Abstract
The formulation of niosomes is influenced by a number of variables, and these variables may eventually affect the formulation’s outcome. One of the elements that can influence the physico-chemical properties of niosomes is the method used in preparation of the formulation. In this study, we established if various methods of preparation have any impact on the prepared vesicles when loaded with 5-fluorouracil. Thereafter, a real-time cell assay (an in vitro cytotoxicity test) against HCT-116 colon cancer cell lines was done on an optimised batch. 5-fluorouracil loaded niosomes were prepared with either Tween 60 or Span 60 by four different methods - namely thin film hydration (TFH), reverse phase evaporation (RPE), evaporation/sonication (EVP/SON), and the ethanol injection method (EIM). In vitro evaluations were done on the formulations, and these included particle size analysis, entrapment efficiency, scanning electron microscopy (SEM), photomicrography, drug release, polydispersity index, and Fourier transform infrared spectroscopy (FTIR). The effects of the preparation method and type of non-ionic surfactants on encapsulation efficiency, particle size, and in vitro drug release of the niosomes at pH 7.4 were evaluated. An in vitro cytotoxicity test (real time cell assay (RTCA)) against HCT-116 cells was carried out using the optimised formulation. Results showed physically stable formulations. The TFH method produced the smallest particle sizes (187 nm and 482 nm), while the EVP/SON method produced the largest particle sizes (4476 nm and 9111 nm). The Tween-based niosomes prepared by TFH or RPE had higher drug entrapment. The FTIR studies of niosomal formulations showed broad peaks at wavenumbers above 3000 cm−1, indicating strong hydrogen bonds. The RTCA showed 5-fluorouracil-loaded niosomes caused more sustained cell death compared to the pure drug and blank niosomes. The methods of preparation affected the particle size, polydispersity index, entrapment efficiency, and the physical stability of the vesicles. The thin film hydration method was more robust in the entrapped 5-fluorouracil and showed lower particle sizes when compared to all the other methods. RTCA showed sustained cell death in real time.
Keywords: 5 Fluorouracil, Niosomes, Cholesterol, Tween 60, Span 60, RTCA
5 Fluorouracil; Niosomes; Cholesterol; Tween 60; Span 60; RTCA
1. Introduction
Novel drug delivery systems have the advantage of delivering drug molecules to sites of action thereby eliciting optimal response. They can act as drug reservoirs, modify drug release and help to minimize adverse effects of drugs by reducing off-target activity [1, 2, 3]. Oral route is the most preferred route for administration of therapeutic agents because of the ease of handling and patient compliance to treatment. Among the existing novel carriers used to deliver drugs orally, niosomes have gained great importance, as they entrap both hydrophilic and hydrophobic drugs 4, 5.
Niosomes, referred to as non-ionic surfactant vesicles is often used as a suitable drug delivery alternative to liposomes. They are known to alleviate the disadvantages associated with liposomes such as instability of the phospholipids and high cost. However the advantages include biodegradability, biocompatibility, non-immunogenicity, long shelf life, controlled drug release, improved stability and low production cost [9, 10]. Niosomes have found extensive usage in the pharmaceutical and food industries, despite their initial investigation as a carrier for cosmetic goods [6, 7, 8]. There are many ways to administer niosomes, including orally, transdermally, sublingually, nasally, and ocularly. The basic components of these vesicles include non-ionic surfactants and cholesterol with or without stabilizers. In niosomes, non-ionic surfactants are referred to as the vesicle formers, whereas phospholipids are the vesicle formers in liposomes. The HLB and type of surfactant used in niosome preparation influence their physicochemical properties [11, 12, 13]. Drugs could be loaded either passively or actively into niosomes. Thin film hydration method, ether injection method, reverse phase evaporation are the most commonly employed passive loading methods. Trans membrane pH gradient method is an active loading technique [14].
5-Fluorouracil (5-FU), the drug of study belongs to the pyrimidine group and it is the backbone chemotherapeutic drug often used in the treatment of colorectal cancer. This drug is also used in the treatment of other malignancies such as breast, head and neck cancers. 5-fluorouracil exerts its mechanism of action by interfering with nucleic acid, inhibiting DNA synthesis and eventually stopping cell growth or proliferation. This chemotherapeutic agent has a very short half-life, therefore it is administered as continuous bolus injection and predisposes to serious adverse effects which are gastrointestinal and cardiac based. In the gastrointestinal tract, 5-FU is known to be degraded by enzymes making oral administration erratic and unreliable [15, 16, 17].
Previous studies have concentrated on the thin film hydration method for passively loading 5-FU into niosomes. The effects of alternative loading strategies on various in vitro properties of 5-FU loaded niosomes are not sufficiently understood. Furthermore, the traditional colometric test (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) is a commonly reported in vitro cytotoxicity test that is based on colour change at the end point and does not provide real-time data on cell death [16, 20]. In this study, we looked into additional passive loading methods and examined how they affected some in vitro characteristics of 5-FU-loaded niosomes. For the in vitro cytotoxicity research, the real-time cell assay (RTCA) was used in place of the colorimetric test. Since the RTCA can capture data in real time, as opposed to the MTT test, we were able to track cell death hourly during the whole experiment [20]. Therefore, the goal of this work is to deliver 5-FU as niosomes using Span 60 and Tween 60 as non-ionic surfactants, stabilised by dicetyl phosphate/Pluronic F68, through various formulation techniques. The specific objectives were to investigate alternative approaches to loading 5-FU into niosomes, make physicochemical comparisons, and perform in vitro cytotoxicity tests (RTCA) against the human colon cell lines HCT-116 using the optimal formulation.
2. Materials and methods
2.1. Materials
5-fluorouracil, Tween 60, Span 60, dicetylphosphate were purchased from Aladdin, China, cholesterol was procured from Abcam, United Kingdom, Pluronic F68 was procured from life technologies, USA, ethanol, hydrochloric acid were procured from Sigma Aldrich, Germany, HCT 116 colon cell lines were purchased from ATCC, USA.
2.2. Solubility studies
Excess amount of 5-fluorouracil (5-FU) was added to each of the solvent (distilled water, simulated gastric fluid pH 1.2, phosphate buffer pH 6.8 and 7.4) and agitated at intervals for 24 h. A 0.1 ml volume of the supernatant was withdrawn from each sample and diluted appropriately. Consequently the solubility of 5-FU in various solvents was determined using spectrophotometer (UV/VIS Spectrulab UK) at the maximum wavelength of absorption (266 nm) [6].
2.3. Selection of process parameter
5-FU loaded niosomes were prepared using the thin film hydration method. Adequate quantities of cholesterol and surfactant (Span 60) at ratio 1:1 were dissolved in 3 ml of ethanol. The preparation was allowed to stir on a magnetic stirrer at temperature of 40 °C and speed of 100 RPM till a thin dry film is formed. The thin film was hydrated with phosphate buffer 7.4 (with 50 mg 5-FU dissolved in it) at various time intervals (30, 45 and 60 min) on the magnetic stirrer at 150 RPM. Each of the formulation produced at the various time interval were sonicated for 0, 5 and 10 min. The effect of the process parameter was determined by calculating the encapsulation efficiency and observing the physical stability of the niosomes. Thereafter, optimized process parameters were selected. Due to physical instabilities seen during the formulation process, dicetylphosphate (5 mg% w/w) and Pluronic F68 (0.1% v/v) were used as stabilisers.
2.4. Preparation of 5 fluorouracil-loaded niosomes using various method of preparation
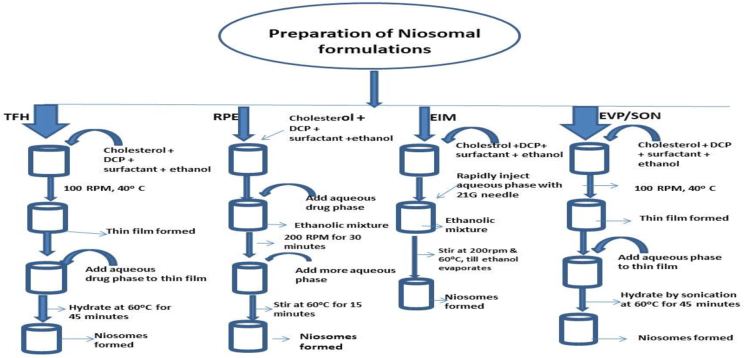
5 fluorouracil-loaded niosomes were produced using the optimized hydration and sonication times obtained from section above. Formulation was done using either a hydrophilic (Tween 60) or hydrophobic (Span 60) surfactant (Table 1). The methods of preparation employed were the thin film hydration method, reverse phase evaporation method, evaporation/sonication method and ethanol injection method (Figure 1).
