic analyses are needed.
Figure 2.
Distribution of antimicrobial sensitivity test results for coagulase-negative staphylococci (CNS), methicillin-resistant CNS (MRS), coliforms and extended-spectrum beta-lactamase (ESBL)-producing coliforms (coliforms (ESBL)) isolated from mastitic milk samples. Broth microdilution method was used to test bacteria for antimicrobial sensitivity. Sensitivity test results categorized as sensitive (blue), intermediate (green), resistant (red), and no data (gray).
A limitation of this study is that we only investigated the association between antimicrobial use and MRS or ESBL-producing coliforms. Potential risk factors other than antimicrobial use that might affect the emergence and selection of AMR were not considered because data were not available. Although the herd size of each farm was a risk factor in this study (Schnitt and Tenhagen, 2020), it was not significantly different between AMR-positive and AMR-negative farms. Other potential risk factors include average herd parity, barn type, age, and body weight (Rajala-Schultz et al., 2004; Sol et al., 2000). Further studies are required to accurately determine the effects of antimicrobial use on AMR.
Declarations
Author contribution statement
Masato Kikuchi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Takuma Okabe, Hideshige Shimizu and Takashi Matsui: Performed the experiments.
Kyoko Fujimoto and Yuko Endo: Analyzed and interpreted the data.
Fuko Matsuda, Takeshi Haga and Katsuaki Sugiura: Conceived and designed the experiments; Wrote the paper.
Funding statement
Prof Katsuaki Sugiura was supported by Japan Racing Association [Project No. 3-Keichikushin-109].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the participating dairy farmers for their cooperation in this study.
References
- Acar J.F., Moulin G. Antimicrobial resistance at farm level. Rev. Sci. Tech. 2006;25:775–792. [PubMed] [Google Scholar]
- Ali T., Ur Rahman S., Zhang L., Shahid M., Zhang S., Liu G., Gao J., Han B. ESBL-producing Escherichia coli from cows suffering mastitis in China contain clinical class 1 integrons with CTX-M linked to ISCR1. Front. Microbiol. 2016;30(7):1931. doi: 10.3389/fmicb.2016.01931. PMID: 27965653; PMCID: PMC5127808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggot J.D. In: Antimicrobial Therapy in Veterinary Medicine. Giguère S., editor. Blackwell Publishing; Ames, IA: 2006. Principles of antimicrobial drug bioavailability and disposition; p. 65. [Google Scholar]
- Barkema H.W., Schukken Y.H., Zadok R.N. Invited review: the role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006;89:1877–1895. doi: 10.3168/jds.S0022-0302(06)72256-1. [DOI] [PubMed] [Google Scholar]
- Boireau C., Cazeau G., Jarrige N., Calavas D., Madec J.Y., Leblond A., Haenni M., Gay É. Antimicrobial resistance in bacteria isolated from mastitis in dairy cattle in France, 2006-2016. J. Dairy Sci. 2018;101(10):9451–9462. doi: 10.3168/jds.2018-14835. [DOI] [PubMed] [Google Scholar]
- Chantziaras I., Boyen F., Callens B., Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J. Antimicrob. Chemother. 2014;69(3):827–834. doi: 10.1093/jac/dkt443. [DOI] [PubMed] [Google Scholar]
- Clinical and laboratory standards institute (CLSI) Vol. 32. 2012. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Information Supplement. M100-S22. Wayne. [Google Scholar]
- Dahmen S., Métayer V., Gay E., Madec J.Y., Haenni M. Characterization of extended-spectrum beta-lactamase (ESBL)-carrying plasmids and clones of Enterobacteriaceae causing cattle mastitis in France. Vet. Microbiol. 2013;162:793–799. doi: 10.1016/j.vetmic.2012.10.015. [DOI] [PubMed] [Google Scholar]
- de Campos J.L., Kates A., Steinberger A., Sethi A., Suen G., Shutske J., Safdar N., Goldberg T., Ruegg P.L. Quantification of antimicrobial usage in adult cows and preweaned calves on 40 large Wisconsin dairy farms using dose-based and mass-based metrics. J. Dairy Sci. 2021;104(4):4727–4745. doi: 10.3168/jds.2020-19315. [DOI] [PubMed] [Google Scholar]