Table 1.
Formula for Span 60 and Tween 60 based 5-FU loaded niosomes.
| Batches | Span based formulations | Tween based formulations |
|---|---|---|
| 5-FU (mg) | 40 | 40 |
| Cholesterol (mg) | 50 | 50 |
| Span 60 (mg) | 50 | - |
| Tween 60 (mg) | - | 50 |
| DCP (mg) | 5 | 5 |
| Pluronic F-68 (ml) | 0.1 | 0.1 |
| Phosphate buffer pH 7.4 (ml) | 10 | 10 |
Figure 1.
Flowchart showing the various methods of preparation of niosomal formulations.
2.4.1. Thin film hydration method for the preparation of 5-fluorouracil-loaded niosomes
An equal amount of cholesterol and surfactant (Span 60, Tween 60) were dissolved in 3 ml of ethanol. 5 mg w/w of dicetylphosphate was added and allowed to dissolve. The mixture was stirred on a magnetic stirrer at temperature of 40 °C, speed of 100 RPM till a thin dry film was formed and the ethanol evaporated. 40 mg of 5-fluorouracil was dissolved in 10 ml of phosphate buffer at pH of 7.4, 0.1 ml of Pluronic F68 (PF68) was added and this dispersion was used to hydrate the thin film for 45 min on a magnetic stirrer at 200 RPM. Sonication was done for 5 min.
2.4.2. Reverse phase evaporation (RPE) method for the preparation of 5- fluorouracil-loaded niosomes
Surfactant (Span 60, Tween 60) and cholesterol in equal ratio were dissolved in 3 ml of ethanol and dicetylphosphate (5 mg% w/w) was added. The aqueous mixture containing 5-fluorouracil and Pluronic F68 in 6 ml of phosphate buffer pH 7.4 was added to the ethanolic mixture. High shear was applied by stirring at 200 RPM for 30 min till evaporation was complete. Then 4 ml of phosphate buffer 7.4 was added and agitated at 60 °C for 15 min to complete the preparation of niosomes. Sonication was done for 5 min. The niosomes formed were stored at 4 °C.
2.4.3. Preparation of 5-fluorouracil-loaded niosomes by ethanol injection method (EIM)
The following substances were dissolved in 3 ml of ethanol: surfactant, cholesterol, and dicetylphosphate. The aqueous medium containing 5-fluorouracil and Pluronic F68 in phosphate buffer pH 7.4 was then rapidly injected with the ethanol solution using a fine needle (21G) on the magnetic stirrer at a temperature of 60 °C and a speed of 200 RPM. Niosomes are generated as a result of ethanol being allowed to evaporate.
2.4.4. Preparation of 5-fluorouracil loaded niosomes by evaporation-sonication method (EVP/SON)
Appropriate amount of surfactant, cholesterol and dicetylphosphate were dissolved in 3 ml of ethanol. The mixture was allowed to stir gently on the magnetic stirrer until a dry thin film was formed. The dried thin film was hydrated in 10 ml of drug solution (together with PF68) by sonication for 45 min at temperature 60 °C. The niosomes formed were left to cool and stored for further studies.
2.5. Freezing and thawing of niosomes
The niosomes formed by thin film hydration method were further subjected to freezing and thawing cycles by freezing in a refrigerator at 0 °C and thawed at room temperature (25 °C). This was repeated three times.
2.6. Entrapment efficiency and drug loading capacity
The entrapment efficiency of the niosomes was determined via centrifugation method. A 1 ml of the niosomal suspension was centrifuged at 12000 RPM for 20 min. Then the supernatant was withdrawn and analysed for free drug at wavelength of 266 nm via the ultraviolent spectrophotometer. Encapsulation efficiency and drug loading capacity was then calculated with Eqs. (1) and (2) respectively:
| (1) |
| (2) |
2.7. Particle size analysis of the 5-fluorouracil-loaded niosomes
Aqueous dispersions of each of the 5-fluorouracil-loaded niosomes were diluted hundredfold with distilled water. The droplet size and polydispersity index were determined using Zeta Sizer (Malvern Instruments, U.K) at a light scattering angle of 90°.
2.8. Morphology of 5-fluorouracil-loaded niosomes
A scanning electron microscope (Hitachi Japan, Model 3400N) was used to observe the surface morphology of 5-fluorouracil-loaded niosomes. A 10 μl of the niosomal suspension was placed on a glass slide and dried at room temperature (30 ± 2 °C). The samples were fixed to the stub and gold sputtered to neutralize the charging effects before scanning in SEM with an acceleration voltage of 20 KV. Furthermore, an optical microscope (Weltzar Germany) was used to capture the niosomes prepared by the thin hydration method.
2.9. In vitro drug release studies
The dialysis technique and magnetic stirrer beaker assembly were used to study the drug release behaviour of the drug-loaded formulations. A mixture of 4 ml of the niosomes and 1 ml of the medium was introduced into a 6 cm long cellulose membrane (molecular weight cut off of 12–14 Kdal) tied at both ends. This was subsequently tied to a vertical spindle and lowered into the phosphate buffer (pH 7.4) dissolution medium. The study was done with 500 ml medium volume at 100 rpm stirrer rotation speed and 37 ± 1 °C. At predetermined intervals, 5 ml aliquots of the dissolution medium were collected and replaced with 5 ml of the corresponding fresh dissolution medium to keep the volume constant. The withdrawn samples were assayed using the UV spectrophotometer (Spectrulab UK) at 266 nm for the amount of drug released. Triplicate determinations were done.
2.10. FTIR of the formulation/drug–excipients compatibility studies
Fourier transform infrared spectrum (FTIR) was performed to ascertain or investigate drug – excipient interaction and to study any incompatibility between the ingredients used in the formulation. Changes in the drug’s distinctive peaks following mixing with the excipients were used to anticipate the incompatibility between the drug and the excipients. FTIR was recorded for the pure drug 5-FU, blank niosomes, 5-fluorouracil loaded niosomes using infrared spectrophotometer (Shimadzu corporation, Japan). The formulations were prepared in KBr disk (2 mg sample/200 mg KBr) with a hydrostatic press at a force of 275790.292 Pa’s for 4 min and the spectrum was produced within the wavelength number of 4000 to 400 cm−1 [18].
2.11. In vitro cytotoxicity studies (real-time cell assay)
HCT-116 colon cell lines were stored in the vapour phase of liquid nitrogen chamber until use. The cells were then rapidly thawed at 37 °C and subsequently suspended in 5 ml of complete medium (supplemented with 10% foetal bovine serum (FBS)). The suspended cells were incubated at 37 °C and 5% CO2 until confluent for passaging. Cells were passaged 3 times before using it for cytotoxicity test. The HCT-116 cells were seeded per well in a 16-well Real-time cell analysis (RTCA) culture plate for overnight to allow for cell adherence. Solutions containing 50 μM of 5-fluorouracil from the optimized niosomal formulation (prepared by TFH method) as well as the pure drugs were added to the plates and incubated at 37 °C in a 5% CO2 incubator. Real–time cell assay was monitored on RTCA software for 24 h and the results were collected and analysed [19, 20].
2.12. Statistical analysis
Data were analysed using SPSS Version 26.0 (SPSS Inc. Chicago, IL, US). Values were presented as mean ± SD (standard deviation). Means were compared via the one-way ANOVA and P < 0.05 was considered statistically significant.
3. Results and discussions
3.1. Solubility studies of 5-FU in various solvents
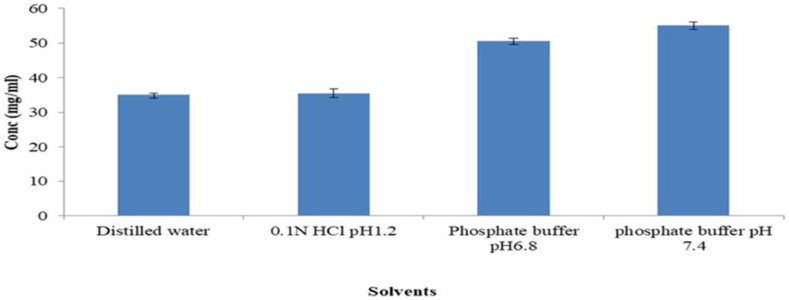
Below is a representation of the solubility study of 5-FU in several solvents (Figure 2). 5-FU showed good solubility in both acidic and alkaline media. The solubility of 5-FU was 35.01, 35.34, 50.50, and 54.98 mg/ml in distilled water, 0.1 N HCl (pH 1.2), phosphate buffer pH 6.8, and pH 7.4, respectively (Table 2). The alkaline media had higher 5-FU solubility values (P < 0.05) than the other media. On the amide N1 and N3 nitrogen, 5-FU has two potential sites for deprotonation that could be used in any interaction, the possibility of salt formation (between the acidic drug and the alkaline environment) at increasing pH values (7.0–8.0) via the potential sites for interaction may be responsible for the higher solubility of 5-FU at alkaline pH. In addition, at alkaline pH, 5-FU is reported to show limited hydrolysis; while at acidic pH, 5-FU shows hydrolysis as a first order reaction [21, 22, 23]. Some researchers have reported higher solubility of 5-FU at elevated pH [22, 46]. 5-FU in alkaline medium is mostly applied in medicine [46]. Our results agreed with these reports. Therefore, phosphate buffer pH 7.4 and 6.8 are the ideal solvent needed for the formulation of 5-FU loaded niosomes in order to achieve optimal 5-FU solubility.
Figure 2.
Solubility studies for 5-fluorouracil (5-FU).
Table 2.
Solubility of 5-FU in various solvents.
| Solvents | Conc (mg/ml) |
|---|---|
| Distilled water | 35.01 ± 0.48 |
| 0.1N HCl pH1.2 | 35.34 ± 1.39 |
| Phosphate buffer pH6.8 | 50.5∗ ±0.81 |
| phosphate buffer pH 7.4 | 54.98∗ ± 1.16 |
Mean values with superscript∗ are considered statistically significant at P < 0.05.
3.2. Optimized selection parameter
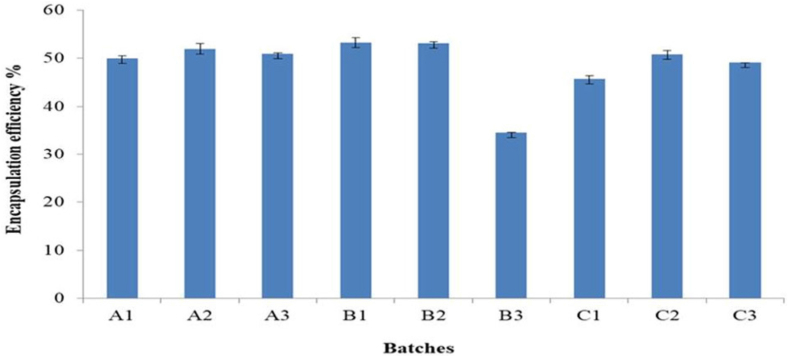
To determine the impact of varied hydration and sonication times on encapsulation efficiency, two process variables (hydration time and sonication time) were examined. Different hydration times (30, 45 and 60 min) and sonication times (0, 5, and 10 min) were investigated using Span 60 based formulated niosomes. The lipid film was hydrated at varied hydration times and sonication times to create niosomes, which were then examined to see how they affected encapsulation efficiency (EE). As the period for hydration and sonication increased, it was observed that the EE (Figure 3) decreased. The EE was found to be at its maximum at the 45-minute hydration point. Additionally, it was shown that 5 min was the ideal amount of time for sonication, as longer periods of sonication resulted in lower drug encapsulation rates. Previous researchers focused on the effects of hydration time & sonication time on particle sizes/PDI of niosomes however our study focuses on the effects of the aforementioned variables on encapsulation efficiency [40, 41, 42]. An inverse association was seen; as hydration and sonication times increased, EE decreased. In order to retain the niosomes' superior physicochemical qualities and stability, previous studies have revealed that an ideal sonication period is needed during the formulation process [24, 25]. Due to the high energy output of sonication at longer sonication times, the vesicles disintegrate, resulting in small particle sizes that are susceptible to particle aggregation and instability. The ideal times for hydration and sonication were 45 min and 5 min, respectively.
Figure 3.
The effect of hydration and sonication time on encapsulation efficiency. ∗∗A1 - 30 min hydration time + 0 min sonication time, A2 - 30 min hydration time + 5 min sonication time, A3 - 30 min hydration time + 10 min sonication time, B1 - 45 min hydration time + 0 min sonication time, B2 - 45 min hydration time + 5 min sonication time, B3 - 45 min hydration time + 10 min sonication time, C1 - 60 min hydration time + 0 min sonication time, C2 - 60 min hydration time + 5 min sonication time, C1 - 60 min hydration time + 10 min sonication time.
3.3. The effects of preparation methods on physical assessment test
The Tween 60 and Span 60 based niosomes were opalescent and homogenous in appearance (Figure 4). The formulations did not exhibit phase separation or caking; however, partial sedimentation did occur during storage and was quickly reversed with gentle agitation. In comparison to niosomes made using the TFH or EIM, those made using the evaporation-sonication method or the reverse phase evaporation approach were more prone to rapid sedimentation. Physical inspection revealed that every niosomal formulation was uniform and opalescent. The RPE approach produces large unilamellar vesicles, but the EVP/SON method may have created aggregated vesicles due to the lengthy sonication duration [8]. All of these elements could be the cause of the niosomes produced by the RPE or EVP/SON techniques having a tendency to silt quickly. The wide variety of particle sizes, reduced electrostatic repulsion between vesicles, and the large energy input necessary during preparation are all common causes of sedimentation in vesicles [26]. The formulations' inclusion of dicetylphosphate and Pluronic F68 significantly increased the stability of the niosomal suspensions. A centre segment of polypropylene oxide (PPO) and two hydrophilic side segments of polyethylene oxide make up the block co-polymer known as Pluronic F68 (PEO). The hydrophilic PEO section that surrounds the pharmaceuticals allows the Pluronic F68 to provide steric hindrance, which stabilises the medications [27]. By exerting stabilising effects through electrical repulsion, dicetylphosphate prevents particle aggregation. The various formulation techniques created physically stable niosomes, although the RPE and EVP/SON techniques were more likely to rapidly silt after storage than the TFH or EIM techniques.
Figure 4.
Pictures of niosomal formulation freshly prepared by a) Thin film hydration method (TFH) b) Ethanol injection method (EIM) c) Evaporation/sonication method (EVP/SON) d) Reverse phase evaporation method (RPE).
3.4. The effect of methods of preparation on Entrapment efficiency and drug loading capacity
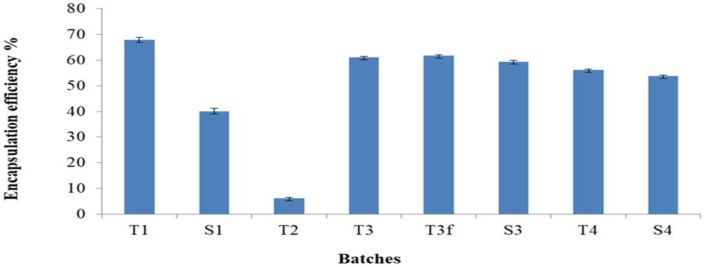
All formulations produced using various techniques had encapsulation efficiencies ranging from 5.34 ± 0.095 to 68.03 ± 1.46% (Figure 5), while drug loading capacities ranged from 2.38 ± 0.15 to 25.88 ± 0.52% (Table 3). The link between the amount of drug entrapped and the overall amount of drug integrated is depicted by the entrapment efficiency (EE). The drug loading capacity (DLC) is defined as the ratio of the amount of drug that is entrapped to the total weight of polymers that were utilised in the formulation [45]. In general, TFH and EVP/SON methods showed higher entrapment (> 50%) for Span-based niosomes (S3 & S4), whereas the RPE and TFH methods indicated higher entrapment (> 60%) for Tween-based niosomes (T1 & T3). The entrapment efficiencies of the Tween-based and Span-based niosomes prepared with the EIM method were 5% and 0% respectively. These values suggest that the EIM was inefficient in the entrapment of 5-FU. Our study further revealed that the RPE, TFH and EVP/SON methods gave significantly higher EE (p < 0.05) compared to the EIM method, regardless of the surfactants used. Freezing and thawing of niosomes have been reported to increase the capacity for more entrapment of hydrophilic drugs [53]. A study by Abdelkader and his team showed that freezing and thawing resulted in a reduction in EE [44] however, our study showed an increment in EE approximately 1%. The Tween and Span – based niosomes prepared using the TFH method showed higher EE compared to most of the other preparation methods. Tween 60 (HLB OF 14.9) and Span 60 (HLB of 4.7) are both non-ionic surfactant with long alkyl chain length (C18) and, have been reported to give higher drug entrapment in niosomal formulations [28]. The drug loading capacities of the RPE, TFH and EVP/SON methods were significantly higher (P < 0.05) than those of EIM method (2.38 ± 0.15%). This result suggest that the EIM method was incapable of trapping good quantities of 5-FU into the niosomes relative to the total weight of polymers used. Whereas the RPE, TFH, and EVP/SON methods had greater capacity to trap in more quantities of 5-FU into the niosomes. We must recall that the niosomal contents and the drug used was the same regardless of the production techniques utilized. A study done by Zatorska et al. showed the effects of various preparation techniques on the drug loading capacities of nanoparticles [54]. In our study it is clear that the drug loading capacity of niosomes is influenced by the production methods. In literature, both the entrapment efficiency and the drug loading capacity can be influenced by a number of variables, including type of surfactant used, drug’s solubility, and production techniques [45]. The molecular weight (MW) of an entrapped drug can also affect the EE. The lower the molecular weight of the drug entrapped, the higher the resulting EE. Mohamed et al. reported a similar trend. In their report, acyclovir which has a lower MW had a higher EE when compared to vancomycin (with a higher MW) when the drugs were used in the preparation of niosomes [43]. The drug of study (5-FU), has a molecular weight of 130.08 g/mol, suggesting a better entrapment in niosomes. Entrapment efficiency was found to be directly correlated with drug loading capacity. Formulations with increased entrapments showed increased drug loading capacities as well. Formulations prepared by EIM were excluded in further studies because of little or no drug entrapment and low drug loading capacity.