- Duse A., Persson Waller K., Emanuelson U., Ericsson Unnerstad H., Persson Y., Bengtsson B. Occurrence and spread of quinolone-resistant Escherichia coli on dairy farms. Appl. Environ. Microbiol. Jun13. 2016;82(13):3765–3773. doi: 10.1128/AEM.03061-15. PMID: 27084013; PMCID: PMC4907179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine R.J., Bartlett P.C., Van Lente J.L., Phipps C.R. Efficacy of systemic ceftiofur as a therapy for severe clinical mastitis in dairy cattle. J. Dairy Sci. 2002;85:2571–2575. doi: 10.3168/jds.S0022-0302(02)74340-3. [DOI] [PubMed] [Google Scholar]
- Erskine R.J., Wagner S., De Graves F.J. Mastitis therapy and pharmacology. Vet. Clin. North Am. Food Anim. Pract. 2003;19:109–138. doi: 10.1016/s0749-0720(02)00067-1. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency . 2015. Committee for Medicinal Products for Veterinary Use (CVMP) Reflection Paper on the Risk of Antimicrobial Resistance Transfer from Companion Animals.https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-risk-antimicrobial-resistance-transfer-companion-animals_en.pdf Available online: [Google Scholar]
- Fessler A.T., Billerbeck C., Kadlec K., Schwarz S. Identification and characterization of methicillin-resistant coagulase-negative staphylococci from bovine mastitis. J. Antimicrob. Chemother. 2010;65:1576–1582. doi: 10.1093/jac/dkq172. [DOI] [PubMed] [Google Scholar]
- Freitag C., Michael G.B., Kadlec K., Hassel M., Schwarz S. Detection of plasmid-borne extended-spectrum β-lactamase (ESBL) genes in Escherichia coli isolates from bovine mastitis. Vet. Microbial. 2017;200:151–156. doi: 10.1016/j.vetmic.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Kawasaki M., Abe R., Yokoyama T., Haga T., Sugiura K. Establishing defined daily doses (DDDs) for antimicrobial agents used in pigs, cattle and poultry in Japan and comparing them with European DDD values. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindonis V., Taponen S., Myllyniemi A., L. Pyörälä S., Nykäsenoja S., Salmenlinna S., Lindholm S., Rantala L.M. Occurrence and characterization of methicillin-resistant staphylococci from bovine mastitis milk samples in Finland. Acta Vet. Scand. 2013;55:1–8. doi: 10.1186/1751-0147-55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonggrijp M.A., Santman-Berends I.M.G.A., Heuvelink A.E., Buter G.J., van Schaik G., Hage J.J., Lam T.J.G.M. Prevalence and risk factors for extended-spectrum β-lactamase- and AmpC-producing Escherichia coli in dairy farms. J. Dairy Sci. 2016;99(11):9001–9013. doi: 10.3168/jds.2016-11134. [DOI] [PubMed] [Google Scholar]
- Hanssen A.M., Sollid J.U. Multiple staphylococcal cassette chromosomes and allelic variants of cassette chromosome recombinases in Staphylococcus aureus and coagulase-negative staphylococci from Norway. Antimicrob. Agents Chemother. 2007;51:1671–1677. doi: 10.1128/AAC.00978-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Moon D.C., Park S.C., Kang H.Y., Na S.H., Lim S.K. Antimicrobial resistance and genetic characterization of coagulase-negative staphylococci from bovine mastitis milk samples in Korea. J. Dairy Sci. 2019;102(12):11439–11448. doi: 10.3168/jds.2019-17028. [DOI] [PubMed] [Google Scholar]
- Larsen J., Clasen J., Hansen J.E., Paulander W., Petersen A., Larsen A.R., Frees D. Copresence of tet(K) and tet(M) in livestock-associated Methicillin-resistant Staphylococcus aureus clonal complex 398 is associated with increased fitness during exposure to sublethal concentrations of Tetracycline. Antimicrob. Agents Chemother. 2016;60(7):4401–4403. doi: 10.1128/AAC.00426-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtolainen T., Shwimmer A., Shpigel N.Y., Honkanen-Buzalski T., Pyörälä S. In vitro antimicrobial susceptibility of Escherichia coli isolates from clinical bovine mastitis in Finland & Israel. J. Dairy Sci. 2003;86:3927–3932. doi: 10.3168/jds.S0022-0302(03)74001-6. [DOI] [PubMed] [Google Scholar]