Figure 5.
Encapsulation efficiency using various methods of preparation. ∗∗∗ T1 - Reverse phase evaporation (RPE) using Tween 60, T2 - Ethanol injection method (EIM) using Tween 60, T3 - Thin film hydration (TFH) using Tween 60, T3f - TFH (freezed & thawed), T4 - Evaporation/sonication (EVP/SON) using Tween 60, S1 - Reverse phase evaporation (RPE) using Span 60, S3 - Thin film hydration (TFH) using Span 60, S4 - Evaporation/sonication (EVP/SON) using Sapn 60.
Table 3.
Drug loading capacity of the 5-FU loaded niosomes.
| Batches | Drug loading capacity (DLC) (%) |
|---|---|
| T1 | 25.88 ± 0.52 |
| S1 | 15.25 ± 0.52 |
| T2 | 2.38 ± 0.15 |
| S2 | - |
| T3 | 23.32 ± 0.10 |
| T3f | 23.55 ± 0.21 |
| S3 | 22.63 ± 0.29 |
| T4 | 21.38 ± 0.20 |
| S4 | 20.46 ± 0.17 |
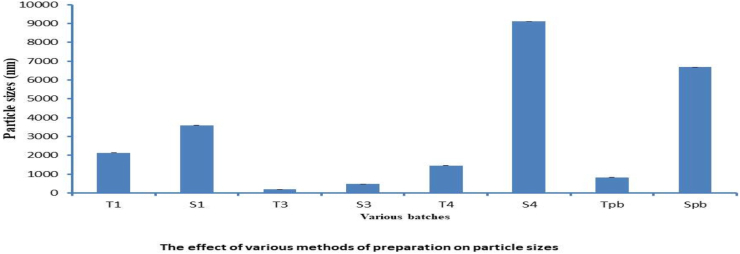
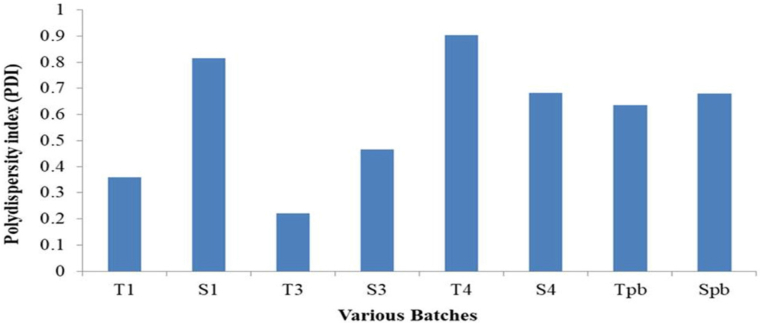
3.5. The effect of various methods of preparations on the particle sizes and polydispersity index (PDI)
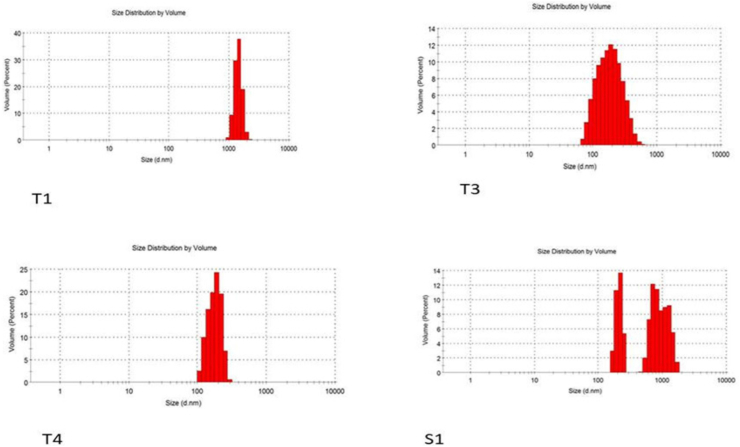
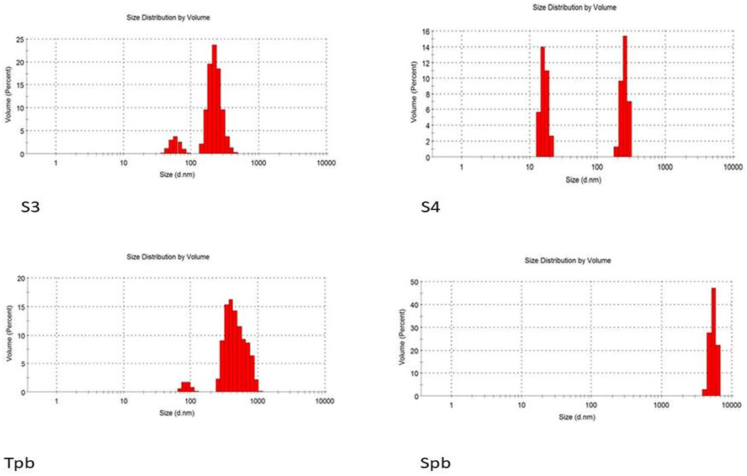
The influence of different methods of preparations on the particle sizes and PDI of the niosomes were studied. While the PDI ranged from 0.22 to 0.904, the particle size of all formulations made using different techniques ranged from 187 to 9111 nm (Figures 6 and 7, Table 4) The Tween 60 and Span 60 niosomes made by the thin film hydration process had the smallest particle sizes, 187 nm and 482 nm, respectively. For all formulations, the particle sizes rose gradually in the following order: TFH < RPE < EVP/SON techniques. The evaporation/sonication approach produced the largest particle sizes for the Tween 60 (4476 nm) and Span 60 (9111 nm). The PDI had a range of 0.22–0.904. The PDI of niosomes made using TFH methods was the lowest (<0.5), whereas the PDI of niosomes made using the EVP/SON method was the greatest. TFH and RPV created niosomes with smaller particles than the other procedures. The enormous energy production from the prolonged sonication period resulted in a thermodynamically unstable system that was prone to particle aggregation, which is why these large-sized niosomes were formed by the EVP/SON approach [29]. The size of the blank noisome created using the thin film hydration technique was 825.3 nm for tween 60-based and 6680 nm for span 60-based niosomes, respectively. The size of nanoparticles is a key factor in how well medications are absorbed through the gastrointestinal tract. The hydrophilicity and HLB of the surfactant, as well as the carbon chain length, have an impact on the particle sizes of vesicles. The presence of hydrophilic PEG in their structure, as was the case with other studies [28, 39], could be the cause of the reduction in particle sizes observed with niosomes made with the Tween surfactant across all of the production techniques investigated. Regardless of the surfactant utilised, it was shown that the inclusion of drugs decreased the formulation’s particle size. This might have happened as a result of the medicine and excipients forming a stronger, more intense lipid bilayer, which in turn caused the particle sizes to decrease [26, 30]. Lower values indicate a tendency toward monodispersity on the PDI scale, which ranges from 0 to 1.0 [31]. High PDI values suggest a significant separation between large and small droplets. Figures 8 and 9 make it evident that there is a significant difference between large and small particles connected with several of the methods used, supporting the high PDI reported with those methods (RPE & EVP/SON). If the particles are more homogeneous the polydispersity value is closer to zero. Formulations made using the thin film hydration process had the lowest PDI.
Figure 6.