- Locatelli C., Scaccabarozzi L., Pisoni G., Moroni P. CTX-M1 ESBL-producing Klebsiella pneumoniae subsp. pneumoniae isolated from cases of bovine mastitis. J. Clin. Microbiol. 2010;48:3822–3823. doi: 10.1128/JCM.00941-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries (MAFF) 2014. Guidelines for Use of Veterinary Antimicrobial Agents for Animals in the Field of Livestock Production.https://www.maff.go.jp/j/keiei/nogyohoken/kokuzi_tsuchi/attach/pdf/index-102.pdf Retrieved from. [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries (MAFF) 2017. Livestock statistics 2016.https://www.maff.go.jp/j/tokei/kouhyou/tikusan/#r Retrieved from. [Google Scholar]
- Mitchell J.M., Griffiths M.W., McEwen S.A., McNab W.B., Yee A.J. Antimicrobial drug residues in milk and meat: causes, concerns, prevalence, regulations, tests, and test performance. J. Food Protect. 1998;61:742–756. doi: 10.4315/0362-028x-61.6.742. [DOI] [PubMed] [Google Scholar]
- National Institute of Infectious Diseases (NIID) 2019. Laboratory Manuals for Pathogen Detection.https://www.niid.go.jp/niid/images/lab-manual/ResistantBacteria20200604.pdf Retrieved from. [Google Scholar]
- Nobrega D.B., De Buck J., Barkema H.W. Antimicrobial resistance in non-aureus staphylococci isolated from milk is associated with systemic but not intramammary administration of antimicrobials in dairy cattle. J. Dairy Sci. 2018;101(8):7425–7436. doi: 10.3168/jds.2018-14540. [DOI] [PubMed] [Google Scholar]
- Ohnishi M., Okatani A.T., Harada K., Sawada T., Marumo K., Murakami M., Sato R., Esaki H., Shimura K., Kato H., Uchida N., Takahashi T. Genetic characteristics of CTX-M-type extended-spectrum-β-lactamase (ESBL)-producing enterobacteriaceae involved in mastitis cases on Japanese dairy farms, 2007 to 2011. J. Clin. Microbiol. 2013;51:3117–3122. doi: 10.1128/JCM.00920-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Riekerink R.G.,M., Barkema H.W., Kelton D.F., Scholl D.T. Incidence rate of clinical mastitis on Canadian dairy farms. J. Dairy Sci. 2008;91:1366–1377. doi: 10.3168/jds.2007-0757. [DOI] [PubMed] [Google Scholar]
- Oliver S.P., Murinda S.E., Jayarao B.M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: a comprehensive review. Foodb. Pathog. Dis. 2011;8(3):337–355. doi: 10.1089/fpd.2010.0730. [DOI] [PubMed] [Google Scholar]
- O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The Review on Antimicrobial Resistance. 2016 https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf Retrieved from. [Google Scholar]
- Pyörälä S. Treatment of mastitis during lactation. Ir. Vet. J. 2009;1(62):S40–44. doi: 10.1186/2046-0481-62-S4-S40. PMID: 22081939; PMCID: PMC3339349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala-Schultz P.J., Smith K., Hogan J.S.L., Love B.C. Antimicrobial susceptibility of mastitis pathogens from first lactation and older cows. Vet. Microbiol. 2004;102:33–42. doi: 10.1016/j.vetmic.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Ruegg P.L. What is success? A narrative review of Research evaluating outcomes of antibiotics used for treatment of clinical mastitis. Front. Vet. Sci. 2021;(8) doi: 10.3389/fvets.2021.639641. PMCID: PMC7884469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini V., McClure J.T., Scholl D.T., DeVries T.J., Barkema H.W. Herd-level association between antimicrobial use and antimicrobial resistance in bovine mastitis Staphylococcus aureus isolates on Canadian dairy farms. J. Dairy Sci. 2012;95(4):1921–1929. doi: 10.3168/jds.2011-5065. [DOI] [PubMed] [Google Scholar]
- Saini V., McClure J.T., Scholl D.T., DeVries T.J., Barkema H.W. Herd-level relationship between antimicrobial use and presence or absence of antimicrobial resistance in gram-negative bovine mastitis pathogens on Canadian dairy farms. J. Dairy Sci. 2013;96(8):4965–4976. doi: 10.3168/jds.2012-5713. [DOI] [PubMed] [Google Scholar]
- Sampimon O.C., Lam T.J., Mevius D.J., Schukken Y.H., Zadoks R.N. Antimicrobial susceptibility of coagulase-negative staphylococci isolated from bovine milk samples. Vet. Microbiol. 2011;150:173–179. doi: 10.1016/j.vetmic.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Santman-Berends I.M.G.A., Gonggrijp M.A., Hage J.J., Heuvelink A.E., Velthuis A., Lam T.J.G.M., van Schaik G. Prevalence and risk factors for extended-spectrum β-lactamase or AmpC-producing Escherichia coli in organic dairy herds in The Netherlands. J. Dairy Sci. 2017;100(1):562–571. doi: 10.3168/jds.2016-11839. [DOI] [PubMed] [Google Scholar]
- Sawant A.A., Gillespie B.E., Oliver S.P. Antimicrobial susceptibility of coagulase-negative Staphylococcus species isolated from bovine milk. Vet. Microbiol. 2009;134:73–81. doi: 10.1016/j.vetmic.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Schnitt A., Tenhagen B.A. Risk factors for the occurrence of methicillin-resistant Staphylococcus aureus in dairy herds: an update. Foodb. Pathog. Dis. 2020;17(10):585–596. doi: 10.1089/fpd.2019.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozuka Y., Kawai K., Takeda A., Yamada M., Kayasaki F., Kondo N., Sasaki Y., Kanai N., Mukai T., Sawaguchi N., Higuchi M., Kondo H., Sugimoto K., Yasuda A., Watanabe A. Randomized clinical trial to evaluate the effectiveness of enrofloxacin as a second-line antibiotic for treatment of acute Escherichia coli mastitis. Anim. Sci. J. 2018;89(7):1033–1039. doi: 10.1111/asj.13025. [DOI] [PubMed] [Google Scholar]
- Silbergeld E.K., Graham J., Price L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Publ. Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- Snow L.C., Warner R.G., Cheney T., Wearing H., Stokes M., Harris K., Teale C.J., Coldham N.G. Risk factors associated with extended spectrum beta-lactamase Escherichia coli (CTX-M) on dairy farms in North West England and North Wales. Prev. Vet. Med. 2012;106(3-4):225–234. doi: 10.1016/j.prevetmed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Sol J., Sampimon O.C., Barkema H.W., Schukken Y.H. Factors associated with cure after therapy of clinical mastitis caused by Staphylococcus aureus. J. Dairy Sci. 2000;83:278–284. doi: 10.3168/jds.S0022-0302(00)74875-2. [DOI] [PubMed] [Google Scholar]
- Stevens M., Piepers S., Supré K., Dewulf J., De Vliegher S. Quantification of antimicrobial consumption in adult cattle on dairy herds in Flanders, Belgium, and associations with udder health, milk quality, and production performance. J. Dairy Sci. 2016;99:2118–2130. doi: 10.3168/jds.2015-10199. [DOI] [PubMed] [Google Scholar]
- Suojala L., Kaartinen L., Pyörälä S. Treatment for bovine Escherichia coli mastitis - an evidence-based approach. J. Vet. Pharmacol. Therapeut. 2013;36(6):521–531. doi: 10.1111/jvp.12057. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Latt K.M., Tarigan E., Yano F., Sato H., Minamino T., Misawa N. A 1-year investigation of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolated from bovine mastitis at a large-scale dairy farm in Japan. Microb. Drug Resist. 2021;27(10):1450–1454. doi: 10.1089/mdr.2020.0481. [DOI] [PubMed] [Google Scholar]
- van Duijkeren E., Ikawaty R., Broekhuizen-Stins M.J., Jansen M.D., Spalburg E.C., de Neeling A.J., Allaart J.G., van Nes A., Wagenaar J.A., Fluit A.C. Transmission of methicillin-resistant Staphylococcus aureus strains between different kinds of pig farms. Vet. Microbiol. 2008;126(4):383–389. doi: 10.1016/j.vetmic.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Weber L.P., Dreyer S., Heppelmann M., Schaufler K., Homeier-Bachmann T., Bachmann L. Prevalence and risk factors for ESBL/AmpC-E. coli in pre-weaned dairy calves on dairy farms in Germany. Microorganisms. 2021;12; 9(10):2135. doi: 10.3390/microorganisms9102135. PMCID: PMC8539614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2015. Global Action Plan on Antimicrobial Resistance.http://www.who.int/iris/handle/10665/193736 Retrieved from. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.