The effect of various preparation methods on particle sizes. ∗∗∗ T1 - Reverse phase evaporation (RPE) using Tween 60, T3 - Thin film hydration (TFH) using Tween 60, T3f - TFH (freezed & thawed), T4 - Evaporation/sonication (EVP/SON) using Tween 60, S1 - Reverse phase evaporation (RPE) using Span 60, S3 - Thin film hydration (TFH) using Span 60, S4 - Evaporation/sonication (EVP/SON) using Sapn 60, Tpb = TFH using Tween 60 (without drug), TFH using Span 60 (without drug).
Figure 7.
The effects of various preparation methods on polydispersity index (PDI). ∗∗∗ T1 - Reverse phase evaporation (RPE) using Tween 60, T3 - Thin film hydration (TFH) using Tween 60, T3f - TFH (freezed & thawed), T4 - Evaporation/sonication (EVP/SON) using Tween 60, S1 - Reverse phase evaporation (RPE) using Span 60, S3 - Thin film hydration (TFH) using Span 60, S4 - Evaporation/sonication (EVP/SON) using Sapn 60, Tpb = TFH using Tween 60 (without drug), TFH using Span 60 (without drug).
Table 4.
Mean particle sizes of the 5-FU loaded niosomes.
| Batches | Mean particle sizes (nm) |
|---|---|
| T1 | 2134 ± 0.81 |
| S1 | 3596 ± 0.48 |
| T3 | 187 ± 0.57 |
| S3 | 482 ± 0.24 |
| T4 | 1458 ± 0.65 |
| S4 | 9111 ± 0.81 |
| Tpb | 825 ± 0.40 |
| Spb | 6688 ± 0.57 |
Figure 8.
Size distribution graph for batches T1, T3, T4 & S1.
Figure 9.
Size distribution graph for batches S3, S4, Tpb & Spb.
3.6. The effects of preparation methods on morphology of the 5-fluorouracil loaded niosomes
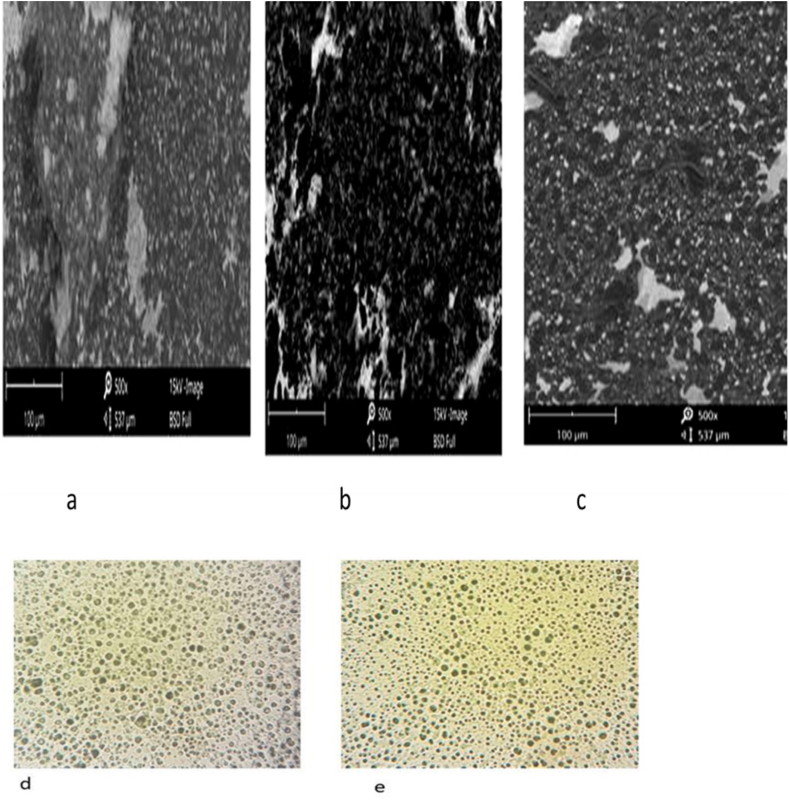
SEM was used to examine the morphology of drug-loaded niosomes made using various techniques (TFH, EVP/SON, and RPE). The TFH and RPE produced spherical vesicles, but the EVP/SON technique failed to produce any discernible vesicles. Refer to Figure 10 (a -e). Compared to the other procedures, the TFH produced vesicles that were more uniform in both shape and distribution. Although rod-shaped or needle-like particles were seen in the EVP/SON method, it appeared that the prolonged sonication time connected with it had disrupted the creation of any discernible spherical vesicles [8]. Recall that the EVP/SON method required 45 min of sonication. Due to the prolonged sonication, smaller-sized particles were created, some of which may be thermodynamically unstable. This instability might result to membrane fusion or particle growth, leading to particles that resemble rods or needles. As a result of potential particle fusion or growth, Pardakhty and his team also discovered comparable needle-like particles in their formulations [47]. The vesicles were more clearly visible in photomicrographs of the drug-loaded niosomes made using TFH techniques.
Figure 10.
Morphology (SEM) of Span 60 based niosomes prepared by a) thin film hydration method, b) Evaporation-sonication method, c) Reverse phase evaporation method (x500) d) photomicrograph of Span 60 based niosomes (TFH), e) Photomicrograph of Tween 60 based niosomes (TFH) (x400).
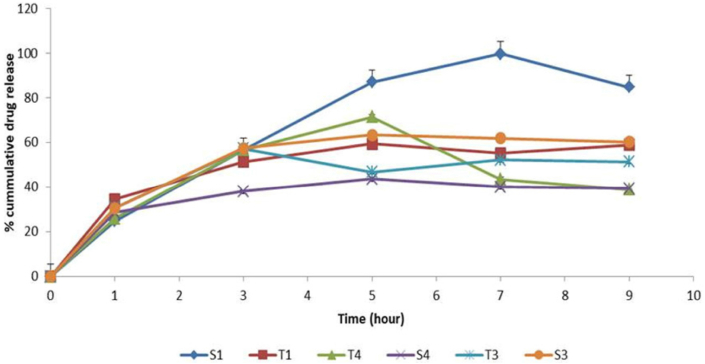
3.7. The effects of preparation methods on In vitro release studies
A study on drug release was conducted for the colloidal drug formulation. When compared to Tween-based formulations, it was observed that the Span-based formulation exhibited a slightly higher drug release (Figure 11). Compared to the other approaches (RPE, Evp/Son), the thin film hydration (TFH) method gave a steady drug release for approximately 9 h for both the Span and Tween based niosomal formulations. A less rigid bilayer in the niosome structure may have resulted from the extended hydrocarbon chain length of either the Span 60 or Tween 60, permitting medication release. The formulations may have exhibited sustained release due to the high phase transition temperature connected to Span 60. Previous researchers have reported on the same trend [33, 34, 35]. As the surfactant chain length extends, drug release can last longer because the transfer temperature can influence the surfactants and make them totally fluid, allowing for increased drug penetration at 37 °C [2]. In general, the in vitro release profile showed sustained release of medicines from the niosomes. For both the Span and Tween based niosomal formulations, the thin film hydration (TFH) method outperformed the other methods (RPE, Evp/Son) in terms of providing a sustained drug release for approximately 9 h. Recall that the smaller vesicles produced by the thin film hydration method (TFH) may be the reason for the improved drug release profile seen.
Figure 11.
In vitro drug release study of 5-FU niosomes prepared by various methods. ∗∗∗ T1 - Reverse phase evaporation (RPE) using Tween 60, S1 - Reverse phase evaporation (RPE) using Span 60, T3 - Thin film hydration (TFH) using Tween 60, T4 - Evaporation/sonication (EVP/SON) using Tween 60, S3 - Thin film hydration (TFH) using Span 60, S4 - Evaporation/sonication (EVP/SON) using Sapn 60.
3.8. Kinetic studies
To identify the dominant release mechanism, the data from the in vitro release investigation was fitted into the various mathematical release models. The results are shown in Table 5. The R2 statistically gives information about how best the formulations fit into the mathematical models. The Higuchi model came second to the Korsmeyer-peppas model in terms of how well each batch fitted. The Korsmeyer-Peppas model describes a system where the fractional release of drugs is exponentially related to time [48], in contrast to the Higuchi release model, which explains that the release of drugs from an insoluble matrix is dependent on the square root of time and grounded in fickian diffusion. When the release mechanism is unclear or there are many release phenomena, the Korsmeyer kinetic model is typically applied. Results are shown in Table 4. The Korsmeyer-Peppas model’s “n” value identifies the release mechanism. While n between 0.5 and 1 indicates mass transfer or anomalous transport, n between 0 and 0.5 suggests fickian diffusion [36]. With the exception of T4, most of the formulations' “n” values were lower than 0.5 (Table 5). This only suggests that the majority of niosomal formulations were released through fickian diffusion, while T4 demonstrated anomalous transport since the n value was greater than 0.5. In their investigation of niosomes containing resveratrol, Pando and his team reported comparable findings [37].
Table 5.
Release Kinetics of niosomal formulations.
| Batches | Zero order R2 |
First order R2 |
Higuchi R2 |
Korsmeyer peppas R2 |
Korsmeyer peppas n |
|---|---|---|---|---|---|
| S1 | 0.739 | 0.963 | 0.923 | 0.923 | 0.492 |
| T1 | 0.267 | 0.618 | 0.828 | 0.975 | 0.218 |
| T4 | 0.921 | 0.996 | 0.988 | 0.995 | 0.603 |
| S4 | 0.609 | 0.740 | 0.940 | 0.981 | 0.189 |
| T3 | 0.481 | 0.691 | 0.833 | 0.907 | 0.256 |
| S3 | 0.790 | 0.938 | 0.983 | 0.987 | 0.433 |
3.9. FTIR of the 5 fluorouracil loaded niosomes
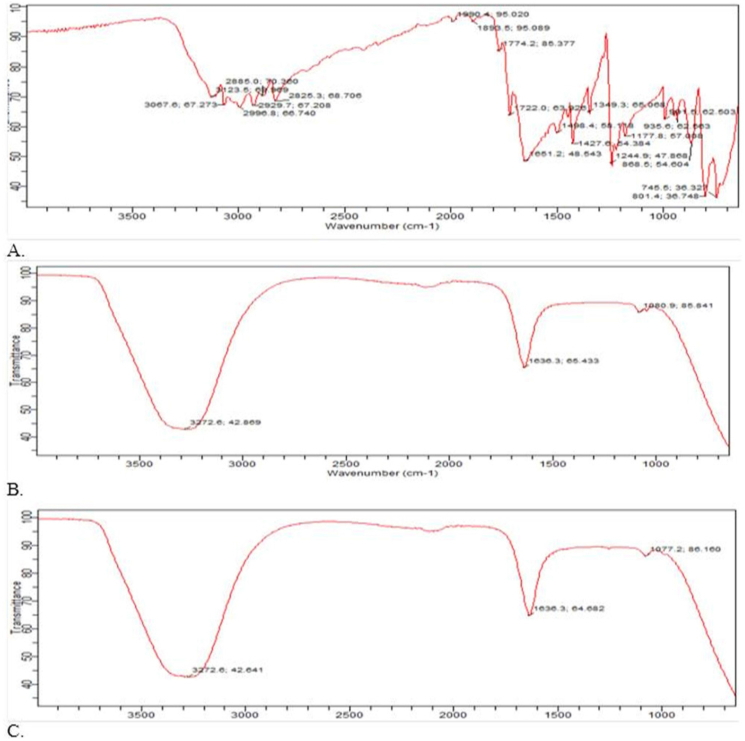
On the formulations created using the thin film hydration technique, FTIR was performed. The FTIR spectra of 5 fluorouracil was characterized by N–H stretch at 3123.5 cm−1, C=O bend at 1651.2 cm−1, C–F stretch at 1427.6 cm−1, C–N stretch at 1244.9 cm−1 and pyrimidine bend at 1349.3 cm−1. Similar findings were also reported by Elkhatib and his team [49]. The presence of the OH group in both the blank and drug-loaded formulations' FTIR spectra resulted in broad peaks between 3261 cm−1 and 3272 cm−1, respectively (Figure 12 (A –C), Table 6). According to the FTIR measurements, there was no discernible difference between the spectra of the formulation containing 5-FU and the blank. The obvious hydroxyl group was caused by intermolecular forces especially hydrogen bonding interactions that took place between the non-ionic surfactant, cholesterol and 5-FU (where applicable). In general, similar patterns have been observed in various niosomal formulations, particularly when the drug moiety comprises potential hydrogen bonding interaction sites. Mohamed and his team found a similar tendency in their investigation, whereas Anbarasan and his colleagues noted obvious hydrogen bonding in the FTIR spectra of capacitabine niosomes [35, 43]. By orienting its OH group toward the aqueous phase and its aliphatic chain toward the hydrocarbon chain of the surfactant, cholesterol creates a hydrogen bond that is known to contribute to the rigid membrane of the niosomes [32]. It is well known that the medication (5-FU) has two deprotonation sites [23]. These locations might also be responsible for the observed hydrogen bonding interaction. The degree of connection or interaction between 5-fluorouracil and the excipients employed is assessed in this investigation using FTIR [16]. The FTIR analysis showed a strong hydrogen bond connection between either the niosomal excipients or the medication and the niosomal carrier.
Figure 12.
FTIR spectra of a) 5-FU b) Blank niosomes c) 5-FU loaded niosomes.
Table 6.
FTIR bands of 5-FU and niosomal formulations.
| 5-FU pure drug |
5-FU loaded niosomes |
Blank Niosomes |
|||
|---|---|---|---|---|---|
| Absorption Band (cm−1) |
Functional Group |
Absorption Band (cm−1) |
Functional Group |
Absorption Band (cm−1) |
Functional Group |
| 3123.5 | N–H stretch | 3272.6 (between 3000 and 3600) | Broad OH stretch | 3272.6 (between 3000 and 3600) | Broad OH stretch |
| 1651.2 | C=O bend | ||||
| 1427.6 | C–F stretch | ||||
| 1244.9 | C–N stretch | 1636.3 | C=O bend | 1636.3 | C=O bend |
| 1349.3 | Pyrimidine bend | ||||
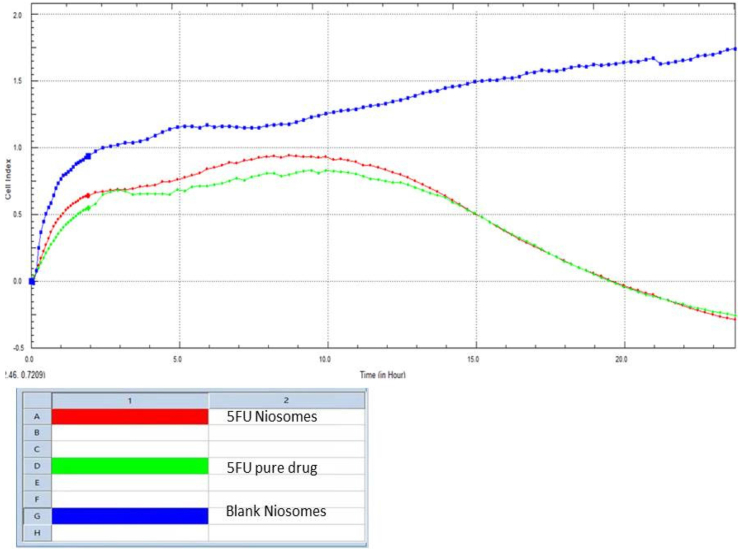
3.10. In vitro cytotoxicity test (RTCA)
Niosomes made using the thin hydration technique (TFH) were used in the RTCA investigation. The RTCA investigation demonstrated that during the 24-hour test period, the 5-FU niosomes operated more persistently and were more cytotoxic. The 5-FU pure medicine initially showed quicker cell death than the niosomal formulations within the first 13 h, but from 15 to 24 h, the niosomal formulations demonstrated higher cell death (Figure 13). Due to the absence of the chemotherapeutic drug, the blank niosomes (without 5-FU) was shown to exhibit increased CI, showing rapid adherence and proliferation of the HCT-116 cells. The RTCA measures the impedance of electric current flow as the cell index and operates on the basis of the principle of cell adhesion on the gold microelectrodes in the RTCA plate (CI). When HCT-116 cells are implanted in an RTCA plate, cell adhesion and growth are observed to obstruct current flow, increasing the CI value. Due to a declining proliferation ratio, reduced CI over 24 h is shown in the presence of either the 5-FU pure medication or niosomes [20]. Increased cell adhesion and proliferation are seen in the increasing CI values of the placebo (niosomes without drug). This demonstrates unequivocally that the 5-FU alone was responsible for the cytotoxicity, not the formulation excipients. Since niosomes have the benefit of controlled drug release over a lengthy period of time, it is possible that the slightly increased cytotoxicity seen in niosomal formulations over the course of the 24-hour research is a result of the medications' progressive release from the vesicles. The 5-FU loaded niosomes had slightly lower cell viability (reduced cell index) than 5-FU pure drug at the end of the study (23–24 h), and this difference might have amplified had the study been conducted over a longer time frame. The similarities between the pure drug and the drug-loaded niosomes may be explained by findings from an earlier study that found that the anti-apoptotic factor translationally controlled tumour protein (TCTP) was significantly overexpressed in HCT116 cells and that its level rose in response to a combination therapy based on 5-FU [38]. Given that the experiment lasted for 24 h, this protein may have contributed to the resistance to 5-FU and may be responsible for the similarity in cytotoxicity. In order to get around some of these difficulties, several researchers have suggested designing delivery systems that can interact at the molecular level. Examples of these strategies include creating ligand-conjugated systems that can interact with specific substrates at tumour sites. In the case of colon cancer, ligands like folic acid overexpressed in tumour site will be of immense benefits. Other strategies include the creation of multifunctional smart drug delivery systems or even the modulation of 5-FU with reduced folates like leucovorin [50, 51, 52]. These approaches are beyond the aim of this study; however, they can form the basis for future research works. Nonetheless, the niosomal formulation produced in this study had the advantages of smaller particle size, potential accumulation in the tumour site, improved 5-FU pharmacokinetic profile, and subsequently fewer side effects.
Figure 13.
RTCA results for 5-FU loaded niosomes, pure drug and blank niosomes (without 5-FU).
4. Conclusion
Among the passive methods studied in the preparation of 5-fluorouracil niosomes, the thin hydration method was most robust as it showed lower particle size, spherical vesicles and good entrapment efficiency compared to all the other methods. The EVP/SON and the RPE methods showed larger particle sizes and were more predisposed to instability. Niosomes produced by EIM did not show large entrapment efficiency so was excluded in further studies. Generally, the presence of the stabilizers (dicetyl phosphate and pluronic F68) in the niosomes contributed greatly to the stability of the niosomal formulation on storage. Sustained cell death (cytotoxicity) was also observed with the niosomal formulation within the 24 h RTCA test period. In conclusion the preparation protocol of niosomes played a major role in the EE, particle size/size distribution and stability of the 5-FU loaded niosomes. The RTCA approach provides hourly information about cell death over 24-hour study period. The design of this study was focused on the influence of various passive loading techniques on some in vitro parameters of niosomes as well as the real time cell assay of optimized formulation against colon cell lines. This study did not cover the scope of active loading strategies or active targeting that is capable of drug delivery at molecular levels. However, to produce interaction at molecular levels, the design of ligand-conjugate systems or multifunctional drug delivery systems can be utilized. These can form the basis of future research works.
Declarations
Author contribution statement
Ugorji Onyinyechi Lydia: Conceived and designed the experiment; Performed the experiment; Analysed and interpreted data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Umeh Ogochukwu Ngozi Chidimma: Analysed and interpreted data; Wrote the paper.
Agubata Chukwuma Obumneme: Contributed reagents, materials, analysis tools or data; Wrote paper.
Adah Dickson: Performed experiment; Contributed reagents, materials, analysis tools or data; Analysed and interpreted data.
Obitte Nicholas Chinedu: Conceived and designed the experiment; Contributed reagents, materials, analysis tools or data.
Chukwu Amarauche: Contributed reagents, materials, analysis tools or data.
Funding statement
Ms Onyinyechi Lydia Ugorji was supported by DAAD in country /in region scholarship [91679770].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We sincerely appreciate Dr Dickson Adah who did the RTCA in vitro cytotoxicity studies. We also appreciate DAAD for funding.
References
- 1.Mokale V.J., Patil H.I., Patil A.P., Shirude P.R., Naik J.B. Formulation and optimisation of famotidine proniosomes: an in vitro and ex vivo study. J. Exp. Nanosci. 2016;11:97–110. [Google Scholar]
- 2.Mansouri M., Khayam N., Jamshidifar E., Pourseif T., Kianian S., Mirzaie A., Akbarzadeh I., Ren Q. Streptomycin sulfate–loaded niosomes enables increased antimicrobial and anti-biofilm activities. Front. Bioeng. Biotechnol. 2021;9:1–11. doi: 10.3389/fbioe.2021.745099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asthana G.S., Sharma P.K., Asthana A. In Vitro and in vivo evaluation of niosomal formulation for controlled delivery of clarithromycin. Sci. Tech. Rep. 2016;2016 doi: 10.1155/2016/6492953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar G.P., Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery - an overview. Acta Pharm. Sin. B. 2011;1(4):208–219. [Google Scholar]
- 5.Sharma V., Anandhakumar S., Sasidharan M. Self-degrading niosomes for encapsulation of hydrophilic and hydrophobic drugs : an ef ficient carrier for cancer multi-drug delivery. Math. Sci. Eng. 2015;56:393–400. doi: 10.1016/j.msec.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 6.Bagheri A., Chu B.S., Yaakob H. Niosomal drug delivery systems: formulation, preparation and applications. World Appl. Sci. J. 2014;32(8):1671–1685. [Google Scholar]
- 7.Rathi J., Tamizharasi S., Dubey A., Rathi V. Development and characterization of niosomal drug delivery of gliclazide. J. Young Pharm. 2009;1(3):205. http://www.jyoungpharm.in/text.asp?2009/1/3/205/57065 Available from: [Google Scholar]
- 8.Mokhtar M., Sammour O.A., Hammad M.A., Megrab N.A. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int. J. Pharm. 2008;361:104–111. doi: 10.1016/j.ijpharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Rajera R., Nagpal K., Singh S.K., Mishra D.N. Niosomes: a controlled and novel drug delivery system. Biol. Pharm. Bull. 2011;34(7):945–953. doi: 10.1248/bpb.34.945. https://www.jstage.jst.go.jp/article/bpb/34/7/34_7_945/_article Available from: [DOI] [PubMed] [Google Scholar]
- 10.Nasir A., Harikumar S.L., Amanpreet K. Niosomes : an excellent tool for drug delivery. IJRPC. 2012;2(2):479–487. [Google Scholar]
- 11.Onochie I.T.O., Nwakile C.D., Umeyor C.E., Uronnachi E.M., Osonwa U.E., Attama A.A., Esimone C.O. Formulation and evaluation of niosomes of benzyl penicillin. J. Appl. Pharmaceut. Sci. 2013;3(12):66–71. [Google Scholar]
- 12.Khan R., Irchhaiya R. Niosomes : a potential tool for novel drug delivery. J. Pharm. Investig. 2016:1–10. [Google Scholar]
- 13.Hao Y., Zhao F., Li N., Yang Y., Li K. Studies on a high encapsulation of colchicine by a niosome system. Int. J. Pharm. 2002;244:73–80. doi: 10.1016/s0378-5173(02)00301-0. [DOI] [PubMed] [Google Scholar]
- 14.Khan R., Irchhaiya R. An overview on niosomes as efficient drug carriers. Int. J. Pharm. Biol. Sci. 2017;8(2):106–116. [Google Scholar]
- 15.Longley D.B., Harkin D.P., Johnston P.G. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 16.Udofot O., Affram K., Israel B., Agyare E. Cytotoxicity of 5-fluorouracil loaded pH sensitive liposomal nanoparticles in colorectal cancer cell line Integr Cancer. Sci. Ther. 2015;2(5):245–252. doi: 10.15761/icst.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaferian S., Negahdari B., Eatemadi A. Colon cancer targeting using conjugates biomaterial 5-fluorouracil. Biomed. Pharmacother. 2016;84:780–788. doi: 10.1016/j.biopha.2016.10.004. https://www.sciencedirect.com/science/article/pii/S0753332216314111 Available from: [DOI] [PubMed] [Google Scholar]
- 18.Patel P.V., Patel H.K., Panchal S.S., Mehta T.A. Self micro-emulsifying drug delivery system of tacrolimus: formulation, in vitro evaluation and stability studies. Int. J. Pharm. Investig. 2013;3(2):95. doi: 10.4103/2230-973X.114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiaoji C.W., Bin L., Song Z.W., Ni M., Mehendale S., Han J.X., Xie J.T., Aung H.H., He T.C., Yuan C.S. Notoginseng enhances anti-cancer effect of 5- Fluorouracil on human colorectal cancer cells. Cancer Chemother. Pharmacol. 2007;60(1):69–79. doi: 10.1007/s00280-006-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Şener L.T., Albeniz G., Dinç B., Albeniz I. iCELLigence real-time cell analysis system for examining the cytotoxicity of drugs to cancer cell lines. Exp. Ther. Med. 2017;14(3):1866–1870. doi: 10.3892/etm.2017.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansova B., Synek S., Opatrilova R. 5-Fluorouracil – characteristics and analytical determination. Curr. Pharmaceut. Anal. 2011;7(1):1–11. [Google Scholar]
- 22.Ibrahim R.A.A.Z., Suhail F.S.A., Al-Hakeim H.K. Stability of anticancer drug 5-fluorouracil in aqueous solution: an assessment of kinetic behavior. Nano Biomed. Eng. 2018;10(3):224–234. [Google Scholar]
- 23.Wielińska J., Nowacki A., Liberek B. 5-Fluorouracil-Complete insight into its neutral and ionised forms. Molecules. 2019;24(20) doi: 10.3390/molecules24203683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owodeha-Ashaka K., Ilomuanya M.O., Iyire A. Evaluation of sonication on stability-indicating properties of optimized pilocarpine hydrochloride-loaded niosomes in ocular drug delivery. Prog. Biomater. 2021;10(3):207–220. doi: 10.1007/s40204-021-00164-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan D.H., Bashir S., Figueiredo P., Santos H.A., Khan M.I., Peltonen L. Process optimization of ecological probe sonication technique for production of rifampicin loaded niosomes. J. Drug Deliv. Sci. Technol. 2019;50:27–33. [Google Scholar]
- 26.Ertekin Z., Bayindir Z., Yuksel N. Stability studies on piroxicam encapsulated niosomes. Curr. Drug Deliv. 2015;12(2):192–199. doi: 10.2174/1567201811666140723115852. [DOI] [PubMed] [Google Scholar]
- 27.Tuomela A., Hirvonen J., Peltonen L. Stabilizing agents for drug nanocrystals : effect on bioavailability. Pharmaceutics. 2016;8(16):1–18. doi: 10.3390/pharmaceutics8020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naderinezhad S., Ghasem A., Haghiralsadat F. Anticancer drugs using biocompatible pH-sensitive. RSC Adv. 2017;7:30008–30019. [Google Scholar]
- 29.Lentz B.R., Carpenter T.J., Alford D.R. Spontaneous fusion of phosphatidylcholine small unilamellar vesicles in the fluid phase. Biochemistry. 1987;26(17):5389–5397. doi: 10.1021/bi00391a026. [DOI] [PubMed] [Google Scholar]
- 30.Liu T., Guo R. Preparation of a highly stable niosome and its hydrotrope-solubilization action to drugs. Langmuir. 2005;(23):11034–11039. doi: 10.1021/la051868b. [DOI] [PubMed] [Google Scholar]
- 31.Obitte N.C., Ofokansi K.C., Kenechukwu F.C. Development and evaluation of novel self-nanoemulsifying drug delivery systems based on a homolipid from Capra hircus and its admixtures with melon oil for the delivery of indomethacin. J. Pharm. 2014;2014 doi: 10.1155/2014/340486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sankhyan A., Pawar P. Recent trends in niosome as vesicular drug delivery system. J. Appl. Pharmaceut. Sci. 2012;2(6):20–32. [Google Scholar]
- 33.Asgharkhani E., Fathi A., Shiva A., Mohsen I., Saffari Z., Norouzian D., Akbarzadeh A., Atyabi S.M. Artemisinin-loaded niosome and pegylated niosome : physico- chemical characterization and effects on MCF-7 cell proliferation. J. Pharm. Investig. 2017:1–6. [Google Scholar]
- 34.Moghassemi S., Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J. Contr. Release. 2014;185(1):22–36. doi: 10.1016/j.jconrel.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Anbarasan B., Rekha S., Elango K., Shriya B., Ramaprabhu S. Optimization of the formulation and in-vitro evaluation of capecitabine niosomes for the treatment of colon cancer. Int. J. Pharma Sci. Res. 2013:1504–1513. [Google Scholar]
- 36.Rodriguez D.P. Doctoral thesis, Universidad de Oviedo; 2014. Formulation and Preparation of Niosomes Containing Bioactive Compounds. [Google Scholar]
- 37.Pando G., Gutierrez G., Coca J., Pazos C. Preparation and characterization of Niosomes containing resveratrol. J. Food Eng. 2013;117(2):227–234. [Google Scholar]
- 38.Bommer U.A., Vine K.L., Puri P., Engel M., Belfiore L., Fildes K., Batterham M., Lochhead A., Aghmesheh M. Translationally controlled tumour protein TCTP is induced early in human colorectal tumours and contributes to the resistance of HCT116 colon cancer cells to 5-FU and oxaliplatin. Cell Commun. Signal. 2017;15:9. doi: 10.1186/s12964-017-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taymouri S., Varshosaz J. Effect of different types of surfactants on the physical properties and stability of carvedilol nano-niosomes. Adv. Biomed. Res. 2016;5:48. doi: 10.4103/2277-9175.178781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeo L.K., Chaw C.S., Elkordy A.A. The effects of hydration parameters and Co-surfactants on methylene blue-loaded niosomes prepared by the thin film hydration method. Pharmaceuticals. 2019;12(46):1–5. doi: 10.3390/ph12020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sezgin-Bayindir Z., Yuksel N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech. 2012;13(3):826–835. doi: 10.1208/s12249-012-9805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Essa E.A. Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosomes. Asian J. Pharm. 2010:227–233. [Google Scholar]
- 43.Mohamed H.B., El-Shanawany S.M., Hamad M.A., Elsabahy M. Niosomes: a strategy toward prevention of clinically significant drug incompatibilities. Sci. Rep. 2017;7(1):1–14. doi: 10.1038/s41598-017-06955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdelkader H., Ismail S., Kamal A., Alany G. Design and evaluation of controlled-release niosomes and discomes for naltrexone hydrochloride ocular delivery. J. Pharmaceut. Sci. 2011;100:1833–1846. doi: 10.1002/jps.22422. [DOI] [PubMed] [Google Scholar]
- 45.Kenechukwu F.C., Kalu C.F., Momoh M.A., Onah I.A., Attama A.A., Okore V.C. Novel Bos indicus fat-based nanoparticulate lipospheres of miconazole nitrate as enhanced mucoadhesive therapy for oral candidiasis. Biointerface Res. Appl. Chem. 2021;13(1):1–19. [Google Scholar]
- 46.Abdrakhimova G.S., Ovchinnikov M.Y., Lobov A.N., Spirikhin L.V., Ivanov S.P., Khursan S.L. 5-Fluorouracil solutions: NMR study of acid–base equilibrium in water and DMSO. J. Phys. Org. Chem. 2014;27:876–883. [Google Scholar]
- 47.Pardakhty A., Shakibaie M., Daneshvar H., Khamesipour A., Mohammadi-Khorsand T., Forootanfar H. Preparation and evaluation of niosomes containing autoclaved Leishmania major: a preliminary study. J. Microencapsul. 2012;29(3):219–224. doi: 10.3109/02652048.2011.642016. [DOI] [PubMed] [Google Scholar]
- 48.Higuchi T. Mechanism of sustained action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharmaceut. Sci. 1963;52:1145–1148. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 49.Elkhatib M.M., Ali A.I., Abdelrazek S.A., Al-Badrawy A.S. Pre-formulation study on 5-fluorouracil and certain lipids for solid lipid nanoparticles preparation. Int. J. Appl. Pharm. 2022;14(2):160–171. [Google Scholar]
- 50.Ullah S., Azad A.K., Nawaz A., Shah K.U., Iqbal M., Albadrani G.M., Al-Joufi F.A., Sayed A.A., Abdel-Daim M.M. 5-Fluorouracil-Loaded folic-acid-fabricated chitosan nanoparticles for site-targeted drug delivery cargo. Polymers. 2022;14(2010):1–14. doi: 10.3390/polym14102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheralayikkal S., Manoj K., Safna Hussan K.P. Formulation and evaluation of a smart drug delivery system of 5-fluorouracil for pH-sensitive chemotherapy. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witika B.A., Bassey K.E., Demana P.H., Siwe-Noundou X., Poka M.S. Current advances in specialised niosomal drug delivery: manufacture, characterization and drug delivery applications. Int. J. Mol. Sci. 2022;23(9668):1–26. doi: 10.3390/ijms23179668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeo P.L., Lim C.L., Chye S.M., Kiong Ling A.P., Koh R.Y. Niosomes: a review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2017;11(4):301–314. [Google Scholar]
- 54.Zatorska M., Łazarski G., Maziarz U., Wilkosz N., Honda T., Yusa S., Bednar J., Jamroz´ D., Kepczynski M. Drug-loading capacity of polylactide-based micro- and nanoparticles – experimental and molecular modeling study. Int. J. Pharm. 2020;591 doi: 10.1016/j.ijpharm.2020.120031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.